Significance
Previous studies localized the hedgehog (HH) signaling system to primary cilia. We discovered that motile cilia on airway epithelia also contain HH signaling proteins, indicating that like primary cilia, these motile cilia have an important sensory function. However, in contrast to the function of HH signaling in most primary cilia, sonic hedgehog (SHH) elicits noncanonical signaling, reducing cellular levels of cAMP. These findings suggest that airway SHH may quiet airway defenses. Involvement of SHH in lung disease and positioning of motile cilia where they sample SHH and other ligands in the airway lumen suggest that noncanonical HH signaling might modulate airway responses to the environment in health and disease.
Keywords: lung, cAMP, host defense, smoothened, sonic hedgehog
Abstract
Differentiated airway epithelia produce sonic hedgehog (SHH), which is found in the thin layer of liquid covering the airway surface. Although previous studies showed that vertebrate HH signaling requires primary cilia, as airway epithelia mature, the cells lose primary cilia and produce hundreds of motile cilia. Thus, whether airway epithelia have apical receptors for SHH has remained unknown. We discovered that motile cilia on airway epithelial cells have HH signaling proteins, including patched and smoothened. These cilia also have proteins affecting cAMP-dependent signaling, including Gαi and adenylyl cyclase 5/6. Apical SHH decreases intracellular levels of cAMP, which reduces ciliary beat frequency and pH in airway surface liquid. These results suggest that apical SHH may mediate noncanonical HH signaling through motile cilia to dampen respiratory defenses at the contact point between the environment and the lung, perhaps counterbalancing processes that stimulate airway defenses.
During embryogenesis and early development and shortly after seeding airway epithelial cultures, airway cells possess primary cilia (1, 2). These single cilia are essential for canonical vertebrate hedgehog (HH) signaling (3, 4). The sonic hedgehog (SHH) ligand is a morphogen that guides development of the lung, regulating pulmonary branching and mesenchyme differentiation (5–9). The receptor/effector system for the HH signaling pathway is complex and involves patched 1 (PTC1), smoothened (SMO), suppressor of fused (SUFU), and the glioma zinc-finger transcription factors GLI1, GLI2, and GLI3 (10).
After development is completed, airway epithelia continue to produce SHH (11–13). SHH is present on the basolateral side of the epithelia, where it signals to primary cilia on mesenchymal cells, restraining their proliferation (12). This paracrine pathway signals via canonical HH signaling to regulate expression of GLI1 in the mesenchyme. SHH is also present on the apical side of the epithelia in the thin layer of liquid covering the epithelial surface (11, 13, 14). However, mature airway epithelia lack primary cilia. As the epithelia polarize and mature, primary cilia disappear, and the cells develop numerous motile cilia on the apical surface (1, 2).
These observations raise the question of whether SHH has apical receptors and what their function might be. Previous studies showed that motile cilia on respiratory epithelia not only perform mechanical work, but also perform sensory functions like primary cilia (15, 16). These findings led us to hypothesize that motile cilia might participate in HH signaling. To test this hypothesis, we studied airway epithelia using human cells because of potential differences in cell-type distribution and HH signaling across species (17, 18), and because HH signaling has been reported to contribute to human lung disease, including interstitial pulmonary fibrosis, chronic obstructive pulmonary disease, and small-cell lung carcinoma (19–22). We examined differentiated primary cultures of human tracheal/bronchial epithelia grown at the air–liquid interface.
Results
HH Signaling Components Are Located in Airway Motile Cilia.
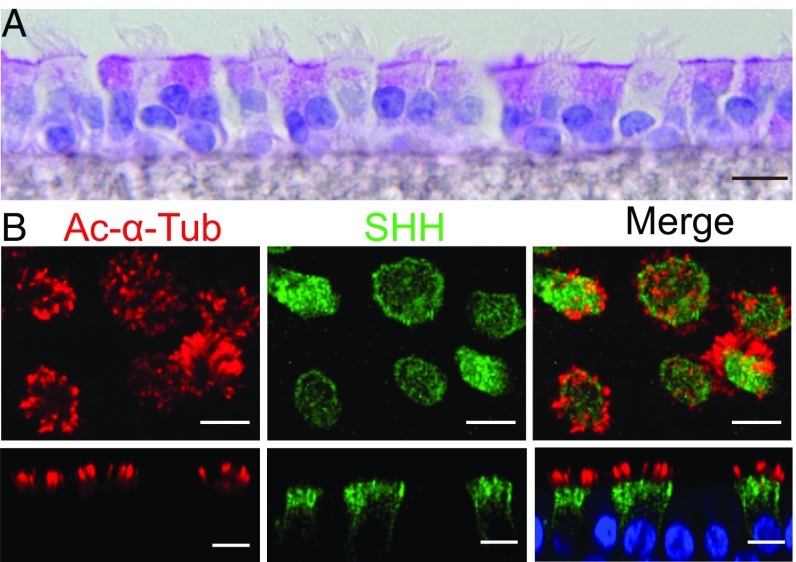
To address the hypothesis that HH signaling involves motile cilia and ciliated airway epithelial cells, we first tested for components of the HH signaling pathway. Human airway epithelia expressed mRNA transcripts for SHH (Fig. S1A). Immunostaining revealed SHH in ciliated airway epithelial cells, located beneath the apical membrane and along the upper lateral membrane (Fig. 1). We detected SHH in airway surface liquid (ASL) and basolateral medium (Fig. S1 B and C). Although the rate of basolateral secretion exceeded apical secretion, the apical SHH concentration was high because of the small volume of liquid. Whether SHH is secreted directly into the basolateral medium and whether basolateral accumulation reflects SHH movement down a concentration gradient remain unknown. These results are consistent with previous reports that ciliated airway epithelial cells produce SHH and that the ligand is present both apically and basolaterally (11–14, 20).
Fig. 1.
SHH is expressed in ciliated airway epithelial cells. Images in this and other figures are from primary cultures of differentiated human airway epithelia, except in Fig. S4, which is lung tissue. (A) Section of periodic acid Schiff-stained epithelia. (B) Immunostaining of acetylated α-tubulin (red), SHH (green), and DAPI (nuclei, blue). Upper images are stacks of X-Y confocal images, and Lower images are X-Z images. (Scale bars, 10 μm.)
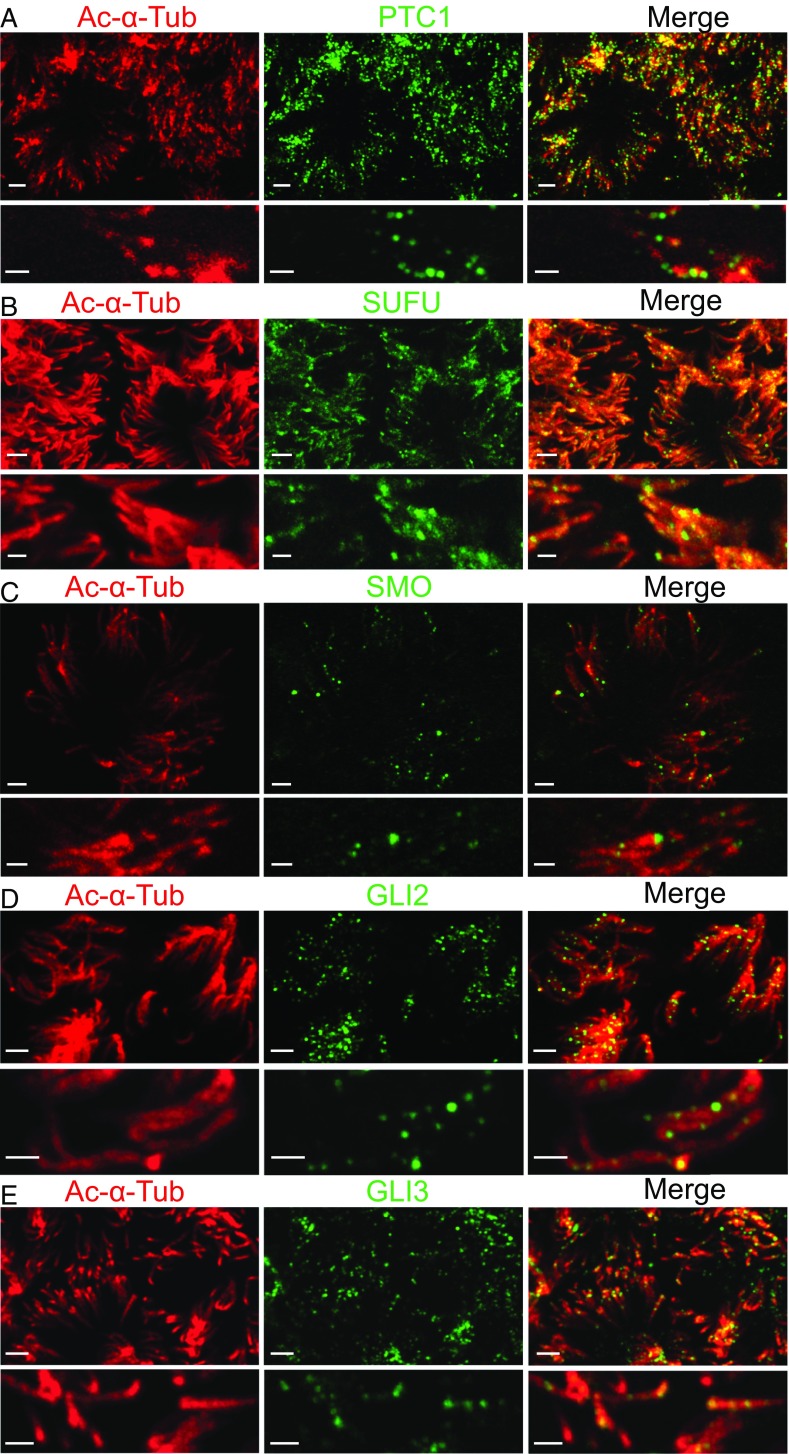
The presence of SHH in apical liquid suggested that it might signal to airway epithelia. Therefore, we tested for components of the HH signaling pathway and found transcripts of PTC1, SMO, SUFU, and the glioma zinc-finger transcription factors GLI2 and GLI3 (Fig. S1A). GLI1 transcripts were detected, but only at very low levels. Moreover, we did not detect GLI1 by immunostaining (Fig. S2 A and B), consistent with previous reports (12). Immunostaining revealed PTC1 located along the length of motile cilia (Fig. 2A and Fig. S3A), which is similar to its location in primary cilia (23). We detected lower levels of patched 2 (PTC2), also in cilia (Fig. S2 C and D). SUFU also localized to cilia, and immunostaining was most prominent distally, consistent with reports that it sits in the distal portion of primary cilia (24) (Fig. 2B and Fig. S3B). In addition, we identified SMO, GLI2, and GLI3 in motile cilia, where they displayed discrete punctate patterns (Fig. 2 C–E and Fig. S3 C–E), similar to their reported localization in primary cilia (25, 26). All of these HH signaling components were expressed in ciliated but not nonciliated cells. Overexpressed SMO confirmed its location in motile cilia (Fig. S3F). Human lung tissue also revealed PTC1, SMO, and GLI2 in motile cilia (Fig. S4 A–C).
Fig. 2.
HH signaling proteins are present in airway motile cilia. Staining of acetylated α-tubulin is red, and staining of other immunolabeled proteins is green. Immunostaining is for PTC1 (A), SUFU (B), SMO (C), GLI2 (D), and GLI3 (E). Lower images are expanded images from Upper. [Scale bars: 2 μm (Upper), 1 μm (Lower).]
Airway epithelial cells have ∼150 motile cilia; two readers counted 147 ± 7 and 160 ± 18 cilia per cell (n = 17 cells). In contrast, cells that have primary cilia have only that one cilium (Fig. S5A) (27–30). Motile cilia have axonemes with nine microtubule doublets around the circumference and two central microtubules: a “9 + 2” axoneme (1, 29). Primary cilia generally lack the two central microtubules: a “9 + 0” axoneme. Airway epithelial cilia exhibit a 9 + 2 structure (29). By transmission electron microscopy, we examined ∼10,000 airway cilia (i.e., the equivalent of ∼65 cells). We found none with a 9 + 0 axoneme (Fig. S5B). These results agree with previous observations (1, 2, 29), and we are not aware of reports that mature airway epithelia have cilia with a 9 + 0 axoneme. Therefore, it is exceedingly likely that the cilia expressing PTC1, SMO, SUFU, GLI2, and GLI3 are motile cilia with a 9 + 2 axoneme. Thus, these data suggest that motile cilia have HH signaling components that might serve as receptors and effectors for SHH in the apical airway liquid.
Airway Motile Cilia Have cAMP Signaling Components.
In canonical HH signaling, SHH binds its transmembrane receptor, PTC1, relieving inhibition of the G protein-coupled receptor SMO, which signals to GLI transcription factors (4, 25). Immunostaining has shown that PTC1 is present in unstimulated primary cilia; adding SHH drives PTC1 to exit primary cilia, SMO then enters (23), and that induces expression of GLI1 and PTC1 genes (31).
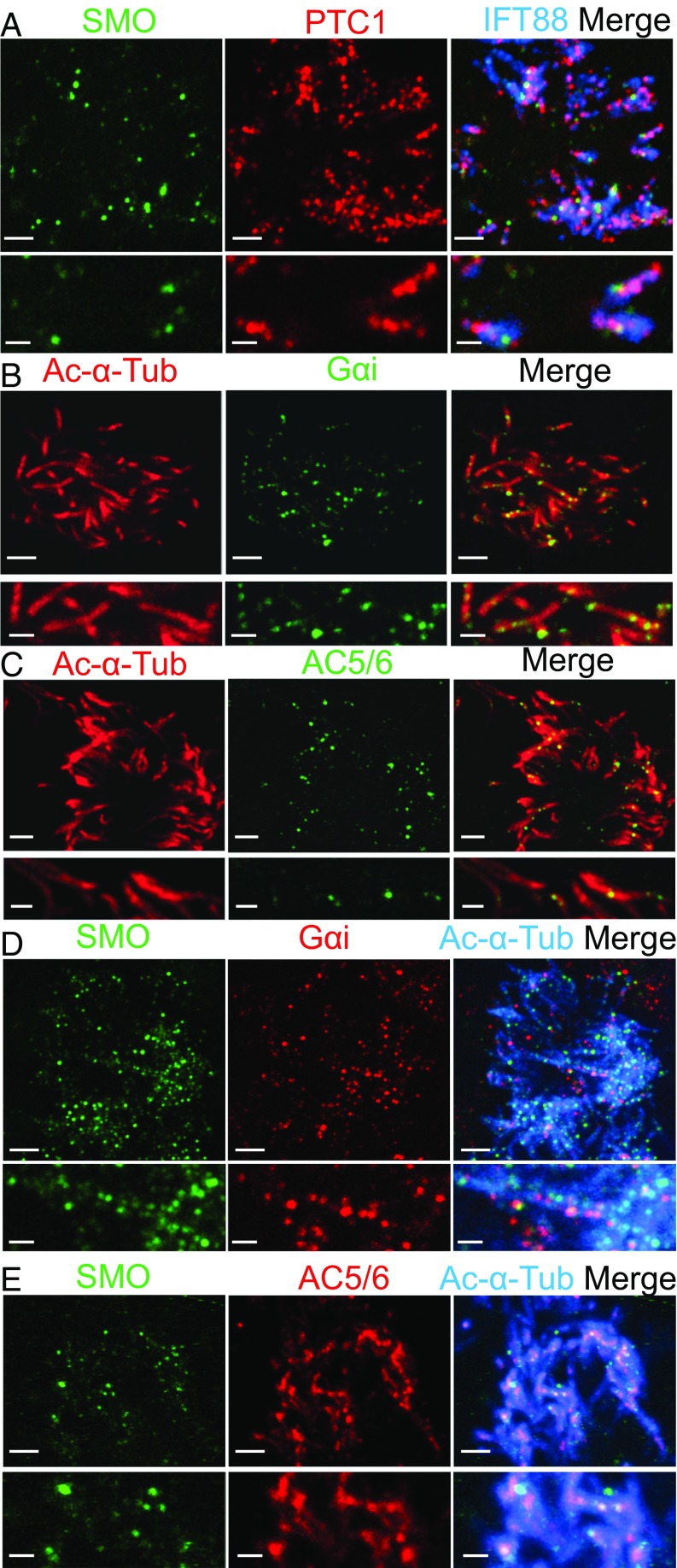
Several observations suggested that canonical HH signaling was not involved in signaling by motile cilia. First, in contrast to most primary cilia, PTC1 and SMO were simultaneously present in airway motile cilia (Fig. 3A). Second, airway cilia and ciliated cells express very little if any GLI1 (Figs. S1A and S2 A and B). Third, applying exogenous human SHH or the SMO agonist SAG did not trigger PTC1 to exit cilia (Fig. S6). Fourth, neither SHH nor SAG increased PTC1 transcripts, and GLI1 transcripts remained very low (Fig. S7A). Fifth, neither SHH, SAG, nor cyclopamine-KAAD affected GLI activity assessed with a GLI-luciferase reporter expressed in airway epithelia (Fig. S7 B and C). Thus, SHH does not appear to activate the canonical HH signaling pathway in airway epithelia.
Fig. 3.
Gαi and AC5/6 are expressed with SMO in motile cilia. (A) Staining of SMO (green), PTC1 (red), and IFT88 (cilia, blue). (B and C) Staining of acetylated α-tubulin is red, and staining of Gαi (B) and AC5/6 (C) is green. (D and E) Staining of SMO is green, staining of Gαi (D) and AC5/6 (E) is red, and staining of acetylated α-tubulin is blue. Lower images are expanded images from Upper. [Scale bars: 2 μm (Upper), 1 μm (Lower).]
SHH can also signal through a noncanonical pathway to decrease intracellular cAMP levels (32, 33). In this context, SMO couples to Gαi (32, 33), which inhibits adenylyl cyclase 5/6. Previous reports identified Gαi and adenylyl cyclase 5/6 in primary cilia (34, 35). We found Gαi and adenylyl cyclase 5/6 localized to airway cilia (Fig. 3 B and C and Fig. S4D) in the same cilia as SMO (Fig. 3 D and E). Consistent with previous reports (15), ciliated airway epithelial cells also expressed the catalytic and regulatory subunits of cAMP-dependent protein kinase (PKA), which localized to multiple cilia and the cytoplasm (Fig. S8).
SHH Signaling Dampens cAMP-Dependent Airway Defense Functions.
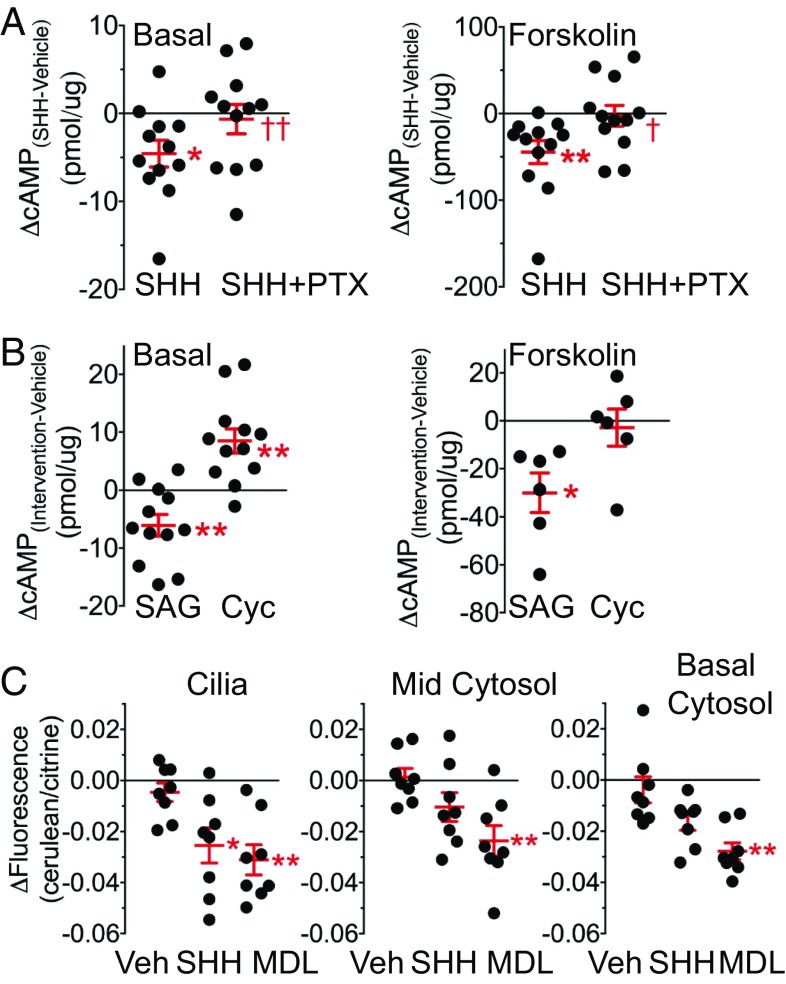
These data place SHH in a position to interact with cilia and regulate intracellular cAMP levels. To test this prediction, we measured cAMP levels in airway epithelia by ELISA. We applied a concentration in the range we observed in ASL (Fig. S1B) and previously reported in vivo (13). SHH reduced cAMP levels under basal conditions and when cAMP was increased by adding forskolin and 3-isobutyl-2-methylxanthine (IBMX) (Fig. 4A and Fig. S9). The SMO agonist SAG had a similar effect (Fig. 4B). Inhibiting Gαi with pertussis toxin (PTX) blunted the effect of SHH (Fig. 4A). In addition, the SMO inhibitor, cyclopamine-KAAD, increased cAMP levels under basal conditions, but had minimal effects when cAMP levels were elevated with forskolin (Fig. 4B).
Fig. 4.
SHH reduces cellular levels of cAMP. (A–C) Each dot is a measurement from epithelia from a different human donor. Bars and whiskers indicate mean ± SEM. (A) Data are changes in cAMP concentrations measured by ELISA in differentiated human airway epithelia. Data for basal conditions (Left) and data obtained 10 min after addition of forskolin (5 μM) and IBMX (100 μM) (Right). Data are the change in cAMP levels compared with vehicle control. For vehicle, basal cAMP level was 11 ± 2 pmol/μg, and for forskolin/IBMX, cAMP level was 219 ± 9 pmol/μg. Epithelia received apical SHH (53 nM) or SHH plus PTX (1.7 nM). n = 12. In A and B, compared with vehicle, **P < 0.01 and *P < 0.05 by paired Student’s t test. In A, compared with SHH, ††P < 0.01 and †P < 0.05 by paired Student’s t test. (B) Data are changes in cAMP concentrations compared with vehicle control following apical addition of the SMO agonist SAG (200 nM) or cyclopamine-KAAD (250 nM) under basal conditions (Left) (n = 12) or with forskolin (5 μM) and IBMX (100 μM) (Right) (n = 6). For vehicle, basal cAMP level was 15 ± 2 pmol/μg and forskolin/IBMX cAMP was 201 ± 11 pmol/μg. (C) Change in cerulean/citrine fluorescence ratio of the FRET-based cAMP sensor in ciliated cells at the level of cilia, in the midportion of the cell, and at the basal region of the cell. Measurements in nonciliated cells in the same microscopic field are in Fig. S10. Fluorescence is reported 15 min after addition of vehicle, SHH (263 nM), or MDL-12330A (100 μM, as a positive control). Each data point is the average of two to four ciliated cells in epithelia from a different donor. *P < 0.05 and **P < 0.01 compared with vehicle by one-way repeated-measures ANOVA with Sidak multiple-comparison posttest.
To test for cAMP in cilia, we expressed a genetically encoded sensor of cAMP levels that localizes to cilia and also cytoplasm (Fig. S10A and Movie S1) (36). In ciliated epithelial cells, SHH reduced cAMP levels in the region of cilia, as did an adenylyl cyclase inhibitor MDL-12330A (a positive control) (Fig. 4C) (35). SHH also tended to reduce cAMP beneath cilia in the cell; a change in cytosolic levels of cAMP might result from cAMP or Gαi diffusion. In contrast, SHH did not reduce cAMP levels in nonciliated cells (Fig. S10B). The presence of HH signaling components in ciliated but not in nonciliated cells and a SHH-induced cAMP reduction in ciliated but not nonciliated cells suggest that SHH regulates cAMP levels via airway cilia. Nevertheless, we cannot exclude some HH signaling via receptors in nonciliated cells.
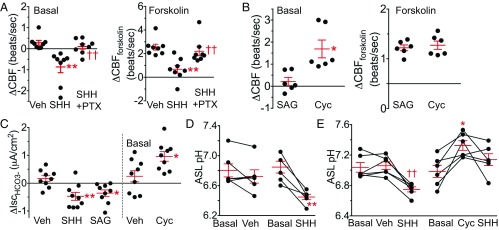
These findings suggest that HH signaling may influence physiological functions at the airway apical surface, the point of contact between the environment and the lung. Two host defenses at that site are mucociliary transport and bacterial killing by antimicrobial proteins. Previous studies showed that increasing cAMP accelerates ciliary beating (37). We found that SHH slowed cilia beat frequency (CBF), and PTX attenuated this effect (Fig. 5A). Conversely, cyclopamine-KAAD increased CBF under basal conditions (Fig. 5B).
Fig. 5.
Apical SHH reduces CBF and ASL pH. Each set of data points is from a different donor. Bars and whiskers indicate mean ± SEM. (A) Change in CBF following addition of vehicle, SHH (263 nM), and SHH plus PTX (1.7 nM) under basal conditions (Left). Data (Right) are change in CBF produced by addition of forskolin (10 μM) and IBMX (100 μM) in presence of vehicle, SHH, or SHH plus PTX. Each data point is average of two to four cells. n = 8. **P < 0.01 vs. vehicle and ††P < 0.01 compared with SHH by one-way repeated-measures ANOVA with Sidak multiple-comparison posttest. (B) Change in CBF after addition of vehicle or cyclopamine-KAAD (250 nM) (Left). Data (Right) are change in CBF produced by addition of forskolin (5 μM) and IBMX (100 μM) in the presence of vehicle or cyclopamine-KAAD. Each data point is average of two to four cells. n = 6. *P < 0.05 vs. vehicle by paired Student’s t test. (C) Change in HCO3− secretion measured as short-circuit current (∆IscHCO3-) with addition of vehicle, SHH (53 nM), and SAG (200 nM). Data on Left, n = 9, **P < 0.01 and *P < 0.05 vs. vehicle by one-way repeated-measures ANOVA with Sidak multiple-comparison posttest. Data (Right) are from a different set of airway epithelia treated with vehicle or cyclopamine-KAAD (250 nM), n = 9, *P < 0.05 by paired Student’s t test. (D) ASL pH measured before (basal) and 30 min after addition of vehicle or SHH (53 nM). Connecting lines indicate paired epithelia from an individual donor. n = 6, **P < 0.01 compared with basal by paired Student’s t test. (E) ASL pH measured before and 45 min after addition of vehicle or cyclopamine-KAAD (250 nM) followed by addition of SHH. n = 6, *P < 0.05 compared with basal and ††P < 0.01 compared with vehicle, both by one-way repeated measures ANOVA with Sidak multiple-comparison posttest.
cAMP also regulates cAMP-dependent PKA, which phosphorylates and activates apical CFTR anion channels (38). CFTR secretes HCO3−, thereby regulating ASL pH (39, 40). Decreased HCO3− secretion acidifies airway liquid, which reduces antimicrobial activity and increases mucus viscosity (40, 41). SHH and SAG reduced and cyclopamine-KAAD increased HCO3− secretion (Fig. 5C). These changes were paralleled by acidification and alkalinization, respectively, of ASL (Fig. 5 D and E).
Discussion
These findings suggest that motile airway cilia serve as a HH signaling center. Thus, in addition to propelling particulate material out of the lung, motile cilia, like primary cilia, may have a sensory function. That conclusion is consistent with previous work showing that motile cilia on airway epithelial cells express bitter taste receptors that increase the intracellular Ca2+ concentration (16). Our results also identify a noncanonical signaling pathway that reduces cAMP levels in ciliated airway epithelial cells.
The HH signaling components and cAMP-dependent signaling proteins were both present in motile cilia. That close proximity may be key for efficient signal transduction, as is the case in primary cilia (3, 42). A ciliary location also positions HH signaling proteins in an optimal location to detect SHH moving through the thin layer of liquid covering the airway surface. Thus, apical HH signaling could involve both cell-autonomous signaling (an individual ciliated cell might release SHH and detect it) and noncell autonomous signaling (SHH released by one ciliated cell might be detected by a different ciliated cell some distance away). This contrasts with basolateral HH signaling, which is paracrine and may be more locally constrained. The apical location also makes HH signaling accessible to other substances in airway liquid and to inhaled therapeutics: for example the SMO agonist, fluticasone, prescribed for asthma (43).
Previous studies reported noncanonical HH signaling mediated through primary cilia. For example, SHH signaling regulates migration of murine embryo fibroblasts and tubulogenesis of endothelial cells in a GLI1-independent manner (44, 45). Previous work in cells with primary cilia has also shown that SMO, the transducer of SHH signaling, can couple with Gi subunits (32, 33), and Gαi can inhibit adenylyl cyclase, thereby reducing cellular levels of cAMP (46).
These results may raise questions about the relationship between primary and motile cilia and HH signaling. Previous studies of vertebrate HH signaling showed that primary cilia are essential for canonical HH signaling (3, 4, 47). Our results now suggest a role for motile cilia in noncanonical HH signaling. In addition, a recent report suggested the HH signaling components localize odorant receptors in mouse olfactory cilia (48); olfactory cilia have a 9 + 2 axoneme, but lack dynein arms and are immotile (30). Thus, during evolution, HH signaling components and cilia may have been adapted for a variety of functions, with variations in cilia type and the function of HH signaling components. In this regard, SUFU, GLI2, and GLI3 are essential regulators in the canonical HH signaling pathway (49, 50), but their potential function in motile airway cilia remains unknown.
Airway injury and chronic lung diseases induce a variety of inflammatory, proliferative, and defense processes (51, 52). They can also increase SHH levels (22, 51, 52). Previous studies showed that SHH in the basolateral compartment may restrain responses to injury by reducing mesenchymal cell proliferation (12). Similarly, SHH in the apical compartment might restrain, at least in part, respiratory defense responses initiated by injury and disease. By reducing intracellular levels of cAMP, SHH might dampen defenses, including cilia beating and CFTR-mediated anion secretion. However, whereas HH signaling in mesenchymal cells is through primary cilia and the canonical HH signaling pathway, in epithelial cells it is through motile cilia and a noncanonical pathway.
Materials and Methods
SI Materials and Methods contains a detailed description of the materials and methods used.
Primary cultures of differentiated human airway epithelia were prepared from trachea and bronchi of nonsmokers, seeded onto collagen-coated semipermeable membranes, grown at the air–liquid interface, and studied after they had differentiated and at least 14 d after seeding (53).
We used standard immunocytochemistry methods. Primary and secondary antibodies are listed in Table S1. Samples were imaged by confocal microscopy and analyzed with NIH Fiji software. All studies were performed at least four times using epithelia prepared from different donors. Studies were approved by the University of Iowa Institutional Review Board.
Methods for transmission electron microscopy and quantitative RT-PCR (Table S2) were standard. The SHH assay used a reporter cell line. cAMP concentrations were measured by ELISA and with a FRET-based cAMP assay (36). CBF was measured with transmitted light line-scans. ASL pH and transepithelial HCO3− secretion were measured as previously described (40, 41).
Statistical significance was tested with an unpaired or paired Student’s t test for comparisons between two samples. For comparisons between more than two samples, statistical significance was tested with a one-way repeated-measures ANOVA with Sidak multiple-comparison posttest. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Lokhoy Brecht and Mary Kenyon for help in preparing the manuscript; and Dr. Andy McMahon (University of Southern California) and Dr. Dagmar Wachten (Münster, Germany) for providing A1::SMO::GFP and cAMP sensor constructs, respectively. This work was supported in part by the National Institutes of Health (Grants HL09184 and HL51670), a Cystic Fibrosis Foundation Research Development Program, and the Roy J. Carver Charitable Trust. M.J.W. is an Investigator of the HHMI.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719177115/-/DCSupplemental.
References
- 1.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 2.Jain R, et al. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43:731–739. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, et al. The role of ciliary trafficking in hedgehog receptor signaling. Sci Signal. 2015;8:ra55. doi: 10.1126/scisignal.aaa5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellusci S, et al. Involvement of sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 7.White AC, et al. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- 8.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 9.Li C, et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Briscoe J, Thérond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 11.Cigna N, et al. The hedgehog system machinery controls transforming growth factor-β-dependent myofibroblastic differentiation in humans: Involvement in idiopathic pulmonary fibrosis. Am J Pathol. 2012;181:2126–2137. doi: 10.1016/j.ajpath.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Peng T, et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526:578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henkin RI, Hosein S, Stateman WA, Knöppel AB, Abdelmeguid M. Improved smell function with increased nasal mucus sonic hedgehog in hyposmic patients after treatment with oral theophylline. Am J Otolaryngol. 2017;38:143–147. doi: 10.1016/j.amjoto.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Henkin RI, Abdelmeguid M, Knöppel AB. On the mechanism of smell loss in patients with type II congenital hyposmia. Am J Otolaryngol. 2016;37:436–441. doi: 10.1016/j.amjoto.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Schmid A, et al. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130:99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Wang H, Teng H, Shi J, Zhang Y. Expression of SHH signaling pathway components in the developing human lung. Histochem Cell Biol. 2010;134:327–335. doi: 10.1007/s00418-010-0738-2. [DOI] [PubMed] [Google Scholar]
- 19.Watkins DN, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 20.Bolaños AL, et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, et al. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet. 2012;21:1325–1335. doi: 10.1093/hmg/ddr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kugler MC, Joyner AL, Loomis CA, Munger JS. Sonic hedgehog signaling in the lung. From development to disease. Am J Respir Cell Mol Biol. 2015;52:1–13. doi: 10.1165/rcmb.2014-0132TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, et al. Dual phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. J Biol Chem. 2011;286:13502–13511. doi: 10.1074/jbc.M110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 26.Kiprilov EN, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singla V, Reiter JF. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 28.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 29.Takeda S, Narita K. Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation. 2012;83:S4–S11. doi: 10.1016/j.diff.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Choksi SP, Lauter G, Swoboda P, Roy S. Switching on cilia: Transcriptional networks regulating ciliogenesis. Development. 2014;141:1427–1441. doi: 10.1242/dev.074666. [DOI] [PubMed] [Google Scholar]
- 31.Chuang PT, McMahon AP. Vertebrate hedgehog signalling modulated by induction of a hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 32.Ogden SK, et al. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456:967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen F, Cheng L, Douglas AE, Riobo NA, Manning DR. Smoothened is a fully competent activator of the heterotrimeric G protein G(i) Mol Pharmacol. 2013;83:691–697. doi: 10.1124/mol.112.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci USA. 2011;108:4482–4487. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore BS, et al. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc Natl Acad Sci USA. 2016;113:13069–13074. doi: 10.1073/pnas.1602393113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee S, et al. A novel biosensor to study cAMP dynamics in cilia and flagella. eLife. 2016;5:e14052. doi: 10.7554/eLife.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Benedetto G, Manara-Shediac FS, Mehta A. Effect of cyclic AMP on ciliary activity of human respiratory epithelium. Eur Respir J. 1991;4:789–795. [PubMed] [Google Scholar]
- 38.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1 Suppl):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 39.Shah VS, et al. Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc Natl Acad Sci USA. 2016;113:5382–5387. doi: 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang XX, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mick DU, et al. Proteomics of primary cilia by proximity labeling. Dev Cell. 2015;35:497–512. doi: 10.1016/j.devcel.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, et al. Identification of select glucocorticoids as Smoothened agonists: Potential utility for regenerative medicine. Proc Natl Acad Sci USA. 2010;107:9323–9328. doi: 10.1073/pnas.0910712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipinski RJ, Bijlsma MF, Gipp JJ, Podhaizer DJ, Bushman W. Establishment and characterization of immortalized Gli-null mouse embryonic fibroblast cell lines. BMC Cell Biol. 2008;9:49. doi: 10.1186/1471-2121-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–579. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 46.Sabbatini ME, Gorelick F, Glaser S. Adenylyl cyclases in the digestive system. Cell Signal. 2014;26:1173–1181. doi: 10.1016/j.cellsig.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maurya DK, Bohm S, Alenius M. Hedgehog signaling regulates ciliary localization of mouse odorant receptors. Proc Natl Acad Sci USA. 2017;114:E9386–E9394. doi: 10.1073/pnas.1708321114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Persson M, et al. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pogach MS, Cao Y, Millien G, Ramirez MI, Williams MC. Key developmental regulators change during hyperoxia-induced injury and recovery in adult mouse lung. J Cell Biochem. 2007;100:1415–1429. doi: 10.1002/jcb.21142. [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Jin Y, Hou X, Liu F, Wang Y. Sonic hedgehog signaling: Evidence for its protective role in endotoxin induced acute lung injury in mouse model. PLoS One. 2015;10:e0140886. doi: 10.1371/journal.pone.0140886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karp PH, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.