Abstract
The aim of this study was to examine the effect of microfiltered and sterilized seawater ingestion on running performance in a hot environment. This cross-over, double-blind randomized trial included 12 experienced male runners. The subjects randomly consumed seawater (SW) or pure water (placebo) in an equivalent amount of 50 ml five minutes prior to running at 40% of their VO2 max for 95.0 ± 18.5 min, at 30°C, until they lost 3% of body weight. Every 20 minutes, a measurement of their body weight was taken and a blood lactate analysis was performed. The concentration of lactate was significantly lower after the running exercise in the SW condition compared to placebo. The results of this study provide evidence supporting the ergogenic effects of microfiltered and sterilized seawater ingestion on running performance and lactate production.
Keywords: Seawater, Sports performance, Fatigue, Runners
INTRODUCTION
Exercise performance can be impaired in a hot environment. The effort and hot temperature increase the metabolic rate and heat production and cause dehydration [1]. Exercise in hot temperature increases the risk of dehydration. The loss of body water resulting in a reduction of blood volume and impaired thermoregulatory function is a major problem encountered by athletes during prolonged exercise. González-Alonso et al. demonstrated that the superimposition of dehydration on hyperthermia during exercise in the heat causes an inability to maintain cardiac output and blood pressure that makes the dehydrated athlete less able to cope with hyperthermia [2]. Dehydration induced by exercise or hot temperature exposure can reduce aerobic performance [3] and impair cognitive function [4]. A recent study provided results confirming that exercise performance in the heat is impaired by hypohydration [5].
Hypohydration is defined as a body water deficit greater than normal daily fluctuation [6] or simply as body water losses of >2% of body mass [7]. The changes in body mass are recognized as the most sensitive and simplest measure to determine acute changes in body water for different kinds of dehydration [6,8]. Adequate fluid intake can reduce or prevent some of the disturbances in metabolic, cardiovascular and thermoregulatory functions as well as performance that follows dehydration. In endurance runners there are different strategies for coping with dehydration [9]. A difference in fluid absorption is a potential source of the exercise impairment seen in the dictated fluid condition. The higher fluid consumption rate presumably would cause greater gastric and oesophageal distention resulting in the diversion of blood flow from working muscles to the gastrointestinal system [10]. Adequate fluid intake can attenuate or prevent many of the disturbances in metabolic, cardiovascular and thermoregulatory functions, as well as performance, that accompany dehydration.
The interest in smaller samples of dedicated fluid for exercising athletes seems to be justified. One new source enhancing performance or recovery could be ocean or sea mineral water [11–14]. It was found that ingestion of deep mineral water could accelerate recovery of aerobic capacity and leg muscle power compared with ingestion of water alone [12]. When compared to typical table water, seawater contains increased amount of minerals such as calcium, magnesium, sodium and potassium, as well as microelements such as cobalt, selenium, chromium and vanadium. For this reason, seawater has received increasing attention from the pharmacological point of view, in order to improve protection of the cardiovascular system, the improvement of metabolic processes, treatment of skin lesions and acceleration in recovery from fatigue. Numerous studies have confirmed that seawater or ocean mineral water has pharmacological effects, including protecting the cardiovascular system, improving metabolic processes, anti-osteoporosis properties, treating atopic dermatitis skin lesions, accelerating recovery from physical fatigue, providing intestinal protection against duodenal ulcers and inhibiting the metastatic potential of human breast cancer cell lines [15]. Despite the increasing number of studies on the effects of administration of seawater on exercise performance, it is still not clear what impact it could have and whether it has favourable effects in athletes or physically active individuals. The aim of this study was to examine the effect of microfiltered and sterilized seawater ingestion on running performance in a hot environment in physically active men.
MATERIALS AND METHODS
Subjects
Twelve experienced men runners (age 32 ± 6.4 years, height 176.5 ±5.5 cm, weight 70.3 ±6.7 kg; VO2max 62.5 ± 4.8 ml • kg−1 • min−1) took part in the study, excluding all nutritional supplements, alcohol intake and tobacco. Both the purpose and the experimental procedure were explained, with informed written consent. The current study was approved by the Ethics Committee of Kinetic Performance Co. (Scientific Park, University of Alicante, Spain) and carried out in accordance with the principles of the Declaration of Helsinki.
During the first meeting, subjects were introduced to the objectives and the design of the study, and they were also instructed in regimes of diet and rest. Subjects were asked not to do any other physical activity during the recovery period and, on the eve of the test, they were instructed to maintain their diet and normal consumption during both trials. In order to achieve this, a 72 h food survey was completed during the first trial, and subjects were asked to follow the same diet during the second trial. The aerobic capacity was tested during a continuous incremental test until exhaustion of not less than 5 days before (VO2max 62.5 ± 4.8 ml • kg−1 • min−1 45.8 ± 8.4 ml kg-1 min-1). Subjects were previously analysed through the ISAK method (International Society for the Advancement of Kinanthropometry), to determine body mass and height, and body composition.
Experimental design
In order to control the possible confounding effect of individual variation, a random double-blind design was applied, with trials separated 7 days from each other. Five minutes before the exercise protocol started, the subjects consumed seawater or pure water in an equivalent amount of 50 ml. In this study the protocol proposed by Hou et al. [16] was applied. Subjects were asked to run on a motorized treadmill at 40% VO2max at room temperature of 30°C until attaining a 3% decrease in body mass. The average speed was 7.4 ± 0.7 km h-1, and the average time of exercise was 95.0 ± 18.5 min. Measurements of body mass and blood lactate concentration were taken every 20 minutes. At the end of the exercise protocol, venous blood samples were analysed to detect the biochemical variables.
Subjects consumed randomly selected samples of water without being informed about the type of water. The microfiltered and sterilised seawater “Totum Sport” was provided by Laboratoires Quinton Co. (Alicante, Spain). This water has been extracted from specific areas of the Atlantic Ocean, in the Bay of Biscay, at a distance of more than 10 miles from the coast and 20 meters deep, at the centre of proliferations or plankton blooms. Once the water is extracted and, in order to guarantee the cold chain, it is transported to the laboratories at low temperatures where, after an initial physical-chemical and microbiological analysis, it follows a double cold microfiltration process in a white room, respecting the regulations established by the European Pharmacopoeia. Immediately after, the finished product is reanalysed and packaged under aseptic conditions.
Physical performance
The maximal oxygen consumption (VO2max) was determined before intervention. VO2max was measured using the incremental stress test. This test comprised a warm-up of 4 minutes followed by a continuous increase in speed of 0.1 km/h each 6 s until volitional fatigue. The criteria used to verify that VO2max was achieved were: a respiratory exchange ratio greater than 1.1, a maximum heart rate of 220 ± 10 beats per minute and a plateau in oxygen uptake with an increasing workload (all criteria had to be met). Samples of expired gases were analyzed using a portable analyser (Metalyzer Sport, CORTEX Biophysik GmbH, Leipzig, Germany). Before each test, the equipment was calibrated according to the manufacturer’s recommendations.
Biochemical analysis
The blood lactate concentration was measured and collected with a capillary blood extraction, which was analysed in order to measure the lactate concentration using the measurement principle (LOD enzyme electrode method), with a lactate analyser (Lactate Pro2, Germany). Samples were assessed by staff of the Immunological Centre of the Valencian Community (Alicante, Spain).
Statistical analysis
The statistical approach used for this study closely follows the one described by Byron and Kenward [17]. Let xijk and y ijk denote the baseline and post-treatment lactate for the kth subject in the ith group within period j (k=1,…,6, i=1,2, and j=1,2). It is assumed that the expected values of the observations will follow the linear model defined in Table 1, where: the parameter μ represents the overall mean; π a period effect; γ a group effect; θ a first order carry-over effect (that is a difference in treatment carry over at the time of the second baseline measurement); λ any direct-by-period interaction that might also be due to a second order carry-over effect (i.e. to difference in carry over at the time of the second post-treatment measurement); and, finally, τ represents the direct treatment effect.
TABLE 1.
Expected values of lactate by group.
| Group 1 (P-SW) | Group 2 (SW-P) |
|---|---|
| E[x11k]= μ − γ − π | E[x21k]= μ + γ − π |
| E[y11k]= μ − γ − π − τ | E[y21k]= μ + γ − π + τ |
| E[x12k]= μ − γ + π − θ | E[x22k]= μ + γ + π + θ |
| E[y12k]= μ − γ + π + τ − λ | E[y22k]= μ + γ + π − τ + λ |
According to the proposed modelling, on average, intake of SW reduces the level of lactate in an athlete after exercise (that is, at dehydration or at 3% weight loss) by τd=-2τ. Our goal is to estimate τd=-2τ, the difference between the direct treatment effect of the placebo and SW. Next we estimate τd in 3 steps: step 1, estimation of the first order carry-over effect θ ; step 2, estimation of the direct-by-period interaction λ; and step 3, estimation of the direct treatment effect τ. At each step a two-sample t-test (or a non-parametric test) and corresponding confidence interval are obtained for the quantity of interest using a suitable contrast and conditioning on the results of the previous steps (for steps 2 and 3).
RESULTS
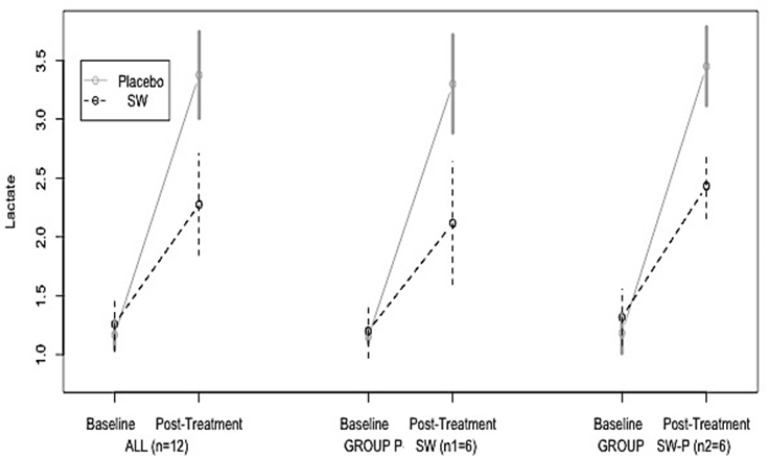
The data of the study (expressed as mean ± one standard deviation) by group and treatment are shown in Table 2 and represented in Figure 1.
TABLE 2.
Lactate at baseline and after exercise (post-treatment) by treatment and group.
| ALL subjects (n=12) | PLACEBO | Lactate |
| Baseline | 1.167 ± 0.123 | |
| Post-treatment | 3.375 ± 0.367 | |
| WS | Lactate | |
| Baseline | 1.258 ± 0.231 | |
| Post-treatment | 2.275 ± 0.433 | |
| GROUP P-SW (n=6) | PLACEBO | Lactate |
| Baseline | 1.150 ± 0.055 | |
| Post-treatment | 3.300 ± 0.415 | |
| WS | Lactate | |
| Baseline | 1.200 ± 0.228 | |
| Post-treatment | 2.117 ± 0.523 | |
| GROUP SW (n=6) | PLACEBO | Lactate |
| Baseline | 1.853 ± 0.172 | |
| Post-treatment | 3.450 ± 0.333 | |
| WS | Lactate | |
| Baseline | 1.317 ± 0.240 | |
| Post-treatment | 2.433 ± 0.280 |
FIG. 1.
Lactate at baseline and after exercise (that is, at dehydration or at 2% weight loss) for placebo and WS for all subjects in the study and separately for the P-SW and the SW-P group. Results are presented as mean ± one standard deviation.
We start by estimating θ using half of the difference between the two baseline measurements from each subject 0.5*(x i1k-x i2k). The mean of such quantities is then compared between the two groups (group 1: P-SW) and (group 2: SW-P) using a two-sample t-test and a 90% confidence interval. From R output we find that the estimate of θ is −0.0917 with a standard error for 10 degrees of freedom of 0.0569. The 90% confidence interval for θ is (-0.191, 0.008) with a two-sample t-test p-value equal to 0.1279, indicating that the first order carry-over effect is not statistically significant even at the 0.1 level (i.e. there is no evidence of difference in treatment carry over at the time of the second baseline measurement).
Assuming θ=0 (i.e. assuming no first order carry-over effect) we next estimate the direct treatment-by-period interaction λ using half of the sum of the subjects change with respect to the baseline, 0.5*[ (y i1k-x i1k) +(y i2k-x i2k)]. Once again, the mean of such quantities is compared between the two groups (P-SW) and (SW-P) using a two-sample t-test and a 90% confidence interval. The point estimate of λ is 0.1583 with a standard error for 10 degrees of freedom of 0.195. The 90% confidence interval for λ is (-0.183, 0.5) with a two-sample t-test p-value equal to 0.4294, indicating that the direct treatment-by-period interaction is not statistically significant even at the 0.1 level (i.e. there is no evidence of direct treatment-by-period interaction).
Finally, assuming θ=λ=0 (i.e. assuming no first order carry-over effect and no direct treatment-by-period interaction) we estimate τ using one fourth of the difference between the two post-treatment measurements from each subject 0.25*(y i2k-y i1k). The point estimate of τ is -0.55 with a standard error for 10 degrees of freedom of 0.0422. The 95% confidence interval for τ is (-0.644 -0.456) with a two-sample t-test p-value smaller than 0.00001, indicating a significant direct treatment effect at the significant level 0.05. Multiplying by minus two we find that the 95 %confidence interval for τd=- 2τ is (0.912,1.288), i.e. with 95% confidence we can say that on average, intake of SW reduces the level of lactate by at least 0.912.
The normality assumptions required by the two-sample t-test and the confidence intervals used to estimate θ, λ and τ were checked using the Shapiro-Wilk test. As an additional assessment of the robustness of the results obtained with respect to the assumption of normality we repeated the analysis using nonparametric tests, obtaining very similar results (not shown) [9].
Robustness with respect to model choice was also addressed, comparing the estimate of τd under the proposed model with the corresponding estimates under alternative models discussed [10]. In particular we compared the estimate of τd under the proposed model (which assuming θ=λ=0 corresponds to model I in [10] with 5 other models (models II, III, IV, V and VI) discussed in the same paper and which make different uses of baseline measurements as the covariate (for a detailed discussion of the alternative models and additional references see [9]). We found that (results not shown) the point estimates of τd and the corresponding standard deviation are very similar under the different models and that the proposed model outperforms the competing models in terms of standard deviation.
DISCUSSION
The aim of this study was to examine the effect of microfiltered and sterilized seawater (SW) ingestion on running performance in a hot environment. The most important finding of this study is that the concentration of lactate was significantly lower after the running exercise in the SW condition compared to pure water. The results of this study suggest the ergogenic effects of microfiltered and sterilized seawater ingestion on running performance and lactate production. This seawater has been extracted from specific areas of the Atlantic Ocean, in the Bay of Biscay, at a distance of more than 10 miles from the coast and 20 meters deep, at the centre of proliferations or plankton blooms. The key mechanism of consuming SW before exercise contributing to the observed ergogenic benefits is not exactly known. It could be supposed that the minerals and trace elements in SW may work cooperatively to maintain exercise performance during hypohydrated conditions.
Sawka et al. [7] stated that hypohydration does not impair submaximal intensity aerobic performance in cold-cool environments, sometimes impairs aerobic performance in temperate environments, and usually impairs aerobic performance in warm-hot environments. A commonality of absolute hypovolaemia (from plasma volume loss) combined with relative hypovolaemia (from tissue vasodilation) is present when aerobic performance is impaired. Hypohydration could impair aerobic exercise performance through multiple physiological mechanisms. These mechanisms include cardiovascular, elevated tissue temperatures, and metabolic changes, which are all integrated through the central nervous system to reduce motor drive to skeletal muscles [7]. In endurance athletes hypohydration may adversely affect the lactate threshold. In some previous studies hypohydration did not significantly alter cardiovascular function or buffering capacity but did cause the lactate threshold to occur at a lower absolute exercise intensity [18]. In the study described by Shou and Ishiko [19] a significant increase in blood lactate concentration during an incremental bicycle exercise under a hot dry environment was observed. Results confirming these were also presented by Powers et al. [20]. Some studies showed the contradictory findings that blood lactate concentrations remained unchanged in a hot environment. Francesconi et al. [21] noted that levels of lactate dehydrogenase, glutamate-oxaloacetate transaminase, and glutamate-pyruvate transaminase were not significantly affected by exercise in the heat either before or after heat acclimatization.
The accumulation of blood lactate acid in muscle and blood is one of the main causes of physical fatigue [22]. The lactate-induced acidosis theory posits that under hypoxic conditions, such as anaerobic exercise, there is increased dissociation of lactic acid into lactate ions and hydrogen (H+) entering skeletal myocytes [23]. This process induces reduction of the internal pH, disrupting the cross-bridge cycle and impairing the contractile capability of such cells, indicative of lactate’s involvement in muscular fatigue. This may also cause an aching muscle pain. The lactate threshold is clearly related to endurance performance potential. It seems that dehydration may alter the lactate threshold. Low levels of dehydration induced a shift in the lactate threshold, in part because of elevated epinephrine concentrations [24]. This shift may have been one cause of the decrease in time to exhaustion for the dehydrated trial. In the study by Moquin and Mazzeo [24], it was observed that the lactate threshold occurred at a significantly lower relative percent of VO2max for the dehydrated trial (72.2 +/- 1.1% for normally hydrated state; 65.5 +/- 1.8% for a dehydrated state gained with 2.6% reduction in body mass) as well as a lower absolute power output when compared with that in the hydrated trial. Thus, preventing dehydration should be reflected by an unincreased blood lactate concentration, which was observed in the present study.
Some previous studies have demonstrated an effect of SW in reducing fatigue in rats and humans, and a possible mechanism responsible for this effect was discussed [16,25]. It should be noted that a great quantity of electrolytes such as sodium, potassium, chlorine, and magnesium will be lost through perspiration during prolonged running [25–27]. Therefore, probably drinking SW rich in minerals can help in maintaining levels of various electrolytes using during exercise and re-establish normal functions of the human body.
Limitations
Despite the use of commercially available solutions and mineral waters to assess their influence on rehydration and running performance in several studies, it is difficult to compare the data because of differences in the magnitude of dehydration and study designs. Also, the conclusion of this study could not be generalized as water sample composition, minerals and trace elements are related to the geographic specificity of seawater and depth of the sea reservoir.
Future research should investigate the effects of different kinds of sea or ocean mineral water and determine the possible mechanism of influence on the human body. Also, hypohydration has an additive effect on impairing aerobic performance in warm-hot high-altitude environments, and it could be interesting to determine the effects of administration of seawater on counteracting the decline in performance in such conditions. On the other hand, it should be investigated whether seawater enhances prolonged exercise performance under non-dehydrating conditions.
CONCLUSIONS
The concentration of lactate was significantly lowered after the running exercise in the seawater condition compared to pure water administration. The results of this study provide evidence supporting the ergogenic effects of microfiltered and sterilized seawater ingestion on running performance and lactate production. Further studies on physiological mechanisms affecting human performance and differences in seawater products are needed.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Tucker R, Rauch L, Harley YR, Noakes T. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflgers Arch - Eur J Physiol. Springer-Verlag. 2004;448(4):422–30. doi: 10.1007/s00424-004-1267-4. [DOI] [PubMed] [Google Scholar]
- 2.González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol. 1997;82(4):1229–36. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- 3.Barr SI. Effects of dehydration on exercise performance. Can J Appl Physiol. 1999;24(2):164–72. doi: 10.1139/h99-014. [DOI] [PubMed] [Google Scholar]
- 4.Nuccio RP, Barnes KA, Carter JM, Baker LB. Fluid Balance in Team Sport Athletes and the Effect of Hypohydration on Cognitive, Technical, and Physical Performance. Sports Med. Springer. 2017;47(10):1951–82. doi: 10.1007/s40279-017-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James LJ, Moss J, Henry J, Papadopoulou C, Mears SA. Hypohydration impairs endurance performance: a blinded study. Physiol Rep. 2017;5(12):e13315. doi: 10.14814/phy2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheuvront SN, Kenefick RW. Comprehensive Physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2014. Dehydration: Physiology, Assessment, and Performance Effects; pp. 257–85. [DOI] [PubMed] [Google Scholar]
- 7.Sawka MN, Cheuvront SN, Kenefick RW. Hypohydration and Human Performance: Impact of Environment and Physiological Mechanisms. Sport Med. 2015;45(S1):51–60. doi: 10.1007/s40279-015-0395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheuvront SN, Kenefick RW, Charkoudian N, Sawka MN. Physiologic basis for understanding quantitative dehydration assessment. Am J Clin Nutr. 2013;97(3):455–62. doi: 10.3945/ajcn.112.044172. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman MD, Stuempfle KJ. Hydration Strategies, Weight Change and Performance in a 161 km Ultramarathon. Res Sport Med. 2014;22(3):213–25. doi: 10.1080/15438627.2014.915838. [DOI] [PubMed] [Google Scholar]
- 10.Backes TP, Fitzgerald K. Fluid consumption, exercise, and cognitive performance. Biol Sport. 2016;33(3):291–6. doi: 10.5604/20831862.1208485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsuda S-I, Yasukawa T, Nakagawa K, Miyake M, Yamasaki M, Katahira K, Mohri M, Shimizu T, Hazama A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi- Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull. 2008;31(1):38–44. doi: 10.1248/bpb.31.38. [DOI] [PubMed] [Google Scholar]
- 12.Stasiule L, Capkauskiene S, Vizbaraite D, Stasiulis A. Deep mineral water accelerates recovery after dehydrating aerobic exercise: a randomized, double-blind, placebo-controlled crossover study. J Int Soc Sports Nutr. 2014;11(1):34. doi: 10.1186/1550-2783-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Liu H, Yang X, Li C, Guan H. What depth should deep-sea water be pumped up from in the South China Sea for medicinal research? J Ocean Univ China. 2013;12(1):134–8. [Google Scholar]
- 14.Wang M-L, Chen Y-J, Cheng F-C. Nigari (deep seawater concentrate) enhances the treadmill exercise performance of gerbils. Biol Sport. 2014;31(1):69–72. doi: 10.5604/20831862.1086735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan H, Tan Z, Hua Y, Huang X, Gao Y, Wu Y, Liu B, Zhou Y. Deep sea water improves exercise and inhibits oxidative stress in a physical fatigue mouse model. Biomed Reports. 2016;4(6):751–7. doi: 10.3892/br.2016.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou C-W, Tsai Y-S, Jean W-H, Chen C-Y, Ivy JL, Huang C-Y, Kuo C-H. Deep ocean mineral water accelerates recovery from physical fatigue. J Int Soc Sports Nutr. 2013;10(1):7. doi: 10.1186/1550-2783-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. In: Byron Jones Michael G., editor. Boca Raton: Taylor & Francis Group, LLC; 2014. Kenward - Google Książki [Internet] [Google Scholar]
- 18.Kenefick RW, Mahood N V, Mattern CO, Kertzer R, Quinn TJ. Hypohydration adversely affects lactate threshold in endurance athletes. J strength Cond Res. 2002;16(1):38–43. [PubMed] [Google Scholar]
- 19.Shou G-C, Ishiko T. The effect of different environmental conditions on blood lactate accumulation, LT and OBLA during incremental exercise. Japanese J Phys Fit Sport Med. The Japanese Society of Physical Fitness and Sports Medicine. 1994;43(1):58–65. [Google Scholar]
- 20.Powers SK, Howley ET, Cox R. Blood lactate concentrations during submaximal work under differing environmental conditions. J Sports Med Phys Fitness. 1985;25(3):84–9. [PubMed] [Google Scholar]
- 21.Francesconi RP, Maher JT, Bynum GD, Mason JW. Recurrent heat exposure: enzymatic responses in resting and exercising men. J Appl Physiol. 1977;43(2):308–11. doi: 10.1152/jappl.1977.43.2.308. [DOI] [PubMed] [Google Scholar]
- 22.White GE, Wells GD. The effect of on-hill active recovery performed between runs on blood lactate concentration and fatigue in alpine ski racers. J strength Cond Res. 2015;29(3):800–6. doi: 10.1519/JSC.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 23.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Integr Comp Physiol. 2004;287(3):R502–16. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 24.Moquin A, Mazzeo RS. Effect of mild dehydration on the lactate threshold in women. Med Sci Sports Exerc. 2000;32(2):396–402. doi: 10.1097/00005768-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Hwang D, Chen R, Chen Y, Liang C, Lin C, Tsai M. Effect of deep sea water on the exercise-induced fatigue of rats. J Food Drug Anal. 2009;17(2):133–41. [Google Scholar]
- 26.Del Coso J, Salinero JJ, Lara B, Abián-Vicén J, Gallo-Salazar C, Areces F. A comparison of the physiological demands imposed by competing in a half-marathon vs. a marathon. J Sports Med Phys Fitness. 2017;57(11):1399–406. doi: 10.23736/S0022-4707.17.07056-6. [DOI] [PubMed] [Google Scholar]
- 27.Lara B, Gallo-Salazar C, Puente C, Areces F, Salinero JJ, Del Coso J. Interindividual variability in sweat electrolyte concentration in marathoners. J Int Soc Sports Nutr. 2016;13(1):31. doi: 10.1186/s12970-016-0141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]