Abstract
This study was designed to investigate the effects of exogenous androgen and resistance exercise on skeletal muscle hypertrophy and the role of the mammalian target of rapamycin (mTOR) signalling during the process. A total of 24 male Sprague-Dawley rats were randomly assigned to sham operation and dihydrotestosterone (DHT) implantation groups with subgroups subjected to sedentary conditions or resistance exercise (SHAM+SED, SHAM+EX, DHT+SED, and DHT+EX). The experimental procedure lasted for 10 days. The mRNA expression of androgen receptor (AR) and insulin-like growth factor I (IGF-I), the expression of myosin heavy chain (MHC), as well as the phosphorylation statuses of AR, mTOR, p70 ribosomal S6 kinase (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) were determined in the white gastrocnemius muscle. The cross sectional area and wet mass of the muscle were also measured. The cross sectional area and MHC expression were significantly higher in SHAM+EX, DHT+SED, and DHT+EX than in SHAM+SED. There was no significant difference among groups in muscle mass. The mRNA expression of AR and IGF-I and the phosphorylation of mTOR, p70S6K, and 4EBP1 were significantly increased in DHT+SED and SHAM+EX and were significantly enhanced in DHT+EX compared with either DHT or exercise alone. These data show that DHT causes hypertrophy in skeletal muscle and that exercise has a synergistic effect on DHT-induced hypertrophy. Exercise enhances androgen-induced rapid anabolic action, which involves activation of the mTOR pathway.
Keywords: Muscle hypertrophy, mTOR, Exercise, Androgen
INTRODUCTION
Muscle atrophy, caused by aging, lack of physical activity, and various diseases, is often treated with exercise and/or hormone replacement therapy. Exercise regimes can be manipulated to improve hormonal responses, thus enhancing skeletal muscle adaptations including physical strength and muscle mass gain. The use of testosterone as an anabolic hormone has been widely studied due to its ability to increase protein synthesis and promote muscle growth, especially in fast-twitch muscle. To increase the significant functional effect of muscle and avoid some side effects of androgen administration, it is advisable to combine exercise with usage of the hormone [1-6]. The effect of testosterone on skeletal muscle is not only explained by activation and regulation of the androgen receptor (AR), but is also the combined result of genomic and non-genomic actions [7]. Dihydrotestosterone (DHT) is considered the terminal active product of androgen biosynthesis [8], and has a greater affinity for AR compared to testosterone [9], which can cross the cell membrane and bind AR to regulate the expression of related genes [10, 11] or locate in the cytoplasm to interact with signal molecules [12, 13]. Cellular signalling pathways, involving surface membrane receptors and second messengers, regulate the non-genomic actions of androgen [14, 15]. Even though the insulin-like growth factor I (IGF-I) / mammalian target of rapamycin (mTOR) pathway has been reported to be involved in crosstalk with the rapid actions, different muscle types from studies have shown various responsiveness to androgen [16-19]. Because of the growing interest in the potential application of exercise as promoting therapy, a better understanding of the molecular action of androgen in conjunction with exercise on skeletal muscle is needed. In this study, we hypothesized that combining DHT with exercise would potentiate muscle growth and activate the mTOR signalling pathway.
MATERIALS AND METHODS
Animals
A total of 24 male Sprague–Dawley rats aged 8 weeks (290.9 ±13.5g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). Four rats were housed in each cage under an artificial 12:12 h light–dark cycle. All animals received a diet of standard pelleted chow and had free access to food and water for the duration of the study. All experimental and training procedures were approved by the Institutional Animal Care and Use Committee at Beijing Sports University and strictly conformed to the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health.
Experimental design
The rats were habituated to treadmill exercise for 4 days and then randomly assigned to sham operation and DHT implantation groups, and each group had 2 subgroups which were subjected to either sedentary conditions or exercise training: SHAM+SED, SHAM+EX, DHT+SED, and DHT+EX (n=6 in each group). A single 21-day release 5α-dihydrotestosterone (DHT) pellet (50 mg/pellet) (Innovative Research of America, USA) was implanted subcutaneously in the neck of each rat in the DHT+SED and DHT+EX groups. Rats in groups SHAM+SED and DHT+SED remained sedentary throughout the experimental period. Rats in groups SHAM+EX and DHT+EX underwent a 10-day training protocol beginning 3 days after the surgery. Rats in these groups exercised on a treadmill at a speed of 20 m/min and a slope of 10% for 60 min/day [20]. All rats were weighed 12 h after the last bout of training and were anaesthetized via an intraperitoneal injection of 20% ethyl carbamate (5 ml/kg body weight). Blood was drained from the abdominal aorta, and the gastrocnemius muscles of the rats were then removed and immediately weighed. The white muscle of the gastrocnemius, which is rich in fast twitch fibres, was dissected out. Each sample was carefully separated into 2 parts; one part was stored at -80°C for mRNA and protein assays and the other was frozen in isopentane, cooled with liquid nitrogen, for histological analysis.
Histological analysis
Frozen sections were stained with haematoxylin and eosin (HE) for the evaluation of muscle fibre size. The images were obtained using an inverted microscope (OLYMPUS, Tokyo, Japan) connected to a computer and analysed by Image-Pro Plus 6.0.
RNA extraction, cDNA synthesis and real-time quantitative polymerase chain reaction (PCR)
Total RNA was isolated from 100 mg samples of white gastrocnemius muscle that were homogenized and purified using TRIzol Reagent (Invitrogen Life Technologies, Massachusetts, USA), according to the manufacturer’s instructions. The RNA was quantified using a spectrophotometer (ND1000 Nanodrop, Massachusetts, USA) by measuring the absorbance at 260 nm (A260) and 280 nm (A280), with A260/A280 ratios above 1.8 indicating high-quality RNA. cDNA was synthesized from 1 μg of total RNA in the presence of Oligo(dT)15, dNTP mix, RNase inhibitor, and reverse transcriptase (Invitrogen Life Technologies, Massachusetts, USA) in a final volume of 20 μl, incubated at 37°C for 50 min and 70°C for 15 min. cDNA was then stored at -20°C until PCR reactions were performed. The primers were designed by Primer Premier 6 and assessed by Oligo 7. The nucleic acid sequences were matched to the PubMed gene database, as shown in Table 1. The PCR mixture (20 μl) consisted of 10 μl of Platinum SYBR Green qPCR SuperMix-UDG, 1 μl of forward primer, 1 μl of reverse primers, 0.1 μl of ROX Reference Dye, 1 μg of cDNA, and DNase-free water. Reactions were incubated for 2 min at 50°C and 2 min at 95°C, followed by 40 cycles consisting of a 15 s denaturing step at 95°C and 1 min annealing step at 60°C. At the end of the PCR reaction, the samples were subjected to 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s to in order to draw the dissociation curve. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize any variations due to inefficiencies of the reverse transcription and PCR. All PCR runs were performed in duplicate. Samples were analysed using an Sequence Detection System (ABI 7500, Applied Biosystems, Massachusetts, USA). Data were analysed using the cycle threshold based on the time at which the fluorescence emission increased beyond a threshold level. Relative expression was determined using the 2-△△ CT method.
TABLE 1.
Quantitative real-time PCR primer.
| Genes | Sequences |
|---|---|
| AR | F: 5′-GGCAGTCATTCAGTATTCC-3′ |
| R: 5′-AGTAGAGCATCCTAGAGTTG-3′ | |
| IGF-I | F: 5′-GGCATTGTGGATGAGTGT-3′ |
| R: 5′-GATGGAACGAGCTGACTT-3′ | |
| GAPDH | F: 5′-CCTGCCAAGTATGATGAC-3′ |
| R: 5′-GGAGTTGCTGTTGAAGTC-3′ |
Western blotting analysis
Frozen gastrocnemius samples were homogenized in urea lysis buffer and centrifuged at 20,000 rpm to remove debris and the protein extractions were transferred to clean tubes. Muscle protein content was measured via bicinchoninic acid assay using bovine serum albumin (BSA) as the standard. Protein extracts were separated by SDS-PAGE gels and transferred to 0.2 μm nitrocellulose membranes (GE Healthcare Amersham Biosciences, Oslo, Norway). Membranes were blocked with 5% BSA in TBS-Tween. The membranes were probed with primary antibodies as follows: myosin heavy chain (MHC), AR, and phosphoSer210-AR from Abcam (Cambridge, UK); phosphoSer2448-mTOR, phosphoThr389-p70 ribosomal S6 kinase (p70S6K), and phosphoThr37/46-eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) from Cell Signal Technology (Massachusetts, USA). β-actin antibody from Santa Cruz Biotechnology (Dallas, USA) was used as the loading control. After washing, membranes were incubated with horseradish-conjugated sheep anti-mouse IgG and sheep anti-rabbit IgG (Jackson ImmunoResearch, Philadelphia, USA) secondary antibodies. Proteins were visualized with ECL plus reagents (Pierce Biotechnology, Rockford, IL, USA), and band images were obtained using the ChemiDoc XRS+ System (Bio-Rad Laboratories, California, USA) and analysed using Quantity One software.
Statistical analysis
Statistical analyses were performed with SPSS 13.0. All data are expressed as mean ± SD. The normal distribution was assessed with the Shapiro-Wilk test. A two-way ANOVA was used to determine significant differences. The statistical significance was set at P<0.05.
RESULTS
Muscle growth after treatment with sham operation or DHT in sedentary or exercise condition
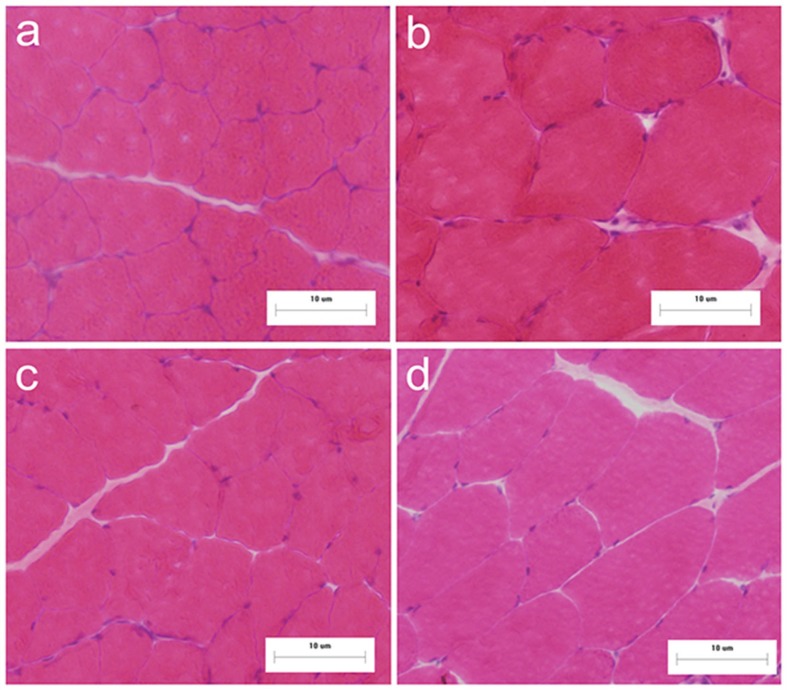
Muscle wet mass (whole gastrocnemius) of SHAM+EX, DHT+SED, SHAM+SED demonstrated ~2.1%, 3.1%, and 6.7% increases, respectively, compared to SHAM+SED (Table 2). Muscle cross sectional area was significantly increased in DHT+SED and DHT+EX (~28% and 27%, respectively; P < 0.05) (Table 2, Fig. 1). MHC expression was significantly increased in DHT+SED, SHAM+EX, and DHT+EX compared to SHAM+SED (~27%, 25%, and 44% respectively; P < 0.01). MHC expression in DHT+EX was ~44%, 15%, and 11% higher than in SHAM+SED (P < 0.01), DHT+SED (P < 0.05), and SHAM+EX (P < 0.05), respectively (Table 2).
TABLE 2.
Muscle growth of the rat gastrocnemius.
| SHAM+SED | DHT+SED | SHAM+EX | DHT+EX | |
|---|---|---|---|---|
| Muscle wet mass (g) | 1.95±0.20 | 2.01±0.12 | 1.99±0.09 | 2.08±0.13 |
| Muscle cross sectional area | 1.00±0.22 | 1.28±0.15*& | 1.05±0.11 | 1.27±0.20*& |
| MHC content | 1.00±0.00 | 1.25±0.09** | 1.27±0.11** | 1.44±0.14**#& |
P<0.05 and
P<0.01 vs. SHAM+SED
P<0.05 vs. DHT+SED
P<0.05 vs. SHAM+EX. Data of cross sectional area were represented as fold of SHAM+SED; data of MHC content were represented as fold of SHAM+SED and normalized to β-actin. All values are means±SD (n=6).
FIG. 1.
Histological appearance of gastrocnemius muscle sustained by HE after DHT and/or exercise (n=6). Groups are SHAM+SED (a), DHT+SED (b), SHAM+EX (c), and DHT+EX (d). (All panels×200).
AR and IGF-I mRNA expression after treatment with sham operation or DHT in sedentary or exercise condition
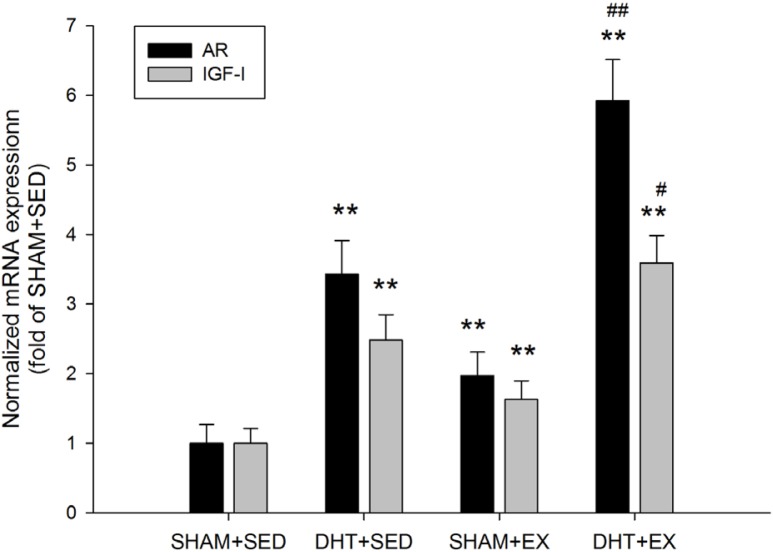
Muscle AR mRNA expression was significantly elevated in DHT+SED and SHAM+EX (~343% and 197%, respectively; P < 0.01). AR mRNA was a significant ~592% higher in DHT+EX vs. DHT+SED (P < 0.01). Likewise, IGF-I mRNA expression was significantly higher in DHT+SED (~248%), SHAM+EX (~163%), and DHT+EX (~359%) compared to SHAM+SED (P < 0.01). Exercise significantly enhanced the effect of DHT on IGF-I mRNA expression (P < 0.05). (Fig. 2)
FIG. 2.
AR and IGF-I mRNA expression normalized to GAPDH in the rat gastrocnemius muscle after DHT and/or exercise measured by real-time PCR. Values are means±SD (n=6). * P<0.05 and ** P<0.01 vs. SHAM+SED; # P<0.05 and ## P<0.01 vs. DHT+SED.
Phosphorylation of AR after treatment with sham operation or DHT in sedentary or exercise condition
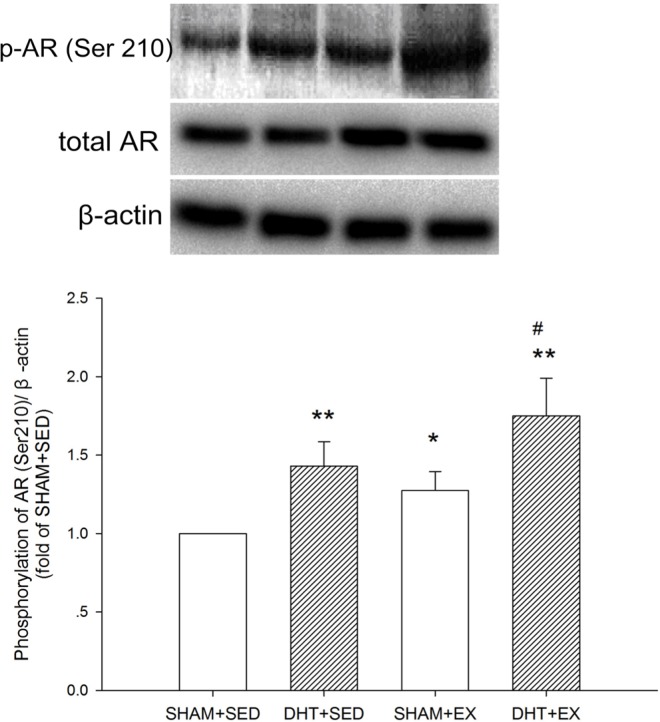
There was no significant difference in total AR between the groups. The phosphorylated status of AR (Ser210) was ~27%, 43%, and 75% higher in SHAM+EX (P < 0.05), DHT+SED (P < 0.05) and DHT+EX (P < 0.01), respectively. (Fig. 3) AR phosphorylation was also significantly higher in DHT+EX vs. DHT+SED (P < 0.05).
FIG. 3.
Phosphorylation of AR in the rat gastrocnemius muscle after DHT and/or exercise measured by Western blot analysis. Data were calculated as percentage of phosphorylated AR (Ser210) to the total form, normalized to β-actin from the same loading, and represented as fold of SHAM+SED. Values are means±SD (n=6). * P<0.05 and ** P<0.01 vs. SHAM+SED; # P<0.05 and ## P<0.01 vs. DHT+SED.
Phosphorylation of mTOR pathway after treatment with sham operation or DHT in sedentary or exercise condition
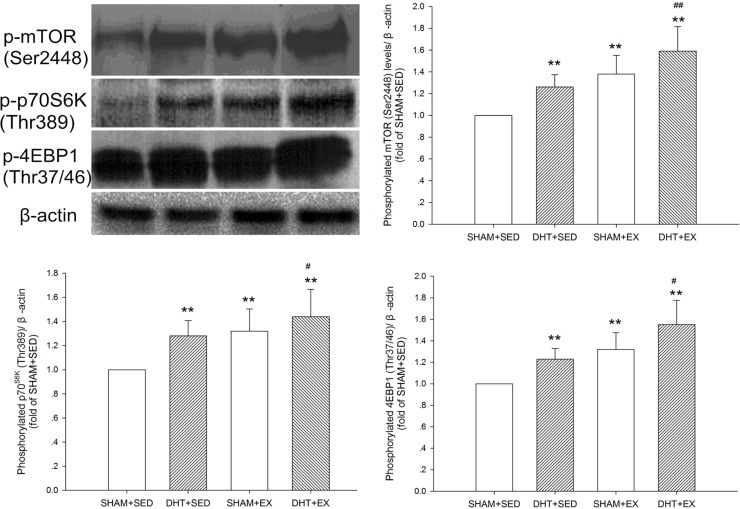
The phosphorylation statuses of mTOR (Ser2448), p70S6K (Thr389), and 4EBP1 (Thr37/46) were significantly increased (P < 0.01) after 10 days of DHT and/or exercise. The combination of DHT and exercise was the most effective at activating the mTOR signalling pathway and led to significantly higher mTOR pathway activation compared with DHT alone. (Fig. 4)
FIG. 4.
The mTOR signalling pathway phosphorylation in the rat gastrocnemius muscle after DHT and/or exercise measured by Western blot analysis. Data of phosphorylated mTOR (Ser2448) (B), phosphorylated p70S6K (Thr389) (C), and phosphorylated 4EBP1 (Thr37/46) (D) were normalized to β-actin from the same loading and represented as fold of SHAM+SED. Values are means±SD (n=6). * P<0.05 and ** P<0.01 vs. SHAM+SED; # P<0.05 and ## P<0.01 vs. DHT+SED.
DISCUSSION
The present study underlines that a combination of androgen and exercise promotes cross section area and protein synthesis in skeletal muscle. Exercise and/or exogenous DHT caused an increase in IGF-I and AR and activated the mTOR signalling pathway; the activity of the hypertrophic pathway induced by androgen would be enhanced by a resistance exercise protocol.
Effects of DHT and/or exercise on muscle mass and protein synthesis
Androgen mainly consists of testosterone and DHT in the human body and has been proven to be able to alleviate muscle atrophy. In addition to pharmacological therapies, regular resistance exercises with rest periods and adaptive loads are often performed to improve or maintain muscle strength and mass. To obtain a significant function effect with gain in muscle mass, it is clinically suggested that the combination of testosterone therapy and strength training results in desirable muscle function, which is usually reflected in muscle strength and physical performance [1-4].
Since different exercise stimuli induce changes in AR content that are specific to skeletal muscle fibre type [21], white gastrocnemius was used in the current study to examine the influence of DHT and resistance exercise in skeletal muscle. In the current study, DHT implantation had an obvious hypertrophic effect by inducing significant increases in both fibre cross-sectional area and MHC content; this result is consistent with previous studies [22,23]. However, exercise only increased the total MHC content by ~27%; thus no significant histological changes were observed. Even though this resistance training model showed a desirable influence on muscle in our previous research [20], the current results may be explained by the limited training period (10 days). In accordance with our hypothesis, rats in DHT+EX had significantly enhanced muscle cross sectional areas compared to rats in SHAM+SED. This effect was paralleled by a significant increase in MHC content in DHT+EX, even when compared to DHT+SED. Muscle mass tended to be higher in DHT+SED, SHAM+EX, and DHT+EX compared to SHAM+SED, though not significantly. The limited increase in muscle mass could have resulted from the fat loss effect of androgen [24, 25]. A previous study reported that testosterone increased the composition of MHC IIa after orchidectomy in mice; strong succinate dehydrogenase reactivity was also observed, which can promote lipid metabolism [19]. Therefore, the discordance between muscle mass and MHC content could be explained by lipid and/or water loss. An increase in fatty-acid binding protein in the soleus was also found after a 2-week testosterone implant, suggesting that testosterone has a direct effect on promoting the metabolism of fatty acids, at least for slow-twitch muscle [26,27]. In addition, resistance training was more effective in fast-twitch muscle, and so was androgen. Frese et al. [23] found that the responses of fibre types differs with the treatment of 19-norandrostenedione, desoxymethyltestosterone, and testosterone propionate (no difference between treated groups); MHC IId/x was significantly up-regulated, IIb was down-regulated, and there was no significant difference in the levels of MHC IIa and MHC I between groups. Research has shown that nearly 100% of the white gastrocnemius is made up of fast-twitch muscle and 21%-25% of the red gastrocnemius is made up of slow twitch muscle, which might be another reason for the limited growth in the mass of the whole gastrocnemius in the subjected groups [28]. After all, androgen combined with resistance exercise would promote muscle growth.
Activity of IGF-I and AR after DHT and/or exercise
The promotion of muscle synthesis by IGF-I has been thoroughly studied and is thought to mainly occur via the mTOR signalling pathway [29]. One study showed that muscle mass was greater in mice with IGF-I overexpression in skeletal muscle compared to control mice [30]. Localized infusion of IGF-I has also been found to induce skeletal muscle hypertrophy in rats [31]. In human skeletal muscle myotubes, testosterone causes an increase in IGF-I mRNA [32]. Lupron, a gonadal steroid suppressor, induced a marked decrease in the concentration of IGF-I mRNA in skeletal muscle and in body protein anabolism in humans [33]. In the current study, it was hypothesized that the rapid physiological function of androgen and exercise might share a similar mechanism, in which IGF-I interacts with AR in skeletal muscle. The results show that both exercise and DHT significantly increased the expression of IGF-I mRNA (~63% and 148%), and this effect was synergistic when DHT was combined with exercise (~259%). During exercise, IGF-I modulates skeletal muscle adaptation to resistance training by stimulating muscle protein synthesis and activating satellite cells to proliferate and differentiate [34, 35], and this process could be under the regulation of multiple hormones including androgen [36]. Importantly, exercise-induced changes in IGF-I mRNA expression in skeletal muscle might be related to serum testosterone level [2,33,37-39]. Although some findings have shown decreased or unchanged IGF-I mRNA expression after resistance exercise [40-42], different recovery times and/or training variables inevitably influence the physiological responses of muscle.
Similarly, AR mRNA expression and the phosphorylation of AR protein were significantly elevated in DHT+SED, SHAM+EX and DHT+EX and were significantly higher in DHT+EX compared with the other two groups. DHT treatment has been shown to strongly increase AR levels in vitro [43]. Previous results from other researchers, along with the data from this study, indicate that DHT binding of AR promotes some phosphorylation of AR, thereby stabilizing AR homodimers and/or influencing transactivation properties [44, 45]. Combining exercise with androgens that enhance AR content will likely optimize muscle anabolism via promoting the interaction between androgens and AR. Bamman et al. also demonstrated that muscle IGF-I and AR mRNA concentration were significantly elevated in vastus lateralis muscle of healthy subjects after they engaged in acute concentric and eccentric loading exercises [46]. These results suggest that resistance training and DHT treatment work together to enhance the activity of the muscle AR and IGF-I system.
IGF-I induces expression, phosphorylation, nuclear translocation and the DNA binding activity of the AR in muscle [12, 47], indicating the existence of a feedback loop between IGF-I and androgen. The IGF-I receptor has been reported to control cellular AR localization and activity in prostate cancer cells [48]; AR transcriptional activity was decreased as a result of IGF-1 receptor down regulation [49]. On the other hand, there is increasing functional evidence in skeletal muscle cells for extra-nuclear AR instead of AR localized exclusively in the nucleus during testosterone action [13]; the PI3K/Akt pathway could then mediate ligand-independent induction of AR nuclear localization [12]. Thus, there could be regulatory crosstalk between IGF-I and AR in skeletal muscle, and the non-genomic pathway, activated by both androgen and resistance exercise, may rely on this mechanism.
mTOR signalling pathway activation induced by DHT and/or exercise
The mTOR signalling pathway plays a central role in the control of protein synthesis [50, 51]. The downstream targets and effectors of the mTOR pathway are p70S6K and 4EBP1. This pathway has been shown to be necessary for regulating skeletal muscle fibre size and inhibiting muscle atrophy induced by disuse [52]. Bodine et al. reported that an inhibitor of the PI3K/Akt/mTOR pathway could suppress 95% of muscle hypertrophy, supporting the idea that the activation of p70S6K and 4EBP1 could inhibit the mechanisms responsible for producing muscle atrophy by preserving muscle fibre size [52]. It has been proven that resistance exercise can activate this pathway [53, 54]. For example, in resistance-trained men, a leg exercise at 70% concentric one repetition maximum demonstrated that the phosphorylation of p70S6K was related to the myofibrillar protein synthesis response [54].
The anabolic effect of androgen is not limited to genomic results, and androgen signalling represents an important target for research aimed at promoting muscle growth. The non-genomic effect of androgen is a rather rapid and reversible process, different from its classic transcriptional effects. The non-genomic effect could be at least partly established through crosstalk with other signalling molecules such as mTOR and IGF-I. Non-genomic AR signalling has been shown to occur in an ERK-independent manner, via activation of the mTOR pathway during cell proliferation in prostate cancer [55]. The central link for muscle hypertrophy and activation of the mTOR pathway was reported in testosterone-induced cardiomyocyte growth. Indeed, protein kinase B (Akt) and mTOR phosphorylation in rat myotubes were significantly increased by testosterone treatment [56]. In addition, the phosphorylation status of Akt, p70S6K and ERK1/2 was abolished by PI3K/Akt and mTOR inhibition, but not by ERK1/2 inhibition in cultured rat skeletal muscle myotubes after testosterone treatment [57]. The important role of the mTOR pathway in androgen-induced skeletal muscle hypertrophy is supported by an in vivo study, in which a rapid increase in the phosphorylation of p70S6K and IGF-I mRNA level was induced by DHT in the androgen-sensitive levator ani muscle of castrated rats [17]. Furthermore, Xu et al. reported that flutamide (an AR antagonist) successfully inhibited DHT-induced p70S6K phosphorylation [17]. Also, the action of AR may require or influence the mTOR signalling [58]. In a previous study, mTOR and Akt signalling were amplified in myotubes treated with testosterone when the testosterone was withdrawn, indicating that AR may play a role in the feedback mechanism mediated by testosterone [59]. Likewise, Peterziel et al. found that DHT did not induce an increase in activity of PI3 kinase (an upstream molecule) in cells lacking AR [60]. Similarly, Wu et al. demonstrated that testosterone failed to induce hypertrophy in rat myoblast cells lacking AR and that the mTOR inhibitor rapamycin also inhibited hypertrophy [61]. These findings indicate that androgen stimulates muscle hypertrophy through a mechanism that requires AR and involves a signalling cascade, which is dependent on the mTOR pathway; this has also been supported by the work of Basualto-Alarcon et al. [57]. Although muscle force was not determined in the current study, both muscle morphology and MHC content showed that 10 days of DHT treatment via DHT implant induced muscle hypertrophy. Furthermore, the three molecules in the mTOR pathway were significantly phosphorylated after stimulation with androgen or a combination of androgen and exercise. These data further support the notion that the mTOR pathway is an important part of a signalling network that is involved in regulation of the androgen- and exercise-induced remodelling processes of skeletal muscle [62-65].
This study has 2 main limitations. First, we did not assess the circulating testosterone and thus lack direct evidence to monitor the exact hormone response induced by exercise and DHT. Second, the discordance between muscle wet mass and increased protein synthesis was due to the limited training period, and a more effective resistance training protocol is required to achieve desirable effects of muscle growth.
CONCLUSIONS
In conclusion, the current study demonstrates that a combination of exercise and DHT may have a more desirable effect on promoting muscle protein synthesis and signalling activities. Exogenous DHT-induced hypertrophy of skeletal muscle is regulated by activation of the mTOR pathway through IGF-I or via the interaction between IGF-I and AR, and this physiological response is promoted by resistance exercise. Further work should investigate to what degree the non-genomic action induced by androgen and exercise contributes to muscle growth. It is also advisable to examine the effectiveness of different prescriptions of androgen combined with exercise.
Acknowledgements
This work was supported by the National Natural Science Fund Project of China (No. 31071034).
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Kvorning T, Christensen LL, Madsen K, Nielsen JL, Gejl KD, Brixen K, et al. Mechanical muscle function and lean body mass during supervised strength training and testosterone therapy in aging men with low-normal testosterone levels. J Am Geriatr Soc. 2013;61:957–962. doi: 10.1111/jgs.12279. [DOI] [PubMed] [Google Scholar]
- 2.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Storer TW, Javanbakht M, Berman N, Yarasheski KE, Phillips J, et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000;283:763–770. doi: 10.1001/jama.283.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56:1991–1999. doi: 10.1111/j.1532-5415.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 7.Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–1667. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med Sci Sports Exerc. 2012;44:818–826. doi: 10.1249/MSS.0b013e31823bfcbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS. 2000;108:838–846. doi: 10.1111/j.1600-0463.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 10.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Lee WJ. Insulin-like growth factor-I-induced androgen receptor activation is mediated by the PI3K/Akt pathway in C2C12 skeletal muscle cells. Mol Cells. 2009;28:495–499. doi: 10.1007/s10059-009-0142-8. [DOI] [PubMed] [Google Scholar]
- 13.Pronsato L, Boland R, Milanesi L. Non-classical localization of androgen receptor in the C2C12 skeletal muscle cell line. Arch Biochem Biophys. 2013;530:13–22. doi: 10.1016/j.abb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 15.Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MI, Horvitz GD, Clemmons DR, Fournier M. Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab. 2002;282:E483–490. doi: 10.1152/ajpendo.00191.2001. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Shen Y, Pink H, Triantafillou J, Stimpson SA, Turnbull P, et al. Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J Steroid Biochem Mol Biol. 2004;92:447–454. doi: 10.1016/j.jsbmb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010;151:3706–3719. doi: 10.1210/en.2010-0150. [DOI] [PubMed] [Google Scholar]
- 19.Ibebunjo C, Eash JK, Li C, Ma Q, Glass DJ. Voluntary running, skeletal muscle gene expression, and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab. 2011;300:E327–340. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- 20.Zeng F, Zhu H, Zhao H. Effects of blocking PI3K and exercise on Akt/mTOR pathway of skeletal muscle in rats. Journal of Beijing Sport University. 2010;33:46–49. [Google Scholar]
- 21.Deschenes MR, Maresh CM, Armstrong LE, Covault J, Kraemer WJ, Crivello JF. Endurance and resistance exercise induce muscle fiber type specific responses in androgen binding capacity. J Steroid Biochem Mol Biol. 1994;50:175–179. doi: 10.1016/0960-0760(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 22.Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, Zhang A, et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152:193–206. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frese S, Velders M, Schleipen B, Schanzer W, Bloch W, Diel P. Myosin heavy chain expression pattern as a marker for anabolic potency: desoxymethyltestosterone (madol), norandrostenedione and testosterone repress MHC-IIb expression and stimulate MHC-IId/x expression in orchiectomized rat gastrocnemius muscle. Arch Toxicol. 2011;85:635–643. doi: 10.1007/s00204-010-0607-8. [DOI] [PubMed] [Google Scholar]
- 24.Sheffield-Moore M. Androgens and the control of skeletal muscle protein synthesis. Ann Med. 2000;32:181–186. doi: 10.3109/07853890008998825. [DOI] [PubMed] [Google Scholar]
- 25.Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Breda E, Keizer HA, Vork MM, Surtel DA, de Jong YF, van der Vusse GJ, et al. Modulation of fatty-acid-binding protein content of rat heart and skeletal muscle by endurance training and testosterone treatment. Pflugers Arch. 1992;421:274–279. doi: 10.1007/BF00374838. [DOI] [PubMed] [Google Scholar]
- 27.Bass NM. The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988;111:143–184. doi: 10.1016/s0074-7696(08)61733-7. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer WJ, Staron RS, Gordon SE, Volek JS, Koziris LP, Duncan ND, et al. The effects of 10 days of spaceflight on the shuttle Endeavor on predominantly fast-twitch muscles in the rat. Histochem Cell Biol. 2000;114:349–355. doi: 10.1007/s004180000193. [DOI] [PubMed] [Google Scholar]
- 29.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 30.Shavlakadze T, Winn N, Rosenthal N, Grounds MD. Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm IGF Res. 2005;15:4–18. doi: 10.1016/j.ghir.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol (1985). 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 32.Sculthorpe N, Solomon AM, Sinanan AC, Bouloux PM, Grace F, Lewis MP. Androgens affect myogenesis in vitro and increase local IGF-1 expression. Med Sci Sports Exerc. 2012;44:610–615. doi: 10.1249/MSS.0b013e318237c5c0. [DOI] [PubMed] [Google Scholar]
- 33.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 34.Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol (1985). 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- 35.Butterfield GE, Thompson J, Rennie MJ, Marcus R, Hintz RL, Hoffman AR. Effect of rhGH and rhIGF-I treatment on protein utilization in elderly women. Am J Physiol. 1997;272:E94–99. doi: 10.1152/ajpendo.1997.272.1.E94. [DOI] [PubMed] [Google Scholar]
- 36.Laviola L, Natalicchio A, Giorgino F. The IGF-I signaling pathway. Curr Pharm Des. 2007;13:663–669. doi: 10.2174/138161207780249146. [DOI] [PubMed] [Google Scholar]
- 37.Montano M, Flanagan JN, Jiang L, Sebastiani P, Rarick M, LeBrasseur NK, et al. Transcriptional profiling of testosterone-regulated genes in the skeletal muscle of human immunodeficiency virus-infected men experiencing weight loss. J Clin Endocrinol Metab. 2007;92:2793–2802. doi: 10.1210/jc.2006-2722. [DOI] [PubMed] [Google Scholar]
- 38.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 39.Aguiar AF, Vechetti-Junior IJ, Alves de Souza RW, Castan EP, Milanezi-Aguiar RC, Padovani CR, et al. Myogenin, MyoD and IGF-I regulate muscle mass but not fiber-type conversion during resistance training in rats. Int J Sports Med. 2013;34:293–301. doi: 10.1055/s-0032-1321895. [DOI] [PubMed] [Google Scholar]
- 40.Aperghis M, Velloso CP, Hameed M, Brothwood T, Bradley L, Bouloux PM, et al. Serum IGF-I levels and IGF-I gene splicing in muscle of healthy young males receiving rhGH. Growth Horm IGF Res. 2009;19:61–67. doi: 10.1016/j.ghir.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Hulmi JJ, Ahtiainen JP, Selanne H, Volek JS, Hakkinen K, Kovanen V, et al. Androgen receptors and testosterone in men--effects of protein ingestion, resistance exercise and fiber type. J Steroid Biochem Mol Biol. 2008;110:130–137. doi: 10.1016/j.jsbmb.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol (1985). 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- 43.Mukherjee B, Mayer D. Dihydrotestosterone interacts with EGFR/MAPK signalling and modulates EGFR levels in androgen receptor-positive LNCaP prostate cancer cells. Int J Oncol. 2008;33:623–629. [PubMed] [Google Scholar]
- 44.Blok LJ, de Ruiter PE, Brinkmann AO. Forskolin-induced dephosphorylation of the androgen receptor impairs ligand binding. Biochemistry. 1998;37:3850–3857. doi: 10.1021/bi9724422. [DOI] [PubMed] [Google Scholar]
- 45.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU Int. 2005;95:1327–1335. doi: 10.1111/j.1464-410X.2005.05527.x. [DOI] [PubMed] [Google Scholar]
- 46.Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:E383–390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ, Lee WJ. Insulin-like growth factor-I induces androgen receptor activation in differentiating C2C12 skeletal muscle cells. Mol Cells. 2009;28:189–194. doi: 10.1007/s10059-009-0118-8. [DOI] [PubMed] [Google Scholar]
- 48.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 49.Sayeed A, Alam N, Trerotola M, Languino LR. Insulin-like growth factor 1 stimulation of androgen receptor activity requires beta(1A) integrins. J Cell Physiol. 2012;227:751–758. doi: 10.1002/jcp.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 52.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 53.Haddad F, Adams GR. Selected contribution: acute cellular and molecular responses to resistance exercise. J Appl Physiol (1985). 2002;93:394–403. doi: 10.1152/japplphysiol.01153.2001. [DOI] [PubMed] [Google Scholar]
- 54.Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, et al. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao RS, Ma S, Miao L, Li R, Yin Y, Raj GV. Androgen receptor-mediated non-genomic regulation of prostate cancer cell proliferation. Transl Androl Urol. 2013;2:187–196. doi: 10.3978/j.issn.2223-4683.2013.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allemand MC, Irving BA, Asmann YW, Klaus KA, Tatpati L, Coddington CC, et al. Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes--a potential model for PCOS-related insulin resistance. PLoS One. 2009;4:e4274. doi: 10.1371/journal.pone.0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basualto-Alarcon C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc. 2013;45:1712–1720. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 58.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, et al. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 59.White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Bauman WA, Blitzer RD, Cardozo C. Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 2010;400:679–683. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- 62.Gibala MJ, Interisano SA, Tarnopolsky MA, Roy BD, MacDonald JR, Yarasheski KE, et al. Myofibrillar disruption following acute concentric and eccentric resistance exercise in strength-trained men. Can J Physiol Pharmacol. 2000;78:656–661. [PubMed] [Google Scholar]
- 63.Goldspink G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda). 2005;20:232–238. doi: 10.1152/physiol.00004.2005. [DOI] [PubMed] [Google Scholar]
- 64.Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kvorning T, Andersen M, Brixen K, Madsen K. Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol Endocrinol Metab. 2006;291:E1325–1332. doi: 10.1152/ajpendo.00143.2006. [DOI] [PubMed] [Google Scholar]