Abstract
Although oxidative stress has long been considered to be a major factor contributing to telomere shortening, recent work has established that oxidative stress and DNA damage are linked to telomere lengthening. Now, Opresko and colleagues resolve this apparent discrepancy by showing that differential modulation of telomerase activity depends on the origin of a common oxidative guanine lesion.
Telomeres are nucleoprotein structures at chromosome ends that, in mammals, consist of double-stranded tandem repeats of (5′-TTAGGG-3′)n ending in a single-stranded 3′ overhang. Maintaining telomere length at or near equilibrium in a species-specific manner is a critical aspect of telomere maintenance, which involves telomerase, a ribonucleoprotein complex that extends the 3′ overhang by using its integral telomerase RNA as a template1. Telomerase activity prevents DNA-replication-dependent telomere loss, known as the ‘end replication problem’, and is essential for the viability of most human cancer cells2. Telomere length maintenance is also influenced by environmental factors, most notably oxidative stress3.
Oxidation of DNA by reactive oxygen species (ROS) constitutes a major source of spontaneous DNA damage. Stacked guanines succumb to oxidation more than other DNA bases or base combinations. 7,8-dihydro-8-oxoguanine (8-oxodG) is one of the widely studied oxidative guanine lesions4, and telomeres are hotspots for the formation or accumulation of 8-oxoGs5,6. A new study by Opresko and colleagues7 reveals the context- and origin-dependent differential effect of 8-oxoG on telomerase’s ability to extend telomeres. Whereas misincorporation of 8-oxodGTP by telomerase has a telomere chain-termination effect that results in telomere shortening, a preexisting 8-oxoG enhances telomerase’s ability to lengthen telomeres by unfolding telomeric G quadruplexes.
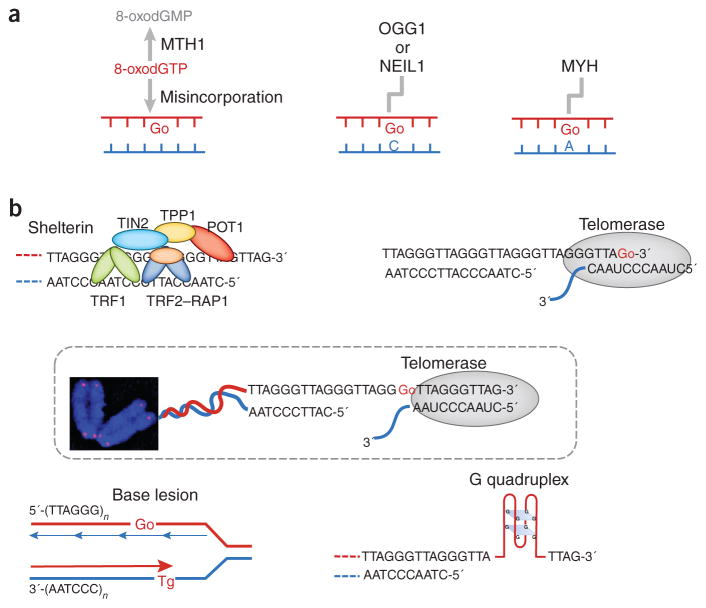
8-oxoG lesions within telomeres can originate in several ways (Fig. 1a): (i) 8-oxodGTP can be incorporated from the free-dNTP pool, and, indeed, the work by Opresko and colleagues7 provides the first demonstration that telomerase incorporates 8-oxodGTP opposite rATP or rCTP in the telomere RNA primer; (ii) a guanine in a regular G-C DNA base pair may be oxidized to 8-oxoG; or (iii) 8-oxodGTP can be misincorporated by DNA polymerase during DNA replication. Hydrolases such as human MTH1 hydrolyze oxidized dNTPs in the free-nucleotide pool, thereby producing the corresponding dNMP; for instance, 8-oxodGTP is converted to 8-oxodGMP8. In contrast, DNA glycosylases such as OGG1, NEIL1 or MYH repair 8-oxoGs within DNA via the base excision repair pathway9. Despite protective antioxidation mechanisms, 8-oxoGs do occur in telomeric DNA during the course of aging, and defective removal of 8-oxoG disrupts telomere length homeostasis, primarily via the telomerase pathway10,11.
Figure 1.
Origin and consequences of oxidative DNA base damage within telomeres. (a) 8-oxoG (Go) in telomeres can arise from misincorporation of 8-oxodGTP or from in situ oxidation of a guanine DNA base. Proteins either hydrolyze the oxidized nucleotide pool or repair the oxidized bases within the DNA. (b) Telomere secondary structures, the telomeric proteins and oxidative DNA lesions influence elongation by telomerase. Top left, telomeric proteins negatively regulate telomerase-dependent elongation through the protein counting model (described in the main text). Oxidized bases compromise the binding of telomeric proteins to telomeres, thereby disrupting the counting mechanism. Top right, telomerase can utilize 8-oxoGTP during substrate extension, but 8-oxoGTP misincorporation terminates telomere elongation. Middle, telomerase uses its integral telomerase RNA as a template (with the sequence CAAUCCCAAUC) and adds the 6-nt repeating sequence 5′-TTAGGG-3′ to the 3′ overhang. Preexisting terminal 8-oxoG does not impair telomerase loading. Lower left, oxidized bases such as thymine glycol (Tg) may cause premature termination of telomere replication, thus leading to telomere shortening. Lower right, 8-oxoG ‘irons out’ G quadruplexes, thereby enhancing substrate accessibility and telomerase loading onto telomeres.
Oxidative stress and 8-oxoG lesions have been linked with puzzling manifestations in studies on telomere length homeostasis. Although oxidative stress is a major factor contributing to telomere shortening3, Saccharomyces cerevisiae or mouse strains that are defective in the major ROS detoxification enzymes or in OGG1 (the enzyme that removes preexisting 8-oxoG from DNA), exhibit oxygen- and/or telomerase-dependent telomere lengthening10–12. It is in this context that the findings by Fouquerel et al.7 are not only novel but also uniquely revealing in their ability to explain this paradox. The biochemical assays in this study demonstrate that telomerase can incorporate an 8-oxodGTP (from the free-nucleotide pool) into the telomere chain but cannot subsequently extend the chain (Fig. 1b, top right). This observation suggests that under acute oxidative stress, conditions that increase 8-oxodGTP levels in the nucleotide pool may induce chain termination by telomerase, thus causing telomeres to shorten. In contrast, the authors’ biochemical and single-molecule fluorescence resonance energy transfer (FRET) assays together provide evidence that although preexisting 8-oxoG in a telomeric DNA chain that is free of secondary structure minimally affects the annealing of telomeric DNA to the telomerase RNA template (Fig. 1b, middle), it stimulates telomerase loading when G quadruplex structures are present, by unfolding and thus removing them from telomeric DNA (Fig. 1b, bottom right). Preexisting 8-oxoG may also alter the affinity of the inherent telomeric proteins for telomeric DNA repeats13 (Fig. 1b, top left). This alteration may in turn disturb the telomeric proteins, negative regulation of telomerase via the ‘protein counting model’14,15. If an 8-oxoG opposite A were to persist after a round of replication, it could give rise to a GC-to-TA transversion9, which might also affect the binding of telomeric proteins. These manifold effects plausibly explain why increased levels of preexisting 8-oxoG (due to defective OGG1 repair and/or ROS detoxification) might contribute to telomere lengthening.
The findings of Fouquerel et al.7 have yet another level of importance because they show that the enhanced levels of 8-oxodGTP in the free-nucleotide pool produced by MTH1 depletion cause cellular senescence and telomere dysfunction in a telomerase-dependent manner, specifically in cancer cells containing short telomeres. Thus, telomerase-positive cancer cells, which generally have short telomeres2, may exhibit increased sensitivity to elevated levels of oxidized dNTPs in the free-nucleotide pool and/or MTH1 depletion. Presumably, both normal and cancer stem cells with long telomeres would show greater tolerance to MTH1 depletion.
Several intriguing questions remain to be resolved. In addition to the source and origin of 8-oxoG in telomeres, it is important to consider the location of 8-oxoG within the telomere. The telomere 3′ overhang is believed to be tucked away in a lariat-like structure called the t loop16, whose folding and unfolding is mediated by telomeric proteins17. Would the G-quadruplex-unfolding ability of 8-oxoG, and hence its effect on telomerase loading, be any different for 8-oxoGs located within the t loop? Another prevalent oxidative base damage that occurs within telomeric DNA is thymine glycol (Tg), and a defect in its removal by NTH1 DNA glycosylase leads to telomere shortening in mice18. Although Tg may block telomere DNA replication19, thereby resulting in a loss of distal telomeric sequences (Fig. 1b), there might be additional layers of modulation of telomerase activity that could explain the telomere length differences between Ogg1- and Nth1-null mice. Recently, a new telomere-length-maintenance model linking telomerase and replication-fork progression has been proposed. In this model, telomerase travels with the lagging-strand replication machinery and remains bound to the fork until it reaches the telomere 3′ overhang for the telomere to be extended20. Thus, replication-fork collapse, for example, when the replication fork encounters Tg lesions, may cause dissociation of telomerase from the replication fork. Failure in telomere-repeat addition by telomerase would lead to telomere shortening. In addition, it is still unclear how Tg affect the telomerase activity, loading, folding and unfolding of telomeric G quadruplexes, and subsequently telomere length. Finally, the effects of telomere length alterations related to oxidative base damage in human disease and aging also remain elusive. Complementary human population, genetic model, biochemical and single-molecule FRET studies are warranted to address these questions in the future.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Blackburn EH. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Shay JW. Cancer Res. 2005;65:3513–3517. doi: 10.1158/0008-5472.CAN-05-0728. [DOI] [PubMed] [Google Scholar]
- 3.von Zglinicki T. Ann NY Acad Sci. 2000;908:99–110. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- 4.Neeley WL, Essigmann JM. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 5.Oikawa S, Kawanishi S. FEBS Lett. 1999;453:365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- 6.Rhee DB, Ghosh A, Lu J, Bohr VA, Liu Y. DNA Repair (Amst) 2011;10:34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouquerel E, et al. Nat Struct Mol Biol. 2016;23:1092–1100. doi: 10.1038/nsmb.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakumi K, et al. J Biol Chem. 1993;268:23524–23530. [PubMed] [Google Scholar]
- 9.Hegde ML, Hazra TK, Mitra S. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, et al. PLoS Genet. 2010;6:e1000951. doi: 10.1371/journal.pgen.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu J, Liu Y. EMBO J. 2010;29:398–409. doi: 10.1038/emboj.2009.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Vallabhaneni H, Yin J, Liu Y. Aging Cell. 2013;12:635–644. doi: 10.1111/acel.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opresko PL, Fan J, Danzy S, Wilson DM, III, Bohr VA. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcand S, Gilson E, Shore D. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 15.Smogorzewska A, de Lange T. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 16.de Lange T. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 17.Opresko PL, et al. Mol Cell. 2004;14:763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Vallabhaneni H, O’Callaghan N, Sidorova J, Liu Y. PLoS Genet. 2013;9:e1003639. doi: 10.1371/journal.pgen.1003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouet P, Essigmann JM. Cancer Res. 1985;45:6113–6118. [PubMed] [Google Scholar]
- 20.Greider CW. Genes Dev. 2016;30:1483–1491. doi: 10.1101/gad.280578.116. [DOI] [PMC free article] [PubMed] [Google Scholar]