Abstract
The startle blink reflex is facilitated during early picture viewing, then inhibited by attention during pleasant and aversive pictures compared to neutral pictures, and finally potentiated during aversive pictures specifically. However, it is unclear whether the postauricular reflex, which is elicited by the same loud acoustic probe as the startle blink reflex but enhanced by appetitive instead of defensive emotion, has the same pattern and time course of emotional modulation. We examined this issue in a sample of 90 undergraduates using serially presented soft acoustic clicks that elicited postauricular (but not startle blink) reflexes in addition to standard startle probes. Postauricular reflexes elicited by both clicks and probes correlated during food and nurturant contents, during which they were potentiated compared to neutral pictures, suggesting clicks effectively elicit emotionally modulated postauricular reflexes. The postauricular reflex was initially facilitated during the first 500 ms of picture processing but was larger during pleasant than neutral pictures throughout picture processing, with larger effect sizes during the latter half of picture processing. Across reflexes and eliciting stimuli, measures of emotional modulation had higher coefficient alphas than magnitudes during specific picture contents within each valence, indicating that only emotional modulation measures assess higher-order appetitive or defensive processing.
Descriptors: Postauricular reflex, Emotion, Time course, Startle blink reflex, Prepulse facilitation, Internal consistency
The postauricular reflex is a vestigial microreflex in humans behind the ear that is greatest during the experience of positive emotion compared to neutral or negative emotion (Benning, Patrick, & Lang, 2004); thus, it serves as an index of positive emotionality. This measure differs from the startle blink reflex measured below the eye, which shows the opposite pattern of modulation (i.e., greater magnitude during the experience of negative emotion) and has been applied in myriad ways to assess defensive emotional processing (see Grillon & Baas, 2003; Vaidyanathan, Patrick, & Bernat, 2009; Vaidyanathan, Patrick, & Cuthbert, 2009). Postauricular and startle blink reflexes are often elicited concomitantly using the same startle probe. Though this approach is methodologically convenient, accumulating evidence suggests that the two reflexes do not share the same neural circuitry (Hackley, 2015), indicating that they measure separate emotional systems. Thus, the time courses of their modulation may be dissimilar, which would indicate that appetitive and defensive processing proceed at different rates.
Emotional Modulation of Startle Blink and Postauricular Reflexes
The picture-viewing paradigm is commonly used to examine emotional effects on a variety of reflexes (e.g., Bradley, Codispoti, & Lang, 2006; Bradley, Cuthbert, & Lang, 1993; Vrana, Spence, & Lang, 1988). In this paradigm, participants view a set of pleasant, neutral, and aversive images, often from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005). During each image, a loud noise probe is played, which elicits the blink component of the startle reflex. This methodology has been regularly employed in startle blink reflex research for decades, consistently yielding a pattern of greatest startle blink reflex during aversive images and lowest startle blink reflex during pleasant images in healthy participants (Bradley et al., 2006; Gard, Gard, Mehta, Kring, & Patrick, 2007; Vrana et al., 1988). More recently, this paradigm has been employed in studies of the postauricular reflex. These studies demonstrated a pattern of greater postauricular reflex magnitude during pleasant images than during neutral or aversive images (e.g., Benning et al., 2004; Dichter, Benning, Holtzclaw, & Bodfish, 2010; Gable & Harmon-Jones, 2009; Hess, Sabourin, & Kleck, 2007; Quevedo, Benning, Gunnar, & Dahl, 2009; Sandt, Sloan, & Johnson, 2009). The postauricular reflex is also potentiated (larger) during pleasant versus neutral or aversive emotional sounds (Benning, 2011), suggesting that the reflex measures positive emotion across sensory modalities.
This reflex may assess appetitive emotional processing more specifically. Postauricular reflexes are more strongly potentiated during pleasant stimuli while people purse their lips (Johnson, Valle-Inclán, Geary, & Hackley, 2012), congruent with primates drawing their ears back while nursing. This pattern is also consistent with research that shows the postauricular reflex is the greatest during images of food and erotic scenes (Sandt et al., 2009), which should directly activate the appetitive system more so than other pleasurable stimuli (such as images of cute animals; Gard et al., 2007). Additionally, postauricular reflexes index appetitive processing in anticipation of consuming chocolate (Hackley, Muñoz, Hebert, Valle-Inclán, & Vila, 2009). However, it is unclear what processes may modulate postauricular reflex magnitude while emotional stimuli are processed and whether these processes operate at the same speed as those for the startle blink reflex. Research on the time course of emotional modulation of the startle blink reflex may provide important clues.
Time Course of Reflex Modulation
Modulation of the startle blink reflex during pictures represents a dynamic interplay between attentional and emotional processes. There is an initial prepulse facilitation (or increase) of startle blink magnitude for all picture contents relative to subsequent time points that represents either cross-modal sensory integration at a common downstream neural location or a muscular priming of the blink response by picture onset (Bradley et al., 2006). Startle blink magnitude then diminishes as pictures are processed more thoroughly; within 300 ms of picture onset, it is particularly inhibited (or decreased) during both pleasant and aversive pictures (Bradley et al., 1993, 2006). This inhibition reflects early attention commanded by pleasant and aversive pictures compared to neutral pictures (Bradley et al., 2006). Between 1,300–2,000 ms, a separate process thought to reflect input from the central nucleus of the amygdala (Davis & Shi, 1999) overcomes this attentional inhibition to potentiate startle blink magnitude during aversive pictures above that during neutral pictures, though startle blink magnitude during pleasant pictures remains inhibited relative to that during neutral pictures (Bradley et al., 1993, 2006). Thus, three processes underpin startle blink modulation: (1) An initial prepulse facilitation, which yields to (2) an attentional inhibition for emotional pictures that operates throughout the picture for pleasant pictures, and (3) after sufficient time has passed to process the picture fully, a final process generates fear-potentiated startle.
At least two of these processes, attentional inhibition and emotional facilitation, would be expected to modulate postauricular reflex magnitude over time. Visual stimuli reduce postauricular reflex magnitude in proportion to the cognitive load needed to evaluate those stimuli throughout a task (Parks, Hilimire, & Corballis, 2009). Thus, because pictures are complex stimuli, it is reasonable to assume that attentional inhibition should reduce postauricular reflex magnitude, particularly during the early processing of emotional versus neutral pictures. As postauricular reflexes are also larger during pleasant than during neutral or aversive stimuli 3–5 s after stimulus onset (Benning, 2011), it is likely that a subsequent process increases postauricular reflex magnitude during pleasant pictures. It is unclear whether postauricular reflexes are initially facilitated by picture onset, as the majority of attentional work on the postauricular reflex has focused on prepulse inhibition (e.g., Hackley, Woldorff, & Hillyard, 1987).
In picture-viewing paradigms, only one startle probe is typically presented during each image. This probe is often a 50-ms, 105 dB white noise burst with nearly instantaneous rise time (see Blumenthal et al., 2005, for guidelines on eliciting startle blink reflexes) Thus, to elucidate the fine-grained time course of emotional modulation of the startle blink reflex, researchers have varied probe presentation both within and between subjects (Bradley et al., 2006). This approach is less feasible for assessing the time course of modulation of the postauricular reflex; as a microreflex, the postauricular reflex requires aggregating multiple trials within each participant to yield interpretable waveforms (Hackley et al., 1987). Thus, applying traditional methods for assessing the time course of the startle blink is not possible for the postauricular reflex. Were the stimulus eliciting the postauricular reflex shorter and less aversive, it would be possible to present multiple clicks throughout each image. Such a procedure would facilitate a time course analysis of the postauricular reflex, allowing a more nuanced examination of emotional response during each picture valence.
Alternative Acoustic Probes to Elicit Startle Blink and Postauricular Reflexes
The audiology literature has used a variety of acoustic stimuli to elicit the postauricular reflex as a screening tool for deafness in infants (Agung, Purdy, Patuzzi, O’Beirne, & Newall, 2005). These noises are shorter (as short as 100 μs; Agung et al., 2005), softer (e.g., 50 dB; see O’Beirne & Patuzzi, 1999), and have varying rise times (see Agung et al., 2005) compared to the standard startle probe. Historically, softer and shorter clicks elicit postauricular reflexes without concerns about having one click alter reactivity to subsequent clicks (Yoshie & Okudaira, 1969). In contrast, postauricular reflexes occasioned by serial startle probes both habituate and are not modulated by emotion after the first probe (Hebert, Valle-Inclán, & Hackley, 2015). Because subsequent startle probes may activate the defensive system (Blumenthal & Berg, 1986) due to their aversive nature (Lang, Bradley, & Cuthbert, 1990), softer and shorter clicks may be more likely to elicit continued emotional postauricular reflex modulation throughout the latter portions of pictures.
Current Study
The primary goal of the current study was to examine whether clicks can elicit the same emotionally modulated postauricular reflex as loud probes by using a click to elicit postauricular reflexes throughout each picture. This design also allowed us to investigate the time course of postauricular reflex modulation during picture processing. We employed a standard picture-viewing paradigm, in which pictures were presented for 6 s each. A single startle probe was presented during each image to elicit postauricular and startle blink reflexes with typical acoustic stimuli. We expected to see postauricular reflexes potentiated during pleasant images and startle blink reflex potentiated during aversive images relative to those during neutral images, consistent with existing literature. In addition, 65 dB, 100 μs white noise clicks similar to those used in the audiology literature (Agung et al., 2005) were played every 100 ms. We reported effect sizes throughout to quantify whether they were similar across probe- and click-elicited reflexes, and whether any observed effects differed in their size throughout the course of picture processing.
We predicted that these clicks would yield a similar pattern of emotional postauricular reflex modulation as typical noise probes, with potentiation to pleasant images compared to aversive and neutral images. We investigated the internal consistency of probe- and click-elicited reflexes during each picture valence along with their emotional modulations to assess whether reflexes elicited by each acoustic stimulus shared similar psychometric properties. Using the clicks to elicit the postauricular reflex also allowed a more nuanced depiction of the time course of its modulation throughout image presentation. We aggregated responses to clicks over 500-ms time bins, for a total of 12 time bins during the 6-s picture presentation. We expected to see a time course similar to the pattern of startle blink reflex modulation found in previous research (Bradley et al., 1993, 2006) with an initial facilitation of the reflex, followed by inhibition during emotional compared to neutral pictures, and ending with a consistent pattern of appetitive modulation.
Method
Participants
Participants were 90 undergraduates recruited from psychology classes at Vanderbilt University. The sample was 52% female, and participants’ mean age was 19.7 years (SD =2.09). In total, 80.2% of participants identified as White, 8.6% as Black, 3.7% as Hispanic, and 7.4% as another race or ethnicity. Because there were no significant interactions of gender or race/ethnicity with emotional modulation of the reflexes (all , median ), we analyzed data across the sample as a whole.
Data for both startle blink and postauricular reflexes were lost for two participants due to equipment failure. Five participants had postauricular reflex data that were either contaminated with artifact or had no discernible response to the startle probe, leaving 83 participants in the analyses of postauricular reflexes. Five participants were excluded from the startle blink analyses for similar reasons, leaving a total of 83 participants in those analyses. Students received course credit for their participation. This study was approved by Vanderbilt University’s Institutional Review Board, and all participants were treated consistent with the principles elaborated in the Declaration of Helsinki.
Stimuli
Pictures
A total of 55 images from the IAPS (Lang et al., 2005) were selected for use in this experiment. Data from the first three images were discarded, as these images were used to habituate participants to the startle probes. Four images were presented without startle probes to reduce probe predictability. The remaining 48 images consisted of 16 pleasant, 16 neutral, and 16 aversive images. There was an equal distribution of specific image categories. Within six of the 12 content categories, some pictures varied between men and women to achieve this balance as described below.
Content categories consisted of the following IAPS pictures. Pictures not in parentheses were presented to participants of either gender; pictures within parentheses to the left of the slash were presented only to men, and those within parentheses to the right of the slash were presented only to women. Adventure: 5623, 8034, 8180, 8210; erotic: 4640, 4660, 4680, (4255/4572); food: 7200, 7230, 7260, 7460; nurturant: (1811, 2071, 2160, 2340/1463, 1722, 2341, 2655); buildings: 5731, 7180, 7490, 7491; humans: 2190, 2393, 2870, 2890; landscapes: 5120, 5390, 5740, 9210; objects: 7002, 7004, 7034, (7031/7038); disgust: 9342, 9520, 9560, 9830; mutilation: (3051, 3061, 9253, 9420/9042, 9265, 9440, 9490); threat: 6250, 6260, 9630, (6243/6190); victim: 6570, 9920, (6312, 6540/6530, 6561). As is evident from the descriptive statistics in Table 1, all image contents were gender balanced on dimensions of normatively rated valence, median t(6) =0.25, p =.810, and arousal, median t(6) =0.09, p =.933.
Table 1.
Normative Valence and Arousal Ratings of Picture Stimuli
| Picture content | Men
|
Women
|
||
|---|---|---|---|---|
| Valence | Arousal | Valence | Arousal | |
| Pleasant | ||||
| adventure | 7.25 | 6.04 | 7.23 | 6.20 |
| erotic | 7.34 | 6.10 | 7.32 | 6.16 |
| food | 7.23 | 5.16 | 7.29 | 5.16 |
| nurturant | 7.30 | 5.08 | 7.47 | 5.02 |
| Neutral | ||||
| buildings | 5.03 | 2.79 | 5.18 | 2.74 |
| humans | 4.90 | 2.77 | 5.06 | 2.87 |
| landscapes | 4.83 | 2.76 | 5.04 | 3.03 |
| objects | 4.94 | 2.79 | 4.97 | 2.64 |
| Aversive | ||||
| disgust | 2.52 | 5.03 | 2.48 | 5.10 |
| mutilation | 2.82 | 6.34 | 2.73 | 6.38 |
| threat | 2.71 | 5.23 | 2.77 | 5.26 |
| victim | 2.58 | 6.04 | 2.52 | 6.01 |
Note. Four pictures were included in each content category.
Images and probe placement were counterbalanced in this study: Two sets of four different serial positions of stimuli were used, one set designed for women and the other for men. In all of the stimulus orders, no more than two stimuli of the same valence were presented next to each other, and images with the same content were never presented one after the other.
Reflex-eliciting acoustic stimuli
The startle probe was a bilateral 50-ms, 105 dB white noise probe with nearly instantaneous rise time. One probe was presented at 3, 4, or 5 s after image onset. The click was a bilateral ~100 μs, 65 dB noise burst with nearly instantaneous rise time and comprised the first 100 μs of the 50-ms noise probe .wav file. This stimulus was used because it was the one in Agung et al. (2005) that was closest to the traditional startle probe. Following image onset, these clicks were presented every 100 ms, except when the startle probe was played. In this case, the startle probe replaced that click, and another click occurred 100 ms after the startle probe. Spacing clicks 100 ms apart allows for recovery of postauricular reflex magnitude between clicks (Yoshie & Okudaira, 1969). During probed intertrial intervals, startle probes were delivered 1.5 s after picture offset.
Physiological Measures
These data were part of a larger psychophysiological battery involving startle blink and postauricular reflexes, corrugator and zygomatic electromyography (EMGs), skin conductance, and EEG collected with the 10–20 system (Benning & Ait Oumeziane, 2016). The postauricular reflex was collected using the optimal locations described in O’Beirne and Patuzzi (1999). A pair of 4-mm Ag-AgCl electrodes was placed over the postauricular muscle of each ear, identified by locating the fibrous strip connecting the pinna (outer ear) and scalp. One electrode was placed over the muscle on the scalp (PAM 5), and the other adjacent to the first on the pinna (PINNA 2). Blink reflexes were also collected using a pair of 4-mm Ag-AgCl electrodes following Blumenthal et al. (2005). One electrode was placed 5 mm below the lid of the right eye, directly under the pupil. The second was placed adjacent to the first, toward the outer canthus.
Startle blink magnitude was recorded from the orbicularis oculi muscle below the right eye, zygomatic EMG was recorded from the right cheek, and corrugator EMG was recorded from above the right eyebrow. EEG was collected using a NeuroScan Quik-Cap, and skin conductance response was collected from the thenar and hypothenar eminences with a UFI 2701 BioDerm skin conductance meter. Prior to placement, all sites were scrubbed with an abrasive gel to reduce impedances below 10 kΩ. Data were sampled at 2000 Hz with a NeuroScan SynAmps2 bioamplifier (Compumedics, Charlotte, NC) at DC with a 500 Hz low-pass filter.
Procedure
Participants arrived and completed a consent form. Next, they were given a verbal description of the psychophysiology setup. They then completed a battery of questionnaires on the computer while seated in a comfortable chair 100 cm away from a 19″ monitor (with a resolution of 1,280 × 1,024 pixels and a refresh rate of 60 Hz) and having electrodes attached to their bodies. Once setup was complete, they were instructed to watch the images that filled the screen and to focus on a fixation cross in the absence of an image. They were also told that they would hear brief noises through headphones, which they could ignore. They were asked to remain as still as possible throughout the data collection portion of the experiment.
They were then presented with three images and noise probes (which served as habituation trials) and given demonstrations of Self-Assessment Manikin ratings (SAM; Bradley & Lang, 1994), which were used to assess valence and arousal after each image. These habituation trials were not included in analyses. Participants were given the opportunity to ask questions, after which the experimenter left the room and the experiment began.
Each image was presented for 6 s followed by a 3-s poststimulus fixation cross. As in the habituation trials, participants completed SAM ratings after each image and then focused on a 3-s fixation cross until the next image appeared. Including SAM ratings, the approximate trial duration was 20 s.
Data Reduction and Analysis
Offline, blink, and postauricular EMGs were epoched from 100 ms preprobe or click to 250 ms postprobe or click. Consistent with published guidelines (Blumenthal et al., 2005), blink data were further bandpass filtered at 28–250 Hz before rectification, whereas postauricular data were rectified after epoching. Blink data for the startle probe were scored trial by trial as the peak activity 30–120 ms following the onset of the probe minus the mean activity 50 ms before probe onset. Postauricular reflexes were scored as aggregate waveforms averaged across each picture content as the peak activity 8–35 ms postprobe or click onset minus the mean activity 50 ms preprobe or click onset. In all cases, negative peaks were set to zero. Data were z scored within subject and electrode for probes and clicks separately, and postauricular data were subsequently averaged over both ears. Rectified zygomatic EMGs were scored trial by trial as the mean activity during the 6-s picture presentation minus the mean activity 1 s before picture presentation with negative means set to zero (Bradley, Codispoti, Cuthbert, & Lang, 2001).
To examine emotional modulation of each reflex to the startle probe, we performed separate within-subject analyses of variance (ANOVAs) on startle blink and postauricular reflexes. We conducted planned follow-up pairwise comparisons of startle blink and postauricular reflex magnitude at each valence. We used within-subject t tests to investigate which emotional contents modulated postauricular reflex magnitude compared to neutral pictures.
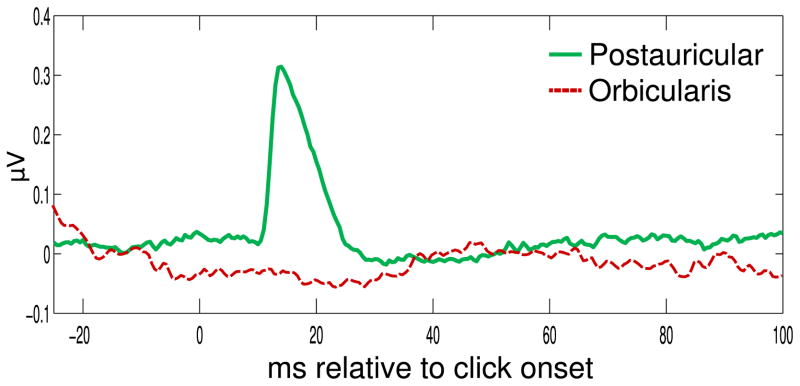
We conducted similar within-subject ANOVA and t tests to examine emotional modulation of postauricular reflexes elicited by clicks. Only postauricular reflexes were included in these analyses because startle blink reflexes were not elicited by clicks in this study (see Figure 1). To map the time course of emotional modulation of the postauricular reflex, we looked at postauricular activity in 500-ms time bins with valence (pleasant, neutral, aversive) and time (12 time bins total) as the factors in a separate within-subject ANOVA. We aggregated the responses to clicks over each 500-ms time bin, such that each bin comprised five clicks. Bins that contained a response to the startle probe were excluded from these aggregates. To assess the time course of potentiation, we conducted planned pairwise comparisons between the three valence categories at each time bin. To investigate whether postauricular reflexes to clicks and probes assessed similar processes, we also correlated probe-elicited postauricular reflexes with click-elicited postauricular reflexes.
Figure 1.
Grand-averaged postauricular and orbicularis oculi muscle responses to 65 dB, 100 μs white noise clicks.
For both probe- and click-elicited reflexes, we computed Cronbach’s alpha (coefficient α) in two ways. In the first set of αs, we used reflex magnitudes during the four contents within each valence as items to compute an α value for each valence. In the second set of αs, we used the differences between reflex magnitudes during the four pleasant or aversive contents and reflex magnitudes during neutral pictures to compute α values for appetitive and defensive modulation of the reflexes. In addition to these summary statistics, we computed the range and median of the intercontent correlations. These correlations should typically range between .15 to .50 to create an adequate measure of a higher-order construct (Clark & Watson, 1995).
All statistical tests were performed using SPSS 22 with an α level of .05. All ANOVAs used the Huynh-Feldt correction for nonsphericity, for which corrected degrees of freedom are reported. Partial eta squared ( ) served as the effect size measure for the ANOVAs, whereas Cohen’s d served as the effect size measure for the various t tests. Following traditional interpretation guidelines (Cohen, 1988), ds of at least 0.2 but less than 0.5 were considered small effect sizes, those of at least 0.5 but less than 0.8 were considered medium effect sizes, and those of 0.8 or greater were considered large effect sizes.
Results
Reflexive Emotional Modulation to Startle Probes
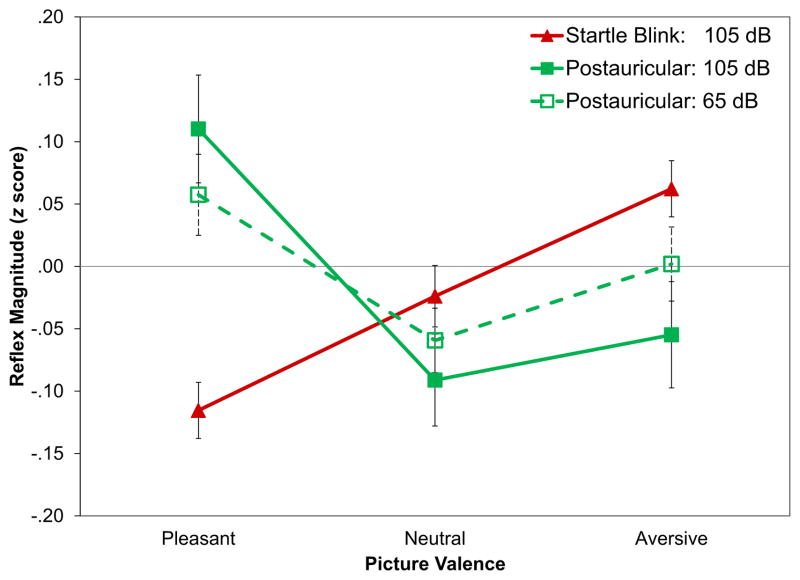
Figure 2 depicts the emotional modulation by valence of startle blink and postauricular reflex magnitude to the startle probes. There was a significant main effect of valence on postauricular reflex magnitude, F(2,164) =4.80, p =.010, . Follow-up pairwise comparisons revealed that postauricular reflexes during pleasant images were greater than those during neutral, t(82) =3.00, p =.003, d =0.33, and aversive images, t(82) =2.20, p =.030, d =0.24. However, postauricular reflexes during neutral and aversive pictures did not differ from each other, t(82) =0.55, p =.580, d =.06. There was also a significant main effect of valence on blink magnitude, F(2,164) =10.3, p <.001, . Follow-up pairwise comparisons revealed that startle blink reflexes during aversive pictures were greater than those during neutral, t(82) =2.17, p =.033, d =0.24, and pleasant images, t(82) =4.82, p <.001, d =0.54. Startle blink reflexes were also greater during neutral pictures than pleasant pictures, t(82) =2.24, p =.028, d =0.25.
Figure 2.
Emotional modulation of startle blink and postauricular reflexes elicited by 105 dB, 50-ms white noise probes or 65 dB, 100 μS white noise clicks. Error bars represent the standard error of each mean.
Table 2 displays the modulation scores for reflex magnitudes, calculated as the difference during each emotional content category from neutral. Startle blink magnitude was potentiated during threatening pictures and inhibited during erotic pictures; contrary to previous studies, it was also inhibited during disgust pictures. Postauricular reflex magnitude was potentiated during erotic, food, and nurturant pictures; it was inhibited during threatening pictures. Unexpectedly, it was also potentiated during mutilation scenes. Across contents, modulation scores for postauricular and startle blink reflexes were generally uncorrelated, |r|s <.20, ps >.08 (median |r| =.14). The lone exception was for victim pictures. However, the difference during victim and neutral pictures was positively rather than inversely correlated between reflexes, r(73) =.38, p <.001.
Table 2.
Emotional Modulation of Startle Blink and Postauricular Reflexes by Picture Content
| Picture content | Startle blink 105 dB
|
Postauricular 105 dB
|
Postauricular 65 dB
|
Postauricular correlations 105 dB/65 dB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SE) | d | Mean | (SE) | d | Mean | (SE) | d | ||
| Pleasant | −0.092* | (0.041) | −0.25 | 0.209** | (0.070) | 0.33 | 0.117* | (0.050) | 0.25 | .49*** |
| adventure | −0.025 | (0.059) | −0.05 | −0.038 | (0.096) | −0.04 | 0.036 | (0.037) | 0.11 | .27* |
| erotic | −0.160** | (0.059) | −0.30 | 0.366** | (0.112) | 0.36 | 0.100 | (0.058) | 0.19 | .32** |
| food | −0.079 | (0.056) | −0.15 | 0.261* | (0.099) | 0.28 | 0.114* | (0.045) | 0.28 | .49*** |
| nurturant | −0.090 | (0.057) | −0.17 | 0.234* | (0.108) | 0.23 | 0.155** | (0.058) | 0.29 | .41*** |
| Aversive | 0.086* | (0.040) | 0.24 | 0.040 | (0.066) | 0.07 | 0.061 | (0.045) | 0.15 | .46*** |
| disgust | −0.112* | (0.051) | −0.24 | −0.123 | (0.090) | −0.17 | 0.044 | (0.041) | 0.12 | .19 |
| mutilation | 0.008 | (0.057) | 0.02 | 0.446*** | (0.098) | 0.49 | 0.083 | (0.050) | 0.18 | .28* |
| threat | 0.408*** | (0.083) | 0.54 | −0.212* | (0.099) | −0.25 | 0.004 | (0.037) | 0.01 | .32** |
| victim | 0.040 | (0.060) | 0.07 | 0.075 | (0.108) | 0.07 | 0.035 | (0.045) | 0.09 | .30** |
Note. Ns range from 80–83. Scores represent the difference between reflexes during pictures with the labeled content and reflexes during neutral pictures. Effect sizes of at least 0.2 SD are presented in bold.
p <.05.
p <.01.
p <.001.
Reliabilities of Startle Blink and Postauricular Reflex Magnitudes and Modulations
Reflex magnitudes analyzed for picture contents within each valence separately had negligible to negative reliability coefficients. This pattern was found for postauricular reflex magnitudes during pleasant contents (α =.01; rintercontent range [−.16, .23], median =−.02), neutral contents (α =−.30; rintercontent range [−.20, .05], median =−.05), and aversive contents (α =−.30; rintercontent range [−.27, .20], median =−.06). Startle blink magnitude during pleasant contents (α =−.16; rintercontent range [−.17, .10], median =−.02), neutral contents (α =−.09; rintercontent range [−.17, .22], median =−.07), and aversive contents (α =−.65; rintercontent range [−.22, .11], median =−.13) showed the same pattern.
In contrast, reliabilities for reflex modulation scores for pleasant or aversive contents minus neutral pictures were positive, albeit lower than desirable for many psychological measures (Clark & Watson, 1995). This pattern held both for postauricular reflex modulation during pleasant contents (α =.58; rintercontent range [.14, .42], median =.24) and aversive contents (α =.45; rintercontent range [−.08, .35], median =.22) and for startle blink modulation during pleasant contents (α =.64; rintercontent range [.21, .40], median = .33) and aversive contents (α =.47; rintercontent range [.09, .38], median =.17).
Time Course of Emotional Modulation of Postauricular Reflexes Elicited by 65 dB Clicks
As evident in Figure 2, there was a significant main effect of valence on postauricular reflex magnitude to the clicks, F(1.89,155) =3.62, p =.032, . Pairwise comparisons revealed that postauricular reflex magnitude was greater during pleasant than neutral images, t(82) =2.67, p =.009, d =0.30. However, postauricular reflexes during aversive pictures did not differ from those during pleasant, t(82) =1.35, p =.179, d =0.15, or neutral pictures, t(82) =1.36, p =.180, d =0.15. Table 2 shows that click-elicited postauricular reflexes during erotic, food, and nurturant pictures were greater than those during neutral pictures; no click-elicited postauricular reflexes during aversive pictures differed from those during neutral pictures.
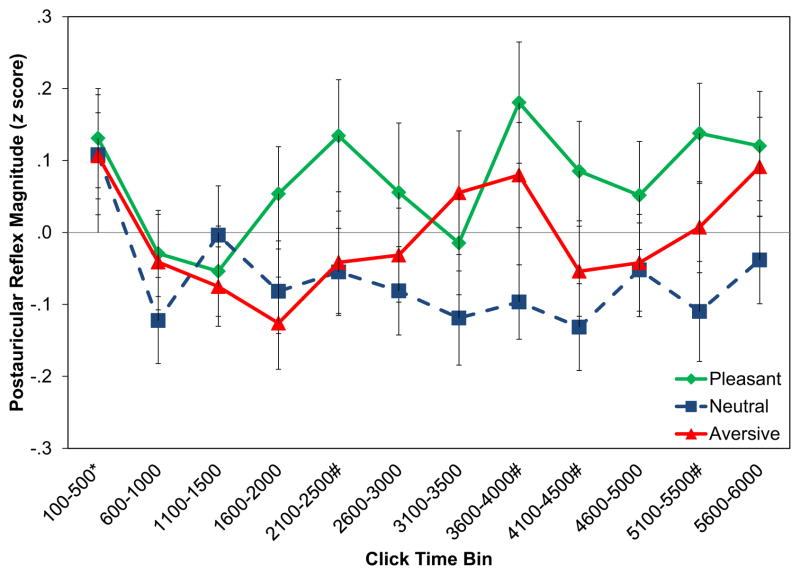
Figure 3 depicts the time course of emotional postauricular reflex modulation over the course of the image. There was a significant main effect of time on postauricular reflex magnitude, F(10.2,839) =1.98, p =.032, . Consistent with prepulse facilitation by pictures, average postauricular reflexes during the 100–500 ms time bin across all valences were significantly larger than the average click-elicited reflex throughout each picture, F(1,82) =9.10, p =.003, . To examine whether this effect was driven only by the first click in this series (as would be expected based on the time course of startle blink modulation; Bradley et al., 2006) or reflected activity throughout all five clicks, we analyzed average postauricular reflex magnitude during each of the five clicks separately within this bin. Reflex magnitude decreased across clicks, F(4,320) =3.26, p =.012, ; specifically, postauricular reflex magnitude decreased across each click in this time bin (Ms =.19, .07, .01, −.13, −.14; all SEs =.07), linear contrast, F(1,80) =12.6, p =.001, . No other time bins exhibited facilitation or inhibition of average postauricular reflex magnitude, Fs(1,82) <3.8, ps >.05, .
Figure 3.
Time course of emotional postauricular reflex modulation. Error bars represent the standard error of each mean. * time bin in which the mean postauricular reflex magnitude across all valences was different from the mean postauricular reflex magnitude across the entire picture; # time bin with a pleasant minus neutral effect size (Cohen’s d) of at least 0.2, representing at least a small effect size.
There was no significant Valence × Time interaction, F(20.8,1700) =0.83, p =.679, . Nevertheless, inspection of Figure 3 suggests substantial variability in the effect sizes of postauricular reflex potentiation during pleasant versus neutral pictures. The following time bins had at least small effect sizes for this effect: 2,100–2,500 ms (d =0.20), 3,600–4,000 ms (d =0.30), 4,100–4,500 ms (d =0.25), and 5,100–5,500 ms (d =0.28). The median effect size across all other time bins was 0.11; the only negative effect size was in the 1,100–1,500 ms time bin (d =−0.05).
Correlations Among Postauricular Reflexes Elicited by Clicks and Probes
Overall, postauricular reflexes during pictures of each valence correlated between clicks and probes: pleasant r(81) =.40, p <.001; neutral r(81) =.46, p <.001; aversive r(81) =.42, p <.001. In particular, postauricular reflex magnitudes during pleasant erotic (r =.32, p =.004), food (r =.45, p <.001), and nurturant contents (r =.40, p <.001) correlated across clicks and probes; those during adventure contents (r =.10, p =.373) did not. Click- and probe-elicited postauricular reflexes during neutral human (r =.37, p =.001), landscape (r =.31, p =.004), and object scenes (r =.24, p =.031) correlated; those during buildings (r =.14, p =.198) did not. Postauricular reflexes during aversive mutilation (r =.26, p =.020) and victim pictures (r =.26, p =.016) correlated across clicks and probes; those during disgust (r =.17, p =.125) and threat pictures (r =.18, p =.111) did not. These eight significant heteromethod–monocontent correlations (out of 12 possible) were more than would be expected given the Type I error rate of this study (binomial sign test p <.001), suggesting that postauricular reflexes during these contents are reasonably similar across methods of eliciting the reflex. However, like their probe-elicited counterparts, click-elicited postauricular reflexes during pleasant (α =−.03; rintercontent range [−.21, .19], median =−.01), neutral (α =.01; rintercontent range [−.09, .17], median =−.02), and aversive (α =−.10; rintercontent range [−.23, .14], median = .00) contents were not reliable measures of each broader valence.
As shown in Table 2, the modulations of postauricular reflexes for each valence and content also correlated between probes and clicks, with the exception of disgust pictures. Consistent with findings for probe-elicited reflexes, the modulations of click-elicited postauricular reflexes during pleasant contents versus neutral pictures (α =.57; rintercontent range [.10, .41], median = .26) and during aversive contents versus neutral pictures (α =.45; rintercontent range [.01, .40], median =.15) were more reliable measures of emotional processing.
Discussion
In this study, we replicated well-established effects of picture valence and content on postauricular and startle blink reflexes elicited by 105 dB, 50-ms white noise acoustic probes. We found that 65 dB, 100 μs clicks also elicited postauricular reflexes—but not startle blinks—and that, unlike the startle blink reflex, emotional postauricular reflex modulation was relatively consistent across time points, albeit with substantially differing effect sizes throughout the course of picture processing. Food and nurturant scenes potentiated both click- and probe-elicited postauricular reflexes. Finally, postauricular reflex magnitude correlated between clicks and probes during pleasant erotic, food, and nurturant images; neutral humans, landscapes, and objects; and aversive mutilation and victim scenes; thus, clicks were effective at eliciting emotionally modulated postauricular reflexes. For all reflexes, emotion versus neutral modulation was a more reliable measure of emotional processing than the reflex magnitudes of various contents within a particular valence.
Comparability of Postauricular Reflexes Elicited by Clicks and Probes
Overall, though our click-elicited postauricular reflexes were smaller than those in the audiology literature (Purdy, Agung, Hartley, Patuzzi, & O’Beirne, 2005), postauricular reflexes elicited by clicks and probes had roughly equivalent effect sizes for the pleasant-neutral difference, though these effect sizes were small. These results comport well with prior work using this set of pictures that found postauricular reflexes are largest during erotic images (Benning, 2011) and provide additional evidence that the postauricular reflex indexes appetitive processes tied to food (Hebert et al., 2015; Quevedo et al., 2009; Sandt et al., 2009). However, postauricular reflexes index positive emotionality beyond appetitive processing, as it was also potentiated during nurturant pictures in the current study, consistent with other studies (Benning, 2011; Quevedo et al., 2009). Contrary to Benning et al. (2004) but in line with Gable and Harmon-Jones (2009), pleasant pictures both high (i.e., erotica) and low in arousal (i.e., food and nurturant) potentiated postauricular reflexes. During each of these contents, probe- and click-elicited postauricular reflexes correlated, indicating that both methods of occasioning the postauricular reflex tap positive emotionality beyond appetitive processing. Probe- and click-elicited postauricular reflexes were not correlated during adventure scenes, indicating that these scenes do not assess the same sort of positive emotion as other picture contents. This is consistent with other studies reporting that adventure scenes do not modulate postauricular reflexes (Benning, 2011; Quevedo et al., 2009; Sandt et al., 2009; cf. Quevedo et al., 2015).
The click- and probe-elicited postauricular reflexes cannot be held as equivalent measures of positive emotion, as the correlations between them were far from 1. Postauricular reflex magnitudes during the various pleasant contents did not correlate significantly, calling into question the unitary nature of the positive emotionality that this reflex assesses without comparison to neutral pictures. Indeed, the negative reliability coefficients indicate that there is more variance within each content than covariance between contents (Henson, 2001). Modulation scores appear to serve as more appropriate measures of broad appetitive and defensive processing with the postauricular and startle blink reflexes, respectively. This study appears to be one of the few that used coefficient α to assess the internal consistency of reflexive measures of emotion (e.g., Amodio, Harmon-Jones, & Devine, 2003); previous studies have typically assessed the reliability of emotion-modulated startle blink reflexes through test-retest correlations (e.g., Larson, Ruffalo, Nietert, & Davidson, 2000) or odd-even trial correlations (e.g., Bradford, Starr, Shackman, & Curtin, 2015). The coefficients α for emotional modulation compare favorably with ERP measures based on four trials per condition (Meyer, Riesel, & Proudfit, 2013), and the middling αs in this study belie the relatively acceptable ranges of interitem correlations for each emotional modulation. Using more contents may help increase reliability estimates of emotional modulation scores, as coefficient α increases with the number of measures used to construct a test (Cortina, 1993) or trials included in an aggregate waveform (Meyer et al., 2013).
There was an aberrant potentiation during mutilation pictures that occurred during probe- but not click-elicited reflexes, a finding that contradicts others in the literature with undergraduates (Benning, 2011; Sandt et al., 2009). However, removing this content would have improved the aversive-only α to −.07 and the aversive modulation α to .49, suggesting that the potentiation for mutilation scenes did not substantially impact the psychometrics of the probe-elicited postauricular reflex. It is possible that, due to the unusual concentration of premedical students in our psychology subject pool, our participants viewed mutilation scenes with curiosity and interest rather than horror (Arráez-Aybar, Castaño-Collado, & Casado-Morales, 2008). Because we did not assess students’ majors in this study, we could not test this possibility empirically, so it remains a purely speculative account of the differences between this study and other studies.
Probe-elicited postauricular reflexes may also be more sensitive to individual differences in positive emotionality. Postauricular reflex potentiation during pleasant pictures is correlated with trait well-being in postpubertal youth (Quevedo et al., 2009), and in this study’s dataset, probe-elicited appetitive postauricular reflex potentiation was negatively associated with depressive symptomatology (Benning & Ait Oumeziane, 2016). However, this study’s click-elicited appetitive postauricular reflex potentiation was not associated with well-being, r =−.04, p =.695, or depression, r =−.12, p =.290. These differences were not due to different reliabilities of appetitive potentiation across eliciting stimuli, so future research may need to use probes to elicit postauricular reflexes if individual differences are of interest.
Finally, with a way to assess postauricular reflex potentiation across picture presentation, these results provide a basis for understanding postauricular reflex potentiation above and beyond treating it as crosstalk from other facial or auricular muscles. In this study, there was no correlation between pleasant versus neutral potentiation of the postauricular reflex and EMG activity during pleasant versus neutral pictures in either the zygomaticus major (r probes =−.02, p =.878; r clicks =−.04, p =.745) or orbicularis oculi (r probes =.17, p =.117; r clicks =−.09, p =.422) muscles that are involved in genuine smiling (Ekman, Davidson, & Friesen, 1990). Emotional postauricular reflex modulation also cannot be due to vestibular evoked myogenic potentials, as those are only evoked by loud probes (Cook & Patuzzi, 2014).
Separability of Postauricular and Startle Blink Reflexes
Both startle blink and postauricular reflex magnitude exhibit prepulse facilitation at the start of picture processing. Our data provided no evidence of postauricular reflexes elicited by picture onset (cf. Bickford, Jacobson, & Cody, 1964). Therefore, it is unlikely that postauricular reflex prepulse facilitation reflects the summation of muscular activity by reflexes elicited by visual and auditory stimuli. Rather, this facilitation may reflect cross-modal sensory summation at a remote neural center. From our follow-up analyses, the summation likely occurs within 100 ms of picture onset and decays linearly throughout the first 500 ms of picture processing, rather than ceasing abruptly within 150 ms of picture onset as is the case for the startle blink reflex (Bradley et al., 2006).
Unlike the startle blink, the postauricular reflex did not exhibit significant attentional inhibition. The absence of this effect may explain why the inhibition of the postauricular reflex across a number of studies has been less robust than its potentiation for pleasant stimuli (e.g., Quevedo et al., 2009; Sandt et al., 2009). Also, unlike the startle blink reflex (which featured a defensive potentiation effect size consistent with that of the appetitive potentiation of the postauricular reflex), postauricular reflex potentiation did not differ significantly across the span of picture processing. Nevertheless, effect sizes for the potentiation of postauricular reflexes varied substantially throughout the picture. To obtain even a small reflexive potentiation, researchers would likely need to present postauricular reflex eliciting stimuli more than 2 s after picture onset. The best results would likely be obtained using clicks or probes in the latter half of the picture.
This study’s results build on previous work (Sandt et al., 2009), demonstrating that across picture contents, emotional startle blink and postauricular reflex modulations are uncorrelated (Sandt et al., 2009), and indicating that orthogonal appetitive and defensive systems modulate these two reflexes (Benning, 2011; Bernat, Patrick, Benning, & Tellegen, 2006). Furthermore, the differential reflexive activity of postauricular and orbicularis oculi muscles to soft, sub-millisecond white noise clicks highlights the neurobiological distinctions between the postauricular and startle blink reflexes (Hackley, 2015). Though 65 dB white noise probes can elicit startle blinks with nearly 50% probability (Blumenthal & Goode, 1991), our clicks were 500 times shorter than those used in previous low-intensity startle probe studies and did not elicit startle blinks. Thus, though postauricular reflexes can be elicited by short, punctate white noise clicks, producing startle reflexes requires longer-lasting probes if the probes are low intensity.
Limitations and Future Directions
Because two properties of the standard startle probe were altered (i.e., duration and intensity), it is unclear how each affected postauricular responses. It is possible that changing one property would have resulted in the desired effect, or that altering both was more effective than isolating only one (cf. Blumenthal, 1988). For example, it is possible that a 65 dB click with a duration of 50 ms would be more successful at eliciting emotionally modulated postauricular reflexes than a 65 dB click with a duration of 100 μs. Alternatively, louder clicks elicit larger postauricular reflexes (O’Beirne & Patuzzi, 1999), suggesting that a 100 μs click at a higher volume would yield more robust postauricular reflexes (Kiziltan, Gündüz, & Şahin, 2010).
The ideal rise time of the postauricular reflex-eliciting stimulus may also differ from that of the startle probe. Many noise probes have nearly instantaneous rise times (Blumenthal et al., 2005), as quicker rise times result in larger and more consistent startle blink reflexes (Blumenthal, 1988) and match the emotional state assessed through the defensive startle response (Lang et al., 1990). However, there are other dimensions on which eliciting acoustic stimuli can vary. Agung et al. (2005) found that 10-ms clicks with rising frequency, referred to as chirps, yielded larger postauricular reflexes than a 100 μs click like that used in the current study. Although it is unclear whether the duration or rising frequency of the chirp drove this effect, using a chirp might result in increased modulation and sensitivity to individual differences. Such a stimulus may also provide a better match to the appetitive system into which the post-auricular reflex is thought to tap (Johnson et al., 2012). Finally, this sample was limited to undergraduate students. More representative samples should be used to generalize these findings.
Nevertheless, this study reinforces the utility of the postauricular reflex as a measure of positive emotional processing, whether it is elicited by clicks or startle probes. Additional work should examine the degree to which click- and probe-elicited postauricular reflexes exhibit similar time courses of modulation, though this study suggests that placing probes more than 3 s after picture onset has fortuitously optimized appetitive postauricular reflex modulation in prior work. It remains to be seen whether click-elicited post-auricular reflexes are sensitive to extremes in individual differences in positive emotion, as in clinical depression (Dichter & Tomarken, 2008) or schizophrenia (Kring & Moran, 2008), in the broader community. Using clicks to elicit postauricular reflexes instead of noise probes also opens up the study of positive emotion in populations that are hypersensitive to loud noises, including participants with bipolar or autism spectrum disorders. It also allows a more continuous measurement of positive emotion throughout an experiment, creating a tool that can assess the course of wanting, liking, and reward learning (Berridge & Kringelbach, 2008).
Acknowledgments
This work was performed in partial fulfillment of a Master of Arts degree in Psychology for RVA. Portions of these data were presented previously at the 51st annual meeting of the Society for Psychophysiological Research.
References
- Agung KB, Purdy SC, Patuzzi RB, O’Beirne GA, Newall P. Rising-frequency chirps and earphones with an extended high-frequency response enhance the post-auricular muscle response. International Journal of Audiology. 2005;44:631–636. doi: 10.1080/14992020500266613. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink response and self-report. Journal of Personality and Social Psychology. 2003;84:738–753. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Arráez-Aybar LA, Castaño-Collado G, Casado-Morales MI. Dissection as a modulator of emotional attitudes of future health professionals. Medical Education. 2008;42:563–571. doi: 10.1111/j.1365-2923.2008.03079.x. [DOI] [PubMed] [Google Scholar]
- Benning SD. Postauricular and superior auricular reflex modulation during emotional pictures and sounds. Psychophysiology. 2011;48:410–414. doi: 10.1111/j.1469-8986.2010.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Ait Oumeziane B. Reduced positive emotionality is uniquely associated with subclinical depression: Evidence from psychophysiology, self-report, and symptom clusters. 2016 doi: 10.1111/psyp.12853. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41:426–432. doi: 10.1111/j.1469-8986.2010.01071. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43:93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford RG, Jacobson JL, Cody TR. Nature of average evoked potentials to sound and other stimuli in man. Annals of the New York Academy of Sciences. 1964;112:204–223. doi: 10.1111/j.1749-6632.1964.tb26749.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. The startle response to acoustic stimuli near startle threshold: Effects of stimulus rise and fall time, duration, and intensity. Psychophysiology. 1988;25:607–611. doi: 10.1111/j.1469-8986.1988.tb01897. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Berg WK. Stimulus rise time, intensity, and bandwidth effects on acoustic startle amplitude and probability. Psychophysiology. 1986;23:635–641. doi: 10.1111/j.1469-8986.1986.tb00682. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Goode CT. The startle blink response to low intensity acoustic stimuli. Psychophysiology. 1991;28(3):296–306. doi: 10.1111/j.1469-8986.1991.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Starr MJ, Shackman AJ, Curtin JJ. Empirically based comparisons of the reliability and validity of common quantification approaches for eyeblink startle potentiation in humans. Psychophysiology. 2015;52:1669–1681. doi: 10.1111/psyp.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:489–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Emotion, novelty, and the startle reflex: Habituation in humans. Behavioral Neuroscience. 1993;107:970–980. doi: 10.1037/0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7:309–319. doi: 10.1037/1040-3590.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cook A, Patuzzi R. Rotation of the eyes (not the head) potentiates the postauricular muscle response. Ear and Hearing. 2014;35:230–235. doi: 10.1097/AUD.0b013e3182a4efdf. [DOI] [PubMed] [Google Scholar]
- Cortina JM. What is coefficient alpha? An examination of theory and applications. Journal of Applied Psychology. 1993;78:98–104. doi: 10.1037/0021-9010.78.1.98. [DOI] [Google Scholar]
- Davis M, Shi C. The extended amygdala: Are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Science. 1999;877:281–291. doi: 10.111/j.1749-6632.1999.tb092273.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Benning SD, Holtzclaw TN, Bodfish JW. Affective modulation of the startle eyeblink and postauricular reflexes in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2010;40:858–869. doi: 10.1007/s10803-009-0925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117:1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58:342–353. doi: 10.1037/0022-3514.58.2.342. [DOI] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Postauricular reflex responses to pictures varying in valence and arousal. Psychophysiology. 2009;46:487–490. doi: 10.1111/j.1469-8986.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Mehta N, Kring AM, Patrick CJ. Impact of motivational salience on affect modulated startle at early and late probe times. International Journal of Psychophysiology. 2007;66:266–270. doi: 10.1016/j.ijpsycho.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Hackley SA. Evidence for a vestigial pinna-orienting system in humans. Psychophysiology. 2015;52:1263–1270. doi: 10.1111/psyp.12501. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Muñoz MA, Hebert K, Valle-Inclán F, Vila J. Reciprocal modulation of eye-blink and pinna-flexion components of startle during reward anticipation. Psychophysiology. 2009;46:1154–1159. doi: 10.1111/j.1469-8986.2009.00867. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Woldorff M, Hillyard SA. Combined use of microreflexes and event-related brain potentials as measures of auditory selective attention. Psychophysiology. 1987;24:632–647. doi: 10.1111/j.1469-8986.1987.tb00343. [DOI] [PubMed] [Google Scholar]
- Hebert KR, Valle-Inclán F, Hackley SA. Modulation of eye-blink and postauricular reflexes during the anticipation and viewing of food images. Psychophysiology. 2015;52:509–517. doi: 10.1111/psyp.12372. [DOI] [PubMed] [Google Scholar]
- Henson RK. Understanding internal consistency reliability estimates: A conceptual primer on coefficient alpha. Measurement and Evaluation in Counseling and Development. 2001;34:177–189. [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44:431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Johnson GM, Valle-Inclán F, Geary DC, Hackley SA. The nursing hypothesis: An evolutionary account of emotional modulation of the postauricular reflex. Psychophysiology. 2012;49:178–185. doi: 10.1111/j.1469-8986.2011.01297. [DOI] [PubMed] [Google Scholar]
- Kiziltan ME, Gündüz A, Şahin R. Auditory evoked blink response and posterior auricular muscle response: Observations in patients with HFS and PFS. Journal of Electromyography and Kinesiology. 2010;20:508–512. doi: 10.1016/j.jelekin.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. [DOI] [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Temporal stability of the emotion-modulated startle response. Psychophysiology. 2000;37:92–101. doi: 10.1111/1469-8986.3710092. [DOI] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Proudfit GH. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology. 2013;50:1220–1225. doi: 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- O’Beirne GA, Patuzzi RB. Properties of the sound-evoked post-auricular muscle response (PAMR) Hearing Research. 1999;138:115–132. doi: 10.1016/S0378-5955(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Parks NA, Hilimire MR, Corballis PM. Visual perceptual load modulates an auditory microreflex. Psychophysiology. 2009;46(2):498–501. doi: 10.1111/j.1469-8986.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Purdy SC, Agung KB, Hartley D, Patuzzi RB, O’Beirne GA. The post-auricular muscle response: An objective electrophysiological method for evaluating hearing sensitivity. International Journal of Audiology. 2005;44:625–630. doi: 10.1080/14992020500266639. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Developmental Psychopathology. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman MM, Lafavor T, Moua B, Gunnar M. The impact of early neglect on defensive and appetitive physiology during the pubertal transition: A study of startle and postauricular reflexes. Developmental Psychobiology. 2015;57:289–304. doi: 10.1002/dev.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive processing with the postauricular reflex. Psychophysiology. 2009;46:491–497. doi: 10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: Affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychological Bulletin. 2009;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037/0021-843X.97.4.487. [DOI] [PubMed] [Google Scholar]
- Yoshie N, Okudaira T. Myogenic evoked potential responses to clicks in man. Acta Oto-laryngologica Supplementum. 1969;252:89–103. doi: 10.3109/00016486909120515. [DOI] [PubMed] [Google Scholar]