Abstract
Methods
357 patients at a free STD clinic in Miami, FL were screened for HCV. Surveys were administered assessing risk factors for infectious disease transmission, and HCV and HIV screening history.
Results
15.1% of participants had been screened for HCV before whereas 83.8% had been screened for HIV (n = 356). Of the patients previously screened for HCV (n = 54), 98.2% of these patients had previously been screened for HIV as well.
Conclusion
This data shows the low prevalence of prior HCV screenings in a high-risk population in Miami, FL. Participants who had previously received an HIV screening test were more likely to report receiving a prior HCV screening. Despite the high prevalence of HCV, most HCV infections are undiagnosed. Mortality from HIV has been declining in the United States while mortality from HCV is increasing. To decrease HCV related mortality, we recommend offering HCV screening in conjunction with HIV screening.
Keywords: HCV, HIV, Screening, Miami, Infectious disease
Introduction
Hepatitis C virus (HCV) infection is the most prevalent chronic blood borne infection in the United States. There are an estimated 130–180 million infected individuals globally, and there are 3–4 million new cases annually [1]. In comparison to HIV, there are 35.9 million people affected worldwide, and 2 million people are infected annually. Despite a higher prevalence of HCV as compared to HIV, most HCV infections are undiagnosed in the United States. In addition, more deaths in the United States are attributed to HCV than HIV [2]. Specifically, 2.7–3.9 million people in the United States are estimated to have chronic HCV infection and 43–72% of these HCV infections are undiagnosed. In comparison to the estimated 1.2 million people living with HIV in the United States, only 14% are presumed undiagnosed. Mortality from HIV has been declining in the United States while mortality from HCV is currently increasing [2].
Low diagnosis rates for HCV infection may result from the fact that acute HCV is usually asymptomatic; however, 55–85% of those infected will develop chronic HCV infection. For those with chronic HCV infection, the risk for liver cirrhosis is 15–30% within 20 years due to the slow, and progressive liver damage that is characteristic of the natural history of this disease. HCV is a leading cause of liver disease, cirrhosis, liver cancer, and the leading reason for liver transplantation. Certain populations are known to be at high risk for HCV infection including current or former injection drug users, recipients of clotting factor concentrates made before 1987, recipients of blood transfusions or solid organ transplants before July 1992, hemodialysis patients, persons with known HCV exposures such as health care workers, persons with HIV infection and children born to HCV infected mothers. The Centers for Disease Control and Prevention (CDC) and the US Preventive Services Task Force (USPSTF) recommend a one-time birth cohort screening for all individuals born between 1945 and 1965.
HCV is now a curable disease. If HCV screening is accompanied by treatment, the risk of hepatocellular carcinoma can be reduced by 70% [3]. The effectiveness of new antiviral medications is measured by patients achieving sustained viral response (SVR). SVR is defined as undetectable HCV RNA at 12–24 weeks after termination of treatment and is equivalent to a durable cure [4]. With these advances in treatment, new policy and screening initiatives are needed to combat rising HCV morbidity and mortality.
Methods
Population
From 06/2014 to 02/2015, 357 participants were screened for HCV in Miami, FL. Participants received free HCV screening at Union Positiva, a community health center offering free HIV testing in Miami-Dade County as part of a county wide screening program. Participants needed to be older than 18 years of age. This study was approved by the University of Miami Institutional Review Board.
Study Design and Methods
Once participants were screened in Miami they were asked to complete a survey assessing their risk factors for HCV. Participants underwent OraQuick HCV rapid antibody testing. Participants were informed of their HCV rapid antibody results at the time of completion, and if positive, blood was drawn for RNA analysis. Participants were subsequently notified of their HCV status, were referred for treatment and were aided in finding providers, regardless of their insurance status.
Measures
Biological Measures
A finger stick blood sample was taken and HCV antibody testing was performed with the OraQuick rapid HCV antibody test. Participants with reactive antibodies were then tested for HCV RNA in a CLIA and CAP certified laboratory.
Self-report Measures
HCV Risk Behavior Interview
Self-report measures were collected using a survey from the Department of Health. This survey assessed sexual practices and drug use.
Self-Report HCV and HIV Status
Participants were asked to report if they had previously been screened for HCV and HIV, and whether they had previously tested positive for HCV or HIV.
Demographics
Study demographic questionnaire included questions regarding gender, race and ethnicity.
Injection Drug Risk Behaviors
Participants were asked if they had a history of intravenous drug use or sharing injection equipment.
Data Analysis
Deidentified data were imported into the University of Miami’s instance of REDCap (Research Electronic Data Capture), a secure, web-based application designed to support data capture for research studies [5]. All data were examined using both numeric and graphical Exploratory Data Analysis Methods (EDA). All data were analyzed with SAS 9.4. Percentages were calculated and counts were compared using contingency table methods. After confirming that the assumptions for Chi square tests were met (e.g., large enough expected cell counts), hypothesis tests for differences in rates were assessed and odds ratios with 95% confidence limits and p-values are reported. Two tailed p-values <0.05 were considered statistically significant. This study was approved by the University of Miami’s Institutional Review Board.
Results
Of the 357 participants, 21 (5.88%) were found to have HCV (confirmed by RNA analysis), a number slightly higher than the estimated national prevalence. 82.9% of participants (n = 296) identified as white, 13.4% identified as Black (n = 48), 2.8% identified as Mixed Black/White (n−=10.) 74.7% of the participants were male and 25% were Female. 4.5% of participants were IV Drug users.
Only 15.1% (n = 54) had been screened for HCV before whereas 83.8% (n = 299) had been screened for HIV. Of the patients who had previously been screened for HCV (n = 54), 98.2% of these patients had previously been screened for HIV as well. Of the patients who had never received a previous HIV test (n = 45), only 1 participant (2.2%) had previously been tested for HCV. Among people who knew their prior testing history, the observed 17% increase in the HCV screening rate, in those who were previously tested for HIV (19%) relative to those without prior testing (2%), is shown in Fig. 1 and is statistically significant (OR: 10.6, 95% CL: 1.4–79, p < 0.004) (Fig. 2).
Fig. 1.
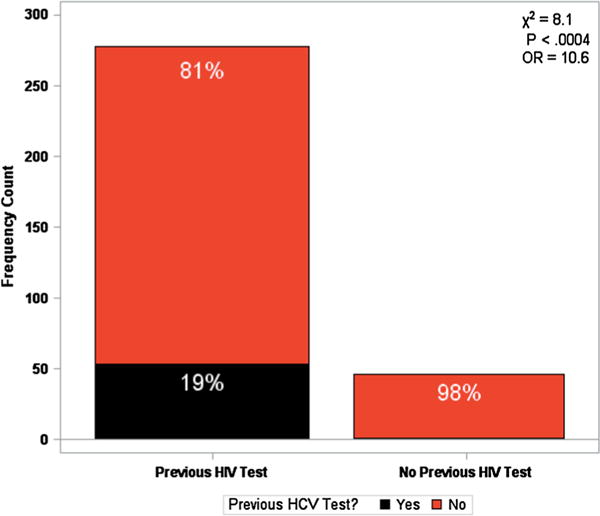
Known HIV and HCV Testing history in a random community population with Risk Factors for HIV/HCV acquisition. 85.8% (n = 278) participants self-reported previously being tested for HIV, while only 19% (n = 53) participant’s self-reported previously being tested for HCV
Fig. 2.
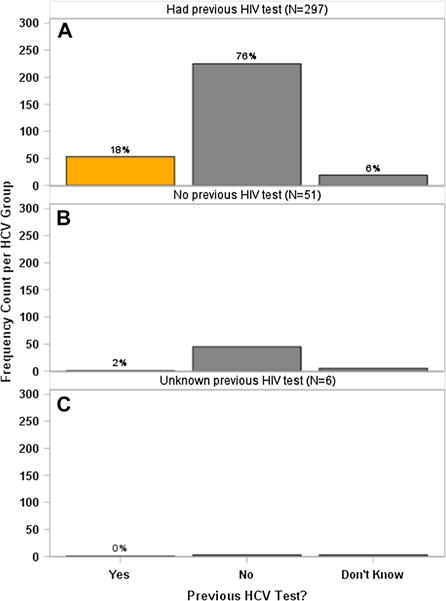
HCV testing status stratified by participants who were previously tested for HIV. a Frequency count of prior HCV screenings among those who had been previously screened for HIV. Of the 54 participants who had been previously screened for HCV, 53 of them (98.2%) had also been screened for HIV. b Frequency count of prior HCV screenings among those who had not been previously screened for HIV. c Frequency count of prior HCV screenings among those who had unknown previous HIV testing history
Discussion and Public Health Implications
HCV and HIV Screening
The findings regarding the discrepancy between previous testing for HCV and HIV were considerable. Of the 357 participants, only 15.1% had been screened for HCV before whereas 83.8% had been screened for HIV. While we observed higher screening rates among those previously tested for HIV, in general these rates are relatively low and suggest that current screening guidelines may not be implemented despite the recent CDC recommendation to screen all adults from high-risk groups. Low screening adherence appears to be the cause of underdiagnoses of HCV. Because HCV is a leading blood borne infection, we must increase our understanding of the reasons underlying underdiagnoses.
One of the primary reasons for lack of HCV screening is most likely the lack of education regarding HCV, specifically regarding transmission and associated mortality [6]. Several studies have demonstrated a significant lack of HCV education in high-risk groups. This knowledge gap was demonstrated by poor knowledge about HCV transmission which inhibits individuals from seeking appropriate testing [7]. In particular, major knowledge gaps have been reported in injection drug users (IDUs) [8], and HCV knowledge among IDUs has been found to be worse than that of knowledge pertaining to HIV infection [9]. If a patient is unaware of the prevalence and mortality associated with HCV, they are unlikely to request screening. Additional health education is needed in order to prevent further transmission of HCV and to help those with HCV infection understand treatment options. Another cause of low screening adherence may be lack of interaction of individuals with the health care system [6]. Certain populations, such as racial and ethnic minorities, immigrant communities and IV drug abusers may lack access to an appropriate health care system limiting their potential to receive screening and putting them at increased risk of undetected HCV infection. Stigma associated with sexually transmitted diseases may also prevent individuals from seeking screening exams. When individuals are unaware of their infection, they are more like to transmit their infection, less likely to benefit from early treatment and thus more prone to the morbidity, mortality and complications of the disease [10].
Barriers to screening can be seen from the physician and provider side as well. Providers may be unaware of the prevalence of Hepatitis C virus, and thus unlikely to suggest screening in asymptomatic patients. One major barrier is the cost of obtaining and implementing HCV screening tests, each test can cost approximately twenty dollars. In addition, the need for confirmatory HCV nucleic acid testing following a positive antibody test is a significant hurdle. If the initial rapid antibody screen comes back positive, resources such as venipuncture and trained phlebotomists are required. Provider uncertainty regarding high treatment costs and insurance coverage may prevent them from offering screening. Particularly if providers believe their patient will not be able to afford treatment they may be hesitant to offer screening. Another barrier to provider instigated screening may be time, a provider may not feel that they can afford to take the time out of their patient visit to educate patients about HCV and suggest screening [11]. Furthermore, providers may feel uncomfortable talking to patients about matters like IV drug use and sexual practices.
Interestingly, when participants were stratified based on if they had previously had an HCV test, nearly all participants who had been previously been screened for HCV had also been screened for HIV. For any participant that had never been screened for HIV, they had also never been screening for HCV. This suggests that HCV screenings are more likely when participants are already being screened for HIV. Additionally, roughly 25% of those in the United States with HIV are co-infected with HCV. Certain populations, such as substance users are more susceptible to being infected by both HIV and HCV. HIV co-infection is associated with increased HCV related liver disease and mortality [10]. This study presents a unique opportunity to improve HCV screening by promoting HIV/HCV co-screening. Bundled screenings could significantly increase HCV testing rates among populations already seeking regular HIV testing. Because HIV and HCV are often transmitted through similar routes including risky sexual behavior or needle sharing, testing positive for either HIV or HCV could serve as an early warning that a person is at increased risk for obtaining additional infections.
Bundled rapid testing assays for HIV and HCV could help improve adherence to HCV screening by reducing both patient and physician centered barriers to screening. The HIV and HCV point-of-care tests offer quick, accessible and affordable testing and should be considered as an option to scale up HIV/HCV co-screening. In our random community sample, HIV screening rates were significantly higher that HCV screening rates. Thus, if individuals are already coming into a facility to get tested for HIV, offering an HCV screening test at the same time as an HIV screening test same time could potentially increase HCV screening rates. These tests do not require laboratory facilities and can be administered without a physician, nurse or phlebotomist. Results are available within 20 min are 98 and 99% accurate with confirmatory nucleic acid testing and western blot for HCV and HIV needed, respectively.
Limitations
Our data was from a primarily white Hispanic cohort. While this represents the population of Miami-Dade County, these results may not be applicable to the wider U.S. population. Different racial and ethnic groups may have varying degrees of stigma regarding sexually transmitted infections; thus, screening rates and screening acceptability may differ between communities. Furthermore, there may be hesitation to administering co- HIV and HCV screenings for psychological and financial reasons. Patients may not be willing to get multiple negative medical assessments about HIV and HCV at same time. Testing centers would need to ensure proper counseling and therapeutic follow up for these patients. Additionally, administering two tests, rather than one may requires additional resources including phlebotomy supplies that are needed for HCV nucleic acid testing on top of an already strained screening infrastructure. However, despite these limitations this study clearly demonstrates a critical need to improve public knowledge of HCV risk factors, the need for screening, and the availability of effective treatment (Table 1).
Table 1.
Racial and Ethnic distribution of random community participants with risk factors for HIV and HCV acquisition
| Hispanic | Non-Hispanic | Total | |
|---|---|---|---|
| White | 266 | 39 | 296 |
| Black/African American | 7 | 41 | 48 |
| Mixed | 9 | 1 | 10 |
| 282 | 72 | 354 |
Acknowledgments
This work was supported by Union Positiva, the Schiff Center for Liver Diseases, MiamiCenter for AIDS Research at the University of Miami Miller School of Medicine,supported by National Institutes of HealthGrant P30AI073961 (to E.T.), and the MiamiClinical and Translational Science Institute KL2 program (to E.T.).
Footnotes
Author contributions: Erica Feldman, B.S. MD/MPH candidate 2019, University of Miami Miller School of Medicine (Contributed new reagents or analytic tools, Analyzed data and Wrote the paper), Raymond Balise, Ph.D. Assistant Professor, Department of Public Health Sciences, University of Miami Miller School of Medicine (Contributed new reagents or analytic tools and Analyzed data), Eugene Schiff, M.D. Director, Schiff Center for Liver Disease, University of Miami Miller School of Medicine (Designed research), Nicole Whitehead, Ph.D. Assistant Professor, College of Public Health and Health Professions, University of Florida (Wrote the paper), Emmanuel Thomas, M.D., Ph.D. Assistant Professor, Schiff Center for Liver Disease, University of Miami Miller School of Medicine (Designed research, Performed research, Contributed new reagents or analytic tools, Analyzed data and Wrote the paper).
Compliance with Ethical Standards
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md) 2013;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of Internal Medicine. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ditah I, Al Bawardy B, Gonzalez HC, et al. Lack of health insurance limits the benefits of hepatitis C virus screening: Insights from the National Health and Nutrition Examination Hepatitis C follow-up study. The American Journal of Gastroenterology. 2015;110(8):1126–1133. doi: 10.1038/ajg.2015.31. [DOI] [PubMed] [Google Scholar]
- 4.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: A cure and so much more. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2011;52(7):889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 5.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead NE, Hearn LE, Marsiske M, Kahn MR, Latimer WW. Awareness of biologically confirmed HCV among a community residing sample of drug users in baltimore city. Journal of Community Health. 2014;39(3):487–493. doi: 10.1007/s10900-013-9782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton BL, Voils CI, Timberlake SH, et al. Community-based HCV screening: Knowledge and attitudes in a high risk urban population. BMC Infectious Diseases. 2014;14:74. doi: 10.1186/1471-2334-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien S, Day C, Black E, Dolan K. Injecting drug users’ understanding of hepatitis C. Addictive Behaviors. 2008;33(12):1602–1605. doi: 10.1016/j.addbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Heimer R, Clair S, Grau LE, Bluthenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction (Abingdon, England) 2002;97(10):1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- 10.Frimpong JA, D’Aunno T, Perlman DC, et al. On-site bundled rapid HIV/HCV testing in substance use disorder treatment programs: Study protocol for a hybrid design randomized controlled trial. Trials. 2016;17(1):117. doi: 10.1186/s13063-016-1225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southern WN, Drainoni ML, Smith BD, et al. Physician nonadherence with a hepatitis C screening program. Quality Management in Health Care. 2014;23(1):1–9. doi: 10.1097/QMH.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]


