Abstract
Antipsychotic drugs are increasingly used in children and adolescents to treat a variety of psychiatric disorders. However, little is known about the long-term effects of early life antipsychotic drug (APD) treatment. Most APDs are potent antagonists or partial agonists of dopamine (DA) D2 receptors ; atypical APDs also have multiple serotonergic activities. DA and serotonin regulate many neurodevelopmental processes. Thus, early life APD treatment can, potentially, perturb these processes, causing long-term behavioural and neurobiological sequelae. We treated adolescent, male rats with olanzapine (Ola) on post-natal days 28–49, under dosing conditions that approximate those employed therapeutically in humans. As adults, they exhibited enhanced conditioned place preference for amphetamine, as compared to vehicle-treated rats. In the nucleus accumbens core, DA D1 receptor binding was reduced, D2 binding was increased and DA release evoked by electrical stimulation of the ventral tegmental area was reduced. Thus, adolescent Ola treatment enduringly alters a key behavioural response to rewarding stimuli and modifies DAergic neurotransmission in the nucleus accumbens. The persistence of these changes suggests that even limited periods of early life Ola treatment may induce enduring changes in other reward-related behaviours and in behavioural and neurobiological responses to therapeutic and illicit psychotropic drugs. These results underscore the importance of improved understanding of the enduring sequelae of paediatric APD treatment as a basis for weighing the benefits and risks of adolescent APD therapy, especially prophylactic treatment in high-risk, asymptomatic patients.
Keywords: Amphetamine, atypical antipsychotic, conditioned place preference, dopamine, reward system
Introduction
There is increasing awareness among the clinical and research communities of the potential for long-term sequelae arising from psychotropic medication of paediatric patients. Antidepressants have been most widely studied in this regard (Oberlander et al., 2009; Simpson et al., 2011), whereas antipsychotic drugs (APDs) have been investigated much less. Although APDs vary in the spectra of their pharmacological actions, nearly all APDs potently inhibit dopamine type 2 (D2) family receptor function; atypical APDs also have a broad spectrum of serotonergic (5-HTergic) and other activities (Bymaster et al., 1996; Baldessarini and Tarazi, 2001). Some of the 5-HTergic activities contribute to the therapeutic efficacy of the atypical APDs clozapine and olanzapine (Ola; Yadav et al., 2011). Atypical APDs, including Ola, are commonly prescribed to treat multiple psychiatric disorders in paediatric patients (Cheng-Shannon et al., 2004; McCracken et al., 2008; Sikich, 2008; Strawn and Delbello, 2008; Weller and Weller, 2009; McKnight and Park, 2010). Patients with psychotic illness often require long-term maintenance therapy, but most paediatric APD treatment is for other, off-label indications (Scheltema Beduin and de Haan, 2010; Alexander et al., 2011), for which consensus guidelines (Pappadopulos et al., 2003, 2011; Thomas et al., 2011) recommend trial off-medication after stability is achieved. Although short-term tolerance of APDs is reasonably good in adult and paediatric patients (Zuddas et al., 2011), the long-term sequelae that follow cessation of APD treatment in paediatric patients are unknown (Cheng-Shannon et al., 2004). In the mature brain, dopaminergic (DAergic) signalling is implicated in behavioural plasticity in response to reward (Willuhn et al., 2010; Gardner, 2011). Dopamine (DA) and serotonin (5-HT) both regulate a variety of neurodevelopmental processes (Levitt et al., 1997; Frost and Cadet, 2000; Azmitia, 2001). Thus, even time-limited APD therapy can, potentially, alter the subsequent developmental trajectory of the brain, resulting in long-term abnormalities of brain function and behaviour. In humans, the cerebral substrates upon which APDs act undergo dramatic ontogenetic changes into the third decade (Giedd et al., 2009; Petanjek et al., 2011). This suggests that the behavioural and neurobiological sequelae of APD therapy could vary with age of treatment and underscores the necessity of independently evaluating the enduring effects of APD administration in humans and in animal models at different ontogenetic stages.
We recently demonstrated that adolescent atypical APD treatment causes enduring deficits in working memory (but not spatial memory) and in extinction of fear conditioning (but not acquisition of fear conditioning; Milstein et al., unpublished data). In the present study, we hypothesized that adolescent APD therapy would also have long-term effects on the brain’s reward system, due to : (1) the DAergic activities of the drugs; (2) the continuing development and plasticity of DAergic transmission during adolescence (Andersen et al., 2000; Brenhouse et al., 2008; Wahlstrom et al., 2010; Andersen and Navalta, 2011); (3) the importance of DA as a regulator of developmental processes; (4) the pivotal role of DA signalling in the mature reward system (Di Ciano et al., 2001; Parkinson et al., 2002). We focused on the nucleus accumbens core (NAcC) because it is a key node in the neural network mediating the acquisition of conditioned appetitive responses (Parkinson et al., 2000; Cardinal et al., 2002), via DA-dependent mechanisms (Parkinson et al., 2002; Liao, 2008; Vidal-Infer et al., 2012). Here, we demonstrate that adult rats treated with Ola as adolescents, using a dosing regimen designed to optimize translational relevance, exhibit enduring, previously unrecognized changes in reward system function and in DAergic neurotransmission in the NAcC.
Method and materials
Subjects
We studied male, colony-bred Long–Evans rats (breeding stock obtained from Charles River, USA). Litters were culled to 10–12 pups on approx. post-natal day (PD)7. Rats were maintained on a light cycle of 14 h light/10 h dark (lights on 07:00 hours). Drinking fluid [water, Ola solution or vehicle (Veh); see below] and food were always available ad libitum. On PD21, subjects were weaned and pair- or triple-housed with same-sex littermates. Different cohorts of rats were used in each experiment. In each cohort, littermates were assigned to the Ola- and Veh-treatment groups, rats in the two groups were age matched and ≥3 litters contributed to each group. All experimental measures were made when the rats were adults (≥90 d; ages for each experiment noted below). All protocols were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by our institutional animal care and use committees.
Drug treatments
All subjects drank tap water except on PD28–49 (PD0=day of birth ; puberty in males is approx. PD42), when the water was replaced with an aqueous solution of Ola in 0.001 M acetic acid (Ola-treated rats) or 0.001 M acetic acid alone (Veh-treated control rats). The Ola solution was mixed fresh daily at a concentration calculated to deliver a target dose of 7.5 mg/kg.d, based on the mean weights of the rats and their fluid consumption over the previous 24 h. (The dose achieved was calculated by measuring actual fluid consumption after 24 h.) On PD49, all rats were switched back to tap water.
Our Ola dosing procedure was designed to reproduce as closely as possible the clinical administration of atypical APDs. In humans, the half-lives of most APDs and their active metabolites are about 1 d (Baldessarini and Tarazi, 2001; Kapur et al., 2003). Thus, daily APD treatment produces plasma drug levels and D2 receptor occupancies whose diurnal variations are limited. In rodents, the half-lives of APDs (including Ola) and their active metabolites are shorter : ≤6 h (Kapur et al., 2003). Consequently, administration of APDs by daily injection (the most common technique) induces only transient ‘pulses’ of drug, rather than a damped oscillation, as in humans. This is functionally important; for example, in adult rats, continuous haloperidol infusion, but not daily injections, enhances the efficacy of amphetamine (Amph) in potentiating conditioned reinforcement (Bedard et al., 2011). The consequences of adolescent APD treatment may similarly depend on the stability of plasma drug levels and receptor occupancy, which are rarely measured in rodent studies. Thus, for many such experiments, the reported effects of APDs may be translationally uninformative.
To address these issues, we administered Ola in the drinking water, rather than by implanted mini-pumps, because the former method allowed us to adjust the daily total dose of Ola to the rapid, developmental weight gain that occurs over PD28–49 in rats. In preliminary studies, six male rats received Ola in the drinking water at a target dose of 7.5 mg/kg.d on PD28–49. On PD49, blood was obtained by tail nick of each subject once at the mid-light phase and once at the mid-dark phase of the light cycle. Plasma Ola concentrations were measured using high-performance liquid chromatography with electrochemical detection (Catlow et al., 1995). Plasma Ola concentrations at the mid-dark and mid-light phases of the diurnal cycle, which correspond, respectively, to periods of high and low activity (and drinking), were 93.0±28.5 and 16.2±12.2 ng/ml (mean±S.D.). These values approximate the broad spectrum of steady state plasma Ola levels (135.7±91.0 ng/ml; mean±S.D.) that, in rats, produces mean D2 receptor occupancies in the human therapeutic range (65–80%; Kapur et al., 2003). In rats, lower or higher Ola doses produce receptor occupancies outside this range (Kapur et al., 2003; Perez-Costas et al., 2008; higher doses could also cause catalepsy), so were not used to obtain our experimental end-points. Ola administration in the drinking water also avoids any potential stress effects of treatment by repeated injections or gavage or surgery to implant and remove infusion pumps.
Conditioned place preference
Rats (22 Ola-treated, 20 Veh-treated) were tested for conditioned place preference (CPP) as young adults (average age 7.8 months, ~6.3 months after termination of treatment). CPP testing [and measurements of DA release in the NAcC evoked by electrical stimulation of the ventral tegmental area (VTA) – see below] was conducted during the light phase of the diurnal cycle due to the unavailability of reverse light cycle facilities. Because rats are naturally active in the dark phase, this would be expected to reduce their responsiveness to the conditioned stimulus. However, we observed an increase in responsiveness in Ola-treated rats, as compared to Veh-treated controls (Results), suggesting that our data provide a conservative estimate of the effects of adolescent Ola treatment. (Further, experience in our lab has shown that, although DA release in awake, behaving rats in response to rewarding external stimuli is modulated by phase of the diurnal cycle, DA release evoked by electrical stimulation of the VTA in anaesthetized rats is not.) For these reasons, it is unlikely that the effects of adolescent Ola treatment reported here were due to experimentation during the light phase. The test apparatus (Med Associates, USA) contained three compartments. One lateral compartment was black, scented with wood chip bedding and had metal rod flooring; the other was white, scented with paper bedding and had metal grid flooring. The central compartment was grey, unscented, connected to the lateral compartments by doors that could be opened or closed prior to each session and had solid flooring.
Each rat was placed in the centre compartment (both doors open) and allowed to explore the apparatus for 10 min. Times in each lateral compartment were recorded to determine baseline preferences. A rat was considered to have entered a new compartment when its shoulders crossed into that compartment. Rats were given their first conditioning session 24 h later. Each rat received Amph in one compartment and saline in the other. Amphetamine-paired compartments were counterbalanced across treatment groups (Ola, Veh) and, for each rat, Amph was paired with the less preferred chamber, as measured at baseline, to minimize type 1 error. For each conditioning session, a rat was injected with either Amph [1 mg/kg (2 mg/ml solution, 0.5 ml/kg) ip) or 0.9% physiological saline (0.5 ml/kg i.p.), then restricted to the appropriate compartment for 30 min. All rats received one daily conditioning session for four consecutive days (two Amph and two saline ; Amph trials 48 h apart) ; half the rats of each treatment group received Amph first, half saline first. Twenty four hours after the fourth conditioning session, the rats were run in a ‘probe’ session (10 min duration) by putting them in the centre compartment, with the doors open so they could explore all three chambers. Time spent in each lateral compartment was recorded by an observer.
Receptor binding
Rats (nine Ola-treated, nine Veh-treated) were euthanized by asphyxiation with CO2 at age 6–8.5 months, ~4.5–7 months after termination of treatment). D1 and D2 receptor binding in the prefrontal cortex reaches mature levels by PD100 (Andersen et al., 2000; Brenhouse et al., 2008; Wahlstrom et al., 2010) and in vivo positron emission tomography imaging shows that D1 and D2 receptor binding levels in adult rats do not begin their age-associated decline until sometime between ages 12 and 24 months (Suzuki et al., 2001). Thus, our data are not confounded by normal developmental- or aging-associated changes in DA receptor binding.
Brains were dissected and punches of NAcC tissue from both sides of the brain were pooled, snap frozen in an isopentane/dry ice mix and stored at −80 °C until processing. Tissue from three rats was pooled for each of the three independent samples studied for each treatment group (i.e. for statistical purposes n=3/group). NAcC cell membranes were prepared at 4 °C (El-Nabawi et al., 2000). Briefly, tissue punches were homogenized Tris- Krebs Ringer (pH 7.4, 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1mM EDTA) and the homogenate was centrifuged (40 000 g, 20 min). The resultant pellet was resuspended in 50 mM Tris-HCl, pH 7.4, then centrifuged (40 000 g, 20 min). The final pellet was resuspended in 50 mM Tris-HCl buffer, pH 7.4 and kept cold until protein content was determined using BCA reagents (bovine serum albumin standard).
Binding of [3H]SCH-23390 (70.30 Ci/mmol; 3 nM) and [3H]YM-09151-2 (85.50 Ci/mmol; 2.5 nM) to D1-family and D2-family receptors, respectively, was determined by filtration assay (El-Nabawi et al., 2000). For D1 binding, membrane homogenates were incubated with [3H]SCH-23390 in 50 mM Tris-HCl buffer, pH 7.4, at 23 °C (1 h). Non-specific binding of [3H]SCH-23390 was measured in the presence of 100 μM flupenthixol. All tubes contained 40 nM ketanserin to preclude binding to 5-HT2 receptors. For D2 binding, homogenates were incubated with [3H]YM-09151-2 in 50 mM Tris-HCl buffer, pH 7.4, containing 120 mM NaCl at 23 °C (1 h). Nonspecific binding of [3H]YM-09151-2 was measured in the presence of 100 μM spiperone. All tubes contain 10 nM ketanserin to preclude binding to 5-HT2 receptors. For GABAA binding, homogenates were incubated with [3H]EBOB (30 Ci/mmol; 6.5 nM) in 50mM Tris-HCl buffer, pH 7.4, containing 300 mM NaCl at 23 °C (1 h). Non-specific binding of [3H]EBOB was measured in the presence of 100 μM picrotoxin. Bound and free ligands were separated by rapid filtration over Whatman GF/B glass-fibre filters (pre-soaked in 0.05% polyethylenimine for ≥20 min), then washed with ice-cold 0.9% NaCl (3×4 ml). Radioactivity was counted by liquid scintillation spectroscopy. All samples were run in triplicate ; the mean of the three measures was used for statistical analyses (two-tailed t test).
DA release
Rats (Ola and Veh treated, n=9/group; aged 110–180 d, ~2.2–4.5 months after termination of treatment) were anaesthetized using urethane (1.0 g/kg i.p.). We used fast-scan cyclic voltammetry (FSCV; Heien et al., 2004) to investigate the effects of adolescent Ola treatment on DA release evoked in the NAcC by electrical stimulation of the VTA. FSCV measures extracellular DA concentration ([DA]EXT) with a temporal resolution of 100 ms. A bipolar stimulating electrode was positioned in the VTA (AP:−5.4 mm; ML: 0.5 mm; DV:−8.7 mm; AP and ML coordinates are relative to bregma). A carbon fibre recording electrode was positioned above the ipsilateral NAcC (AP: +1.3 mm; ML: 1.3 mm). Every 100 μM along the electrode track, from DV −6.0 to −7.0 mm (relative to the pial surface), we recorded DA transients evoked by trains of 60 rectangular, biphasic pulses (2 ms/phase; one pulse train/3 min), 300 μA amplitude, 60 Hz, delivered in the VTA. At DV=−6.4 mm, additional responses were recorded for 60 Hz stimulation at intensities of 100, 200, 400 and 500 μA (two pulse trains at each intensity, in order of increasing intensity). FSCV used a triangular input waveform (−0.4 to +1.3 V vs. an Ag/AgCl reference electrode ; 400 V/s ramps), repeated every 100 ms. When the applied voltage reaches +600 mV, DA adsorbed to the surface of the electrode is oxidized and the current produced is recorded. Background current measured without VTA stimulation was subtracted from the ‘faradaic’ current, measured during VTA stimulation, to calculate the electrically induced current component. Post-recording electrode calibration curves were used to convert this current to [DA]EXT. After each experiment, rats were euthanized by an overdose of urethane.
The small size of the carbon-fibre electrodes used for electrochemical measurements in this study is particularly useful in making measurements of DA release and uptake in subregions of the corpus striatum without overlap. It is well established that both the release and uptake rate of DA decrease along a trajectory from dorsolateral to ventromedial in the caudate-putamen and continuing into the core then the shell of the NAc. The spatial gradient of release and uptake correlates well with the relative density of DAergic axon terminals on which the DAT is located (Jones et al., 1996; Calipari et al., 2012). Thus, voltammetric studies have traditionally relied on the magnitude and decay constant of DA transients to confirm that recordings are being made in the structures targeted stereotaxically. The DA uptake rates obtained in our experiment are consistent with those seen in the core of the NAc (Wightman et al., 2007; Aragona et al., 2009). Determination of recording site location in this way allows us to maintain our recording electrode intact – a requirement for post-recording calibration that could not be met were we to make a lesion using the electrode before removing it from the brain.
Results
Subject development
In a large cohort of male rats from which our subjects were drawn, the mean actual Ola dose over the 3-wk treatment period was 7.22±0.02 mg/kg.d. Ola-treated and control rats gained weight steadily and there was no significant overall effect of Ola on weight over the treatment period (Fig. 1a; F1,79=0.275; p=0.602). The weights of the Ola-treated rats increased marginally slower than those of Veh-treated littermates during the last week of treatment, although there was no single day on which the Ola-treated rats were significantly lighter (Fig. 1a). Thus, there was a small but significant interaction of age and treatment on weight [F7.47, 224.09=4.55; p<0.0001; Huynh–Feldt corrected (ε=0.526)]. When the Veh-treated (n=21) and Ola-treated (n=22) rats began CPP training, their mean weights (Fig. 1b; 709±21.3 g and 674±22.8 g, respectively) did not differ significantly (t41=1.12, p=0.27). Others (Llorente-Berzal et al., 2011) have obtained similar body weight data in rats treated with Ola as adolescents. They also showed that, in contrast to humans and rats treated with Ola as adults, rats treated as adolescents have normal plasma glucose levels during and after treatment and that triglyceride levels are unchanged during adolescent Ola treatment and fall following termination of treatment (Llorente-Berzal et al., 2011). Thus, the behavioural and neurobiological effects reported here for rats treated with Ola as adolescents occur in the absence of the metabolic changes induced by atypical APDs in humans and adult rats.
Fig. 1.
Effects of adolescent olanzapine (Ola) treatment on body weight. (a) Body weight as a function of age during the treatment period [based on n=47 Ola-treated and 39 vehicle (Veh)-treated rats]. (b). Mature body weight of rats treated with Veh or Ola (based on n=22 Ola-treated and 21 Veh-treated rats). Error bars are S.E.M.
Conditioned place preference
Two-way analysis of variance (ANOVA) of the time spent in the Amph-associated chamber demonstrated effects of test session (Fig. 2a; F1,40=62.02; p<0.0001) and its interaction with treatment (F1,40=6.547; p=0.014), but not of treatment alone (F1,40=1.68; p=0.202). At baseline, neither Ola- nor Veh-treated rats preferred one chamber to the other (t21=0.84, p>0.41 and t19=1.87, p>0.05, respectively) and the two groups did not differ significantly in their preference for the Amph-paired chamber (Fig. 2a; t40=1.093; p=0.281). In both groups, preference for that chamber was increased by conditioning, as measured in the probe session (Fig. 2a ; Ola: t21=−10.508; p<0.0001; Veh: t19=−2.966 ; p=0.008). Post-conditioning, Ola-treated rats showed a significantly greater preference than the Veh-treated rats for the Amph-paired chamber (Fig. 2a; t40=2.393; p=0.022). The increase in preference for the Amph-paired chamber following conditioning was significantly greater for Ola- than for Veh-treated rats (Fig. 2b; t40=2.56 ; p=0.014).
Fig. 2.
Effects of adolescent olanzapine (Ola) treatment on conditioned place preference (CPP) for amphetamine (Amph) at maturity. (a) Time spent in the Amph-associated chamber expressed as a percentage of the total time spent in both chambers. At baseline, neither Ola-treated (n=22) nor vehicle (Veh)-treated (n=20) rats preferred one chamber to the other and the two groups did not differ significantly in their baseline preference for the Amph-paired chamber. Both Ola- and Veh-treated rats showed a significant post-conditioning increase in preference for the Amph-paired chamber (# indicates p<0.01). Post-conditioning, Ola-treated rats had a greater preference for the Amph-paired chamber than Veh-treated controls (* indicates p=0.022). (b) CPP, measured by the increase from baseline, was greater in Ola- than in Veh-treated rats ($ indicates p=0.014). Error bars are S.E.M.
Receptor binding
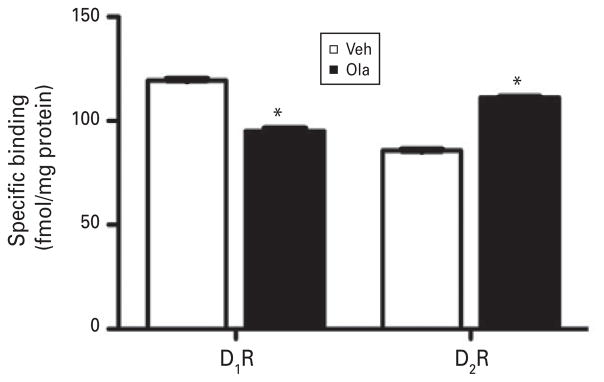
Adolescent Ola treatment reduced specific D1-family binding in the adult NAcC by 20% (Fig. 3; t4=2.6 ; p=0.001), whereas it increased specific D2-family binding by 30% (Fig. 3; t4=16.22; p<0.001). There was no significant difference in total GABAA receptor binding between Ola- and Veh-treated rats (data not shown; 257.8±1.9 and 253.2±2.0 fmol/mg protein, respectively; t4=1.545, p=0.197).
Fig. 3.
Effects of adolescent olanzapine (Ola) treatment on specific dopamine D1 and D2 receptor binding (D1R and D2R, respectively) in the nucleus accumbens core at maturity. D1 binding is reduced by 20% and D2 binding is increased by 30%. * Indicates p≤0.001. Error bars are S.E.M. [based on n=9 for Ola- and for vehicle (Veh)-treated rats ; statistics based on n=3 independent samples in each group; each sample consists of pooled tissue from three cases].
DA release
In all rats, DA release evoked by VTA stimulation at 300 μA, 60 Hz changed with depth in the NAcC (Fig 4a, b) and peaked between−6.4 and−6.6 mm. Adolescent Ola treatment reduced stimulation-evoked DA release in the NAcC, as shown for typical Veh- and Ola-treated cases in Fig. 4b. In each Veh- (Fig. 4c) and Ola-treated (Fig. 4d) rat, the amplitude and duration of DA transients increased monotonically with increasing stimulus pulse amplitude (constant frequency, 60 Hz). We used a normalization procedure to analyse treatment effects across rats (Fig. 4 legend). With increasing stimulation amplitude from 100 to 500 μA, normalized [DA]MAX increased approximately linearly in both treatment groups, but the slope was steeper in Veh- than in Ola-treated rats (Fig. 4e). Two-way ANOVA showed significant main effects of treatment (F1,16=8.76 ; p=0.009) and stimulation amplitude (F1.708,27.3=57.8 ;p<0.001) on normalized [DA]MAX but no significant interaction between the two [F1.708,27.33=2.96, p=0.076 ; Huynh–Feldt corrected (ε=0.569)]. Normalized [DA]MAX, averaged over the range of 200–500 μA, is reduced by adolescent Ola treatment to ~63% of control values.
Fig. 4.
Effects of adolescent olanzapine (Ola) treatment on dopamine (DA) release in the nucleus accumbens core (NAcC) at maturity [n=9 for Ola- and for vehicle (Veh)-treated rats]. Stimulation frequency is 60 Hz and error bars are S.E.M. (a) Changes in [DA]MAX with depth along the electrode track in the NAcC (300 μA stimulation amplitude). To allow comparison of data across cases, for each rat, [DA]MAX at each depth was normalized to the smallest value of [DA]MAX at the depths shown (‘Depth normalized’). (b) ‘Surface plots’ showing the time-course of extracellular DA concentration ([DA]EXT) along the entire electrode penetration are shown for typical Veh- and Ola-treated cases (stimulation amplitude is 300 μA). (c, d) Effects of increasing stimulus amplitude on [DA]EXT in typical Veh- and Ola-treated cases respectively. Data illustrated in (c) and (d) are from the same rats as the data shown in (b). (e) Changes in [DA]MAX with stimulation amplitude. We used a normalization procedure to analyse treatment effects across rats : At DV 6.4 mm, [DA]MAX, the maximum value of [DA]EXT at each stimulation amplitude was divided by [DA]MAX at the lowest stimulation amplitude (100 μA), to obtain ‘normalized [DA]MAX’. Insert : Average concentration vs. time plots of [DA]EXT as a function of time following ventral tegmental area (VTA) stimulation at 500 μA, 60 Hz. Arrow marks the time of VTA stimulation ; shading indicates S.E. Similar effects are obtained at other stimulation amplitudes. ( f ) Decay of [DA]EXT (normalized to [DA]MAX) following VTA stimulation at 500 μA, 60 Hz. Insert : Distribution of decay constant values calculated from single exponential curve fits of the data from individual rats.
We analysed the temporal decay of [DA]EXT at 500 μA amplitude, 60 Hz. For each subject, starting at the time of [DA]MAX, [DA]EXT was divided by [DA]MAX to obtain ‘normalized [DA]EXT’. The decay constant was calculated for each rat by fitting the falling phase of normalized [DA]EXT to a single exponential function. This decay constant (τ) was not significantly altered by adolescent Ola treatment (Fig. 4 f; τ=1.003 and 0.904 s for controls for Ola-and VET-treated rats, respectively ; t1=0.05; p=0.618).
Discussion
This study demonstrates for the first time that adolescent Ola therapy causes enduring changes in reward behaviour and DAergic transmission in the NAcC, including: (1) enhancement of CPP for Amph; (2) reduced D1 binding and increased D2 binding in the NAcC; (3) reduced VTA stimulation-induced DA release in the NAcC, without affecting the rate of clearance of DA from the extracellular space. These data raise important questions concerning the benefits and risks of atypical APD therapy in the treatment of adolescent patients and define future areas of inquiry for informing the resolution of those issues.
Behavioural sequelae of adolescent Ola treatment
Selectivity of behavioural responses
CPP, a form of Pavlovian learning, measures increases in the salience of environments associated with rewarding stimuli. In parallel studies, we have demonstrated that adult rats treated with Ola as adolescents exhibit impaired working memory and reduced extinction of fear conditioning, but normal spatial memory (Milstein et al., unpublished data). Performance in other domains, such as motor activity and skill, habituation to an open field, and affect are also normal in Ola-treated rats (Milstein et al., unpublished data). The normalcy of these behavioural measures demonstrates that the deficits induced by adolescent Ola therapy are specific and not due to global, drug-induced defects of brain development or function. Our data are consistent with the sparing of spatial memory, latent inhibition and reversal learning in adult animals treated as adolescents with either of two other atypical APDs, clozapine (Piontkewitz et al., 2009; Meyer et al., 2010) and risperidone (Piontkewitz et al., 2011) and with the impairment of novel object recognition ~2 wk after the termination of adolescent Ola treatment (Llorente-Berzal et al., 2011). The bias of the effects of adolescent atypical APD treatment towards reward behaviour and certain types of learning, as suggested by our data and others, may be due to the substantial overlap of the neural networks that mediate these abilities.
Adolescent vs. adult Ola treatment
The behavioural deficits that occur in adult rats after an extended period of withdrawal from chronic, adolescent Ola treatment might also occur in rats treated as adults for the same amount of time and studied at a similar post-treatment interval. We are unaware of any data that address this possibility. Adult rats studied at various intervals during chronic Ola treatment at doses similar to ours exhibit a different spectrum of behavioural effects: short term memory deficits ; impaired acquisition and retention of spatial learning in the Morris water maze; motor deficits (Terry et al., 2008). However, even if data obtained after withdrawal from Ola treatment of adult rats were available, they would do little to inform the interpretation of the enduring effects of adolescent Ola treatment or the development of an improved therapeutic approach.
Behavioural and neurobiological phenotypes induced by chronic psychotropic drug therapy often depend on homeostatic responses to treatment. These responses can vary greatly depending on the developmental status of the brain at the time of treatment and at the time the effects are assessed. Furthermore, a single behavioural phenotype can be associated with different underlying neurobiological phenotypes or mechanisms. For example, our rats that have increased D2 receptor binding and reduced DA release in the NAcC and mice that have mechanistic abnormalities of opposite sign – targeted knockout of D2 autoreceptors (beginning on gestational day 15) and increased DA release in the NAcC both exhibit enhanced CPP as adults (Bello et al., 2011). Thus, regardless of whether or not the enduring behavioural sequelae of a limited course of chronic Ola treatment are similar or different in adolescents and adults, one still would not know whether those effects reflect the action of similar or different mechanistic pathways. From the translational perspective, our data suggest that adolescent Ola treatment induces enduring behavioural deficits and changes in the neural networks that mediate the affected behaviours. If confirmed in humans, improved therapeutic strategies will be required, regardless of whether or not similar effects occur when the drug is administered to adults.
Brain regions mediating behavioural effects
In which brain regions might Ola act to produce the observed spectrum of effects? CPP acquisition depends upon the integrity of the NAcC (Cardinal et al., 2002) and contextual information derived from the ventral hippocampal area (Grace et al., 2007), whereas the medial prefrontal cortex (MPC) contributes to behavioural flexibility, which is required in assays of working memory (e.g. Uylings et al., 2003) and extinction of conditioning (Morgan and LeDoux, 1995; Peters et al., 2009). Information from both structures converges upon the NAcC (Grace et al., 2007). Neural activity in the NAcC shifts between synchrony with the hippocampus and synchrony with the MPC in a task-dependent manner (Gruber et al., 2009), suggesting the weighting of inputs from those structures according to situational requirements. Hippocampus×MPC interactions appear to contribute to these shifts. Thus, adolescent Ola treatment could exert its behavioural effects directly, by targeting the MPC or hippocampus and/or indirectly by targeting intra-NAc mechanisms that mediate those interactions.
Effects of adolescent Ola treatment on DAergic neurotransmission in the NAcC
Receptor binding
Acute APD treatment antagonizes D2 receptors. However, in mature animals (Tarazi et al., 2001; Vasconcelos et al., 2003; Samaha et al., 2007; Ginovart et al., 2009) and human patients (Silvestri et al., 2000; Remington and Kapur, 2010), chronic treatment with typical and atypical APDs (including Ola) over a protracted period can upregulate D2 binding, depending on the duration and percentage of D2 receptor blockade. This causes a loss of therapeutic efficacy in patients (Silvestri et al., 2000; Remington and Kapur, 2010). D2 binding returns to normal levels within a few weeks following withdrawal of treatment, with (Vasconcelos et al., 2003; Ginovart et al., 2009) or without (Baron et al., 1989) D2 up-regulation. Chronic, adolescent Ola treatment for 3 wk increases D2-family binding and decreases D1-family binding in the NAcC (and the MPC; Milstein et al., unpublished data) at maturity. The opposite sign of the changes in D1 and D2 binding and the absence of a significant effect on GABAA binding demonstrate the specificity of each result. Thus, the long-term effects of 3 wk adolescent Ola treatment on D2-family binding have the same sign as the effects of chronic APD treatment in adulthood. However, in contrast to the effects of chronic Ola therapy in adults, D2 binding does not normalize following termination of chronic, adolescent Ola treatment.
In the NAc (Andersen et al., 1997) and other brain regions (Andersen et al., 2000; Brenhouse et al., 2008; Wahlstrom et al., 2010), DAergic signalling undergoes rapid, ontogenetic changes during adolescence. A key issue in linking our behavioural and receptor binding data is to understand how adolescent Ola treatment alters the developmental trajectory and function of NAc circuitry. This is potentially important for the design of new therapeutic regimens for minimizing the adverse impacts of adolescent APD therapy.
DA release
The reduction of stimulation-induced [DA]MAX in rats treated with Ola as adolescents (Fig. 4e), suggests several, non-exclusive, potential abnormalities of DAergic function in the NAcC: (1) augmenting stimulus amplitude at a fixed frequency increases current spread and recruits more VTA neurons to release DA in the NAcC. Thus, adolescent Ola treatment might enduringly diminish spatial summation; (2) for a fixed frequency and amplitude of VTA stimulation, [DA]MAX depends primarily on the amount of DA released per impulse arriving at VTA axon terminals and the rate of clearance of DA from the extracellular space. DA release is negatively regulated by presynaptic D2 autoreceptors whereas clearance from the extracellular space is performed by the DA transporter (DAT). Thus, adolescent Ola treatment might alter the density and function of presynaptic D2 receptors and DATs, as well as the density of projections from the VTA. Investigation of these possibilities will be required in order to understand the cellular mechanism of Ola-induced changes in DA release.
The effects of DA in the NAc depend on the magnitude and timing of DA release (Grace et al., 2007), which vary according to the task being performed and the degree to which it has been acquired (e.g. Cheer et al., 2007; Day et al., 2007). In addition, VTA activity is modulated by input from other neural structures (Grace et al., 2007; Koob and Volkow, 2010), all of which may be directly or indirectly affected by adolescent Ola treatment. The consequences of treatment with stimulants (and, perhaps, other rewarding stimuli) can vary depending on the environment in which the drugs are administered (Li et al., 2004), response contingencies and whether subjects actively administer the drugs or receive them passively (Lecca et al., 2007). Thus, assessment of the behavioural significance of our DA release data requires measurement of DA release in the NAcC during the performance of appetitive behaviours under multiple experimental conditions, including CPP.
Translational implications
Responsiveness to drugs
The changes in reward system function induced by adolescent Ola treatment may resemble some of those that underlie the development of addiction (Koob and Volkow, 2010). We are studying the effects of adolescent Ola treatment on additional responses to drugs of abuse, to determine if, like CPP, they are enhanced. Early life APD treatment might also alter the mature brain’s responses to therapeutic drugs, especially those that have DAergic or 5-HTergic activities.
Psychiatric disorders
In rodent models of schizophrenia, adolescent atypical APD treatment can attenuate or prevent the emergence of some disease-associated behavioural phenotypes, notably abnormalities of latent inhibition (Piontkewitz et al., 2009, 2011; Meyer et al., 2010), pre-pulse inhibition (Meyer et al., 2010), Amph-induced locomotor activity (Richtand et al., 2006, 2011, 2012; Piontkewitz et al., 2009, 2011) and discrimination reversal (Piontkewitz et al., 2011). To our knowledge, the effects of adolescent atypical APD treatment on working memory and extinction of conditioned responses have not been assessed in these models. Together, these data and ours (current results ; Milstein et al., unpublished data) suggest that, although preventive APD therapy may be beneficial in some individuals with an elevated risk for psychosis-related behavioural dysfunction, prophylactic treatment of patients who might not otherwise develop psychotic symptoms, carries substantial risk of long-term behavioural pathology.
Acknowledgments
Supported by: NIMH grant MH07083 to DOF; NIDA grants DA25890 and DA022340 to J.C. ; a CIHR grant to B.K.; olanzapine from Eli Lilly & Co. and NIMH. J.M. was supported by NINDS training grant T32 NS007375. We thank Drs Michal Arad, Todd Gould, Peg McCarthy, Patricio O’Donnell, Rosy Roberts and Leo Tonelli for critiques of the manuscript and Drs Paul Fishman and Gloria Reeves for helpful discussions. Olanzapine for this study was supplied free of charge by Eli Lilly & Co. and by NIMH. These experiments were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Our protocols were approved by the Institutional Animal Care and Use Committees of the University of Maryland, Baltimore (Protocol #0411003), St. Mary’s College of Maryland (Protocol #R010907) and the University of Lethbridge (Protocol #0712).
Footnotes
Statement of Interest
None.
References
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf. 2011;20:177–184. doi: 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Annual research review: new frontiers in developmental neuropharmacology: can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry. 2011;52:476–503. doi: 10.1111/j.1469-7610.2011.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tarazi FI. Drugs and the treatment of psychiatric disorders : psychosis and mania. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s The pharmacological basis of therapeutics. 10. New York: McGraw Hill; 2001. pp. 485–520. [Google Scholar]
- Baron JC, Martinot JL, Cambon H, Boulenger JP, Poirier MF, Caillard V, Blin J, Huret JD, Loc’h C, Maziere B. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment : correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology (Berl) 1989;99:463–472. doi: 10.1007/BF00589893. [DOI] [PubMed] [Google Scholar]
- Bedard AM, Maheux J, Levesque D, Samaha AN. Continuous, but not intermittent, antipsychotic drug delivery intensifies the pursuit of reward cues. Neuropsychopharmacology. 2011;36:1248–1259. doi: 10.1038/npp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons : relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Huggins KN, Mathews TA, Jones SR. Conserved dorsal-ventral gradient of dopamine release and uptake rate in mice, rats and rhesus macaques. Neurochem Int. 2012;60:986–991. doi: 10.1016/j.neuint.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Catlow JT, Barton RD, Clemens M, Gillespie TA, Goodwin M, Swanson SP. Analysis of olanzapine in human plasma utilizing reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1995;668:85–90. doi: 10.1016/0378-4347(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Cheng-Shannon J, McGough JJ, Pataki C, McCracken JT. Second-generation antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14:372–394. doi: 10.1089/cap.2004.14.372. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nabawi A, Quesenberry M, Saito K, Silbergeld E, Vasta G, Eldefrawi A. The N-methyl-D-aspartate neurotransmitter receptor is a mammalian brain target for the dinoflagellate Pfiesteria piscicida toxin. Toxicol Appl Pharmacol. 2000;169:84–93. doi: 10.1006/taap.2000.9042. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of drug-induced neurotoxicity on development of neural circuitry : a hypothesis. Br Res Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Hussey D, Houle S, Kapur S. D2- receptor upregulation is dependent upon temporal course of D2-occupancy: a longitudinal [11C]-raclopride PET study in cats. Neuropsychopharmacology. 2009;34:662–671. doi: 10.1038/npp.2008.116. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O’Donnell P. The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS ONE. 2009;4:e5062. doi: 10.1371/journal.pone.0005062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Jones SR, O’Dell SJ, Marshall JF, Wightman RM. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse. 1996;23:224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition : a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell vs. core dopamine in the rat : a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Liao RM. Development of conditioned place preference induced by intra-accumbens infusion of amphetamine is attenuated by co-infusion of dopamine D1 and D2 receptor antagonists. Pharmacol Biochem Behav. 2008;89:367–373. doi: 10.1016/j.pbb.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Llorente-Berzal A, Mela V, Borcel E, Valero M, Lopez-Gallardo M, Viveros MP, Marco EM. Neurobehavioral and metabolic long-term consequences of neonatal maternal deprivation stress and adolescent olanzapine treatment in male and female rats. Neuropharmacology. 2011;62:1332–1341. doi: 10.1016/j.neuropharm.2011.07.031. [DOI] [PubMed] [Google Scholar]
- McCracken JT, Suddath R, Chang S, Thakur S, Piacentini J. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol. 2008;18:501–508. doi: 10.1089/cap.2007.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight RF, Park RJ. Atypical antipsychotics and anorexia nervosa: a review. Eur Eat Disord Rev. 2010;18:10–21. doi: 10.1002/erv.988. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development : molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappadopulos E, Macintyre JC, II, Crismon ML, Findling RL, Malone RP, Derivan A, Schooler N, Sikich L, Greenhill L, Schur SB, Felton CJ, Kranzler H, Rube DM, Sverd J, Finnerty M, Ketner S, Siennick SE, Jensen PS. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry. 2003;42:145–161. doi: 10.1097/00004583-200302000-00008. [DOI] [PubMed] [Google Scholar]
- Pappadopulos E, Rosato NS, Correll CU, Findling RL, Lucas J, Crystal S, Jensen PS. Experts’ recommendations for treating maladaptive aggression in youth. J Child Adolesc Psychopharmacol. 2011;21:505–515. doi: 10.1089/cap.2010.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical–ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115:745–753. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2011;37:1257–1269. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S. Antipsychotic dosing: how much but also how often? Schizophr Bull. 2010;36:900–903. doi: 10.1093/schbul/sbq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn P, Stanford K, Bronson SL, McNamara RK. Effects of risperidone and paliperidone pre-treatment on locomotor response following prenatal immune activation. J Psychiatr Res. 2011;45:1194–1201. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn P, Tambyraja R, Grainger M, Bronson SL, McNamara RK. Fluoxetine and aripiprazole treatment following prenatal immune activation exert longstanding effects on rat locomotor response. Physiol Behav. 2012;106:171–177. doi: 10.1016/j.physbeh.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, Hayes S, Coolen LM, Pritchard LM, Logue A, Herman JP, McNamara RK. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. ‘Breakthrough’ dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–2986. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltema Beduin A, de Haan L. Off-label second generation antipsychotics for impulse regulation disorders : a review. Psychopharmacol Bull. 2010;43:45–81. [PubMed] [Google Scholar]
- Sikich L. Efficacy of atypical antipsychotics in early-onset schizophrenia and other psychotic disorders. J Clin Psychiatry. 2008;69(Suppl 4):21–25. [PubMed] [Google Scholar]
- Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, Kapur S, Zipursky RB, Wilson AA, Christensen BK, Seeman P. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152:174–180. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci USA. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Delbello MP. Olanzapine for the treatment of bipolar disorder in children and adolescents. Expert Opin Pharmacother. 2008;9:467–474. doi: 10.1517/14656566.9.3.467. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hatano K, Sakiyama Y, Kawasumi Y, Kato T, Ito K. Age-related changes of dopamine D1-like and D2-like receptor binding in the F344/N rat striatum revealed by positron emission tomography and in vitro receptor autoradiography. Synapse. 2001;41:285–293. doi: 10.1002/syn.1085. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain : implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2001;297:711–717. [PubMed] [Google Scholar]
- Terry AV, Jr, Warner SE, Vandenhuerk L, Pillai A, Mahadik SP, Zhang G, Bartlett MG. Negative effects of chronic oral chlorpromazine and olanzapine treatment on the performance of tasks designed to assess spatial learning and working memory in rats. Neuroscience. 2008;156:1005–1016. doi: 10.1016/j.neuroscience.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Stansifer L, Findling RL. Psychopharmacology of pediatric bipolar disorders in children and adolescents. Pediatr Clin North Am. 2011;58:173–187. xii. doi: 10.1016/j.pcl.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vasconcelos SM, Nascimento VS, Nogueira CR, Vieira CM, Sousa FC, Fonteles MM, Viana GS. Effects of haloperidol on rat behavior and density of dopaminergic D2-like receptors. Behav Processes. 2003;63:45–52. doi: 10.1016/s0376-6357(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Vidal-Infer A, Roger-Sanchez C, Daza-Losada M, Aguilar MA, Minarro J, Rodriguez-Arias M. Role of the dopaminergic system in the acquisition, expression and reinstatement of MDMA-Induced conditioned place preference in adolescent mice. PLoS ONE. 2012;7:e43107. doi: 10.1371/journal.pone.0043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosc Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller EB, Weller RA. Olanzapine as treatment for children and adolescents with Tourette’s syndrome. Curr Psychiatry Rep. 2009;11:95–96. doi: 10.1007/s11920-009-0015-z. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PN, Abbas AI, Farrell MS, Setola V, Sciaky N, Huang XP, Kroeze WK, Crawford LK, Piel DA, Keiser MJ, Irwin JJ, Shoichet BK, Deneris ES, Gingrich J, Beck SG, Roth BL. The presynaptic component of the serotonergic system is required for clozapine’s efficacy. Neuropsychopharmacology. 2011;36:638–651. doi: 10.1038/npp.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents : a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21:600–620. doi: 10.1016/j.euroneuro.2011.04.001. [DOI] [PubMed] [Google Scholar]