Abstract
Purpose
Assess the association between geographic access to mammography facilities and Women’s mammography utilization frequency.
Methods
Using data from the population based 1995–2007 Wisconsin Women’s Health study, we used proportional odds and logistic regression to test whether driving times to mammography facilities and the number of mammography facilities within 10 km of Women’s homes were associated with mammography frequency among women aged 50-74, and whether associations differed between Rural Urban Commuting Areas and income and education groups.
Results
We found evidence for nonlinear relationships between geographic access and mammography utilization (nonlinear effects of driving times and facility density, P-values 0.01 and 0.005, respectively). Having at least one nearby mammography facility was associated with greater mammography frequency among urban women (1 vs. 0 facilities, OR 1.26, 95% CI 1.09-1.47), with similar effects among rural women. Adding more facilities had decreasing marginal effects. Long driving times tended to be associated with lower mammography frequency. We found no effect modification by income, education, or urbanicity. In rural settings, mammography non-use was higher, facility density smaller, and driving times to facilities were longer.
Conclusion
Having at least one mammography facility near one’s home may increase mammography utilization, with decreasing effects per each additional facility.
Keywords: Mammography, Spatial Epidemiology, Health Services Accessibility
INTRODUCTION
While there has been a recent controversy about benefits and harms, optimal starting age and frequency of mammography screening1–3, there is broad consensus that mammography screening can detect breast cancer early and reduce mortality4. Specific breast cancer screening recommendations have changed over time, but for women aged 50-74 mammography screening every 1-2 years has been consistently recommended since the 1980s5–7. Screening uptake varies by social characteristics; for example racial minorities and low-socioeconomic status women use mammography screening less often than white, middle-class women8,9.
Since the 1990s, mammography utilization appears to have plateaued or even decreased10,11. At the same time, there was a decrease in the number of mammography facilities, potentially reducing geographic access to mammography services12. Urban sprawl and limited public transportation make most Americans dependent on personal vehicle use13,14. Among lowest income groups in the US, there is a disproportionate number of households without a car15. Social gradients in geographic and transportation access have been recognized as determinants of health care utilization, and may reinforce existing social disparities in mammography utilization16,17.
In this analysis, we aimed to assess how driving times and geographic availability of mammography facilities relate to frequency of mammography screening among women without breast cancer, and whether these associations differ between rural and urban settings. We hypothesized that decreased geographic access would be related to lower frequency of mammography screening use, and that this relationship would be strongest for low income and low education groups. We additionally hypothesized that long driving times and fewer geographically available mammography facilities would impact rural more than urban populations.
MATERIALS AND METHODS
Study Population
The Wisconsin Women’s Health Study was a series of population based case control studies at the University of Wisconsin. Women with a history of breast cancer were the cases and women without the controls18. We only included controls in this analysis because our outcome was mammography screening frequency before any breast cancer diagnosis. We obtained informed consent from study participants, and the University of Wisconsin Health Sciences Institutional Review Board approved the study.
Subject eligibility has been described in detail elsewhere18–21. 70.2% of eligible Wisconsin participants enrolled in the study. Briefly, women randomly selected from lists of licensed drivers were eligible to participate as controls if they had no personal history of breast cancer, had a listed telephone number, and if they were able to complete a standardized telephone interview. We restricted our analysis to women aged 50-74 at baseline who participated between 1995-2007 because screening recommendations existed for this age group consistently during the study period, and because we had access to data on mammography facilities since 1995. During recruitment, not all questionnaire versions contained questions on income. Income is likely a confounder of geographic access in relation to mammography use. To avoid potential misclassification for a large number of participants, we excluded women who were not asked about income at baseline (N=1,184) rather than imputing these incomes.
6,075 women were eligible for our analysis. Of these, we excluded 145 women (2.4 %) because of missing data on mammography use. Comparing these women with the women in the final sample, excluded women were more likely to be postmenopausal (95% vs. 87%), less likely to have used postmenopausal hormones (24% vs. 47%), less likely to have a family history of breast cancer (13% vs. 16%), less likely to be white (93% vs. 96%), and more likely to have missing data across all variables (data not shown).
Data Collection
In telephone interviews, we collected information on family history of breast cancer, frequency of mammography screening, postmenopausal hormone use, race, education, income, and household size. Information on the number and locations of mammography facilities between 1995 and 2007 was obtained from the FDA which has maintained administrative records on certified mammography facilities in the US since 199422. Although all participants lived in Wisconsin, we accounted for mammography facilities in adjacent states close to the Wisconsin border, because some participants may have traveled to surrounding states for screening.
Measures
The outcome in our analytical models was mammography frequency, measured as the self-reported number of screening mammograms in the past 5 years translated into an annual frequency. Primary exposures were driving times to nearby mammography facilities and the number of mammography facilities near a woman’s home. Driving times were measured as the shortest driving time to a mammography facility near a woman’s home and were determined in two steps: Using ArcGIS 10.0 Generate Near Table functionality, for each woman we identified the two closest (Euclidean distance) certified mammography facilities in the year of her study participation. Afterwards, we used the Googlemaps Distance Matrix API via the R-package httr23 to determine for which of the two facilities driving time was the shortest. Googlemaps Distance Matrix API limits the number of requests to 2,500 per 24 hours. Therefore, driving times were calculated over 10 week days, each day around the same time to make traffic conditions comparable. Mammography facility density was defined as the number of certified mammography facilities within a 10 km radius around each woman’s residential address in the year of her participation, and estimated using ArcGIS 10.0 buffer/intersect functionalities.
We used DAGitty24 to draw our conceptual model as a directed acyclic graph (DAG) (Figure 1) in order to decide which covariates to include in our analysis. DAGs are depictions of researchers’ beliefs to qualitatively explain outcomes by potential determinants25. If a DAG correctly depicts the relationships, it identifies confounders and mediators, i.e. which variables to include and exclude from statistical models. Based on our DAG, we chose two confounder models. One included the minimal sufficient adjustment set for estimating the total effects of our exposures, which would sufficiently control for confounding if our DAG correctly identified all relationships. The minimal sufficient adjustment set included: family income, persons per household, education, race, and mammography capacity, i.e. the number of mammography machines available per 10,000 women aged 40 and older in a county. The second model additionally adjusted for age, family history, and indicators of neighborhood deprivation by Census Tract: population fraction below the poverty line, median 1999 income, population fraction without a vehicle, population fraction without a high school degree and with at least a college degree; and by County: the population fraction without health insurance. Data on health insurance were only collected for a minority of participants and therefore not included in our main models, but only in our sensitivity analyses.
Figure 1.
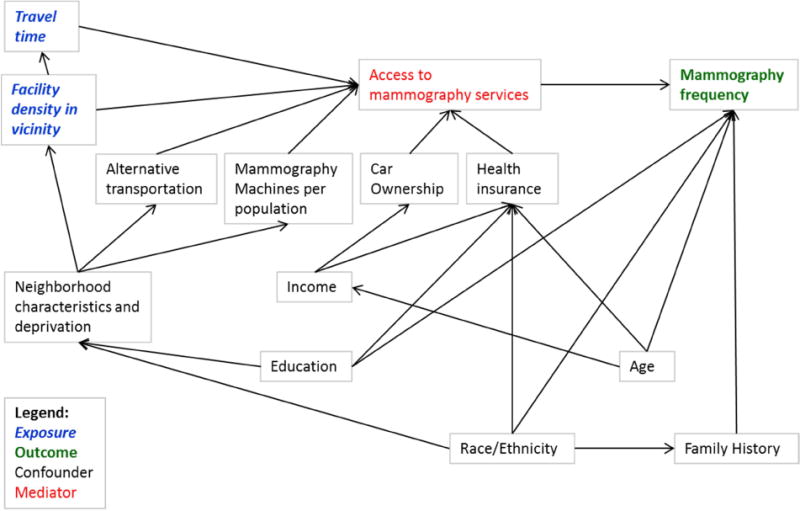
Directed Acyclic Graph of the associations between driving time and mammography facility density within residential neighborhood, and frequency of mammography screening use
Geospatial Analysis
Methods for geocoding have been described elsewhere22,26. Each participant was assigned a corresponding 2000 Census Tracts, county and Rural Urban Commuting Area (RUCA) code27. Mammography facilities were geocoded by street address, using ArcGIS software (Version 9.2, ESRI, Redlands, CA). Mobile mammography facilities were assigned to the county of their mailing address22. To describe regional patterns, we fit a semivariogram model and a kriging interpolation of mammography utilization frequency in Wisconsin using the R packages sp, gstat, spatstat, maps, and geoR28–32. Using the R jitter() function33, we jittered the coordinates of 24 randomly selected participants out of 48 participants who had duplicate geocoordinates due to rounding.
Statistical Analysis
We excluded women with missing outcome data, but used multiple imputation (5 datasets) in the SAS MI procedure34 to impute missing main exposure and confounder values, i.e. education (16 missing), race (28 missing), family history (130 missing), and income (660 missing), and in the case of 38 women who could not be geocoded: driving time and facility density, and their Census Tract and County level confounders.
To test the associations of driving time and mammography facility density with mammography frequency, we used ordered multinomial regression (proportional odds model) in the SAS 9.3 GLIMMIX and MIANALYZE procedures35,36. We ran the regressions as multilevel models, grouping participants by residential 2000 Census Tracts to account for potential neighborhood clustering. We tested for nonlinear relationships between our main exposures and the outcome by including cubic splines of driving time and facility density. Splines can capture nonlinear effects that are not easily captured by polynomials. We tested for interaction between our main exposures and RUCA codes, income, and education levels, and re-ran the final model stratified by these same variables. Additionally, we ran a logistic regression, modeling the odds of a mammography frequency greater or equal 0.5 (equivalent to at least one mammogram in two years) compared to a frequency below 0.5. Model selection was carried out on the proportional odds model; only the final model was also run as logistic regression.
RESULTS
Table 1 shows selected characteristics at baseline of the study population. Most participants were non-Hispanic white. When comparing women reporting no screening in the past 5 years (non-users) to women reporting at least annual mammography screening, non-users had fewer mammography facilities near their homes, were less likely to be white, to have a family history of breast cancer, to have gone through menopause, and to have used postmenopausal hormones, and they had lower income and education levels. The proportion of women from rural areas was similar in all utilization groups, except for non-users, which were disproportionally rural (X2 test, P < 0.0001). Table 2 summarizes the distribution of driving times and facility densities in rural vs. urban settings. Urban women had more facilities near their homes and shorter driving times. Almost half (48%) of rural women had no facility within a 10km radius around their home.
Table 1.
Baseline characteristics of participants by mammography screening frequency, (N=5,930), Wisconsin Women’s Health Study, 1995-2007
| Annual Frequency of Mammography Screening
|
||||||
|---|---|---|---|---|---|---|
| Characteristics | Total % (N=5,930) |
0 Times % (N=596) |
< 0.5 Times % (N=968) |
0.5 – 0.9 Times % (N=894) |
≥ 1 Times % (N=3,472) |
P |
| Age, Years | ||||||
| 50–59 | 49.9 | 46.8 | 50.5 | 55.6 | 48.8 | |
| 60–69 | 47.0 | 48.2 | 45.4 | 40.2 | 49.0 | |
| ≥ 70 | 3.1 | 5.0 | 4.1 | 4.3 | 2.1 | < 0.00001 |
| Driving Time to Closest Mammography Facility, Minutes | ||||||
| < 5 | 27.4 | 27.5 | 27.5 | 25.6 | 27.8 | |
| 5 – 10 | 31.7 | 30.2 | 31.2 | 31.8 | 32.1 | |
| 11–20 | 29.7 | 29.7 | 28.8 | 30.8 | 29.6 | |
| > 20 | 11.2 | 12.6 | 12.5 | 11.9 | 10.4 | 0.61 |
| Number of Mammography Facilities within 10km Radius, N | ||||||
| 0 | 28.1 | 31.0 | 31.6 | 28.6 | 26.4 | |
| 1 – 2 | 29.8 | 33.4 | 28.5 | 29.6 | 29.5 | |
| 3 | 7.8 | 6.5 | 7.2 | 6.3 | 8.6 | |
| > 3 | 34.4 | 29.0 | 32.6 | 35.5 | 35.5 | 0.0014 |
| Number of Mammography Machines per 10,000 Women Aged 40+ in County, N | ||||||
| 0 – 1 | 24.6 | 29.2 | 26.0 | 26.0 | 23.0 | |
| 2 – 3 | 42.9 | 43.3 | 42.4 | 42.8 | 43.0 | |
| > 3 | 32.5 | 27.5 | 31.6 | 31.2 | 34.0 | 0.0070 |
| Race/Ethnicity | ||||||
| White, Non-Hispanic | 95.8 | 94.3 | 95.1 | 94.7 | 96.5 | |
| Other | 4.2 | 5.7 | 4.9 | 5.3 | 3.5 | 0.010 |
| Menopausal Status | ||||||
| Premenopausal | 14.2 | 16.4 | 18.2 | 18.6 | 11.6 | |
| Postmenopausal | 85.8 | 83.6 | 81.8 | 81.4 | 88.4 | < 0.00001 |
| Family History of Breast Cancer | ||||||
| Yes | 16.4 | 12.2 | 13.8 | 15.0 | 18.1 | |
| No | 83.6 | 87.8 | 86.2 | 85.0 | 81.9 | 0.000098 |
| Postmenopausal Hormone Use | ||||||
| Never | 53.1 | 84.9 | 70.7 | 57.6 | 41.6 | |
| Ever | 46.9 | 15.1 | 29.3 | 42.4 | 58.4 | < 0.00001 |
| Family Income | ||||||
| ≤ 30,000 | 33.0 | 53.5 | 41.4 | 32.0 | 27.4 | |
| $30,001 – 50,000 | 30.2 | 25.8 | 29.2 | 29.1 | 31.5 | |
| $50,001 – 100,000 | 27.9 | 16.9 | 24.4 | 27.3 | 30.8 | |
| > $100,000 | 8.9 | 3.7 | 5.0 | 11.6 | 10.2 | < 0.00001 |
| Education | ||||||
| No High School Degree | 7.3 | 15.4 | 8.4 | 7.5 | 5.6 | |
| High School Degree | 42.8 | 49.8 | 42.7 | 38.9 | 42.6 | |
| Some College | 26.6 | 23.3 | 29.0 | 28.4 | 26.0 | |
| College Degree | 23.3 | 11.4 | 19.9 | 25.2 | 25.8 | < 0.00001 |
| Urbanicity | ||||||
| Urban | 64.2 | 56.4 | 62.1 | 65.2 | 65.8 | |
| Rural | 35.8 | 43.6 | 37.9 | 34.8 | 34.2 | 0.000063 |
Table 2.
Distributions of driving times and facility density, urban vs. rural, (N=5,930), Wisconsin Women’s Health Study, 1995-2007
| Main Exposures | Minimum | 1st Quartile | Median | Mean | 3rd Quartile | Maximum |
|---|---|---|---|---|---|---|
| Number of Mammography Facilities within 10km Radius, N | ||||||
| Urban | 0 | 1 | 4 | 6.9 | 10 | 39 |
| Rural | 0 | 0 | 1 | 0.8 | 1 | 14 |
| Driving Time to Closest Mammography Facility, Minutes | ||||||
| Urban | 0.02 | 4.4 | 6.9 | 8.4 | 11.4 | 37.4 |
| Rural | 0.2 | 5.8 | 12.2 | 13.7 | 19.7 | 138.8 |
The heat map from our kriging analysis in Figure 2 visualizes average utilization patterns in Wisconsin. Utilization above average is shaded in blue, and below average in red. Utilization is consistently above average where many facilities are regionally clustered, especially in large urban areas (Milwaukee, Madison, Greenbay), and below average where facility density is sparse.
Figure 2.
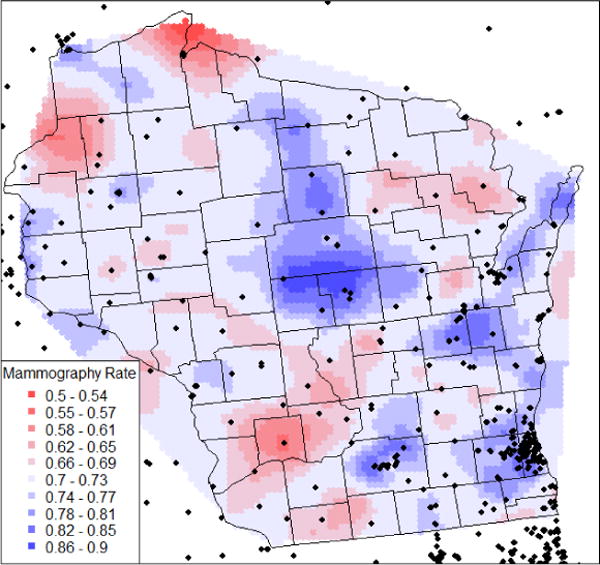
Estimated spatial pattern of average annual mammography utilization in Wisconsin relative to location of mammography facilities (black points) (N=5,930), Wisconsin Women’s Health Study, 1995–2007
Odds ratios (OR) of more frequent mammography utilization from the proportional odds models are shown in Table 3, with results from both the minimal adjustment set model and the model with more potential confounders. Nonlinear effects of driving time and facility density were significant in both models (nonlinear effects of driving time, P-values 0.003 and 0.01, respectively; nonlinear effects of facility density, P-values 0.003 and 0.005, respectively). After stratifying the model with more confounders by urban vs. rural RUCA codes, driving time remained significant among rural women (nonlinear effects of driving time, P-value 0.02), and facility density remained significant among urban women (nonlinear effects of facility density, P-value 0.005). Interactions of the main exposures with urban vs. rural RUCA codes, and with income and education were not significant.
Table 3.
Odds ratios of more frequent mammography screening use by geographic access, (N=5,930), Wisconsin Women’s Health Study, 1995-2007
| Main Exposures | Minimal Confounder Model§, Nonlinear Effects, Urban | Minimal Confounder Model§, Nonlinear Effects, Rural | Full Confounder Model†, Nonlinear Effects, Urban | Full Confounder Model†, Nonlinear Effects, Rural | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Driving Time | ||||||||
| Marginal Effects | ||||||||
| 20 vs. 10 Minutes | 1.09 | 0.77–1.54 | 1.39 | 1.03–1.87 | 1.09 | 0.77–1.55 | 1.46 | 1.09–1.97 |
| 30 vs. 20 Minutes | 0.64 | 0.37–1.08 | 0.89 | 0.76–1.04 | 0.64 | 0.38–1.09 | 0.93 | 0.79–1.08 |
| 40 vs. 30 Minutes | 0.48 | 0.20–1.15 | 0.69 | 0.53–0.89 | 0.49 | 0.20–1.16 | 0.71 | 0.54–0.92 |
| Short Driving Times as Reference | ||||||||
| 30 vs. 10 Minutes | 0.69 | 0.38–1.26 | 1.24 | 0.86–1.78 | 0.70 | 0.38–1.28 | 1.35 | 0.94–1.96 |
| 40 vs. 10 Minutes | 0.34 | 0.09–1.28 | 0.85 | 0.55–1.31 | 0.34 | 0.09–1.31 | 0.96 | 0.61–1.49 |
| Number of Mammography Facilities within 10km Radius | ||||||||
| Marginal Effects | ||||||||
| 1 vs. 0 Facilities | 1.22 | 1.05–1.42 | 1.17 | 0.92–1.49 | 1.26 | 1.08–1.47 | 1.21 | 0.95–1.53 |
| 2 vs. 1 Facilities | 1.15 | 1.03–1.29 | 1.10 | 0.94–1.30 | 1.18 | 1.05–1.33 | 1.12 | 0.96–1.32 |
| 3 vs. 2 Facilities | 1.05 | 0.99–1.11 | 1.00 | 0.87–1.15 | 1.06 | 1.00–1.12 | 1.00 | 0.87–1.15 |
| 4 vs. 3 Facilities | 0.98 | 0.93–1.02 | 0.93 | 0.76–1.13 | 0.97 | 0.92–1.02 | 0.92 | 0.75–1.12 |
| No Nearby Facilities as Reference | ||||||||
| 2 vs. 0 Facilities | 1.40 | 1.08–1.83 | 1.29 | 0.88–1.91 | 1.49 | 1.14–1.96 | 1.35 | 0.91–2.01 |
| 3 vs. 0 Facilities | 1.48 | 1.07–2.03 | 1.29 | 0.84–2.00 | 1.58 | 1.15–2.18 | 1.35 | 0.87–2.10 |
| 4 vs. 0 Facilities | 1.44 | 1.04–2.00 | 1.20 | 0.75–1.91 | 1.53 | 1.10–2.13 | 1.24 | 0.77–2.00 |
Model adjusts for education, income, number of household members, race, and mammography capacity.
Model adjusts for education, income, number of household members, race, and mammography capacity, age, family history of breast cancer, and indicators of neighborhood deprivation by Census Tract: population fraction below the poverty line, median 1999 income, population fraction without a vehicle, population education levels, population fraction without health insurance (by county).
The final model included more potential confounders beyond the minimal adjustment set, nonlinear effects of the main exposures, no interaction terms, and was stratified by urban vs. rural RUCA codes. Among urban women, having one mammography facility within a 10km radius was associated with more frequent mammography utilization (1 vs. 0 facilities, OR 1.26, 95% CI 1.08-1.47). More facilities increased the odds of more frequent screening, but the effect decreased with each added facility and became insignificant with 2-3 nearby facilities (2 vs. 1 facilities, OR 1.18, 95% CI 1.05-1.33; 3 vs. 2 facilities, OR 1.06, 95% CI 1.00-1.12). When comparing the effects of 2 vs. 0, 3 vs. 0, and 4 vs. 0 nearby facilities, the OR were essentially identical, indicating a plateau effect when adding more facilities if there are already one or two facilities in the vicinity. In the rural model, effects of facility density were similar, albeit not statistically significant. Contrary to our hypothesis, driving times of 20 vs. 10 minutes were associated with increased odds of more frequent screening among rural women (OR 1.46, 95% CI 1.09-1.97). There was generally a trend towards decreased ORs with long driving times, but odds ratios for moderate vs. short driving times were inconsistent. OR of having had at least 1 mammogram in two years (logistic regression, Table 4) were close to the OR from the proportional odds model. Estimated effects stratified by income (Q1 vs. higher incomes) and education (no vs. at least college degree) were also similar (supplemental Tables 1 and 2).
Table 4.
Odds ratios of at least one mammogram in two years by geographic access, (N=5,930), Wisconsin Women’s Health Study, 1995-2007
| Main Exposures | Full Confounder Model, Nonlinear Effects, Urban | Full Confounder Model, Nonlinear Effects, Rural | ||
|---|---|---|---|---|
|
|
||||
| OR | 95% CI | OR | 95% CI | |
| Driving Time | ||||
| Marginal Effects | ||||
| 20 vs. 10 Minutes | 1.07 | 0.71–1.64 | 1.56 | 1.10–2.23 |
| 30 vs. 20 Minutes | 0.68 | 0.35–1.31 | 0.94 | 0.78–1.14 |
| 40 vs. 30 Minutes | 0.54 | 0.18–1.57 | 0.70 | 0.51–0.96 |
| Short Driving Times as Reference | ||||
| 30 vs. 10 Minutes | 0.73 | 0.35–1.51 | 1.47 | 0.96–2.27 |
| 40 vs. 10 Minutes | 0.39 | 0.08–2.04 | 1.03 | 0.61–1.75 |
| Number of Mammography Facilities within 10km Radius | ||||
| Marginal Effects | ||||
| 1 vs. 0 Facilities | 1.29 | 1.06–1.55 | 1.26 | 0.95–1.68 |
| 2 vs. 1 Facilities | 1.20 | 1.04–1.38 | 1.19 | 0.98–1.45 |
| 3 vs. 2 Facilities | 1.06 | 0.98–1.14 | 1.08 | 0.90–1.30 |
| 4 vs. 3 Facilities | 0.96 | 0.91–1.02 | 1.00 | 0.78–1.29 |
| No Nearby Facilities as Reference | ||||
| 2 vs. 0 Facilities | 1.54 | 1.10–2.15 | 1.51 | 0.93–2.43 |
| 3 vs. 0 Facilities | 1.63 | 1.09–2.43 | 1.63 | 0.95–2.80 |
| 4 vs. 0 Facilities | 1.57 | 1.04–2.37 | 1.63 | 0.90–2.97 |
Models adjust for education, income, number of household members, race, and mammography capacity, age, family history of breast cancer, and indicators of neighborhood deprivation by Census Tract: population fraction below the poverty line, median 1999 income, population fraction without a vehicle, population education levels, population fraction without health insurance (by county).
We conducted sensitivity analyses on our final model (data not shown). Although postmenopausal hormone use and marital status were not confounders in our DAG, we reran the final model including an indicator of postmenopausal hormone use and an indicator of living with a partner. We also reran the final model without imputing missing variables, and another version excluding mammography facilities outside Wisconsin. Neither of these changes substantially altered the estimated effects of our main exposures. We reran the final model for a reduced sample of women (N=759) with and without an indicator of having health insurance. Having health insurance slightly attenuated the effect of facility density among urban women (1 vs. 0 facilities while controlling for insurance, OR 1.18, 95% CI 0.74-1.88, vs. without controlling for health insurance, OR 1.24, 95% CI 0.78-1.99), but in both model versions, the pattern still indicated increased odds of screening use with higher facility density, with decreasing marginal effects per added facility. Finally, in a model with only linear effects, effects of driving time were not significant, and the OR for driving time and facility density were close to 1.
DISCUSSION
We found that having one or two mammography facilities within a 10 km radius may increase mammography frequency among women without breast cancer, increasing the odds of more frequent utilization by 15-50%, but adding more than two facilities had decreasing marginal effects. Our kriging interpolation confirmed that mammography utilization was highest where facilities were regionally clustered. With long driving times, there was a tendency towards decreased odds of screening among rural women, but other driving time comparisons were inconsistent. We found no evidence of effect modification of geographic access by income or education, or by urbanicity. However, rural women had fewer mammography facilities near their homes and longer driving times to facilities, and were more likely to have reported no mammogram in the past 5 years.
Findings from similar studies have been inconsistent. Khan-Gates37 reviewed articles relating geographic access variables to mammography use. Among those, Meersman et al38 found reduced odds of a recent mammogram if only 0-1 mammography facilities were located near a woman’s home, but the odds of screening hardly changed for 2-10 nearby facilities. We similarly found that higher facility density within a 10km radius was nonlinearly associated with greater odds of more frequent utilization, without measurable marginal benefits when more than 2-3 facilities were available. This correlation between high facility density and greater utilization frequency was also confirmed by our kriging analysis.
Other studies examined the relationships between driving distance to mammography facilities and utilization. Meersman et al38 found no association between distance to facilities and mammography utilization; Engleman et al39 found that with each additional 5 miles of distance, odds of screening use decreased by 3%; Khang found that women living closer to mammography facilities were more likely to complete work-up after an abnormal mammogram16. Some40 but not all41–43 studies have suggested that longer driving distances or driving times may be related to later stage of breast cancer at diagnosis, which is associated with poorer survival. These inconsistent findings parallel our inconclusive results with regards to driving time. We found some evidence of a nonlinear effect of driving times on mammography utilization, and a tendency towards reduced mammography utilization with long driving times among rural women, but inconsistent odds ratios when comparing moderate to short driving times.
Other studies evaluated the number of mammography machines per population as main exposure. Elkin et al22 found that in counties with inadequate availability of mammography screening machines, odds of screening use was reduced by 13-15%. Elting et al44 found that the presence of a mammography facility in the county was associated with increased odds of mammography utilization. Other studies found no association45–47. Included as a confounder in our study, mammography capacity per population was not significantly associated with mammography utilization in our models (data not shown).
A strength of our study is that we allowed for nonlinear effects of our main exposures, which was not done in previous studies we reviewed and may have captured effects more accurately. We found evidence that driving times and facility density were nonlinearly related to mammography utilization, while a strictly linear model did not detect any statistically or clinically significant effects. Our study also has limitations. All women in our study had a driver’s license because of the sampling frame, but we had no data on individual car access. Pucher et al15 showed that owning at least one car per household is common in the US, but becomes less common for lowest income groups. These lowest income groups may be less likely to have participated in our study, which could have prevented us from detecting effect modification by income. Furthermore, 50-74 year-old women may be more likely to have access to a car compared with younger age groups. Mammography is used infrequently, which may make geographic access less relevant compared with other considerations regarding mammography utilization, e.g. time off work and child care. We did not include health insurance in our main models. In a sensitivity analysis, including health insurance slightly attenuated but generally confirmed our findings. Our findings cannot be generalized to other medical services. Another limitation in our study is a historic discrepancy between driving times which we estimated in 2015, and our mammography use measures that we collected 1995-2007. This could have created non-differential exposure misclassification, possibly biasing our effect estimates towards the null.
CONCLUSIONS
Our analysis emphasizes that relationships can be nonlinear. According to our findings, the availability of one or two mammography facility near Women’s homes may indeed increase mammography screening utilization, but effects plateau with more than two nearby facilities. While we found no evidence of effect modification by urbanicity, we did find that geographic access to mammography facilities was more restricted in rural than in urban areas, and that mammography utilization was below average where facility density was sparse. From a public health perspective, identifying areas without any nearby mammography facilities may be one means to address under- and non-use of mammography services among eligible women, especially in rural areas.
Supplementary Material
Supplemental Figure 1 Semivariogram of annual mammography utilization in Wisconsin (N=5,930), Wisconsin Women’s Health Study, 1995–2007
Supplemental Table 1 Odds ratios of more frequent mammography screening use by geographic access, stratified by income and education (N=5,930), Wisconsin Women’s Health Study, 1995-2007
Supplemental Table 2 Odds ratios of at least one mammogram in two years by geographic access, stratified by income and education (N=5,930), Wisconsin Women’s Health Study, 1995-2007
Acknowledgments
This work was supported by the National Institutes of Health grants R01 CA067264, P30 CA014520, and R01 CA047147. We would like to thank Julie McGregor and Kathy Peck for study support and data collection, Felix Elwert for directions on using directed acyclic graphs, and Jaime Martindale for her ArcGIS support.
LIST OF ABBREVIATIONS
- CI
Confidence Interval
- DAG
Directed Acyclic Graph
- OR
Odds Ratio
- RUCA
Rural Urban Commuting Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. The Lancet Oncology. 2014;15(6):e234–e242. doi: 10.1016/S1470-2045(13)70598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss SM, Cuckle H, Evans A, Johns L, Waller M, Bobrow L. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: a randomised controlled trial. Lancet. 2006;368(9552):2053–2060. doi: 10.1016/S0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- 3.Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up: a randomized screening trial of mammography in women age 40 to 49 years. Annals of Internal Medicine. 2002;137(5 Part 1):305–312. doi: 10.7326/0003-4819-137-5_part_1-200209030-00005. [DOI] [PubMed] [Google Scholar]
- 4.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909–919. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald P, Sondik EJ. Cancer control objectives for the nation, 1985–2000. 1986 [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for Breast Cancer. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Accessed June 10 2015.
- 7.Dodd GD. American Cancer Society guidelines on screening for breast cancer: an overview. CA: A Cancer Journal for Clinicians. 1992;42(3):177–180. doi: 10.1002/1097-0142(19920401)69:7+<1885::aid-cncr2820691702>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Sabatino SA, Coates RJ, Uhler RJ, Breen N, Tangka F, Shaw KM. Disparities in mammography use among US women aged 40–64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Medical care. 2008;46(7):692–700. doi: 10.1097/MLR.0b013e31817893b1. [DOI] [PubMed] [Google Scholar]
- 9.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States. Cancer. 2003;97(6):1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 10.Breen N, A Cronin K, Meissner HI, et al. Reported drop in mammography. Cancer. 2007;109(12):2405–2409. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 11.Breen N, Gentleman JF, Schiller JS. Update on mammography trends. Cancer. 2011;117(10):2209–2218. doi: 10.1002/cncr.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkin EB, Atoria CL, Leoce N, Bach PB, Schrag D. Changes in the availability of screening mammography, 2000-2010. Cancer. 2013 Nov 1;119(21):3847–3853. doi: 10.1002/cncr.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frumkin H. Urban sprawl and public health. Public Health Reports. 2002 May-Jun;117(3):201–217. doi: 10.1093/phr/117.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing R, Pendall R, Chen D. Measuring sprawl and its transportation impacts. Transportation Research Record: Journal of the Transportation Research Board. 2003;(1831):175–183. [Google Scholar]
- 15.Pucher J, Renne JL. Socioeconomics of urban travel: evidence from the 2001 NHTS. Transportation Quarterly. 2003;57(3):49–77. [Google Scholar]
- 16.Khang L, Adams SA, Steck SE, Zhang J, Xirasagar S, Daguise VG. Travel distance to screening facilities and completion of abnormal mammographic follow-up among disadvantaged women. Annals of epidemiology. 2016 doi: 10.1016/j.annepidem.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Kirby RS, Delmelle E, Eberth JM. Advances in spatial epidemiology and geographic information systems. Annals of epidemiology. 2016 doi: 10.1016/j.annepidem.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Sprague BL, Gangnon RE, Hampton JM, et al. Variation in Breast Cancer–Risk Factor Associations by Method of Detection: Results From a Series of Case-Control Studies. Am J Epidemiol. 2015;181(12):956–969. doi: 10.1093/aje/kwu474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson SE, Gould MN, Hampton JM, Trentham-Dietz A. A case-control study of the HER2 Ile655Val polymorphism in relation to risk of invasive breast cancer. Breast cancer research. 2005;7(3):1. doi: 10.1186/bcr1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newcomb P, Trentham-Dietz A, Hampton J. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. British journal of cancer. 2010;102(5):799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer Epidemiology Biomarkers & Prevention. 2007;16(2):236–243. doi: 10.1158/1055-9965.EPI-06-0713. [DOI] [PubMed] [Google Scholar]
- 22.Elkin EB, Ishill NM, Snow JG, et al. Geographic access and the use of screening mammography. Medical care. 2010 Apr;48(4):349–356. doi: 10.1097/MLR.0b013e3181ca3ecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickham H. R Package ‘httr’. https://cran.r-project.org/web/packages/httr/httr.pdf. Accessed December 18, 2015.
- 24.Textor J. DAGitty. http://www.dagitty.net/. Accessed December 5, 2014.
- 25.Elwert F. Graphical Causal Models. In: Morgan SL, editor. Handbook of Causal Analysis for Social Research. Springer; Netherlands: 2013. pp. 245–273. [Google Scholar]
- 26.McElroy JA, Remington PL, Trentham-Dietz A, Robert SA, Newcomb PA. Geocoding addresses from a large population-based study: lessons learned. Epidemiology (Cambridge, Mass) 2003 Jul;14(4):399–407. doi: 10.1097/01.EDE.0000073160.79633.c1. [DOI] [PubMed] [Google Scholar]
- 27.Center RHR. Rural Health Research Center. Using RUCA Data. http://depts.washington.edu/uwruca/ruca-uses.php.
- 28.Adrian Baddeley, Rolf Turner, Rubak E. R spatstat package. https://cran.r-project.org/web/packages/spatstat/index.html. Accessed 7 June 2017.
- 29.Edzer Pebesma, Graeler B. R gstat package. https://cran.r-project.org/web/packages/gstat/gstat.pdf. Accessed 7 June 2017.
- 30.Edzer Pebesma, Roger Bivand, Barry Rowlingson, et al. R sp package. https://cran.r-project.org/web/packages/sp/sp.pdf. Accessed 7 June 2017.
- 31.Ribeiro Paulo J, Diggle Peter J. R geoR package. https://cran.r-project.org/web/packages/geoR/geoR.pdf. Accessed 7 June 2017.
- 32.Becker Richard A, Wilks Allan R, Brownrigg Ray, Minka Thomas P, Deckmyn Alex. R maps package. https://cran.r-project.org/web/packages/maps/maps.pdf. Accessed 7 June 2017.
- 33.Werner Stahel, Maechler M. R jitter() function. https://stat.ethz.ch/R-manual/R-devel/library/base/html/jitter.html. Accessed 7 June 2017.
- 34.SAS MI Procedure. https://support.sas.com/documentation/onlinedoc/stat/141/mi.pdf. Accessed 7 June 2017.
- 35.SAS GLIMMIX Procedure. https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#glimmix_toc.htm. Accessed Accessed 7 June 2017.
- 36.SAS MIANALYZE Procedure. https://support.sas.com/rnd/app/stat/procedures/mianalyze.html. Accessed 7 June 2017.
- 37.Khan-Gates JA, Ersek JL, Eberth JM, Adams SA, Pruitt SL. Geographic Access to Mammography and Its Relationship to Breast Cancer Screening and Stage at Diagnosis: A Systematic Review. Women’s Health Issues. 2015;25(5):482–493. doi: 10.1016/j.whi.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meersman SC, Breen N, Pickle LW, Meissner HI, Simon P. Access to mammography screening in a large urban population: a multi-level analysis. Cancer Causes & Control. 2009;20(8):1469–1482. doi: 10.1007/s10552-009-9373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman KK, Hawley DB, Gazaway R, Mosier MC, Ahluwalia JS, Ellerbeck EF. Impact of geographic barriers on the utilization of mammograms by older rural women. Journal of the American Geriatrics Society. 2002;50(1):62–68. doi: 10.1046/j.1532-5415.2002.50009.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Dignan M, Han D, Johnson O. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 2009 Fall;25(4):366–371. doi: 10.1111/j.1748-0361.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- 41.Celaya M, Berke E, Onega T, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998–2004) Rural Remote Health. 2010;10(2):1361. [PMC free article] [PubMed] [Google Scholar]
- 42.Henry KA, Sherman R, Farber S, Cockburn M, Goldberg DW, Stroup AM. The joint effects of census tract poverty and geographic access on late-stage breast cancer diagnosis in 10 US States. Health & place. 2013 May;21:110–121. doi: 10.1016/j.healthplace.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Onega T, Cook A, Kirlin B, et al. The influence of travel time on breast cancer characteristics, receipt of primary therapy, and surveillance mammography. Breast cancer research and treatment. 2011;129(1):269–275. doi: 10.1007/s10549-011-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elting LS, Cooksley CD, Bekele BN, et al. Mammography capacity: Impact on screening rates and breast cancer stage at diagnosis. American journal of preventive medicine. 2009;37(2):102–108. doi: 10.1016/j.amepre.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Rahman S, Price JH, Dignan M, Rahman S, Lindquist PS, Jordan TR. Access to mammography facilities and detection of breast cancer by screening mammography: a GIS approach. International journal of cancer prevention. 2009;2(6):403. [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Social Science & Medicine. 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Marchick J, Henson DE. Correlations between access to mammography and breast cancer stage at diagnosis. Cancer. 2005;103(8):1571–1580. doi: 10.1002/cncr.20915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Semivariogram of annual mammography utilization in Wisconsin (N=5,930), Wisconsin Women’s Health Study, 1995–2007
Supplemental Table 1 Odds ratios of more frequent mammography screening use by geographic access, stratified by income and education (N=5,930), Wisconsin Women’s Health Study, 1995-2007
Supplemental Table 2 Odds ratios of at least one mammogram in two years by geographic access, stratified by income and education (N=5,930), Wisconsin Women’s Health Study, 1995-2007


