Abstract
Due to the importance of fluid flow during thrombotic episodes, it is quite appropriate to study clotting and bleeding processes in devices that have well-defined fluid shear environments. Two common devices for applying these defined shear stresses include the cone-and-plate viscometer and parallel-plate flow chamber. While such tools have many salient features, they require large amounts of blood or other protein components. With growth in the area of microfluidics over the last two decades, it has become feasible to miniaturize such flow devices. Such miniaturization not only enables saving of precious samples but also increases the throughput of fluid shear devices, thus, enabling the design of combinatorial experiments and making the technique more accessible to the larger scientific community. In addition to simple flows that are common in traditional flow apparatus, more complex geometries that mimic stenosed arteries and the human microvasculature can also be generated. The composition of the microfluidics cell substrate can also be varied for diverse basic science investigations, and clinical investigations that aim to assay either individual patient coagulopathy or response to anti-coagulation treatment. This review summarizes the current state of the art for such microfluidic devices and their applications in the field of thrombosis and hemostasis.
Introduction
Human vascular networks are complex in that vessel size scales from 1–3 cm in the aorta and pulmonary arteries down to 3–10 μm in capillaries [1]. The applied wall shear stress (=viscosity × local shear rate for Newtonian fluids) also varies dramatically from ~0.7–9 dyn/cm2 in the venous circulation to 20–70 dyn/cm2 in arterioles, and up to >450 dyn/cm2 in stenosed arteries [2]. The range of wall shear rates (a measure of velocity gradient) corresponding to these fluid shear stresses, ranges over three orders of magnitude from 15–10,000/s. This complex biophysical milieu is a host to a variety of biological processes that regulate platelet adhesion to damaged vessels walls, leukocyte adhesion at sites of inflammation, and coagulation processes that eventually plug the flow of blood [3]. The different biophysical and biochemical processes that participate in these processes are all part of the Virchow’s triad [4]. These include: i. Blood components including the various coagulation factors and associated risk factors that control hypercoagulability like natural single nucleotide polymorphisms (SNPs) that alter protein function; ii. Injuries or trauma that contribute to vessel/endothelium dysfunction during wall injury, hypertension, medical devices or chronic inflammation; and iii. Alterations in fluid flow either due to stasis in local regions of the circulation or abnormal shear profiles due to vessel constrictions or stenosis.
The above processes are primarily regulated by biophysical (flow, morphology, and gradients) and biochemical (molecules, matrixes, and cells) components. While biochemical factors including cell surface receptors and vessel wall composition have been extensively characterized [5, 6], the roles of biophysical components is less characterized in part due to the failure to reproduce physiological fluid shear in many in vitro assays. Traditional parallel plate flow chambers and newer microfluidic devices have emerged as a promising technology to fill this gap. They enable the study of biological processes under fluid shear, both for gathering basic science knowledge and for point-of-care diagnostic applications. This review presents selected applications of microfluidics to investigate processes regulating thrombosis and hemostasis.
Complex flows and combinatorial experiments enabled by microfluidics technology
Parallel plate flow chambers and cone-plate viscometer shearing devices were introduced in the 1980s to study the role of fluid shear in regulating thrombosis. Typical flow chambers are transparent. They require a syringe pump to inject/perfuse a suspension of cells at low Reynolds numbers (low flow conditions that typically result in laminar flow, without turbulence) over a desired range of shear rate to mimic in vivo hemodynamics. By seeding cells or immobilizing protein substrates on the chamber substrate to mimic the blood vessel wall, such flow devices have been applied to study leukocyte-endothelial cell interactions [7, 8] and thrombus formation under flow [9]. While valuable for studying simple cell interactions under constant shear in a square cross-section device, classical flow chamber fabrication techniques are unable to mimic flow dynamics in circular vessels with convergent or divergent flows, and 3D vascular networks where bifurcation or stenosis is prevalent. Additionally, they require a large amount of reagents for a single run.
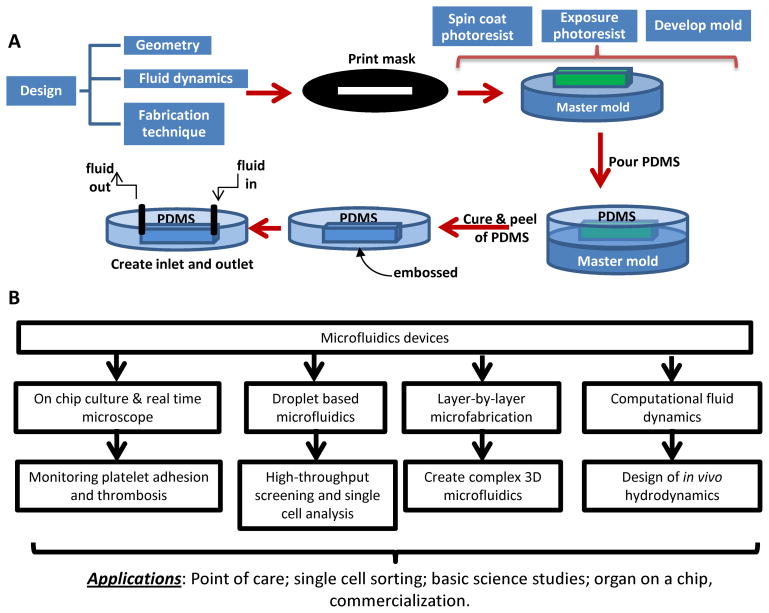
The design of microfluidic devices enables the creation of more complex geometries that more accurately mimic μm-size features observed in the vasculature. Under the most standard condition, photolithography methods originally developed in the 1960s for building integrated circuits, have been used to build microfluidic devices (Figure 1). This method allows the creation of features down to 100nm. To achieve this, first, a photoresist (often SU-8) of defined thickness is spin-coated under well-controlled speeds onto a silicon wafer. UV light is then exposed on the thin photoresist layer through a high-resolution mask, printed with a pattern of interest. This operation crosslinks all regions of the photoresists, save the area protected by the mask. Chemical removal of the non-crosslinked material results in a template master mold. Beginning in the 1990s, a range of techniques were developed, using ‘soft’ elastomers often composed of PDMS (polydimethysiloxane) [10]. In one variant of this ‘soft lithography’ method, negative templates of the rigid master mold were created in PDMS and these were either chemically bonded or vacuum-sealed onto glass/PDMS substrates to create simple flow cells (Figure 1A). PDMS templates generated from the original master can also be used to as a secondary master to fabricate additional devices using other biocompatible polymers, a method called ‘replica molding’. Proteins mixed in an ink, and soaked into the PDMS mold, can also be ‘microcontact printed’ onto surfaces for easy template duplication. In this case, the ink is often made of alkanethiols which form self-assembled monolayers (SAMs) on gold. The interaction of these alkanethiols with proteins then enables high-resolution patterning of proteins on the gold substrate. Additionally, the above soft lithography techniques can be augmented with Computational Fluid Dynamic (CFD) based modeling, to create more complex microfluidic device designs and networks. Studies with these new devices can enhance our understanding of vascular disease processes and result in the development of next generation diagnostics/point-of-care devices (Figure 1B).
Figure 1. Fabrication of microfluidics flow cells using polydimethylsiloxane replica molding.
A. Fabrication starts with the design of the geometry and fluid dynamics that are of interest. The photolithography steps make use of the negative photoresist, e.g. SU-8. Exposure of this resist to UV radiation in the presence of the pattern mask results in the master mold. Then polydimethylsiloxane (PDMS) is used to cast microfluidic devices containing pre-designed structures reflected by the mold. In the final step, the PDMS mold is peeled from the master template and assembled into a flow device. B. Design and applications of microfluidics. Online figure is available in color.
Platelet GpIbα-VWF interactions leading to platelet accumulation and thrombus growth
The adhesion of platelets onto denuded vessel walls is a shear dependent process that is fundamental to thrombosis and hemostasis. This process is highly regulated by complex flow. This is exemplified by earlier work that shows that platelet-rich thrombi are found in regions of high fluid flow such as arteries. When the flow velocity is lower such as in the venous regime, red cells in addition to platelets reside in the fibrin clots [11]. Cell adhesion at the injured vessel site is initiated by the interaction of glycoprotein receptors expressed on platelet surface such as GpIbα and αIIbβ3, with extracellular matrix molecules such as collagen and vitronectin [12]. The plasma glycoprotein von Willebrand factor (VWF) is critical for thrombus formation under arterial flow conditions, with fibronectin and fibrinogen also being important at lower shears. VWF and fibrinogen form important substrates that augment clot formation in various prosthetics, including left ventricular assist devices [13].
Among the molecular interactions regulating platelet adhesion, there is considerable interest in studying the effect of fluid shear in controlling platelet GpIbα binding to von Willebrand factor (VWF) immobilized on collagen at sites of vessel denudation. Here, the A3-domain of VWF primarily binds extracellular matrix proteins, with VWF A1-domain also recognized collagen-VI [14]. Once captured onto substrates, the A1-domain of VWF captures platelet GpIbα from flow. Such cell adhesion is a transient, stop-go, shear-dependent interaction that is best studied under flow [12, 15]. During such platelet recruitment, shear rate, controls the contact time between platelet membrane receptors and immobilized VWF at sites of vessel denudation [16]. This flow parameter thus controls the on-rate of interaction or the cell capture rate from flow. As increasing flow rate results in reduced contact time, the platelet capture efficiency is greater at lower wall shear rates. Shear stress affects the off-rate of the interaction by controlling the lifetime of adhesive bonds, and consequently platelet detachment is augmented upon increasing shear stress. A number of investigators have studied the shear-stress dependent kinetics of platelet GpIbα-VWF interactions using ligand-coated microspheres [17, 18]. In such work, Yago et al. [18] suggest that rather than resulting in a monotonic increase in cell rolling velocity with wall shear stress, the platelet GpIbα-VWF bond rather exhibits a ‘catch bond’ property. Due to such bond mechanics, platelet rolling velocity is minimal at an intermediate shear. Besides, catch bond formation, studies show that platelet deformation also likely controls the rate of cell capture [17]. Together, the unique GpIbα-VWF bond mechanics and platelet deformation regulate the stability of the cell rolling/translocation interaction.
Besides natural VWF binding to platelet GpIbα, microfluidics also aids structure-function relationship studies using mutant proteins. For example, Madabhushi et al. [19] created a series of VWF variants and applied microfluidics to demonstrate that VWF propeptide binding to the VWF D′D3 domain reduces VWF-platelet adhesion interactions. This may represent a mechanism by which the propeptide in blood regulates thrombosis in circulation. To elaborate on this concept, the authors also made anti-D′D3 monoclonal antibodies that sterically inhibit the A1 domain of VWF from binding platelet GpIbα in flow based assays. Additionally, to demonstrate the importance of structural features N-terminal to the A1-domain in regulating VWF-GpIbα binding, various mutants were created [20]. Functional studies performed with these constructs using microfluidics show that the D′D3 domain of VWF is a key regulator that inhibits the binding of platelet GpIbα to the VWF-A1 domain and thrombus formation under physiological shear. In addition to D′D3, using VWF mutants lacking the A2-domain, Aponte-Santamaria et al. [21] show increased binding of platelets to VWF in the absence of A2. Thus, both the domains that flank the A1 domain at the N- (D′D3) and C- (A2) terminals may control platelet binding under shear.
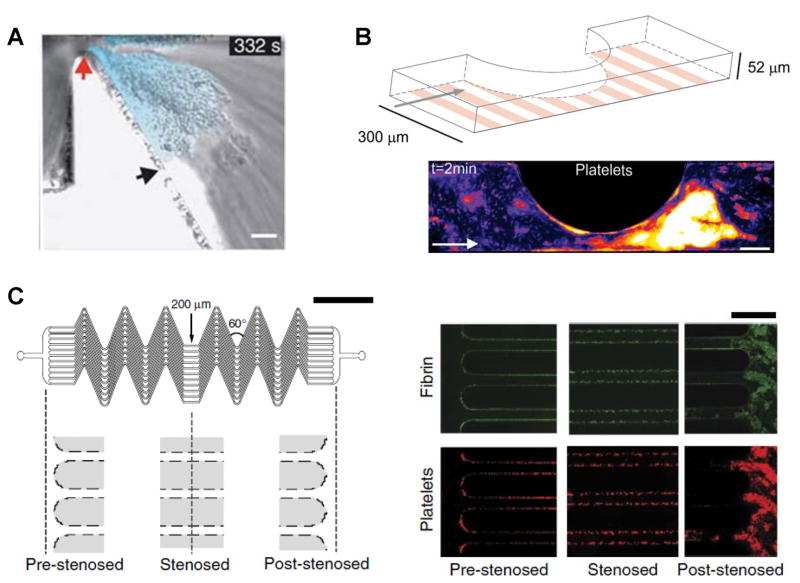
The newly recruited platelets on the denuded vessel wall are activated by local soluble secreted agonists, receptor mediated outside-in signaling (particularly via platelet GP-VI and VLA-2), and other shear-dependent processes that elicit cytosolic calcium flux. Here, the binding of GpIbα to the VWF A1 domain elicits signals that contribute to platelet activation [22]. Such cell activation occurs both in the context of platelets bound to substrates, and during shear induced platelet activation in suspension [23, 24]. In this context, initial platelet binding to immobilized VWF elicits a relatively small calcium peak (α/β) due to cation release from intracellular stores. The second larger Ca2+ signal (γ peak) occurs when platelets firmly attached via activated αIIbβ3 [25] and the VWF C1 domain [26]. Interestingly, such activation is optional for thrombosis at high shear rates exceeding 10–20,000 s−1, since the VWF-A1 domains become ‘active’ in this regime and interacts with un-activated platelets to form aggregate [27]. Here, VWF forms elongated fibers and become the core of the aggregates. Using a tapering microfluidic flow channel that mimics stenotic flow, Nesbitt et al. [28] suggest that VWF-dependent, activation-independent platelet aggregation is specifically initiated at the stenosis apex in the context of atherosclerosis, with further aggregate growth occurring downstream in the expansion zone (Figure 2A). They propose that, rheology rather than cell activation controls platelet aggregation. More refined studies by Westein et al. [29] show that beyond rheological factors, endothelial secretion of VWF and autocrine platelet stimulation are also necessary for aggregation downstream of vessel stenosis (Figure 2B). Under high shear conditions, platelets bind to immobilized VWF via discrete ligands and withstand tensile forces resulting from hydrodynamic drag. This drag force transmitted through the GpIbα receptor triggers cell signaling and the exposure of phophatidylserine (coagulation reactions sites) on the platelet membrane [23, 24]. At the same time, the pulling force also results in elongated membrane tethers and microparticles [30]. Mechanisms contributing to platelet microparticle formation include biochemical stimulation via thrombin and collagen [31], and biomechanical forces [32, 33].
Figure 2. Microfluidic devices to study platelet adhesion in post-stenotic region.
In all examples, platelet adhesion and thrombus formation is observed in the post-stenotic downstream region, in the ‘shear deceleration zone’. All studies measure platelet aggregation using whole blood. A. Model microfluidics channel incorporating fixed 90% stenosis. Wall shear rate changes abruptly from 1800/s upstream to 200/s in the shear deceleration expansion zone. Scale bar=10μm (ref. [28]). B. 300 × 52 μm flow chamber with confluent endothelial/HUVEC substrate. Platelet aggregates formed in whole blood, in a shear dependent manner, are shown using pseudo coloring of fluorescent platelets. Scale bar=100μm (ref. [29]). C. Schematic to left showing a region of flow acceleration to the left (‘pre-stenosed’) and flow deceleration to the right (‘post-stenosed’). In between are 12 parallel 200 × 75 μm channels with a series of 60° angle turns. Right panel are representative images showing fibrin (top, green) and adhered platelets (bottom, red), prominently in the post-stenotic region (ref. [50]). Online figure is available in color. All figures are reproduced with permission.
Intracellular signaling is necessary for stable platelet adhesion, and subsequent platelet recruitment via activated platelet αIIbβ3 receptor. In addition to platelet adhesion, the stoppage of blood also depends on coagulation related fibrin network formation, clot retraction and fibrinolysis [34]. Casa et al. used different geometry microfluidic devices to quantify the relative impact of platelet-platelet to platelet-surface interactions on cell adhesion [35]. Their results show that larger circular vessel geometries and increasing rectangular test section aspect ratios favor platelet-platelet interactions compared to platelet-substrate binding, which is more common in constricted vessels. Consistent with this, surface-bound inhibitors are more efficacious in smaller channels, while the ideal mode of clot inhibition is dependent on local shear rate in larger channels [36]. Overall, studies using microfluidic devices revealed important details on the mechanism of platelet adhesion particularly the role of VWF, and the impact of shear forces in clot propagation.
Microfluidic devices for measuring bleeding risk
The balance between pro- and anti-thrombotic processes in normal physiology is critical, and perturbation of this balance leads to clinical pathology or bleeding diseases. To diagnose hemostatic dysfunction, clinical tests focus on the evaluation of platelet concentration and function, coagulation factor activity, and ancillary thrombotic or fibrinolytic proteins. Ideally, in addition to measuring each of these parameters individually, it would also be beneficial to quantify the integration between these distinct biochemical processes in a shear and time dependent manner, in assays that include the endothelium. In this regard, it is known that platelet adhesion, aggregation and related VWF interactions are shear dependent [37]. Additionally, the nature of fibrin mesh formation during coagulation is also dependent on local thrombin concentrations, and this is flow regulated [38]. Activated platelets regulate coagulation via three major mechanisms: exposure of phosphatidylserine on activated surface to enhance sites for coagulation [39]; providing thrombin and fibrin for these sites [40]; and regulating fibrin clot retraction [41]. Endothelial cells also regulate clotting through the secretion of VWF, prostacyclin, ADPases, thrombomodulin, and selectins that recruit white cells [42]. Thus, microfluidic devices that mimic blood components and flow dependent vessel wall interactions can aid diagnostics.
Unlike commercial devices like PFA-100® (Platelet Function Analyzer 100), VerifyNow® and Cone and Plate analyzers that provide end-point analyses, microfluidic assays incorporating fluorescence microscopy has been widely used to follow the temporal evolution of thrombi under shear. Depending on the nature of the measurements, these assays can monitor multiple output parameters including: immobilized platelet number and aggregate morphology, platelet activation via cell surface P-selectin, cell surface phosphatidylserine via Annexin-V measurements, fibrin formation and also local thrombin production [20, 43, 44]. Using blood from FVIII−/− mice or human blood supplemented with anti-FIXa aptamer, early studies by Ogawa et al. [45], showed that thrombus formation patterns in haemophilia are regulated by fluid shear. Upon application of a novel microfluidic device containing 8 channels to evaluate hemophiliac blood clotting, Colace et al. also demonstrated a correlation between low coagulation factor activity (less than 1%) and deficiency in both fibrin formation and platelet deposition [43]. The contribution of recombinant Factor VIII in hemophilia A patients to fibrin formation has also been evaluated [46]. Flow devices have also been used to evaluate von Willebrand Disease (VWD). Using a flow chambers with varying linear shear rates, Sugimoto et al. compared mural thrombus formation under physiologic blood flow among type 2A, 2B and healthy controls [47]. Their study confirms impaired thrombus formation in type 2B VWD. In addition, microfluidic devices have also been used to study thrombus formation in a range of VWD Type 2A mutants and also using Type 3 VWD blood [48, 49]. While fibrillar collagen is typically the substrate for the evaluation of thrombus formation, recently de Witt et al. [44] extended this approach by creating 52 different substrates composed of various collagen and collagen mimetic peptides, decorin, fibrinogen, fibronectin, laminin, thrombospondin-1, vitronectin and VWF. In addition to evaluating the relative roles of adhesion and signaling molecules (glycoprotein VI, C-type lectin-like receptor-2 (CLEC-2), GPIbα, α6β1, αIIbβ3 and α2β1), the authors also developed a 9-microspot test set to evaluate blood from individuals with severe clotting deficiency compared with normal blood [44]. Most recently, microfluidic devices with shear gradients have been integrated into extracorporeal circuits in pig models to enable real-time surveillance of coagulopathy in stenotic vessels [50] (Figure 2C). Together, this body of literature supports the possibility that flow devices may be a useful tool to complement classical biochemical assays for the evaluation of individuals with suspected bleeding disorders or pro-thrombotic tendency.
In order to make microfluidic devices suitable for clinical diagnostics, however, a number of challenges remain. While the fabrication costs for the flow device itself is low, instruments (like microscopes) needed for data acquisition and analysis are expensive. As the microfluidics technology is also a relatively new methodology, its standardization is challenging. In this regard, the output measured depends on the nature of the substrate used in the assay, flow conditions, chamber geometry and measurement technique [51]. While laboratory scale devices require intensive maintenance efforts to pattern and setup the system, the fabrication of disposable standardized microfluidic devices is likely to enhance usage of this method. Additionally, clinical trials and standardization efforts are needed to ensure reproducibility and ease of handling. With this goal in mind, a number of companies focused on the application of microfluidics for hemostasis diagnostics/management have emerged. Their success is now awaited.
More complex flow patterns in microfluidic devices
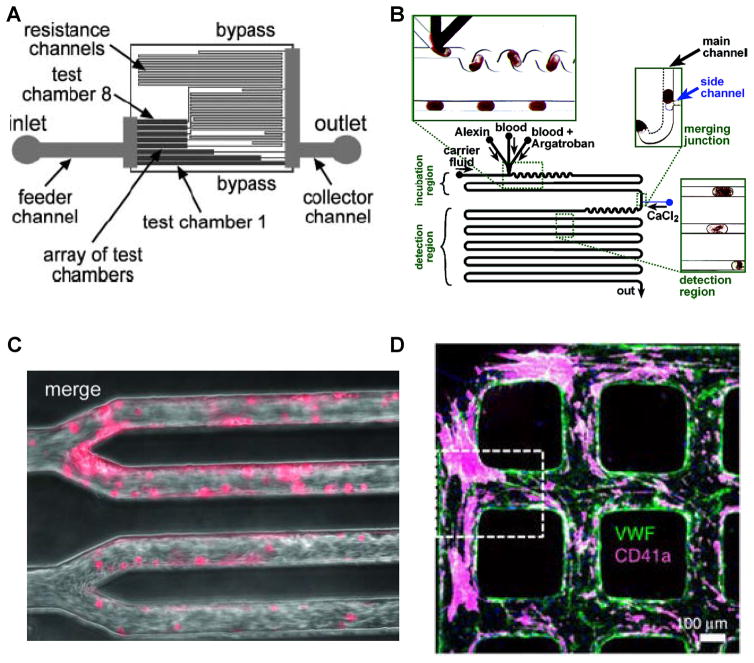
Besides straight-channel flow devices with or without constrictions, there is growing interest in creating channels with more complex geometries since this enables diverse applications not possible using other means. In this regard, some investigators have created flow cells with different resistance channels in series as this enables the simulation of different shear stresses in a single, compact microfluidic device ([52], Figure 3A). Using this, comprehensive platelet adhesion tests can be carried out under multiple conditions using small volumes of blood available from transgenic mice. In another example, the activated partial thromboplastin time (APTT) test was performed in small plugs containing blood or platelet poor plasma (PPP) separated from each other by fluorocarbon fluids (Figure 3B, [53]). Here, bright field microscopy was used to measure fibrin clots, and fluorogenic substrate was applied to monitor thrombin production. Results from microfluidics assays were compared with clinical tests.
Figure 3. Different geometries in advanced microfluidic devices.
A. Inclusion of resistance channels to simulate different shear stresses on a single chip (ref. [52]). B. Testing of anti-coagulant (argatroban) in small plugs (ref. [53]). C. Microvascular clots (pink) formed upon perfusing blood into channels containing TNF-α activated endothelial cells (ref. [57]). D. VWF deposited in 3D endothelialized microfluidic channels. Platelet thrombi (pink) are seen decorated on VWF strings (green) (ref. [56]). Online figure is available in color. All figures are taken from cited references, with permission.
There is also growing interest in developing flow cells containing microvascular networks as they are sites of vasculogenesis [54], thrombosis [55] and VWF size regulation by the metalloprotease ADAMTS13 [56]. In one example, to investigate hematologic diseases involving microvascular occlusion and thrombosis, Tsai et al. created an “endothelialized” microfluidics based flow network to resemble pathophysiological processes involved in hemolytic uremic syndrome and sickle cell disease (Figure 3C, [57]). Their results show that shear stress influences microvascular thrombosis and obstruction. Zheng et al. [55] created non-thrombotic 3D endothelialized microfluidic vessel based tissue scaffolds and characterized their morphology, mass transfer properties and stability. Using this device, the authors show that endothelial-secreted VWF can assemble into thick bundles and complex meshes in microvessels (Figure 3D, [56]). These VWF deposits can be cleaved by the ADAMTS13, support microvascular thrombosis, and even contribute to erythrocyte fragmentation (schistocytes) [56].
The ability to generate highly mono-dispersed droplets in microfluidic systems in immiscible carrier phases allows the separation of single or discrete events [58]. Such systems are resulting in next generation techniques for enzymatic assays, single cell assays, blood clotting studies and diagnostics [59]. Here, droplet-based microfluidics can handle complex biological fluids such as blood, and measure clotting time using small volumes of blood or plasma in the presence of specific anticoagulants. This is important in the clinic, in order to determine the anticoagulant drug dose required for individual patients. In one example, Song et al, performed such drug titrations using a droplet-based microfluidic device and argatroban, a direct thrombin inhibitor. In this study, microfluidics enabled the titration of different doses of argatroban in a series of well separated blood drops, with inline measurement of clot formation and thrombin generation using fluorescent microscopy [53]. In a second example, Kline et al, applied droplet-based microfluidics to perform ABO and D blood typing, using both antibodies and lectins [60]. The scale of these droplet based studies is small currently, and considerable refinement is required before such methods find practical applications in the clinic.
Finally, to overcome the problem of platelet shortage in transfusion medicine, investigators are creating platelet bioreactors that enable megakaryocyte fragmentation to produce thrombocytes. In one example, 3D bioreactors were shown to be superior to 2D cultures for the generation of platelets from CD34+ hematopoietic progenitors [61]. In another case, microfluidic reactors mimicking various features of the bone marrow niche including extracellular matrix, channel size and fluid shear have been constructed in order to synthesize functional platelets from induced pluripotent stem cells [62]. Functional platelets have also been created using a 3D silk based synthetic bone marrow niche [63]. Even though these bioreactor systems can mimic the in vivo conditions to some extent and improve the production of platelets in vitro, technical hurdles remain since the economical scale-up of these miniaturized technologies is challenging.
Conclusions
Microfluidic devices are important analysis tools that can be used for diverse tests with human blood. These tests may measure thrombus formation in whole blood, secretion of specific cell products, fibrin deposition or titration of anticoagulants for precision medicine applications. The exact design features can be varied depending on the specific application. Further, these devices can be rapidly cast at low cost using PDMS, and the technology is primed for rapid prototyping. These devices create controlled hemodynamic conditions that mimic in vivo flows using simple syringe pumps to create desired pressure gradients. The ability to micropattern complex geometries using PDMS enables the ready recreation of physiologically relevant flows. Thus, they are ideally suited for a range of laboratory scale experiments and next-generation clinical applications.
Footnotes
Declaration of Interest
This work was supported by the National Institutes of Health grant HL77258, and American Heart Association award 161RG27770071.
References
- 1.Enderle J, Blanchard S, Bronzino J. Introduction to Biomedical Engineering. San Diego, CA: 2000. Cardiovascular mechanics; pp. 467–535. [Google Scholar]
- 2.Gogia S, Neelamegham S. Role of fluid shear stress in regulating VWF structure, function and related blood disorders. Biorheology. 2015;52(5–6):319–35. doi: 10.3233/BIR-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423–36. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 4.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. 2008;143(2):180–90. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 5.Pugh N, et al. Dynamic analysis of platelet deposition to resolve platelet adhesion receptor activity in whole blood at arterial shear rate. Platelets. 2015;26(3):216–9. doi: 10.3109/09537104.2014.893289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94(5):657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakarpandian B, et al. Expression and functional significance of adhesion molecules on cultured endothelial cells in response to ionizing radiation. Microcirculation. 2001;8(5):355–64. doi: 10.1038/sj/mn/7800105. [DOI] [PubMed] [Google Scholar]
- 8.Crutchfield KL, et al. CD11b/CD18-coated microspheres attach to E-selectin under flow. J Leukoc Biol. 2000;67(2):196–205. doi: 10.1002/jlb.67.2.196. [DOI] [PubMed] [Google Scholar]
- 9.Alevriadou BR, et al. Real-Time Analysis of Shear-Dependent Thrombus Formation and Its Blockade by Inhibitors of Vonwillebrand-Factor Binding to Platelets. Blood. 1993;81(5):1263–1276. [PubMed] [Google Scholar]
- 10.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner HR. The role of blood flow in platelet adhesion, fibrin deposition, and formation of mural thrombi. Microvasc Res. 1973;5(2):167–79. doi: 10.1016/0026-2862(73)90069-1. [DOI] [PubMed] [Google Scholar]
- 12.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 13.Nascimbene A, et al. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127(25):3133–41. doi: 10.1182/blood-2015-10-636480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flood VH, et al. Crucial role for the VWF A1 domain in binding to type IV collagen. Blood. 2015;125(14):2297–304. doi: 10.1182/blood-2014-11-610824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moroi M, et al. Analysis of the involvement of the von Willebrand factor-glycoprotein Ib interaction in platelet adhesion to a collagen-coated surface under flow conditions. Blood. 1997;90(11):4413–24. [PubMed] [Google Scholar]
- 16.Zhang Y, Neelamegham S. Estimating the efficiency of cell capture and arrest in flow chambers: study of neutrophil binding via E-selectin and ICAM-1. Biophysical Journal. 2002;83(4):1934–52. doi: 10.1016/S0006-3495(02)73956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doggett TA, et al. Alterations in the intrinsic properties of the GPIbalpha-VWF tether bond define the kinetics of the platelet-type von Willebrand disease mutation, Gly233Val. Blood. 2003;102(1):152–60. doi: 10.1182/blood-2003-01-0072. [DOI] [PubMed] [Google Scholar]
- 18.Yago T, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118(9):3195–207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madabhushi SR, et al. von Willebrand factor (VWF) propeptide binding to VWF D′D3 domain attenuates platelet activation and adhesion. Blood. 2012;119(20):4769–78. doi: 10.1182/blood-2011-10-387548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madabhushi SR, et al. Platelet GpIba binding to von Willebrand Factor under fluid shear:contributions of the D′D3-domain, A1-domain flanking peptide and O-linked glycans. J Am Heart Assoc. 2014;3(5):e001420. doi: 10.1161/JAHA.114.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aponte-Santamaria C, et al. Force-sensitive autoinhibition of the von Willebrand factor is mediated by interdomain interactions. Biophys J. 2015;108(9):2312–21. doi: 10.1016/j.bpj.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nesbitt WS, et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277(4):2965–72. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 23.Dayananda KM, et al. von Willebrand factor self-association on platelet GpIbalpha under hydrodynamic shear: effect on shear-induced platelet activation. Blood. 2010;116(19):3990–8. doi: 10.1182/blood-2010-02-269266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood. 2003;101(7):2637–45. doi: 10.1182/blood-2002-05-1550. [DOI] [PubMed] [Google Scholar]
- 25.Kasirer-Friede A, et al. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 2004;103(9):3403–11. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri ZM, et al. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri ZM, et al. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903–10. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesbitt WS, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–73. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 29.Westein E, et al. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci U S A. 2013;110(4):1357–62. doi: 10.1073/pnas.1209905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis. 2006;36(2):182–7. doi: 10.1016/j.bcmd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Muller I, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17(3):476–8. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 32.Holme PA, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol. 1997;17(4):646–53. doi: 10.1161/01.atv.17.4.646. [DOI] [PubMed] [Google Scholar]
- 33.Shankaran H, Neelamegham S. Hydrodynamic forces applied on intercellular bonds, soluble molecules, and cell-surface receptors. Biophys J. 2004;86(1 Pt 1):576–88. doi: 10.1016/S0006-3495(04)74136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tynngard N, Lindahl TL, Ramstrom S. Assays of different aspects of haemostasis - what do they measure? Thromb J. 2015;13:8. doi: 10.1186/s12959-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casa LD, Ku DN. Geometric design of microfluidic chambers: platelet adhesion versus accumulation. Biomed Microdevices. 2014;16(1):115–26. doi: 10.1007/s10544-013-9811-7. [DOI] [PubMed] [Google Scholar]
- 36.Runyon MK, et al. Effects of shear rate on propagation of blood clotting determined using microfluidics and numerical simulations. J Am Chem Soc. 2008;130(11):3458–64. doi: 10.1021/ja076301r. [DOI] [PubMed] [Google Scholar]
- 37.Kroll MH, et al. Platelets and shear stress. Blood. 1996;88(5):1525–41. [PubMed] [Google Scholar]
- 38.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98(7):1344–52. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brzoska T, et al. Binding of thrombin-activated platelets to a fibrin scaffold through alpha(IIb)beta(3) evokes phosphatidylserine exposure on their cell surface. PLoS One. 2013;8(2):e55466. doi: 10.1371/journal.pone.0055466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci. 2008;38(1):15–23. doi: 10.1016/j.transci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osdoit S, Rosa JP. Fibrin clot retraction by human platelets correlates with alpha(IIb)beta(3) integrin-dependent protein tyrosine dephosphorylation. J Biol Chem. 2001;276(9):6703–10. doi: 10.1074/jbc.M008945200. [DOI] [PubMed] [Google Scholar]
- 42.Wu KK, Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med. 1996;47:315–31. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]
- 43.Colace TV, et al. Microfluidic assay of hemophilic blood clotting: distinct deficits in platelet and fibrin deposition at low factor levels. J Thromb Haemost. 2014;12(2):147–58. doi: 10.1111/jth.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Witt SM, et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat Commun. 2014;5:4257. doi: 10.1038/ncomms5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa S, et al. Evaluation of a novel flow chamber system to assess clot formation in factor VIII-deficient mouse and anti-factor IXa-treated human blood. Haemophilia. 2012;18(6):926–32. doi: 10.1111/j.1365-2516.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 46.Onasoga-Jarvis AA, et al. The effect of factor VIII deficiencies and replacement and bypass therapies on thrombus formation under venous flow conditions in microfluidic and computational models. PLoS One. 2013;8(11):e78732. doi: 10.1371/journal.pone.0078732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugimoto M, et al. Mural thrombus generation in type 2A and 2B von Willebrand disease under flow conditions. Blood. 2003;101(3):915–20. doi: 10.1182/blood-2002-03-0944. [DOI] [PubMed] [Google Scholar]
- 48.Brehm MA, et al. von Willebrand disease type 2A phenotypes IIC, IID and IIE: A day in the life of shear-stressed mutant von Willebrand factor. Thromb Haemost. 2014;112(1):96–108. doi: 10.1160/TH13-11-0902. [DOI] [PubMed] [Google Scholar]
- 49.Zwaginga JJ, et al. Can blood flow assays help to identify clinically relevant differences in von Willebrand factor functionality in von Willebrand disease types 1–3? J Thromb Haemost. 2007;5(12):2547–9. doi: 10.1111/j.1538-7836.2007.02807.x. [DOI] [PubMed] [Google Scholar]
- 50.Jain A, et al. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun. 2016;7:10176. doi: 10.1038/ncomms10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roest M, et al. Flow chamber-based assays to measure thrombus formation in vitro: requirements for standardization. J Thromb Haemost. 2011;9(11):2322–4. doi: 10.1111/j.1538-7836.2011.04492.x. [DOI] [PubMed] [Google Scholar]
- 52.Gutierrez E, et al. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip. 2008;8(9):1486–95. doi: 10.1039/b804795b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H, et al. On-chip titration of an anticoagulant argatroban and determination of the clotting time within whole blood or plasma using a plug-based microfluidic system. Anal Chem. 2006;78(14):4839–49. doi: 10.1021/ac0601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeon JS, et al. Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb) 2014;6(5):555–63. doi: 10.1039/c3ib40267c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Y, Chen J, Lopez JA. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat Commun. 2015;6:7858. doi: 10.1038/ncomms8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai M, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440(7082):358–62. doi: 10.1038/nature04599. [DOI] [PubMed] [Google Scholar]
- 59.Theberge AB, et al. Microdroplets in microfluidics: an evolving platform for discoveries in chemistry and biology. Angew Chem Int Ed Engl. 2010;49(34):5846–68. doi: 10.1002/anie.200906653. [DOI] [PubMed] [Google Scholar]
- 60.Kline TR, et al. ABO, D blood typing and subtyping using plug-based microfluidics. Anal Chem. 2008;80(16):6190–7. doi: 10.1021/ac800485q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullenbarger B, et al. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37(1):101–10. doi: 10.1016/j.exphem.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thon JN, et al. Platelet bioreactor-on-a-chip. Blood. 2014;124(12):1857–67. doi: 10.1182/blood-2014-05-574913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Di Buduo CA, et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood. 2015;125(14):2254–64. doi: 10.1182/blood-2014-08-595561. [DOI] [PMC free article] [PubMed] [Google Scholar]