Abstract
Chimpanzees are important referential models for the study of life history in hominin evolution. Age at sexual maturity and first reproduction are key life history milestones that mark the diversion of energy from growth to reproduction and are essential in comparing life history trajectories between chimpanzees and humans. Yet, accurate information on ages at these milestones in wild chimpanzees are difficult to determine because most females transfer before breeding. Precise age at first birth is only known from a relatively small number of non-dispersing individuals. Moreover, due to small sample sizes, the degree to which age at maturation milestones varies is unknown. Here we report maturation milestones and explore sources of variance for 36 wild female chimpanzees of known age, including eight dispersing females born in Gombe National Park, Tanzania. Using Kaplan-Meier survival analysis, including censored intervals, we find an average age of 11.5 years (range 8.5–13.9) at sexual maturity and 14.9 years (range 11.1–22.1) at first birth. These values exceed previously published averages for wild chimpanzees by one or more years. Even in this larger sample, age at first birth is likely underestimated due to the disproportionate number of non-dispersing females, which, on average, give birth two years earlier than dispersing females. Model selection using Cox Proportional Hazards models shows that age at sexual maturity is delayed in females orphaned before age eight years old and those born to low-ranking mothers. Age at first birth is most delayed in dispersing females and those orphaned before eight years. These data provide improved estimates of maturation milestones in a population of wild female chimpanzees and indicate the importance of maternal factors in development.
Keywords: Life history, First birth, Sexual maturity, Hominin, Dispersal, Maternal effects
Introduction
Human life history consists of an unusual suite of characteristics including slow growth, delayed maturation, short interbirth intervals (IBI), long periods of offspring dependency, and cessation of reproduction prior to death, all of which differentiate humans from other primate taxa (Bogin and Smith, 1996; Leigh, 2001; Gurven, 2012; Alberts et al., 2013). How, why, and when a modern human-like life history pattern evolved, however, is unresolved and partly dependent on a fossil record preserving only indirect evidence. Life history patterns in the closely related extant great apes are of particular importance for understanding the origin of hominin life history. Data on chimpanzees (Pan troglodytes) and bonobos (Pan paniscus), above all, are often used in conjunction with human data to reconstruct the life history pattern of the Pan/Homo last common ancestor (LCA; e.g., Robson and Wood, 2008; Gurven, 2012; Kelley and Schwartz, 2012).
Two important female maturational events used in comparative life history analysis are age at menarche and age at first birth. These signal the end of the developmental period and the diversion of energy from growth to reproduction (Stearns, 1992; Charnov and Berrigan, 1993). The ages at which these milestones are reached are used to compare the length of the developmental period between species, and reviews comparing the age at first birth for chimpanzees and humans variously report that human ages exceed those of chimpanzees by 38% to 80% (or five to eight years; Schultz, 1969; Bogin, 1997; Robson and Wood, 2008; Gurven, 2012; Kelley and Schwartz, 2012). These data are often interpreted as being indicative of a lengthening of the developmental period in humans after the Pan/Homo split. However, a number of factors complicate the determination of maturational milestones in chimpanzees and bonobos for use in these comparisons. First, accumulating a sufficient sample size of known aged females requires the continuous observation of numerous females, from birth until ages of up to 25 years, to encompass the full range of variability in the age of first birth in chimpanzees and bonobos (and even longer for humans). Such records exist for some captive individuals, and several long-term studies of wild chimpanzees have now surpassed this length. Nevertheless, because most of these field studies have focused on only one social group, the number of known aged females reaching menarche is still small and no records of known aged bonobos have been published. The problem of sample size is greatly exacerbated for the determination of age at first birth in wild groups because of the dispersal patterns of Pan species (Kano, 1992; Tutin, 1994). In social primates, it is common for the members of one sex or the other to leave their natal group before breeding, at least partly as a mechanism to avoid inbreeding (Pusey and Packer, 1987). Unlike many primates, where males leave but females remain in their natal group, the common pattern in chimpanzees and bonobos is for females to leave their natal community around menarche and settle in new communities before breeding (Pusey, 1979; Kano, 1992; Boesch and Boesch-Achermann, 2000; Nishida et al., 2003; Reynolds, 2005; Kahlenberg et al., 2008; Langergraber et al., 2009). This means that unless more than one community is under observation, which is rarely the case, the majority of known aged females is lost to follow-up. However, across chimpanzee sites, some proportion of females remain and breed in their natal community for life. This proportion varies considerably from ~50% in the Kasekela community of Gombe National Park to less than 10% in most other studied communities (Pusey and Schroepfer-Walker, 2013). The small number of non-dispersing female chimpanzees at a few sites is, currently, the only source of data for accurate information on age at first birth in wild Pan populations.
A second problem concerns the treatment of right-censored data in calculating mean ages at menarche or first birth. In any prospective study, there will always be females for whom the interval between birth and the maturation milestone is completed (closed) and those in which the interval is still open (right-censored): that is, they have not yet experienced the milestone at the end of the study, or they were lost to follow-up through death or emigration. Failure to include right-censored intervals in calculations of the mean length of intervals introduces a systematic downward bias (Sheps and Menken, 1972; Galdikas and Wood, 1990; Jones et al., 2010), which will be particularly marked in studies of short duration with many right-censored intervals. Although techniques of survival analysis exist that handle right-censoring, the published data on chimpanzee age at menarche and first birth are based only on closed intervals. Because human datasets generally have larger samples sizes and are more likely to be retrospective, the proportion of intervals that are right-censored is much smaller. Thus, comparisons of published figures for the two species are likely to amplify the differences between them.
A third issue to take into account in choosing appropriate data for species comparisons is the extent and causes of variability in developmental rates and, hence, the age at which reproductive milestones are reached. It is well known from studies of non-human mammals (e.g., ring-tailed lemurs [Parga and Lessnau, 2005], long-tailed macaques [van Noordwijk and van Schaik, 1999], baboons [Bronikowski et al., 2002], chimpanzees [Tutin, 1994]) that rates of development are often greatly accelerated in captive, compared to wild, populations; an effect that is thought to be largely the result of access to a high quality, predictable diet (Sadlier, 1969; Lee and Kappeler, 2003). An analogous pattern is also found in humans maturing in industrial societies compared to those maturing in resource-limited societies (Bentley, 1999). For this reason, it is important to compare populations experiencing similar conditions in studies of life history evolution and, accordingly, in recent reviews, data from wild chimpanzee populations have been compared to data from modern hunter-gatherer and foraging populations (Robson and Wood, 2008; Gurven, 2012). More generally, measurement of the magnitude of variation in age at sexual maturity and examination of the factors influencing inter- and intra-population variability are important considerations when selecting the appropriate metrics for species comparisons and for understanding the selective forces in life history evolution.
Accurate data are available from a small number of female chimpanzees in Gombe National Park, Tanzania (Wallis, 1997); Mahale National Park, Tanzania (Nishida et al., 2003; Nakamura, 2015); the Kanyawara community in Kibale National Park, Uganda (Stumpf et al. 2009); and Bossou, Guinea (Sugiyama, 2004) where non-dispersing known aged females have been observed to sexual maturity, emigration, or first birth (summarized in Table 1). While studies are currently underway, little is known about maturation milestones in bonobos and values reported to date are derived from a small number of females with estimated ages. Nevertheless, the published data show little difference from wild chimpanzees except for the age at emigration (Furuichi, 1989; Kuroda, 1989;Table 1). Reported ages at first birth for non-dispersing female chimpanzees (~13 years) are undoubtedly low compared to the ages at which emigrants give birth. When assigning estimated ages to immigrant females, based on the average age at emigration of known aged females and general body size and condition of immigrating individuals, researchers at Tai National Park, Ivory Coast, and Mahale estimated that immigrants give birth closer to or in their 14th year of life (Boesch and Boesch-Achermann, 2000; Nishida et al., 2003; Table 1). In Mahale, the difference in age at first birth in non-dispersing and dispersing females is estimated to exceed one year (Nishida et al., 2003), though this difference diminished when more data became available (Nakamura, 2015).
Table 1.
Comparison of mean maturation milestones (in years) for wild chimpanzees and bonobos from previously published data and the current study.a
| Chimpanzees | Bonobos | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gombe (this study) | Gombe1 | Kanyawara2 | Mahale4 | Bossou5 | Tai6 | Wamba | |
| Age at sexual maturity | |||||||
| Known age females | 11.47 (36*) | 10.8 (8) | – | 10.63 (5)3 | – | – | – |
| Estimated age females | – | – | – | – | – | – | – |
| Unknown or mixed | – | – | – | – | 8.5 (5) | – | – |
| Age at emigration/immigration | |||||||
| Known age females | 12.26 (12) | – | 12.6 (9*) | 11.38 (24) | – | – | – |
| Estimated age females | – | – | – | 11.24 (41) | – | 11.42 (7) | ~9.4†† (12)8 |
| Unknown or mixed | – | – | 12.9 (13*) | – | – | – | – |
| Age at first birth | |||||||
| Non-dispersing known age females | 13.72 (12) | 13.3 (4) | – | 13.57 (6) | 10.1** (4) | – | – |
| Dispersing known age females | 16.21 (7) | – | – | – | – | – | – |
| Dispersing estimated age females | – | – | – | 14.50 (28) | – | 13.7 (7) | 14.2 (6)9 |
| Interval between immigration and first birth | 3.75 (39*) | – | 2.24 (8*) | 3.11 (28) | – | 2.65 (7) | – |
| 2.3† (7)7 | |||||||
Sample size is indicated in parentheses.
Includes censored intervals
Definition of known aged individuals included females or offspring whose birthdate was known to within one year, a wider margin than for most other studies.
Initially reported as time from immigration to conception; 226 days (gestation length) added to the mean duration.
Includes nine individuals (with no infants) who were assigned an age range rather than a single age
Deschner and Boesch (2007),
The timing of life history events varies, not only between species, but also between and within populations. Extensive investigation of human growth rates and the onset of reproduction reveals tremendous variation among traditional societies (Walker et al., 2006). Typically, humans raised under better environmental conditions (i.e., in areas of high food availability) grow faster and experience menarche and first birth earlier (Hill and Hurtado, 1996; Bentley, 1999), however, in some cases accelerated maturation may also be associated with poor conditions and high mortality (Walker et al., 2006). This phenomenon has been best studied among modern humans, but non-human primates are subject to similar variation. In baboons, females mature faster in periods with more rainfall and when group size is smaller and competition is presumably alleviated (Altmann and Alberts, 2003; Charpentier et al., 2008). Maternal effects, especially dominance rank, also affect maturation rates in some primates. For example, in many cercopithecoid species (both in the wild and captivity), daughters born to higher ranking mothers tend to give birth earlier than those born to lower ranking mothers (Harcourt, 1987; Ellis, 1995; Pusey, 2012). In many primates, infants born to nulliparous mothers suffer high mortality due to a combination of factors including small maternal size, inexperience, and poor resource access (Bercovitch et al., 1998; Setchell et al., 2002; Pusey, 2012). In savannah baboons, first born infants are smaller than later born infants, a disadvantage that leads to a delay in age at sexual maturity (Altmann and Alberts, 2005). Overall, in female baboons, maternal and kin effects explain most of the variance in age at maturity (Charpentier et al., 2008).
Maturation milestones in chimpanzees are expected to be similarly affected by environmental and social conditions, but limited sample sizes have impeded such analyses. Nevertheless, substantial variation in maturation milestones and reproductive parameters have been documented across (Emery Thompson, 2013) and within (Pusey et al., 1997; Nishida et al., 2003; Stumpf et al., 2009; Jones et al., 2010) field sites. Dispersal is predicted to delay age at first birth due to the costly nature of this transition where females must integrate socially into a new group in an unfamiliar area (Nishida et al., 2003). Previous work suggests maternal effects may also alter the attainment of maturation milestones. In a sample of Gombe chimpanzees, females born to higher-ranking mothers reached sexual maturity earlier (Pusey et al., 1997) and anecdotal reports from two field sites suggest maturation is delayed in females orphaned at a young age (Goodall, 1986; Boesch and Boesch-Achermann, 2000). Unlike many primates, chimpanzee infants born to nulliparous mothers at Gombe National Park do not have higher infant mortality but are more attentively nursed, groomed, and played with than infants born to multiparous mothers (Stanton et al., 2014). Nevertheless, the interbirth interval following the birth of the first infant is longer than that following subsequent infants (Jones et al., 2010), indicating that first born females may experience slower growth and thus later sexual maturity and first birth. Finally, as in humans and baboons, food availability and favorable environmental conditions should accelerate maturation.
In this paper, we present extensive new data on maturational milestones from the longest running study of wild chimpanzees. We utilize over 50 years of data from Gombe National Park on two chimpanzee communities and we include in our calculations censored intervals where individuals died, disappeared, or had not yet attained the milestone to more accurately determine age at sexual maturity, emigration, and first birth. We place our results in the context of previously published data, document the magnitude of the variability, examine factors influencing this, and discuss how these new data may alter views of the evolution of human life histories.
Methods
Study site
Gombe National Park is located in western Tanzania. It consists of a rugged 35 km2 strip of land along the eastern shore of Lake Tanganyika, containing a mosaic of evergreen forest in the valleys, woodland on the valley sides, and grassland on the hills (Goodall, 1986; Wilson, 2012). Three chimpanzee communities are found within the park; Kasekela, Mitumba, and Kalande. A fourth community, Kahama, fissioned from the Kasekela community in 1972 but ceased to exist in 1978 following the death of all the adult males (Goodall, 1986). Observations on the Kasekela community began in 1960 and, by 1964, most chimpanzees were known individually. Systematic data collection in the Kasekela community began in 1963 at an artificial feeding area at which chimpanzees were provisioned with bananas. Provisioning was reduced in 1968, but continued at low levels until 2000 (Wrangham, 1974; Goodall, 1986; Pusey et al., 2008a). From 1972, Tanzanian field assistants have conducted near-daily full day focal follows on most adult individuals. In 1985, researchers began to periodically follow the Mitumba community and an artificial feeding station was established in 1990. Provisioning was reduced in 1996 and ceased in 2000. All Mitumba individuals were known individually by 1995 and systematic focal follows similar to those in Kasekela have been conducted since then. The Kalande community remains unhabituated but individuals are known genetically through the analysis of non-invasively collected fecal samples. Beginning in 1985, females who transferred between Kasekela and Mitumba could be followed continuously throughout their lives. While substantial provisioning is likely to accelerate maturation rates, we do not address the effects of provisioning here because the majority of females in the current sample matured during a time of low-level provisioning, and most of those born after the cessation of provisioning have yet to mature and none have given birth.
Determination of age
Party association changes frequently within the fission-fusion society of a chimpanzee community and individuals are not necessarily seen every day. In particular, pregnant females are sometimes absent from the group for long periods around parturition (Pusey et al., 2008b). For this reason, infants are usually not seen on the day of their birth. Infants are assigned a minimum birthday corresponding to the last day their mother was observed with no infant and a maximum birthday corresponding to the first day their mother was observed with an infant. The midpoint between these dates is used as the date of birth, unless, when first seen, there are signs of a more recent birth such as an attached umbilicus (Strier et al., 2010). To be included in our sample of known aged females, individuals had to be observed within five months of birth (median span between minimum and maximum birthdate = 7 days, range 0–131 days, n = 36). The date of birth for the first infant born to each female of known age was also required to be known to within 5 months (median span = 15.5 days, range 0–125 days, n = 19). For the 19 females of known age who gave birth, the total uncertainty around their age at first birth (taking into account uncertainty around their own birthdate and that of their first offspring) ranged from 0–162 days (median = 23 days).
Ethics statement
This research adhered to all laws and guidelines of Tanzania and was approved by the Institutional Animal Care and Use Committees at the University of Minnesota and Duke University.
Survival analysis
To allow for the inclusion of censored intervals, we used survival analysis to calculate average age at sexual maturity, emigration, and first birth and the interval between immigration and first birth. To facilitate comparison with previous studies and to examine the extent to which the exclusion of censored intervals biases estimates, we also calculated means derived only from completed intervals. We employed univariate Cox Proportional Hazards models to investigate differences in age at first birth in dispersing and non-dispersing females and differences between communities in the interval between immigration and first birth. Multivariate Cox Proportional Hazards models were used to investigate sources of variance in age at sexual maturity and first birth. Main effects in each model were first checked for correlation using variance inflation factors, none of which indicated correlations among any main effects. In our multivariate analyses, we used a multi model inference paradigm with AICc as our selection criterion, considering models within 2 AICc points to have equal support. All statistical analyses were conducted in R v. 3.2.4 (R Core Team, 2014) using the survival package (Therneau, 2015).
Age at sexual maturity
Adult females experience regular sexual cycles of approximately 35 days, during which their ano-genital region is inflated or swollen for 10–14 days (Wallis, 1997). Females are only sexually receptive when fully swollen and mate promiscuously during this period. In wild chimpanzees, the onset of menarche is difficult to detect. In contrast, mating is conspicuous and, in the few cases in the Kasekela community where age at menarche was recorded, it closely followed the commencement of mating with adult males (Pusey, 1980, 1990; Goodall, 1986). For this reason, we used the first time a female with a full estrous swelling mated with an adult male to assign age at sexual maturity, which is roughly comparable to age at menarche in humans. Among known aged females, the minimum age at first maximal anogenital swelling and first mating with a sexually mature male (aged >12 years) was eight and a half years, so we only included females that reached eight years of age in our sample, yielding a sample of 36 known age females.
To identify maternal factors that influence female age at sexual maturity we included mother’s rank at her daughter’s birth (hereafter mother’s rank), mother’s presence when her daughter was eight years old (hereafter mother’s presence), and mother’s parity at her daughter’s birth (hereafter mother’s parity) as main effects in a Cox Proportional Hazards model, each of which are defined below. Nine females who reached eight years of age were excluded from this analysis because information on maternal rank or parity was not available, leaving a total sample size of 27 known age females.
Mother’s rank
Rank can be assigned to chimpanzees based on the direction of submissive pant grunt vocalizations and decided aggressive interactions. We used a modification of the standard elo ranking to assign standardized cardinal elo scores to mothers on the day of birth of each offspring (Foerster et al., 2016). For visualization purposes, females were placed in three rank categories (high, middle, and low) using Jenks natural breaks.
Mother’s presence
We compared females whose mothers died before they reached eight years of age to those whose mothers remained alive. Prior to the onset of sexual cycles and an interest in mating, which in this population minimally occurred at eight and a half years of age, juvenile females travel almost exclusively with their mothers (Pusey, 1983, 1990). Therefore, females who lost their mothers prior to age eight years were considered orphans. The mother of one female, Dilly, emigrated to a new community when Dilly was six years of age. Dilly did not accompany her mother and was effectively orphaned, and is classified as such in these analyses.
Mother’s parity
Females born to nulliparous mothers were classified as firstborns and those born to parous mothers as laterborns. Mother’s parity status was determined from the long-term demographic data. If the mother was an immigrant from an unknown community, she was assumed to be nulliparous at entry unless accompanied by a juvenile or estimated to be of advanced age.
Age at emigration
Seven nulliparous females of known age born into the Kasekela community transferred to Mitumba following the attainment of sexual maturity and one nulliparous female transferred from Mitumba to Kasekela. A further four females, three in Kasekela and one in Mitumba, disappeared around the attainment of sexual maturity in good health and are presumed to have emigrated. We could not definitively assign a residence status to 11 females who survived to age eight because they either died prior to giving birth/emigrating or had not yet given birth or emigrated by the end of the analysis period (12/31/2016). These females are consequently excluded from these analyses, leaving a total sample size of 12 known age emigrant females, eight of whom were confirmed to have dispersed to a new community.
Because resident females are not seen every day and young females sometimes make prolonged visits to neighboring communities before transferring permanently, it is difficult to pinpoint the date of emigration from a community. To simplify analyses, we considered the last date a female was seen in her natal community as the date of emigration. Because of the small sample size, we only report descriptive statistics and do not analyze sources of variance for age at emigration.
Interval between immigration and first birth
For 39 immigrant females to Kasekela and Mitumba, we calculated the interval between immigration (date first seen in the new community) and first live birth to assess the length of the sterile window post-transfer, while controlling for potential differences between communities. Two immigrant females to Kasekela, Nasa and Sifa, have not given birth in over 15 years despite cycling and mating regularly. They are presumed sterile and are excluded from this analysis.
Age at first birth
To be included in analyses determining age at first live birth, we required females to have survived to 11 years or later because the earliest age at first birth was 11.1 years, yielding a total sample size of 24 known age females. Two females that reached age 11 were excluded: Skosha died at age 33 without giving birth, despite cycling and mating regularly, and was assumed to be sterile. Honey Bee was born in Kasekela, but joined the newly formed Kahama community prior to sexual maturity, after which she was seen intermittently in Kasekela, but too infrequently to clarify her residence status or parity. Survival analysis was used to calculate the mean age at first birth.
Cox Proportional Hazards models were used to identify factors contributing to variance in age at first birth. In addition to the fixed effects examined in age at sexual maturity (mother’s rank, mother’s presence, and mother’s parity), residence status (non-dispersing vs. dispersing) was also included as a fixed effect. In six cases, mother’s rank at the daughter’s birth was unknown and/or a residence status could not be definitively assigned because the female had died prior to emigration/reproduction or had not yet emigrated/reproduced. These six females are excluded from this analysis leaving a total sample size of 18 known age females.
Results
Maturation milestones
The mean ages at maturation milestones are summarized in Table 2. According to survival analysis, females reached sexual maturity at a mean age of 11.47 years (Fig. 1) and emigrated at a mean age of 12.26 years. The mean age at first birth was 14.89 years (Fig. 1). When censored intervals were excluded, the mean age at sexual maturity was 11.22 years and at first birth was 14.63 years. In an examination of mean age at first birth by residence status, using Cox Proportional Hazards models, we found that dispersing females gave birth at 16.21 years, significantly later than non-dispersing females who gave birth at 13.72 years (β = 1.15, SE(β) = 0.57, p = 0.044). For 39 immigrants to Mitumba and Kasekela, the average duration from immigration to first birth was 3.75 years. Time to first birth did not differ by transfer community (β = −0.17, SE(β) = 0.19, p = 0.39). When censored intervals were excluded (n = 6), mean time to first birth was 3.29 years.
Table 2.
Mean age (years) at maturation milestones as calculated using Kaplan-Meier survival analysis (including censored intervals) in female chimpanzees of known age.
| Milestone | n | Mean (yrs) | SE | Range* |
|---|---|---|---|---|
| Age at sexual maturity | 36 (10 censored) | 11.47 | 0.27 | 8.50–13.86 |
| Age at emigration | ||||
| All females | 12 (0 censored) | 12.26 | 0.54 | 10.38–15.44 |
| Confirmed dispersers | 8 (0 censored) | 12.89 | 0.71 | 10.38–15.44 |
| Age at first birth | ||||
| All females | 24 (5 censored) | 14.90 | 0.65 | 11.11–22.08 |
| Non-dispersing females | 12 (0 censored) | 13.72 | 0.59 | 11.11–17.25 |
| Dispersing females | 7 (0 censored) | 16.21 | 1.35 | 11.21–22.08 |
| Immigration to first birth | 39 (6 censored) | 3.75 | 0.39 | 0.55–8.16 |
Only includes completed intervals
Figure 1.
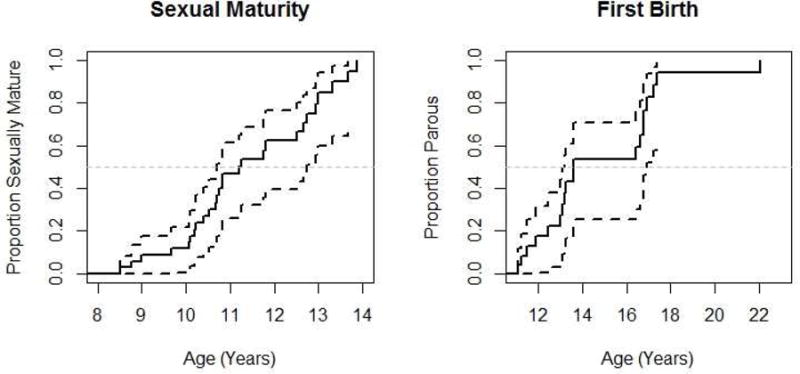
Survival curve of age at sexual maturity and age at first birth for known aged Gombe females. Dark dashed lines indicate 95% confidence intervals. Age at which the survival curve crosses the light dashed line denotes when 50% of females have reached that milestone.
Factors influencing age at sexual maturity
Model selection revealed support for a single model that included mother’s rank and mother’s presence to explain age at sexual maturity (Table 3, Fig. 2) but excluded mother’s parity. Mother’s rank had a large effect on the maturation hazard, increasing the likelihood of reaching maturity by a factor of 2.96 for each standard deviation increase in a female’s rank, indicating a female born to a higher ranking mother has a 75% chance of maturing faster than a female of lower rank. The model predicted median age at which a female born to a high ranking mother matured versus a female born to a low ranking mother, all else being equal, was 9.0 years versus 12.8 years. The effect of being orphaned also had a large effect on the maturation hazard, reducing the likelihood of reaching maturity at a particular age by a factor of 0.07, indicating females without mothers only have a 6.5% chance of maturing faster than a non-orphan. The model predicted median age at maturity, all else being equal, was 10.7 years for females with a mother present and 13.3 years for those without a mother.
Table 3.
Results of the best fit model for age at sexual maturity using AICc selection criterion.
| Covariate | β | Exp(β) | SE(β) |
|---|---|---|---|
| Mother’s rank (z-transformed) | 1.085 | 2.96 | 0.382 |
| Mother absent v. mother present | −2.604 | 0.07 | 1.126 |
Figure 2.
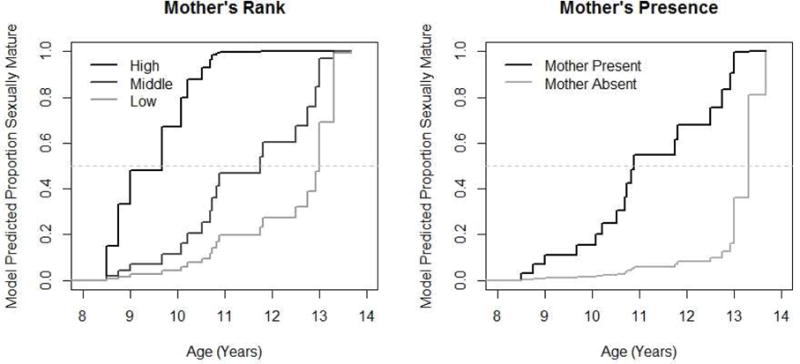
Survival curves showing model predicted outcomes in comparing age at sexual maturity in a) daughters born to females that are high ranking (high), middle ranking (mid), and low-ranking (low) as demarcated using Jenks natural breaks, and b) females whose mother was absent at age eight and those whose mother was present at age eight. Age at which the survival curve crosses the light dashed line denotes when 50% of females have reached that milestone.
Factors influencing age at first birth
Model selection revealed equivalent support for 10 models predicting age at first birth. Each variable was retained in some of the best fit models and was, therefore, associated with age at first birth. According to the Akaike weights of each model, no model had an Akaike weight above 0.15, so we employed full model averaging to interpret the results (Table 4). Among the categorical variables, residence status had the largest effect on the hazard of giving birth. Remaining in her natal community increased the likelihood of a female giving birth at a particular age by a factor of 2.86, indicating non-dispersing females had a 74% chance of giving birth earlier than dispersing females. Mother’s presence and parity had similarly large effects on the hazard ratio; being orphaned decreased the hazard of giving birth at any given age by a factor of 0.45, indicating that non-orphans had a 69% greater chance of giving birth earlier than orphans. Being born to a parous mother increased the likelihood of giving birth at a given age by a factor of 1.95, indicating later born daughters had a 66% chance of giving birth earlier than first-born daughters. For each standard deviation increase in mother’s rank score, the likelihood of giving birth by a given age was increased by a factor of 1.38, indicating females born to higher ranking mothers had a 58% greater chance of giving birth earlier than did average females. We summed Akaike weights for each variable to interpret their relative importance in contributing to variance in age at first birth. Dispersal status and mother’s presence had the largest summed Akaike weights at 0.67 and 0.64, respectively, indicating they are more important predictors of age at first birth than are mother’s rank and parity with respective weights of 0.49 and 0.48.
Table 4.
Results from model selection using AICc as selection criterion.a
| Model | Mother’s rank | Mother’s parity | Mother’s presence | Dispersal | AICc | ΔAICc | Akaike weight |
|---|---|---|---|---|---|---|---|
| 1 | 2.09 | 0.15 | 3.53 | 65.48 | – | 0.13 | |
| 2 | 3.96 | 0.14 | 2.91 | 65.60 | 0.11 | 0.12 | |
| 3 | 1.64 | 5.04 | 65.77 | 0.29 | 0.11 | ||
| 4 | 3.33 | 0.10 | 65.92 | 0.44 | 0.10 | ||
| 5 | 2.53 | 4.35 | 66.49 | 1.01 | 0.08 | ||
| 6 | 3.16 | 66.51 | 1.03 | 0.08 | |||
| 7 | 1.81 | 0.11 | 66.61 | 1.13 | 0.07 | ||
| 8 | 1.81 | 2.72 | 0.11 | 3.67 | 66.62 | 1.14 | 0.07 |
| 9 | 0.21 | 67.43 | 1.96 | 0.05 | |||
| 10 | 1.55 | 2.53 | 0.08 | 67.47 | 1.99 | 0.05 | |
|
| |||||||
| Mean | 1.38 | 1.95 | 0.45 | 2.86 | |||
Hazard ratios (exp(β)) for each term are denoted.
We next calculated the median age at first birth for females in each category, all else being equal, using values obtained from the full model. The model predicted median age at which females born to high ranking mothers gave birth was 14.2 years and for those born to low ranking mothers was 16.4 years. For females whose mother was present, the model predicted median age at first birth was 13.6 years and for those whose mother was absent was 16.8 years. For females born to parous mothers, the model predicted median age at first birth was 13.6 years, and for females born to nulliparous mothers, it was 16.6 years. For non-dispersing females, the model predicted median age at first birth was 13.0 years, and for dispersing females, it was 16.75 years.
Discussion
We present data on the largest sample of known aged female chimpanzees currently available for the study of maturation milestones. We found that females in this sample matured and gave birth at later ages than previously reported, either from Gombe National Park (Wallis, 1997) or other studied populations (Boesch and Boesch-Achermann, 2000; Nishida et al., 2003; Sugiyama, 2004; Nakamura, 2015), expanding the range of variation in maturation milestones in P. troglodytes. On average, Gombe females, regardless of dispersal status, attained sexual maturity at 11.6 years and gave birth for the first time at 14.9 years. Dispersing females emigrated at 12.3 years and, following an interval of 3.7 years, gave birth for the first time at 16.2 years, significantly later than non-dispersing females. As expected, excluding censored intervals reduced the estimates of age at sexual maturity and first birth by 3–4 months. When compared to the previous Gombe sample (Wallis, 1997), we find that the range of variation within a single population for age at sexual maturity is increased by about four months from 5.00 to 5.36 years, while the range of variation for age at first birth is increased by five years from 6.10 to 10.98 years. The latter extension comes largely from our ability, for the first time, to include data from females that emigrated. These patterns suggest that age at sexual maturity may be more constrained than age at first birth and support the hypothesis that dispersal has profound effects on the onset of reproduction.
Further evidence of the importance of dispersal comes from comparing the patterns in our data with those from other populations. The mean ages at sexual maturity and emigration in Gombe females exceed those reported from Mahale by less than a year (Nishida et al., 2003), a difference that could be partly owing to the inclusion of censored intervals for Gombe but not Mahale. The age of emigration at Gombe is very similar to that reported at Kanyawara, whose calculation, like Gombe, included censored intervals (Stumpf et al., 2009). Similarly, age at first reproduction in non-dispersing known aged females in our expanded sample is only marginally later than that reported for non-dispersing known aged females at Mahale National Park (Nishida et al., 2003) and falls within the range of the downward bias we detected when excluding censored intervals. However, mean age at first reproduction at Gombe, with all females included, is over a year later than the means reported at other field sites, and the mean based only on dispersing females is over 2.5 years later, suggesting that the large difference in our new estimate is primarily driven by the inclusion of dispersing females. In these new data, the average age at emigration and the interval between immigration and first birth at Gombe sum to 16.01 years, a value nearly equivalent to the average age at first birth for known aged dispersing females. The age at emigration and the interval between immigration and first birth are also known for females at Kanyawara (Stumpf et al., 2009) and Mahale (Nakamura, 2015), where they sum to 14.84 and 14.49 years, respectively, and may be indicative of a somewhat younger expected age at first birth for dispersing females at these sites. Given that the majority of females disperse at most field sites, a better estimate of the age at first birth for a typical wild female is likely between 14.5 and 16.5 years, rather than the widely used estimate of 13–14 years.
These results also provide evidence for social and ecological mediation of development (as related to attainment of sexual maturity) in female chimpanzees. A large body of research on growth and development in primates, including humans, emphasizes the plasticity around the attainment of sexual maturity in both males and females (Harcourt, 1987; Ellis, 1995; Hill and Hurtado, 1996; Bentley, 1999; Altmann and Alberts, 2003; Walker et al., 2006; Charpentier et al., 2008). We report similar findings in Gombe female chimpanzees. Variation in age at sexual maturity encompasses a range equivalent to one interbirth interval (five years), while variation in age at first birth encompasses a range equivalent to two interbirth intervals (11 years). As in baboons, maternal effects have substantial effects on the attainment of maturation milestones in chimpanzees. Being orphaned prior to eight years of age delays both the attainment of sexual maturity and age at first birth. Similarly, daughters born to high ranking mothers mature and give birth earlier than those born to low ranking mothers. Females born to nulliparous mothers gave birth later than females born to parous mothers.
The negative effect of being orphaned is not surprising given the long dependence of chimpanzees on their mothers. Chimpanzees are weaned around age five (Plooij and van de Rijt-Plooij, 1987), but daughters still travel almost exclusively with their mother until they experience their first sexual swelling (Pusey, 1983, 1990). Grooming and association bonds between mother-daughter pairs are strong (Pusey, 1983, 1990; Goodall, 1986; Watts and Pusey, 2002; Langergraber et al., 2009; Foerster et al., 2015), and daughters presumably benefit from the near constant presence of a higher-ranking ally. Indeed, mothers occasionally intervene on behalf of their juvenile female offspring in disputes with other individuals (van Lawick-Goodall, 1968; Goodall, 1971; Pusey, 1983; Markham et al., 2015). Moreover, mothers provide foraging benefits to their offspring indirectly via opportunities for social learning (van Lawick-Goodall, 1968; Matsuzawa et al., 2001; Biro and Rikako, 2003; Lonsdorf et al., 2004) and directly through communication and occasional food sharing (Silk, 1978; Pusey, 1983). Orphans lack these advantages.
The negative relationship between mother’s rank and age at maturity in this expanded sample corroborates previous results from Gombe females (Pusey et al., 1997). This advantage to daughters of high ranking mothers likely results at least partly from better access to food resources. High-ranking females forage primarily in small, centrally located, high quality core areas (Williams et al., 2002; Murray et al., 2007) and, accordingly, spend less time foraging and eat fewer low-quality food items than low-ranking females (Murray et al., 2006), all of which should confer energetic advantages on their maturing daughters. Finally, the later maturation of offspring born to first-time mothers likely results from a combination of the competing demands of continued maternal growth with reproduction (Bercovitch et al., 1998), low social status, and poor access to resources (Pusey and Schroepfer-Walker, 2013). In female chimpanzees, rank increases with age and young nulliparous females are typically, though not always, low ranking (Foerster et al., 2016). Here, we concentrated on the importance of maternal effects in determining age at sexual maturity, but further work should address environmental and demographic effects such as rainfall, provisioning, and female density.
While females are likely to benefit from dispersal by avoiding the costs of inbreeding with their close male relatives (Pusey, 1980; Walker et al., 2017), our results reveal the costs of dispersal: compared to females that remain in their natal community, females that disperse at Gombe start their reproductive lives at a disadvantage of one half interbirth interval (~2.5 years). Several factors probably contribute to this delay. Dispersing females must leave close social associates and familiar foraging areas, and immigrant females are under considerable social stress (Pusey, 1979; Pusey et al., 2008b); across field sites, resident females rebuff newcomers and behave aggressively towards them for an extended period of time after entry (Pusey, 1979; Nishida, 1989; Townsend et al., 2007; Kahlenberg et al., 2008; Pusey et al., 2008b). Immigrant females may be further stressed by dietary deficiencies induced via changes in their foraging patterns. Although the delay in first birth at Gombe is of greater magnitude than that estimated among Mahale females (Nishida et al., 2003), we expect that, as more data become available on known aged females, reproduction will be found to occur later than previously reported in most populations of P. troglodytes when dispersing individuals are included.
In a broader context, these data add to a growing body of research revealing the diversity and complexity of chimpanzee life history patterns in different populations. Of particular note is the emergence of patterns showing high intra- and inter-population variability in life history milestones, including maturation (this study), interbirth interval (Emery Thompson et al., 2007, 2016; Jones et al., 2010), and longevity (Hill et al., 2001; Muller and Wrangham, 2014; Wood et al., 2017). While this variation is likely driven in part by differences in resource availability and accessibility, variation at the population level may also be affected by differences in body size and mortality, two factors known to influence life history parameters (Harvey and Clutton-Brock, 1985; Stearns, 1992; Charnov and Berrigan, 1993; Walker et al., 2006). The available evidence suggests that the Gombe chimpanzees are smaller than chimpanzees at other sites (Pusey et al., 2005; Carter et al., 2008), and they also appear to have higher mortality than Kanyawara and Ngogo chimpanzees (Muller and Wrangham, 2014; Wood et al. 2017). Because these factors are generally correlated with accelerated maturation (Stearns, 1992; Charnov and Berrigan, 1993), we might expect the Gombe chimpanzees to mature more rapidly than those at Kanyawara or Mahale, yet our results, if anything, suggest that the Gombe chimpanzees experience first birth later. Alternatively, increased female competition among Gombe females may act as an opposing selective force on the speed of maturation in dispersing females, inhibiting the onset of reproduction (Muller and Mitani, 2005; Pusey and Schroepfer-Walker, 2013), which may inhibit the onset of reproduction in dispersing females. Clearly, more study of the pace of life histories in different populations is required, along with measurement of body size, resource availability, population density, and agents of mortality, to make sense of the complexity of these relationships.
The implications of our amended female chimpanzee maturation schedules (and associated maternal effects) for understanding the evolution of hominin life history are unclear. Compared to other apes, humans are oft-cited to have a unique, prolonged period of growth and development associated with delayed attainment of maturity (Bogin, 1997; Kaplan et al., 2000; Jones and Marlowe, 2002). In small-scale human societies, the average age at menarche is 14.7 years and the average age at first birth is 19.1 years (Walker et al., 2006). Previous reviews have suggested that human values exceed those of chimpanzees by as much as 50% (Bogin, 1997; Robson and Wood, 2008; Gurven, 2012). Yet in comparison with the values for wild chimpanzees that we report here, age at first reproduction in these equivalent human populations only exceeds that in dispersing Gombe chimpanzees by 3–4 years, or 23%. As such, our findings on chimpanzee maturation timing diminish the distinctiveness of humans relative to chimpanzees. Theoretically, if these new data are taken as representative of the Pan genus, they may be interpreted as preliminary evidence that a prolonged period of development typified the Pan/Homo LCA and is the ancestral condition in hominins. Such a claim, however, is still tentative given the limited and specific nature of our findings, and, while less distinct, the general narrative of human delayed maturation still stands. Furthermore, the evolutionary significance of a small, but nevertheless present, gap in the maturation schedules between these genera remains unknown. Lastly, while the maturation variables in this work are central to understanding life history, they cannot yet be directly applied to the hominin fossil record. Therefore, we echo Dean (2016) in advocating for future work that relates life history variables to somatic markers, such as investigations involving age at first reproduction and dental parturition lines.
Despite these caveats, the results presented here do have consequences for future studies and for the interpretation of past studies of hominin life history evolution. A more human-like period of immaturity in chimpanzees may call into question hominin life history reconstructions built upon the notion of a marked divergence in maturation schedules. Most importantly, the finding of high intrapopulation variation and potential interpopulation variation, with respect to peri-reproductive female life history, diminishes the significance of earlier reports relying on population or species’ means based on small and biased samples. Here, we demonstrate the strong effect of socioecological influences in the mediation of life history patterns. Though the effect of food resources was not directly tested, it appears to us likely that the maternal effects influencing life history in our sample are at least partially related to dietary quality and/or abundance. However, much work remains to understand these influences and the impact they have on life history across populations. Acknowledging this plasticity in maturation timelines is particularly important for fossil hominin life history reconstructions, for which a few individuals often serve as representatives of an entire species or population.
Figure 3.
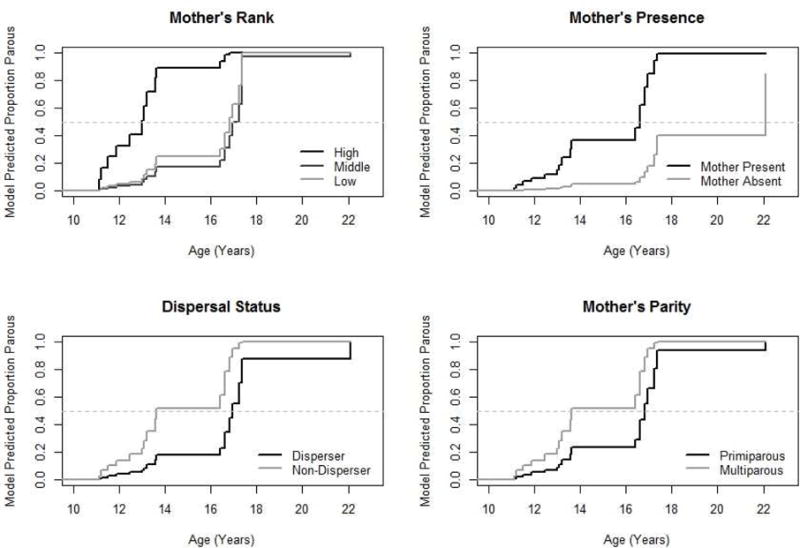
Survival curves showing model predicted outcomes in comparing age at first birth in a) daughters born to females that are high ranking (high), middle ranking (mid), and low-ranking (low) as demarcated using Jenks natural breaks; b) females whose mother was absent at age eight and those whose mother was present at age eight; c) dispersing and non-dispersing females; and d) first born and later born females. Age at which the survival curve crosses the light dashed line denotes when 50% of females are predicted to have reached that milestone.
Acknowledgments
We thank Tanzania National Parks, the Tanzania Wildlife Research Institute, and the Tanzanian Commission for Science and Technology for granting us permission to work on this project in Gombe National Park and the Gombe Stream Research Center staff for data collection. We thank S. Churchill, Z. Cofran, E. Lonsdorf, and C. Murray for comments on an earlier draft.
Funding
We thank the Jane Goodall Institute for funding data collection at Gombe. Additional funding was provided by National Science Foundation grants DBS-9021946, SBR-9319909, BCS-0452315, IOS-LTREB-1052693, and DGE-1106401, National Institutes of Health grant R01 AI 058715 and grants from the Leakey Foundation and Margot Marsh Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts SC, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A. Reproductive aging patterns in primates reveal that humans are distinct. Proc Natl Acad Sci. 2013;110:13440–13445. doi: 10.1073/pnas.1311857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Variability in reproductive success viewed from a life-history perspective in baboons. Am J Hum Biol. 2003;15:401–409. doi: 10.1002/ajhb.10157. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57:490–501. [Google Scholar]
- Bentley GR. Aping our ancestors: comparative aspects of reproductive ecology. Evol Anthropol. 1999;7:175–185. [Google Scholar]
- Bercovitch FB, Lebron MR, Martinez HS, Kessler MJ. Primigravidity, body weight, and costs of rearing first offspring in rhesus macaques. Am J Primatol. 1998;144:135–144. doi: 10.1002/(SICI)1098-2345(1998)46:2<135::AID-AJP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Biro D, Rikako NI. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Tai Forest: Behavioral Ecology and Evolution. Oxford University Press; New York: 2000. [Google Scholar]
- Bogin B. Evolutionary hypotheses for human childhood. Yearb Phys Anthropol. 1997;40:63–89. [Google Scholar]
- Bogin B, Smith BH. Evolution of the human life cycle. In: Stinson S, Bogin B, O’Rourke D, editors. Human Biology: An Evolutionary and Biocultural Perspective. John Wiley and Sons, Inc; New York: 1996. [Google Scholar]
- Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: comparative demography in a non-human primate. Proc Natl Acad Sci. 2002;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ML, Pontzer H, Wrangham RW, Peterhans JK. Skeletal pathology in Pan troglodytes schweinfurthii in Kibale National Park, Uganda. Am J Phys Anthropol. 2008;403:389–403. doi: 10.1002/ajpa.20758. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol Anthropol. 1993;1:191–194. [Google Scholar]
- Charpentier MJE, Tung J, Altmann J, Alberts SC. Age at maturity in wild baboons: genetic, environmental and demographic influences. Mole Ecol. 2008;17:2026–2040. doi: 10.1111/j.1365-294X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- Dean MC. Measures of maturation in early fossil hominins: events at the first transition from australopiths to early Homo. Phil Trans R Soc B. 2016;371:20150234. doi: 10.1098/rstb.2015.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol Sociobiol. 1995;16:257–333. [Google Scholar]
- Emery Thompson M. Reproductive ecology of female chimpanzees. Am J Primatol. 2013;75:222–237. doi: 10.1002/ajp.22084. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Sabbi K, Machanda ZP, Otali E, Wrangham RW. Faster reproductive rates trade off against offspring growth in wild chimpanzees. Proc Natl Acad Sci. 2016;113:7780–7785. doi: 10.1073/pnas.1522168113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, McLellan K, Schroepfer-Walker K, Murray CM, Gilby I, Krupenye C, Pusey AE. Social bonds in the dispersing sex: Partner preferences among adult female chimpanzees. Anim Behav. 2015;105:139–152. doi: 10.1016/j.anbehav.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, Franz M, Murray C, Gilby I, Feldblum J, Walker K, Pusey A. Chimpanzee females queue but males compete for social status. Sci Rep. 2016;6:35404. doi: 10.1038/srep35404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T. Social interactions and life history of female Pan paniscus in Wamba, Zaire. Intl J Primatol. 1989;10:173–197. [Google Scholar]
- Galdikas B, Wood J. Birth spacing patterns in humans and apes. Am J Phys Anthropol. 1990;83:185–191. doi: 10.1002/ajpa.1330830207. [DOI] [PubMed] [Google Scholar]
- Goodall J. In the Shadow of Man. Collins; London: 1971. [Google Scholar]
- Goodall J. The Chimpanzees of Gombe. Belknap Press; Cambridge: 1986. [Google Scholar]
- Gurven M. Human survival and life history in evolutionary perspective. In: Mitani J, Call J, Kappeler P, Palombit R, Silk J, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 293–314. [Google Scholar]
- Harcourt AH. Dominance and fertility among female primates. J Zool. 1987;213:471–487. [Google Scholar]
- Harvey P, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Hill K, Hurtado AM. Ache Life History. Aldine de Gruyter; New York: 1996. [Google Scholar]
- Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- Jones JH, Wilson ML, Murray CM, Pusey AE. Phenotypic quality influences fertility in Gombe chimpanzees. J Anim Ecol. 2010;79:1262–1269. doi: 10.1111/j.1365-2656.2010.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NB, Marlowe FW. Does it take 20 years to learn to hunt and gather? Hum Nat. 2002;13:199–238. doi: 10.1007/s12110-002-1008-3. [DOI] [PubMed] [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Anim Behav. 2008;76:1497–1509. [Google Scholar]
- Kano T. The Last Ape: Pygmy Chimpanzee Behavior and Ecology. Stanford University Press; Stanford: 1992. [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- Kelley J, Schwartz GT. Life-history inference in the early Hominins Australopithecus and Paranthropus. Intl J Primatol. 2012;33:1332–1363. [Google Scholar]
- Kuroda S. Developmental retardation and behavioral characteristics of pygmy chimpanzees. In: Heltne P, Marquardt L, editors. Understanding Chimpanzees. Harvard University Press; Cambridge: 1989. pp. 184–193. [Google Scholar]
- Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) Am J Primatol. 2009;71:840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- Lee P, Kappeler P. Socioecological correlates of phenotypic plasticity of primate life histories. In: Kappeler P, Pereira M, editors. Primate Life Histories and Socioecology. University of Chicago Press; Chicago: 2003. pp. 41–65. [Google Scholar]
- Leigh SR. Evolution of human growth. Evol Anthropol. 2001;10:223–236. [Google Scholar]
- Lonsdorf E, Eberly L, Pusey A. Sex differences in learning in chimpanzees. Nature. 2004;428:715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- Markham AC, Lonsdorf EV, Pusey AE, Murray CM. Maternal rank in fluences the outcome of aggressive interactions between immature chimpanzees. Anim Behav. 2015;100:192–198. doi: 10.1016/j.anbehav.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T, Biro D, Humle T, Inoue-Nakamura N, Tonooka R, Yamakosi G. Emergence of culture in wild chimpanzees: Education by master-apprenticeship. In: Matsuzawa T, editor. Primate Origins of Human Cognition and Behavior. Springer; Berlin: 2001. pp. 557–574. [Google Scholar]
- Muller MN, Mitani JC. Conflict and Cooperation in Wild Chimpanzees. Adv Study Behav. 2005;35:275–331. [Google Scholar]
- Muller MN, Wrangham RW. Mortality rates among Kanyawara chimpanzees. J Hum Evol. 2014;66:107–114. doi: 10.1016/j.jhevol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behav Ecol. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Anim Behav. 2007;74:1795–1804. [Google Scholar]
- Nakamura M. Demography of the M Group. In: Nakamura M, Hosaka K, Itoh N, Zamma K, editors. Mahale Chimpanzees. Cambridge University Press; Cambridge: 2015. pp. 82–93. [Google Scholar]
- Nishida T. Social interactions between resident and immigrant female chimpanzees. In: Heltne P, Marquardt L, editors. Understanding Chimpanzees. Harvard University Press; Cambridge: 1989. pp. 68–89. [Google Scholar]
- Nishida T, Corp N, Hamai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, Mitani JC, Nakamura M, Norikoshi K, Sakamaki T, Turner L, Uehara S, Zamma K. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- Parga JA, Lessnau RG. Female age-specific reproductive rates, birth seasonality, and infant mortality of Ring-Tailed Lemurs on St. Catherines Island: 17-Year reproductive history of a free-ranging colony. Zoo Biol. 2005;24:295–309. [Google Scholar]
- Plooij FX, van de Rijt-Plooij HHC. Growing independence, conflict and learning in mother-infant relations in free-ranging chimpanzees. Behaviour. 1987;101:1–86. [Google Scholar]
- Pusey A. Magnitude and sources of variation in female reproductive performance. In: Mitani J, Call J, Kappeler P, Palombit R, Silk J, editors. The Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 344–366. [Google Scholar]
- Pusey AE. Intercommunity transfer of chimpanzees in Gombe National Park. In: Hamburg D, McCown E, editors. The Great Apes. Benjamin Cummings; Menlo Park: 1979. pp. 465–479. [Google Scholar]
- Pusey AE. Inbreeding avoidance in chimpanzees. Anim Behav. 1980;28:543–552. [Google Scholar]
- Pusey AE. Mother-offspring relationships in chimpanzees after weaning. Anim Behav. 1983;31:363–377. [Google Scholar]
- Pusey AE. Behavioural changes at adolescence in chimpanzees. Behaviour. 1990;115:203–246. [Google Scholar]
- Pusey AE, Packer C. Dispersal and Philopatry. In: Smuts B, Cheney D, Seyfarth R, Wrangham R, Struhsaker T, editors. Primate Societies. University of Chicago Press; Chicago: 1987. pp. 250–266. [Google Scholar]
- Pusey AE, Schroepfer-Walker KK. Female-female competition in chimpanzees. Phil Trans R Soc B. 2013;368:20130077. doi: 10.1098/rstb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey AE, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Oehlert G, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. Intl J Primatol. 2005;26:3–31. [Google Scholar]
- Pusey AE, Wilson ML, Collins DA. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. Am J Primatol. 2008a;70:738–744. doi: 10.1002/ajp.20567. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Murray CM, Wallauer W, Wilson ML, Wroblewski EE, Goodall J. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. Intl J Primatol. 2008b;29:949–973. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2017. Vienna, Austria. URL https://www.R-project.org/ [Google Scholar]
- Reynolds V. The Chimpanzees of the Budongo Forest: Ecology, Behavior and Conservation. Oxford University Press; Oxford: 2005. [Google Scholar]
- Robson SL, Wood B. Hominin life history: reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlier R. The Ecology of Reproduction in Wild and Domestic Mammals. Methuen; London: 1969. [Google Scholar]
- Schultz A. The Life of Primates. Weidenfeld and Nicolson; London: 1969. [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. Reproductive parameters and maternal investment in Mandrills (Mandrillus sphinx) Intl J Primatol. 2002;23:51–68. [Google Scholar]
- Sheps M, Menken J. Distribution of birth intervals according to the sampling time frame. Theoretic Pop Biol. 1972;3:1–26. doi: 10.1016/0040-5809(72)90031-7. [DOI] [PubMed] [Google Scholar]
- Silk J. Patterns of food sharing among mother and infant chimpanzees at Gombe National Park, Tanzania. Folia Primatol. 1978;29:129–141. doi: 10.1159/000155835. [DOI] [PubMed] [Google Scholar]
- Stanton MA, Lonsdorf EV, Pusey AE, Goodall J, Murray CM. Maternal behavior by birth order in wild chimpanzees (Pan troglodytes) increased investment by first-time mothers. Curr Anthropol. 2014;55:483–489. doi: 10.1086/677053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- Strier KB, Altmann J, Brockman DK, Bronikowski AM, Cords M, Fedigan LM, Lapp H, Liu X, Morris WF, Pusey AE, Stoinski TS, Alberts SC. The primate life history database: a unique shared ecological data resource. Methods Ecol Evol. 2010;1:199–211. doi: 10.1111/j.2041-210X.2010.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf RM, Emery Thompson M, Muller MN, Wrangham RW. The context of female dispersal in Kanyawara chimpanzees. Behaviour. 2009;146:629–656. [Google Scholar]
- Sugiyama Y. Demographic parameters and life history of chimpanzees at Bossou, Guinea. Am J Phys Anthropol. 2004;124:154–165. doi: 10.1002/ajpa.10345. [DOI] [PubMed] [Google Scholar]
- Therneau T. A Package for Survival Analysis in S. 2015 version 2.38 <URL: https://CRAN.R-project.org/package=survival>.
- Townsend SW, Slocombe KE, Emery Thompson M, Zuberbühler K. Female-led infanticide in wild chimpanzees. Curr Biol. 2007;17:355–356. doi: 10.1016/j.cub.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Tutin CEG. Reproductive success story: variability among chimpanzees and comparisons with gorillas. In: Wrangham RW, McGrew W, de Waal FBM, Heltne P, editors. Chimpanzee Cultures. Harvard University Press; Cambridge: 1994. pp. 181–194. [Google Scholar]
- van Lawick-Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav Monogr. 1968;1:161–311. [Google Scholar]
- van Noordwijk MA, van Schaik CP. The effects of dominance rank and group size on female lifetime reproductive success in wild Long-tailed Macaques, Macaca fascicularis. Primates. 1999;40:105–130. doi: 10.1007/BF02557705. [DOI] [PubMed] [Google Scholar]
- Walker KK, Rudicell RS, Li Y, Hahn BH, Wroblewski E, Pusey AE, Walker KK. Chimpanzees breed with genetically dissimilar mates. R Soc Open sci. 2017;4:160422. doi: 10.1098/rsos.160422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Gurven M, Hill K, Migliano A, Chagnon N, de Souza R, Djurovic G, Hames R, Hurtado AM, Kaplan H, Kramer K, Oliver WJ, Valeggia C, Yamauchi T. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Bio. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- Wallis J. A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. J Reproduction Fertility. 1997;109:297–307. doi: 10.1530/jrf.0.1090297. [DOI] [PubMed] [Google Scholar]
- Watts D, Pusey A. Behavior of juvenile and adolescent great apes. In: Pereira M, Fairbanks L, editors. Juvenile Primates. University of Chicago Press; Chicago: 2002. pp. 148–167. [Google Scholar]
- Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. Female competition and male territorial behaviour influence female chimpanzees’ ranging patterns. Anim Behav. 2002;63:347–360. [Google Scholar]
- Wilson ML. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In: Kappeler P, Watts DP, editors. Long-Term Field Studies of Primates. Springer; Berlin: 2012. pp. 357–384. [Google Scholar]
- Wood BM, Watts DP, Mitani JC, Langergraber KE. Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. Journal of Human Evolution. 2017;105:41–56. doi: 10.1016/j.jhevol.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham R. Artificial feeding of chimpanzees and baboons in their natural habitat. Anim Behav. 1974;22:83–93. [Google Scholar]


