‘Efferocytosis’ (Greek: to carry the dead to the grave) is the term used to describe the phagocytic removal of apoptotic cells. Impaired efferocytosis was recently shown to be causal for atherosclerosis, where an imbalance in so-called ‘eat me’ ligands allows uncleared cells to accumulate in the plaque and potentiate lesion expansion. We showed that this defect could be reversed with pro-efferocytic therapies, which led to marked reductions in disease burden1. The majority of the anti-atherosclerotic benefit appeared to occur via specific targeting of apoptotic debris, and regression of the necrotic core2.
It is important to note, however, that the consequences of efferocytic signaling extend beyond the simple engulfment of diseased cells. For example, macrophages which have successfully phagocytosed an apoptotic body are known to secrete a number of anti-inflammatory factors3. Some of these could theoretically influence vessel integrity, medial degeneration and/or macrophage recruitment. Accordingly, we hypothesized that efferocytosis could have relevance to vascular disorders other than atherosclerosis, including those not driven by growth of the necrotic core, such as abdominal aortic aneurysm (AAA).
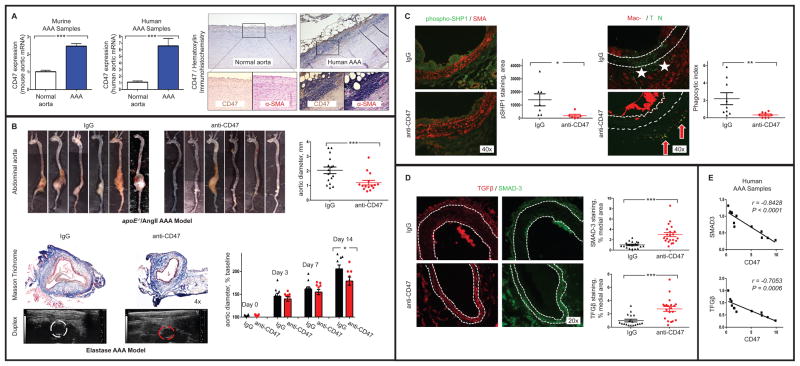
To test this hypothesis, we performed genomic profiling in aortic samples harvested from mice and humans with AAA disease4. In both species, the key ‘don’t eat me’ molecule, CD47, was found to be upregulated in aneurysmal tissue compared to control (Figure A). These data suggest that a defect in programmed cell removal may contribute to the high prevalence of apoptotic cells observed in AAA disease. We then assessed whether restoration of efferocytosis could ameliorate vascular degeneration in two murine models of AAA disease, including the apoE−/−/AngII model and the porcine pancreatic elastase model (as described in detail in4). In each case, mice treated with pro-efferocytic anti-CD47 antibodies developed significantly smaller aortic diameters, relative to IgG control (Figure B).
Figure. The ‘don’t eat me’ molecule, CD47, is a new translational target for AAA.
A. mRNA analysis revealed that CD47 is upregulated in murine (2.4×, left) and human (6.5×, middle) AAA samples, relative to control aortae (N=5–6/condition). Immunohistochemical staining (right) confirmed the upregulation of CD47 on inflammatory cells in the media of human AAA samples (Abcam B6H12.2). B. apoE−/− mice infused with 1000 ng/kg/min of AngII via minipumps were protected from AAA disease when treated with 200 μg of anti-CD47 Ab (MIAP410, BioXcell) QOD (N=15–16/condition, top). Similar findings were observed for C57BL/6 mice in the elastase model, where serial ultrasounds confirmed a protective effect at the terminal endpoint (MOPC-21, BioXcell, N=9/condition, bottom). C. Immunoflourescence staining for phosphorylated SHP1 (CD47’s downstream effector molecule) was significantly reduced in lesional macrophages in mice receiving therapy (green, Abcam ab131500). Co-localization studies revealed that anti-CD47 antibody reduced the ratio of ‘free’ apoptotic bodies (‘AB’, indicated by TUNEL staining; Roche Cell Death Detection Kit) to those co-localizing with macrophages (indicated by Mac-2 staining; BD-550292), indicative of an increase in the ‘phagocytic index’ (stars indicate ‘free’, arrows indicate ‘associated’ AB). The overall burden of AB and adventitial macrophages was also reduced (P < 0.01 and 0.05, respectively). D. Medial staining for the key anti-aneurysmal factors, TGFβ (red, Proteintech #18978-1) and SMAD-3 (green, Proteintech #25494-1) were significantly increased by pro-efferocytic anti-CD47 Ab therapy. E. CD47 and TGFβ are inversely correlated in human AAA samples. * P<0.05, ** P<0.03, *** P<0.01 via two-tailed Student’s t-tests. Error bars represent the SEM.
We next evaluated the mechanism by which this therapy prevented aneurysm disease. While anti-CD47 Ab had no effect on macrophage polarization, endothelial function, TIMP-activity or metalloproteinase elaboration (not shown), we did observe a significant reduction in vascular phospho-SHP1 staining (a factor known to inhibit efferocytosis when phosphorylated1). This change was associated with a reduction in free apoptotic cells and adventitial macrophages, along with an increase in the co-localization of the remaining TUNEL+ and Mac-2+ cells (Figure C). These data indicate an improvement in the ‘phagocytic index’ in the vessel wall1, and suggest that diseased tissues were cleared before they could undergo secondary necrosis (which promotes recruitment of additional inflammatory cells). In keeping with prior reports3, this augmentation in efferocytosis was associated with increased staining for the potent anti-aneurysmal cytokine, TGFβ5, as well as its downstream signaling factor, SMAD3 (Figure D). In support of a mechanistic association, CD47 and TGFβ were found to be anti-correlated in human AAA samples (Figure E).
To date, no medical therapy has been conclusively shown to prevent AAA disease, and this highly morbid condition continues to affect more than 10% of men over 755. This study is the first to associate disease pathogenesis with a defect in efferocytosis, and suggests that restorative therapy could promote fibrotic processes which may stabilize the enlarging aorta. Pro-phagocytic antibodies are currently being investigated in human oncology trials for cancers which upregulate ‘don’t eat me’ molecules to evade tumoricidal macrophages (ClinicalTrials.gov: NCT02216409). Assuming they prove safe and effective, efferocytosis-stimulating therapies could represent a new non-surgical approach for aortic disease.
Acknowledgments
Ethics and IRB approvals were provided by Stanford University (protocol 27279), the Karolinska Institute (protocols N48/16 and N30/16) and the Munich Vascular Biobank (protocol 2799/10); informed consent was received.
Funding Sources:
This study was supported by the National Institutes of Health (R01HL12522401 and R01HL12337001 to NJL).
Footnotes
Data Sharing: The data, analytic methods, and study materials will be maintained by the corresponding author and made available to other researchers upon request.
Disclosures:
NJL and ILW are cofounders and hold equity interest in 47 Incorporated.
References
- 1.Kojima Y, Volkmer JP, McKenna K, Civelek M, Lusis AJ, Miller CL, Direnzo D, Nanda V, Ye J, Connolly AJ, Schadt EE, Quertermous T, Betancur P, Maegdefessel L, Matic LP, Hedin U, Weissman IL, Leeper NJ. Cd47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima Y, Weissman IL, Leeper NJ. The role of efferocytosis in atherosclerosis. Circulation. 2017;135:476–489. doi: 10.1161/CIRCULATIONAHA.116.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving tgf-beta, pge2, and paf. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS. Mir-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Krishna S, Walker PJ, Norman P, Golledge J. Transforming growth factor-beta and abdominal aortic aneurysms. Cardiovascular pathology. 2013;22:126–132. doi: 10.1016/j.carpath.2012.07.005. [DOI] [PubMed] [Google Scholar]