Abstract
Chronic exposure to fine particulate matter (PM2.5) is accepted as a causal risk factor for coronary heart disease (CHD). However, most of the evidence for this hypothesis is based upon cohort studies in whites, comprised of either only males or females who live in urban areas. It is possible that many estimates of the effect of chronic exposure to PM2.5 on risk for CHD do not generalize to more diverse samples. Therefore, we estimated the relationship between chronic exposure to PM2.5 and risk for CHD in among participants in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort who were free from CHD at baseline (n = 17,126). REGARDS is a sample of whites and blacks of both genders living across the continental US. We fit Cox proportional hazards models for time to CHD to estimate the hazard ratio (HR) for baseline 1-year mean PM2.5 exposure, adjusting for environmental variables, demographics, and other risk factors for CHD including the Framingham Risk Score (FRS). The HR (95% confidence interval) for a 2.7 μg/m3 increase (interquartile range) 1-year mean concentration of PM2.5 was 0.94 (0.83, 1.06) for combined CHD death and nonfatal MI, 1.13 (0.92, 1.40) for CHD death, and 0.85 (0.73, 0.99) for nonfatal MI. We also did not find evidence that these associations depended upon overall CHD risk factor burden. Our results do not provide strong evidence for an association between PM2.5 and incident CHD in a heterogeneous cohort, and we conclude that the effects of chronic exposure to fine particulate matter on CHD require further evaluation.
Keywords: fine particulate matter, coronary heart disease, hazard ratio, risk factor
Introduction
Increased chronic exposure to airborne particulate matter with aerodynamic diameter smaller than 2.5 microns (PM2.5) increases risk for coronary heart disease (CHD).1 Many studies using a variety of epidemiologic designs have replicated this finding. Hypothesized mechanisms for the effect of chronic exposure to ambient air pollution on cardiovascular health include systemic inflammation, oxidative stress, and other pro-atherosclerotic mechanisms.1
Despite this large number of studies, most of the evidence for the relationship between fine particulate matter and incident CHD is based upon cohort studies in whites,2,3 comprised of either only males4 or only females5,6 who live in urban areas.7–10 Restriction of study samples to these populations have often been a function of the large cohort data that was available at the time the study was conducted, or the need to sample participants near air quality monitoring stations operated by the Environmental Protection Agency (EPA) in order to obtain robust exposure assessments. The effects of PM2.5 on CHD may not generalize to populations who were not represented in these study samples.
Therefore, we assessed the association between chronic PM2.5 exposure and incident CHD in a national cohort of white and black participants of both genders, living in both urban and rural areas across the continental US.
Methods
Study population
We used a subsample of the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort who were free of CHD at baseline. REGARDS is a nationwide study of 30,239 black and white participants age 45 years and older of both genders recruited between 2003 and 2007, designed to investigate the causes for increased stroke mortality in eight southeastern states (i.e., the “Stroke Belt”), as well as for the increased stroke mortality in blacks compared to whites. Participants were recruited by mail and phone, with oversampling of participants from the Stroke Belt and Stroke Buckle (coastal plains of North Carolina, South Carolina, and Georgia), as well as oversampling of blacks. A baseline Computer Assisted Telephone Interview (CATI) was conducted to collect demographic and health-related variables. After the baseline CATI, an in-home visit was performed by a trained health professional who collected anthropometric measurements such as height, weight, and blood pressure, as well as collected blood and urine samples and an electrocardiogram.
Outcomes
Potential CHD events were detected during a follow-up CATI conducted every 6 months. A report of a hospitalization or Emergency Department visit for any reason related to the heart triggered medical record retrieval. If proxies reported the participant was deceased, or if national database searches revealed the participant was deceased, medical records, autopsy reports, and death certificates were retrieved, and the proxy was interviewed about the circumstances immediately contiguous with the demise. Cases were then adjudicated by experts using all available materials following national consensus guidelines 11,12. A definite or probable myocardial infarction (MI) was present based on the presence of ischemic signs and symptoms; a rising and falling pattern of cardiac biomarkers, most often troponin, to a peak of at least twice the upper limit of normal over at least 6 hours; and electrocardiogram (ECG) or other imaging evidence consistent with ischemia, following the Minnesota code. Independent adjudication had a kappa statistic for agreement >0.80. CHD death was present if a person died within 28 days of a definite or probable MI, or evidence was incomplete to adjudicate an MI but the circumstances were highly suggestive of CHD as the underlying cause of death.
We investigated the impact of PM2.5 on three outcomes: incident total CHD, incident CHD death, and incident nonfatal MI. Incident total CHD was defined as definite or probable MI or acute CHD death, whichever occurred first. Incident CHD death was defined as a CHD death without an antecedent nonfatal MI. Incident nonfatal MIs were definite or probable MIs that did not result in death within 28 days. Events through December 31, 2012 were available for this analysis.
Exposure assessment
One-year mean PM2.5 concentrations were estimated for each participant at baseline. Geocoding was performed using SAS (Cary, NC) and the participant’s street address, and we assumed that participants resided at their baseline address throughout follow up. Approximately 20% of the REGARDS participants did not live in urban areas, so traditional methods of spatially smoothing only ground-level monitoring stations could have resulted in biased exposure assessments. Therefore, the estimates of chronic exposure were created using daily PM2.5 exposures for each participant that were estimated using a combination of EPA Air Quality System (AQS) ground-level measurements of PM2.5 concentration and satellite measurements of Aerosol Optical Depth (AOD) from the MODerate resolution Imaging Spectroradiometer (MODIS) instrument on the National Aeronautics and Space Administration (NASA) Aqua satellite, which estimates the amount of particulate matter in the air column 13,14. The AOD and AQS measurements were combined by using previously published regression models for each US EPA region and season to predict ground-level PM2.5 concentrations from MODIS satellite AOD measurements 15. Then, the national dataset of AQS measurements was combined with the predicted PM2.5 concentrations from the regression models. Finally, a b-spline smoothing algorithm was used to spatially smooth the ground-level PM2.5 estimates, with greater weight given to the AQS measurements 13,14. The final spatial resolution of the estimated surface was 10 km x 10 km and covered the continental US. This combination of ground-level and satellite data helped to overcome the limited spatial coverage of AQS monitoring stations, particularly for rural participants in REGARDS, as well as the indirect nature of MODIS-derived measurements.
Measurement of covariates
Framingham Risk Score
Using the self-reported variables and lab values from blood samples collected during the baseline CATI and in-home visit, respectively, the Framingham Risk Score (FRS) for CHD was calculated for each participant 16. The FRS consists of the following risk factors: age, high density lipoprotein cholesterol (HDLC), total cholesterol (TC), systolic blood pressure (SBP), diastolic blood pressure (DBP), diabetes status, and smoking status. HDLC and TC were measured from the blood samples taken at the baseline in-home visit. Blood pressure values were an average of two measurements taken after the participant had been seated for five minutes. Participants were considered to have diabetes if their non-fasting glucose was ≥ 200 mg/dL, or if their fasting glucose was ≥ 126 mg/dL, or if the participant self-reported use of insulin or medication to treat diabetes. Participants were classified as smokers if they reported current smoking. After calculating the FRS for each individual, participants were classified as “low risk factor burden” if their FRS was < 10% and as “high risk factor burden” if their FRS was ≥ 10%. We used this classification, as opposed to other more common classifications with more categories, because of the limited number of events in each combination of risk category.
Other covariates
The following variables were included as potential confounders of the relationship between PM2.5 and risk for CHD: 1-year mean baseline temperature (degrees Celsius), season at baseline, urbanicity (rural, mixed, or urban), and calendar year of the baseline visit. Temperature was assessed using the North American Land Data Assimilation System (NLDAS), which provides daily estimates of several meteorological variables on an approximately 12 km by 12 km grid across the continental US 14,17. Urbanicity was defined based on Census data, describing the percentage of the participant’s census tract that was classified as urban area: no more than 25% urban area was rural, 25% – 75% was mixed, and at least 75% urban area was urban.
Other variables were included as potential confounders of the relationship between FRS and risk for CHD: age (years), race (black or white), region of residence (Stroke Buckle [coastal plains of North Carolina, South Carolina, and Georgia], Stroke Belt [North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas, and Louisiana], or rest of the US), income (< $20,000, $20,000 – $34,000, $35,000 – $74,000, at least $75,000, and declined to report), education (less than high school, high school graduate, some college, and college graduate and above), diabetes (as defined above), current use of antihypertensive medications, current use of statins, body mass index (BMI; kg/m2), pack-years of cigarette smoking, alcohol use (none, moderate [≤14 drinks/week for men and ≤7 drinks/week for women], and heavy [>14 drinks/week for men and >7 drinks/week for women]), and physical activity (none, 1 – 3 times per week, and 4 or more times per week). Another reason for including age, gender, pack-years of smoking, and diabetes was to account for possible residual confounding, even though they are included in the FRS. Race, age, gender, income, education, pack-years of smoking, alcohol use, physical activity, and current use of antihypertensive medications were self-reported during the CATI at baseline, region of residence was assigned based upon the participant’s geocoded street address, and current statin use was assessed using pill bottle review during the in-home visit. BMI and diabetes classification were determined using measurements and blood samples collected during the in-home visit.
Statistical methods
We first summarized the risk factor burden, potential confounders, and outcomes of the cohort by quartile of baseline 1-year mean PM2.5 exposure. We then calculated event rates and 95% confidence intervals for total CHD for each combination of baseline risk factor burden (low or high) and quartile of baseline 1-year mean PM2.5 exposure.
We used Cox proportional hazards models to estimate the relationship between PM2.5 exposure and incident CHD, first without an interaction between PM2.5 exposure and risk factor burden summarized by the FRS, and then including an interaction between PM2.5 exposure and risk factor burden. This analysis was prespecified, given the recent work focusing on identifying subpopulations that might have differential risk for CHD due to PM2.5 exposure.18
We fit models for three separate outcomes: (1) total CHD (combined CHD death and nonfatal MI); (2) CHD death, with nonfatal MIs censored; and (3) nonfatal MI, with CHD death censored. Model 1 for each outcome included 1-year mean temperature, season, race, region, urbanicity, income, education, age, gender, pack-years, BMI, alcohol use, physical activity, and calendar year, while Model 2 added terms for statin use, antihypertensive medication use, and diabetes. The three additional covariates in Model 2 were left out of Model 1 because they could potentially be a part of the causal pathway between PM2.5 and risk for CHD. We investigated modeling PM2.5 and temperature using restricted cubic splines, but found that linear modeling was sufficient (data not shown).
We also conducted sensitivity analyses to test for interactions between PM2.5 and gender, PM2.5 and race, and PM2.5 and urbanicity each with the same covariates as in Model 2. These post hoc sensitivity analyses were conducted to pursue possible discrepancies between our results and results from previous studies.
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Additional funding was provided by an investigator-initiated grant-in-aid from the National Heart, Lung, and Blood Institute (NHLBI 5R01HL080477). Additional funding was provided by NASA NNX09AV81G, NHBLI 5T32HL079888, American Heart Association 2014 Predoctoral Fellowship (14PRE18830073), and NHLBI 5T32HL00745734.
The authors would like to thank Mr. Andy Westfall and Mr. Chris Gamboa for assistance in preparing the datasets for analysis.
Results
The present study excluded those with anomalous data (n = 56), were missing the date of their in-home visit (n = 14), were missing location data (n = 11), were missing baseline CHD variable (n = 567), had baseline CHD (n = 5,310), had no follow-up data in REGARDS (n = 395), were missing their pill bottle review data (n = 63), had low certainty in their geocoded location of residence (n = 3,671), were missing education status (n = 14), were missing their FRS (n = 1,127), were missing self-reported use of antihypertensive medications (n = 749), were missing alcohol use (n = 322), were missing physical activity (n = 257), were missing BMI (n = 78), or were missing pack years (n = 479), leaving a final analytic sample size of 17,126.
Of the 17,126 participants included in this study, 43% were black and 58% were female. The median(25th percentile, 75th percentile) age at baseline was 63(57, 71) years, and the median follow up time was 6 years. Ten percent of the participants lived in rural areas, 10% lived in mixed areas, and 80% lived in urban areas. Thirty-four percent of the cohort had a high baseline risk factor burden. There were 628 total CHD events (215 CHD deaths and 413 nonfatal MIs). The median(25th percentile, 75th percentile) 1-year mean PM2.5 concentration was 13.6 μg/m3 (12.1, 14.8). Descriptive statistics by quartile of PM2.5 baseline concentration are presented in Table 1. Figure 1 and Supplemental Table 1 present crude total CHD event rates per 1,000 person-years for each combination of baseline risk factor burden and quartile of baseline PM2.5 exposure. The highest event rate was 12.9 CHD events per 1,000 person-years, among participants with high risk factor burden in the second quartile of baseline exposure to PM2.5, while the lowest event rate was 3.2 CHD events per 1,000 person-years, among participants with low risk factor burden in the highest quartile of PM2.5 exposure.
Table 1.
Summary statistics of overall study sample, and by quartile of PM2.5 exposure.
| PM2.5 quartile (μg/m3)
| |||||
|---|---|---|---|---|---|
| 6.5–12.1 N=4,282 |
12.2–13.6 N=4,282 |
13.6–14.8 N=4,283 |
14.8–20.8 N=4,279 |
Combined N=17,126 |
|
| Age (years) | 64 (58,71) | 63 (57,71) | 63 (57,70) | 63 (57,70) | 63 (57,71) |
| Blacks | 32.6% (1,394) |
40.4% (1,728) | 46.2% (1,980) |
51.5% (2,205) |
42.7% (7,307) |
| Women | 54.5% (2,332) |
58.5% (2,505) | 59.2% (2,536) |
58.8% (2,518) |
57.8% (9,891) |
| High risk for CHD (≥10%) | 33.7% (1,443) |
34.2% (1,463) | 34.8% (1,489) |
34.5% (1,475) |
34.3% (5,870) |
| Education level | |||||
| Less than high school | 8.5% (366) | 12.5% (535) | 10.9% (467) |
10.4% (446) | 10.6% (1,814) |
| High school graduate | 21.7% (929) | 26.4% (1,130) | 26.9% (1,150) |
25.0% (1,069) |
25.0% (4,278) |
| Some college | 26.6% (1,139) |
26.4% (1,131) | 26.8% (1,149) |
28.2% (1,205) |
27.0% (4,624) |
| College graduate and above | 43.2% (1,848) |
34.7% (1,486) | 35.4% (1,517) |
36.4% (1,559) |
37.4% (6,410) |
| Income | |||||
| < $20,000 | 14.7% (629) | 18.4% (787) | 16.9% (723) |
16.0% (684) | 16.5% (2,823) |
| $20,000–$34,000 | 22.2% (949) | 23.4% (1,002) | 23.3% (999) |
25.0% (1,071) |
23.5% (4,021) |
| $35,000–$74,000 | 32.5% (1,390) |
30.2% (1,293) | 30.7% (1,313) |
31.5% (1,347) |
31.2% (5,343) |
| >=$75,000 | 19.6% (841) | 16.0% (683) | 17.7% (756) |
16.8% (719) | 17.5% (2,999) |
| Refused | 11.0% (473) | 12.1% (517) | 11.5% (492) |
10.7% (458) | 11.3% (1,940) |
| Region | |||||
| Rest of US | 68.4% (2,928) |
28.0% (1,198) | 42.7% (1,830) |
44.3% (1,895) |
45.8% (7,851) |
| Stroke Belt | 20.8% (892) | 35.7% (1,527) | 35.2% (1,507) |
46.5% (1,989) |
34.5% (5,915) |
| Stroke Buckle | 10.8% (462) | 36.4% (1,557) | 22.1% (946) |
9.2% (395) | 19.6% (3,360) |
| Urbanicity | |||||
| Rural(<=25% urban) | 8.6% (367) | 12.8% (548) | 10.5% (449) |
6.8% (291) | 9.7% (1,655) |
| Mixed(25–75% urban) | 8.7% (371) | 14.2% (609) | 10.3% (443) |
7.8% (333) | 10.3% (1,756) |
| Urban(>=75% urban) | 82.8% (3,544) |
73.0% (3,125) | 79.2% (3,391) |
85.4% (3,655) |
80.1% (13,715) |
| Physical activity | |||||
| None | 31.2% (1,338) |
32.5% (1,393) | 33.8% (1,447) |
33.9% (1,450) |
32.9% (5,628) |
| 1 to 3 time per week | 37.1% (1,590) |
37.0% (1,583) | 38.5% (1,647) |
38.1% (1,630) |
37.7% (6,450) |
| 4 or more per week | 31.6% (1,354) |
30.5% (1,306) | 27.8% (1,189) |
28.0% (1,199) |
29.5% (5,048) |
| Alcohol use | |||||
| None | 55.5% (2,377) |
63.4% (2,713) | 63.4% (2,716) |
63.5% (2,719) |
61.5% (10,525) |
| Moderate | 39.6% (1,694) |
32.2% (1,378) | 32.8% (1,405) |
33.0% (1,410) |
34.4% (5,887) |
| Heavy | 4.9% (211) | 4.5% (191) | 3.8% (162) |
3.5% (150) | 4.2% (714) |
| Packyears | 0.2 (0.0, 18.0) | 0.1 (0.0,16.0) | 0.1 (0.0,16.5) |
0.07 (0.0,15.0) |
0.1 (0.0,16.5) |
| Antihypertensive | 47.3% (2,025) |
50.4% (2,157) | 52.3% (2,240) |
51.8% (2,216) |
50.4% (8,638) |
| Statin use | 26.4% (1,131) |
27.0% (1,154) | 26.1% (1,119) |
26.3% (1,126) |
26.5% (4,530) |
| Diabetes | 15.7% (673) | 19.2% (822) | 20.2% (864) |
18.3% (782) | 18.3% (3,141) |
|
Body mass index (kg/m2) |
28.1 (25.0,32.1) |
28.3 (25.0,32.6) |
28.3 (25.0,32.7) |
28.2 (25.1,32.4) |
28.3 (25.0,32.4) |
| Temperature (Celsius) | 18.3 (14.0,21.5) |
18.6 (16.7,20.7) |
16.6 (13.1,18.8) |
17.3 (13.9,18.4) |
17.6 (14.2,19.7) |
| Season | |||||
| Fall | 19.9% (854) | 23.3% (999) | 21.0% (898) |
27.5% (1,175) |
22.9% (3,926) |
| Spring | 25.7% (25.0,32.4)(1,099) |
23.6% (1,011) | 25.9% (25.0,32.4)(1,109) |
19.9% (853) | 23.8% (25.0,32.4)(4,072) |
| Summer | 33.5% (25.0,32.4)(1,434) |
29.9% (1,281) | 29.6% (1,268) |
26.9% (1,150) |
30.0% (5,133) |
| Winter | 20.9% (895) | 23.1% (991) | 23.5% (1,008) |
25.7% (1,101) |
23.3% (3,995) |
| Year of baseline visit | |||||
| 2003 | 18.1% (773) | 16.5% (706) | 11.1% (25.0,32.4)(477) |
19.9% (851) | 16.4% (25.0,32.4)(2,807) |
| 2004 | 31.7% (25.0,32.4)(1,359) |
27.9% (1,195) | 37.3% (1,596) |
26.0% (1,112) |
30.7% (5,262) |
| 2005 | 17.6% (752) | 24.1% (1,032) | 22.4% (960) |
27.2% (1,164) |
22.8% (3,908) |
| 2006 | 10.4% (444) | 16.8% (721) | 19.6% (25.0,32.4)(840) |
19.5% (835) | 16.6% (25.0,32.4)(2,840) |
| 2007 | 22.3% (954) | 14.7% (628) | 9.6% (25.0,32.4)(410) |
7.4% (317) | 13.5% (2,309) |
a (b, c) represent the median a, the lower quartile b, and the upper quartile c for continuous variables. Numbers after proportions are frequencies.
Figure 1.
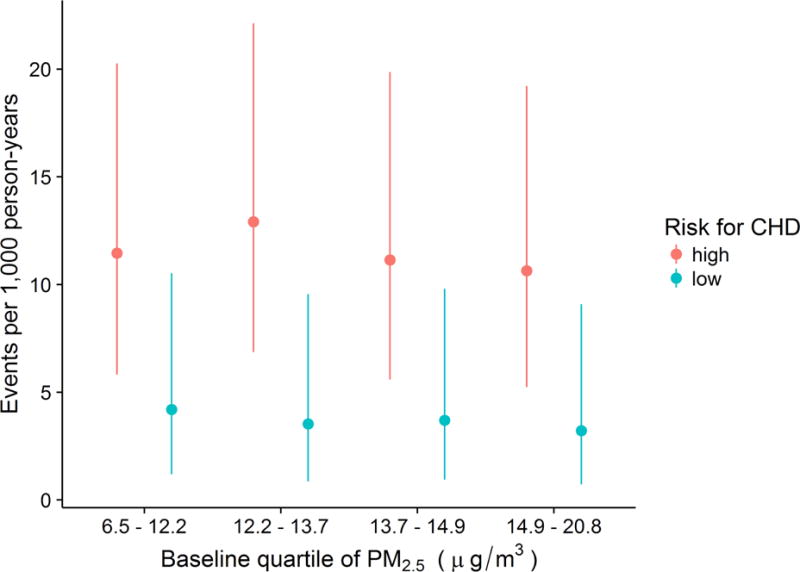
Event rates and 95% confidence intervals of incident coronary heart disease (CHD) by baseline 1-year mean PM2.5 concentration and baseline risk factor burden for CHD in REGARDS sample
The Cox proportional hazards models were fit and proportional hazards were assessed using Schoenfeld residuals 19. The proportional hazards assumption was deemed satisfied through visual inspection of scatterplots of residuals of each variable versus log time. Estimated hazard ratios (HRs) for all model terms for total CHD are presented in Table 2, for CHD death in Supplemental Table 2, and for nonfatal MI in Supplemental Table 3. In fully adjusted models for total CHD (Model 2), the HR for a 2.7 μg/m3 increase (interquartile range) PM2.5 exposure modelled linearly was 0.94 (95% confidence interval: 0.83, 1.06), indicating no statistically significant evidence of a relationship between total CHD and chronic exposure to PM2.5. The HR for the same increase in PM2.5 was similar in the model for CHD death (1.13 [0.92, 1.40]) in Model 2. However, Model 2 for nonfatal MI had a statistically significant HR of 0.85 (0.73, 0.99) for the same increase in PM2.5, indicating an inverse relationship between chronic PM2.5 exposure and nonfatal MI. Figure 2 shows the predicted log hazard for each value of PM2.5 for each outcome.
Table 2.
Hazard ratios for Cox proportional hazards model for total CHD, with no interaction between PM2.5 and Framingham Risk Score.
| Model term | Model 1 | Model 2 |
|---|---|---|
| PM2.5 (μg/m3) - 14.8 vs. 12.1 | 0.93 (0.82–1.06) | 0.94 (0.83–1.06) |
| Age (years) - 71.0 vs. 57.0 | 1.71 (1.49–1.96) | 1.71 (1.49–1.97) |
| Packyears - 16.5 vs. 0.0 | 1.10 (1.05–1.16) | 1.11 (1.05–1.16) |
| Body mass index (kg/m2) - 32.4 vs. 25.0 | 1.13 (1.02–1.25) | 1.06 (0.95–1.18) |
| Temperature (Celsius) - 19.7 vs. 14.2 | 1.00 (0.88–1.15) | 1.01 (0.88–1.15) |
| Race - Black vs. White | 1.01 (0.85–1.21) | 0.94 (0.78–1.13) |
| Gender - Women vs. Men | 0.56 (0.46–0.68) | 0.54 (0.44–0.65) |
| Income - $20,000 – $34,000 vs. < $20,000 | 0.91 (0.72–1.15) | 0.91 (0.72–1.16) |
| Income - $35,000 – $74,000 vs. < $20,000 | 0.81 (0.63–1.05) | 0.82 (0.63–1.06) |
| Income - >=$75,000 vs. < $20,000 | 0.85 (0.61–1.19) | 0.87 (0.62–1.21) |
| Income - Refused vs. < $20,000 | 0.84 (0.62–1.13) | 0.85 (0.63–1.14) |
| Education level - High school graduate vs. Less than high school | 0.78 (0.61–1.00) | 0.79 (0.61–1.01) |
| Education level - Some college vs. Less than high school | 0.78 (0.61–1.01) | 0.79 (0.61–1.03) |
| Education level - College graduate and above vs. Less than high school | 0.63 (0.48–0.82) | 0.64 (0.48–0.84) |
| Region - Stroke Belt vs. Rest of US | 1.33 (1.08–1.64) | 1.31 (1.06–1.60) |
| Region - Stroke Buckle vs. Rest of US | 1.15 (0.88–1.49) | 1.11 (0.86–1.44) |
| Urbanicity - Mixed vs. Urban | 0.75 (0.55–1.01) | 0.75 (0.55–1.02) |
| Urbanicity - Rural vs. Urban | 1.00 (0.76–1.32) | 1.00 (0.76–1.32) |
| Alcohol use - Heavy vs. None | 0.79 (0.51–1.22) | 0.80 (0.52–1.24) |
| Alcohol use - Moderate vs. None | 0.82 (0.69–0.99) | 0.84 (0.70–1.01) |
| Physical activity - 1 to 3 time per week vs. None | 0.78 (0.65–0.94) | 0.79 (0.65–0.95) |
| Physical activity - 4 or more per week vs. None | 0.72 (0.58–0.88) | 0.72 (0.59–0.88) |
| Diabetes - Yes vs. No | – | 1.48 (1.22–1.80) |
| Risk for CHD - High vs. Low | 1.89 (1.57–2.28) | 1.66 (1.36–2.01) |
| Antihypertensive medication use - Yes vs. No | – | 1.21 (1.01–1.43) |
| Statin use - Yes vs. No | – | 0.92 (0.77–1.11) |
| Season - Spring vs. Fall | 1.14 (0.90–1.46) | 1.14 (0.89–1.45) |
| Season - Summer vs. Fall | 1.07 (0.85–1.34) | 1.07 (0.85–1.34) |
| Season - Winter vs. Fall | 1.08 (0.84–1.40) | 1.08 (0.84–1.39) |
| Year of baseline visit - 2004 vs. 2003 | 0.95 (0.76–1.19) | 0.94 (0.75–1.18) |
| Year of baseline visit - 2005 vs. 2003 | 0.99 (0.75–1.30) | 0.97 (0.74–1.28) |
| Year of baseline visit - 2006 vs. 2003 | 0.98 (0.72–1.33) | 0.97 (0.71–1.32) |
| Year of baseline visit - 2007 vs. 2003 | 1.05 (0.75–1.47) | 1.04 (0.74–1.47) |
Model 1 included all of the covariates listed in the rows of the table, except diabetes, antihypertensive medication use, and statin use. Model 2 added these three variables to model 1.
Figure 2. Predicted log hazard for incident total CHD, CHD death, and nonfatal MI versus previous 1-year mean ambient PM2.5 concentration.
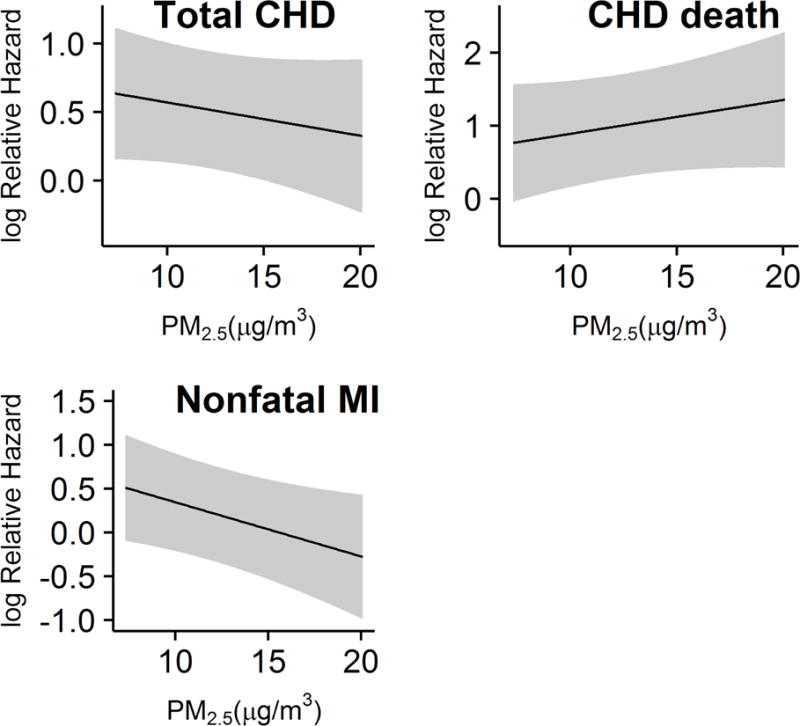
Grey bands are 95% prediction intervals. In models that included an interaction between PM2.5 and risk factor burden, the interaction was not statistically significant for total CHD (p = 0.44), for CHD death (p = 0.47), nor for nonfatal MI (p = 0.09). In models for total CHD, sensitivity analyses did not identify statistically significant interactions between PM2.5 exposure and gender (p = 0.998), race (p = 0.85), or urbanicity (p = 0.40). None of these interactions were significant for either CHD death or nonfatal MI (data not shown).
Discussion
Previous studies of the relationship between chronic exposure to ambient fine particulate matter and CHD have usually been restricted to one gender, whites, and/or participants living in urban areas. These restrictions could indicate that many estimates of the relationship between chronic exposure to fine particulate matter and CHD risk are not generalizable. In a cohort of whites and blacks of both genders, living in both rural and urban areas across the US, we were not able to identify statistically significant associations between 1-year mean concentrations of PM2.5 and total CHD or CHD death. We did identify a slightly statistically significant inverse relationship between PM2.5 exposure and nonfatal MI. We were not able to identify statistically significant effect modification of these relationships by baseline risk factor burden, gender, race, or urbanicity. In summary, we were not able to identify any definitive evidence for a relationship between 1-year mean concentration of fine particulate matter and incident CHD in a geographically and demographically heterogeneous national cohort.
Previous studies have found associations between increased chronic PM2.5 exposure and increased risk for CHD, and the cardiovascular disease epidemiology community currently accepts that chronic exposure to high concentrations of PM2.5 has a causal effect on CVD outcomes.1 The association with CHD has tended to be found in women2,6 and not men,2,4 with the estimated HRs varying from 0.90 to 2.02 for CHD death for a 10 μg/m3 increase in chronic PM2.5 exposure (See Supplemental Table 4). Although we reported HRs for a 2.7 μg/m3 increase in PM2.5 concentration given the limited variability in our data, a 10 μg/m3 increase in our study would have had an associated HR of approximately 1.6. Thus, our estimated association for CHD death falls within a reasonable range, given prior studies’ results. The inverse association we detected between PM2.5 exposure and nonfatal MI was also seen in the Nurses’ Health Study, although it was not statistically significant in that sample.6 We conclude that the results from our study fall into the highly variable range of estimated associations between chronic exposure to fine particulate matter and incident CHD. Although we hypothesized that the limited heterogeneity of previous studies in terms of race, gender, and urban residence might affect generalizability, we found no evidence that our results depended upon race, gender, or type of residence (rural, mixed, or urban).
There were some important differences between our study and previous studies that require mention, most notably the method of estimating PM2.5. We integrated both ground-level measurements of PM2.5 and satellite measurements of AOD to predict ambient PM2.5 over a large spatial area (continental US). Al-Hamdan et al. (2009) showed that merging these remotely-sensed data with surface observations of PM2.5 not only provided a more complete daily representation of PM2.5 than either dataset alone would allow, but it also resulted in an average of 16% reduction in the root mean squared difference between the predicted/smoothed and observed PM2.5 concentrations, compared to predicted PM2.5 surfaces that used only observed values from AQS monitoring stations.13 We have used this same method for estimating PM2.5 exposure in studies of incident cognitive impairment20 and stroke,21 for which we also did not identify associations with PM2.5 concentration. Additionally, given the large area over which spatial prediction was performed (i.e., the continental US), we could justify prediction at 10 km by 10 km grid blocks only, meaning that participants in the same city had a high probability of having identical PM2.5 values for each day they were in the study. Previous studies in smaller urban areas could justify prediction at much higher spatial resolutions, which resulted in more variability in predicted PM2.5 concentrations. However, a recent study in Canada using the same spatial resolution as our study found associations with chronic PM2.5 exposure and incident fatal CHD, indicating that the larger spatial scale we used did not preclude the identification of statistically significant associations per se.3 Another potential cause of our slightly different inference compared to previous studies is the secular decrease in the mean and variance of PM2.5 concentration levels in the US. Finally, PM2.5 is a heterogeneous mixture, and the mixture could vary between rural and urban areas, leading to associations that depend on location, as have been seen for PM2.5 and term low birth weight.22
Our study had a few limitations worth noting. We used an estimated value for PM2.5 concentration, with the assumption that participants did not relocate from their baseline address, creating uncertainty in the estimations of exposure for a given participant. These estimates included information from ground-level measurements from AQS stations potentially far away from a participant, leading to the possible induction of a Berkson error, which can bias estimates of association between an exposure and an outcome.23 However, the inclusion of estimated PM2.5 values from AOD measurements much nearer to the participant’s residence likely reduced the effect of this exposure-outcome spatial misalignment, but we concede that exposure misclassification could still be present, particularly because we could not compare these predicted PM2.5 values with observed values from AQS stations given the lack of coverage of these stations in rural areas. MODIS measurements are also subject to limited spatial coverage depending upon the season, land surface terrain, and cloud cover. Estimates of ambient PM2.5 exposure are only a proxy for an individual’s actual daily exposure, which depends on his or her amount of outdoor physical activity or occupational exposure. We excluded participants who did not have sufficiently high certainty in the participant’s geocoded location, which preferentially excluded rural participants and might limit the generalizability of our results.
Despite the limitations, there are also several strengths to our study. As opposed to previous studies, we were able to expand our participant population outside metropolitan areas, as well as expand the population to cover the entire continental US. This larger population and more geographically diverse sample allowed us to achieve greater generalizability than previous studies and also allowed us to control for confounding by urbanicity of residence (rural, mixed, or urban). We also had a large cohort of black and white participants of both genders, while some previous studies had only one gender and/or race. The in-home visit in the REGARDS study provided measured variables for all participants, such as blood pressure, which reduced the potential for misclassification of risk factor burden due to geographic variation in self-report bias, which has been documented for self-reported BMI 24. Finally, because blood samples were obtained from all participants, we were able to calculate the FRS, a summary of risk factor burden for CHD.
In conclusion, we did not find evidence that chronic PM2.5 exposure was associated with incident CHD, except for a possible inverse association with nonfatal MI. We cannot conclude from our study that there is no relationship between PM2.5 exposure and incident CHD among REGARDS participants, but we do hypothesize that the generalizability of estimates of the effects of chronic exposure to ambient fine particulate matter and CHD requires further study, particularly among populations consisting of multiple races and persons living in non-urban areas. A lack of generalizability could lead to policies on air pollution that are not optimal for all persons.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Additional funding was provided by an investigator-initiated grant-in-aid from the National Heart, Lung, and Blood Institute (NHLBI 5R01HL080477). Representatives from the NHLBI did not have any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript. Additional funding was provided by NASA NNX09AV81G, NHBLI 5T32HL079888, American Heart Association 2014 Predoctoral Fellowship (14PRE18830073), and NHLBI 5T32HL00745734. The authors would like to thank Mr. Andy Westfall and Mr. Chris Gamboa for assistance in preparing the datasets for analysis.
This work was partially funded by NHLBI T32HL079888, AHA 14PRE18830073, NHLBI 5T32HL00745734, NINDS U01NS41588, and NASA NNX09AV81G.
Footnotes
Conflict of interest statement
Dr. Safford has served as a consultant to diaDexus.
Author contributions
MSL conceived the study, performed the statistical analysis, and wrote the paper. LAM conceived the study, oversaw the statistical analysis, and wrote the paper. MMS conceived the study and wrote the paper. EBL provided scientific input on epidemiologic design and statistical analysis and wrote the paper. MZ-AH and WLC provided scientific input on exposure assessment and wrote the paper. All authors have approved the final version of the manuscript.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1.. [DOI] [PubMed] [Google Scholar]
- 2.Chen LH, Knutsen SF, Shavlik D, et al. The association between fatal coronary heart disease and ambient particulate air pollution: Are females at greater risk? Environ Health Perspect. 2005;113(12):1723–1729. doi: 10.1289/ehp.8190. http://www.ncbi.nlm.nih.gov/pubmed/16330354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crouse DL, Peters PA, van Donkelaar A, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: a Canadian national-level cohort study. Environ Health Perspect. 2012;120(5):708–714. doi: 10.1289/ehp.1104049. http://ehp.niehs.nih.gov/1104049/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puett RC, Hart JE, Suh H, Mittleman M, Laden F. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect. 2011;119(8):1130–1135. doi: 10.1289/ehp.1002921.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–458. doi: 10.1056/NEJMoa054409.. [DOI] [PubMed] [Google Scholar]
- 6.Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117(11):1697–1701. doi: 10.1289/ehp.0900572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnat SE, Winquist A, Schauer JJ, Turner JR, Sarnat JA. Fine particulate matter components and emergency department visits for cardiovascular and respiratory diseases in the St. Louis, Missouri-Illinois, metropolitan area. Environ Health Perspect. 2015;123(5):437–444. doi: 10.1289/ehp.1307776.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.CIR.103.23.2810.. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16(1):41–48. doi: 10.1097/01.ede.0000147116.34813.56. https://www.ncbi.nlm.nih.gov/pubmed/15613944. [DOI] [PubMed] [Google Scholar]
- 11.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies a statement from the AHA council on epidemiology and prevention; AHA statistics committee; World heart federation council on epidemiology and prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. http://circ.ahajournals.org/content/108/20/2543.short. [DOI] [PubMed] [Google Scholar]
- 12.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–1774. doi: 10.1001/jama.2012.14306.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hamdan MZ, Crosson WL, Limaye AS, et al. Methods for characterizing fine particulate matter using ground observations and remotely sensed data: potential use for environmental public health surveillance. J Air Waste Manag Assoc. 2009;59(7):865–881. doi: 10.3155/1047-3289.59.7.865. http://www.ncbi.nlm.nih.gov/pubmed/19645271. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hamdan MZ, Crosson WL, Economou SA, et al. Environmental public health applications using remotely sensed data. Geocarto Int. 2014;29(1):85–98. doi: 10.1080/10106049.2012.715209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Hoff RM, Engel-Cox JA. The relation between Moderate Resolution Imaging Spectroradiometer (MODIS) aerosol optical depth and PM2.5 over the United States: a geographical comparison by U.S. Environmental Protection Agency regions. J Air Waste Manag Assoc. 2009;59(11):1358–1369. doi: 10.3155/1047-3289.59.11.1358. http://www.ncbi.nlm.nih.gov/pubmed/19947117. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. http://www.ncbi.nlm.nih.gov/pubmed/9603539. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove BA, Lohmann D, Mitchell KE, et al. Real-time and retrospective forcing in the North American Land Data Assimilation System (NLDAS) project. J Geophys Res D: Atmos. 2003;108(D22) http://onlinelibrary.wiley.com/doi/10.1029/2002JD003118/pdf. [Google Scholar]
- 18.Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119(4):446–454. doi: 10.1289/ehp.1002255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison PD. Survival Analysis Using SAS: A Practical Guide. Sas Institute; 2010. https://books.google.com/books?hl=en&lr=&id=RmbZ2y1KLwUC&oi=fnd&pg=PR3&dq=paul+allison+survival&ots=yQ4Z9lDkzF&sig=fAKpzOAvAr6Uh5ylfYYQJCYYI4A. [Google Scholar]
- 20.Loop MS, Kent ST, Al-Hamdan MZ, et al. Fine particulate matter and incident cognitive impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. PLoS One. 2013;8(9):e75001. doi: 10.1371/journal.pone.0075001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClure LA, Loop MS, Crosson W, Kleindorfer D, Kissela B, Al-Hamdan M. Fine Particulate Matter (PM25) and the Risk of Stroke in the REGARDS Cohort. J Stroke Cerebrovasc Dis. 2017 Apr; doi: 10.1016/j.jstrokecerebrovasdis.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Y, Strosnider H, Balluz L, Qualters JR. Geographic Variation in the Association between Ambient Fine Particulate Matter (PM2.5) and Term Low Birth Weight in the United States. Environ Health Perspect. 2016;124(2):250–255. doi: 10.1289/ehp.1408798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gryparis A, Paciorek CJ, Zeka A, Schwartz J, Coull BA. Measurement error caused by spatial misalignment in environmental epidemiology. Biostatistics. 2009;10(2):258–274. doi: 10.1093/biostatistics/kxn033.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300–306. doi: 10.1002/oby.20451.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann B, Weinmayr G, Hennig F, et al. Air quality, stroke, and coronary events: results of the Heinz Nixdorf Recall Study from the Ruhr Region. Dtsch Arztebl Int. 2015;112(12):195–201. doi: 10.3238/arztebl.2015.0195.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


