Abstract
Background
We hypothesized that hippocampal-sparing radiotherapy via volumetric modulated arc therapy (VMAT) could preserve the neurocognitive function (NCF) of patients with primary brain tumors treated with radiotherapy.
Methods
We reviewed data from patients with primary brain tumors who underwent hippocampal-sparing brain radiotherapy via VMAT between February 2014 and December 2015. The optimization criteria for the contralateral hippocampus was a maximum dose (Dmax) of less than 17 Gy. For NCF evaluations, the Seoul Verbal Learning Test for total recall, delayed recall, and recognition (SVLT-TR, DR, and Recognition) was performed at baseline and at seven months after radiotherapy.
Results
A total of 26 patients underwent NCF testing seven months after radiotherapy. Their median age was 49.5 years (range 26–77 years), and 14 (53.8%) had grade III/IV tumors. The median Dmax to the contralateral hippocampus was 16.4 Gy (range 3.5-63.4). The median mean dose to the contralateral hippocampus, expressed as equivalent to a 2-Gy dose (EQD2/2), was 7.4 Gy2 (0.7–13.1). The mean relative changes in SVLT-TR, SVLT-DR, and SVLT-Recognition at seven months compared to the baseline were − 7.7% (95% confidence interval [CI], − 19.6% to 4.2%), − 9.2% (95% CI, − 25.4% to 7.0%), and − 3.4% (− 12.7% to 5.8%), respectively. Two patients (7.7%) showed deteriorated NCF in the SVLT-TR and SVLT-DR, and three (11.5%) in the SVLT-Recognition. The mean dose of the left hippocampus and bilateral hippocampi were significantly higher in patients showing deterioration of the SVLT-TR and SVLT-Recognition than in those without deterioration.
Conclusions
The contralateral hippocampus could be effectively spared in patients with primary brain tumor via VMAT to preserve the verbal memory function. Further investigation is needed to identify those patients who will most benefit from hippocampal-sparing radiotherapy of the primary brain tumor.
Electronic supplementary material
The online version of this article (10.1186/s13014-018-0975-4) contains supplementary material, which is available to authorized users.
Keywords: Primary brain tumor, Brain radiotherapy, Volumetric modulated arc therapy, Hippocampus, Neurocognitive function test
Background
Radiotherapy is an integral part of brain cancer treatment. It improves the progression-free survival (PFS) of patients with low-grade glioma and is also a standard treatment after surgery with or without chemotherapy in cases of high-grade glioma.
While the tumor itself may affect the neurocognitive function (NCF) of patients, radiotherapy is also associated with declined NCF. In particular, due to the association between the hippocampal neural stem and memory function, radiation therapy of the hippocampal area is associated with deteriorated cognitive and memory functions [1–3].
Effective hippocampal sparing was made possible with the development of sophisticated radiotherapy delivering techniques such as intensity modulated radiotherapy (IMRT) [2, 4, 5]. Hippocampal-sparing whole brain radiotherapy (WBRT) for brain metastases was proven to be effective in a recent clinical trial. The Radiation Therapy Oncology Group (RTOG) 0933 trial enrolled 113 patients with brain metastases treated with hippocampal-sparing WBRT, showing promising results in the preservation of memory function, compared to historical data [6].
However, unlike WBRT, the hippocampal-sparing strategy for the radiotherapy treatment of primary brain tumor has not been thoroughly evaluated. Although the dosimetric feasibility has been reported in a number studies [2, 7–17], to our knowledge, there has been no report on the association between NCF and hippocampal-sparing radiotherapy.
Therefore, we report a dosimetric profile of hippocampal-sparing radiotherapy for the treatment of primary brain tumor as well as the change in NCF of the patients.
Methods
Patient Selection
Hippocampal-sparing radiotherapy to the brain was delivered using the volumetric modulated arc therapy (VMAT) technique between February 2014 and December 2015 at Seoul National University Bundang Hospital. A total of 74 patients have received partial brain irradiation for primary brain tumor, 69 of whom agreed to undergo NCF testing at baseline. Among them, 26 patients also underwent NCF testing 7 months after radiotherapy. After obtaining approval from the Institutional Review Board (No. B-1411/276-105), we analyzed the medical records and dosimetric parameters of these patients.
Radiotherapy Simulation
All patients were positioned using a Variable Axis Baseplate ™ (CIVCO Medical Instruments, Kalona, IA, USA). The head was inclined as previously described [18]. The computed tomography (CT) scans were acquired by using a Brilliance CT Big Bore™ CT simulator (Philips, Cleveland, OH, USA) with a slice thickness of 2 mm.
VMAT Plan Technique
All CT images of the patients were fused with their recent magnetic resonance (MR) images. The hippocampus was delineated according to RTOG guidelines [19]. All contours were delineated by the same radiation oncologist (I.A.K) and each delineation was peer-reviewed by K.S.K and J.Y.S. An optimization criterion for the hippocampus was a maximum dose (Dmax) of less than 17 Gy. However, we did not compromise the coverage of the planning target volume (PTV). In cases where the ipsilateral hippocampus was close to the PTV, we tried to meet the dosimetric criteria for the contralateral hippocampus. The brain stem, optic chiasm, and optic apparatus were also delineated. The other organs at risk were prioritized over the hippocampal dose constraint.
For primary brain tumors, the clinical target volume (CTV) was calculated with an adequate margin of 1.5 – 2.0 cm from the tumor bed or gross tumor. The prescription dose was 60 Gy to the PTV for high-grade glioma and 40 – 56 Gy for low-grade glioma. The mean dose (Dmean) hippocampus was calculated as equivalent to a 2-Gy dose (EQD2/2) with α/β = 2.
NCF
NCF was assessed using the Mini-Mental State Examination (MMSE); Seoul Verbal Learning Test (SVLT); and Rey Complex Figure Test, and Recognition Trial (RCFT) [20–23]. The SVLT is used to assess the verbal memory system using a list of 12 nouns with four words drawn from each of three semantic categories. The total recall (SVLT-TR) trial is the sum of the three learning trials. The SVLT also includes a 20-min delayed recall trial (SVLT-DR) and a yes/no delayed recognition trial (SVLT-Recognition). This last trial consists of a randomized list of 12 target words and 12 non-target words, six of which are drawn from the same categories as those of the targets. This study was standardized and norms that have been adjusted for age, education, and gender were developed for the elderly Korean population [22]. The NCF test was conducted at baseline and 7 months after radiotherapy. The relative differences were measured as ΔNCF = (NCFB-NCFF)/NCFB, where B = baseline, F = follow-up, and the deterioration in the NCF test from baseline was defined as a z-score drop of 1.5 (drop of 1.5 standard deviations).
Statistical analysis
The doses administered to the bilateral hippocampi and right and left hippocampus of the two groups were compared using Student’s t-tests. P-values less than 0.05 were considered to indicate statistically significant differences. Analyses were performed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL).
Results
Patients’ Characteristics
Of the 69 patients who agreed to undergo NCF testing at baseline, 26 also underwent the test 7 months after radiotherapy. Their median age was 49.5 years (range 26-77 years) and 57.7% of the patients were female. Twelve patients (46.2%) had WHO grade I or II tumor, whereas 14 patients (53.8%) had grade III or IV tumor. The median PTV volume was 173.1 cm3 (range 30.3-493.6) and the median prescribed dose was 60 Gy (range 40-60). Concurrent chemotherapy was administered to eight patients (30.8%) diagnosed with glioblastoma (Table 1).
Table 1.
Patients’ and tumor characteristics
| Number | Percent | ||
|---|---|---|---|
| Median Age (range) | 49.5 yrs. (26-77) | ||
| Sex | |||
| Male | 11 | 42.3 | |
| Female | 15 | 57.7 | |
| Diagnosis | |||
| WHO I | |||
| Pituitary adenoma | 2 | 4 | 15.4 |
| Megingioma | 1 | ||
| Craniopharyngioma | 1 | ||
| WHO II | |||
| Oligodendroglioma | 3 | 8 | 30.8 |
| Atypical meningioma | 3 | ||
| Diffuse astrocytoma | 1 | ||
| Ependymoma | 1 | ||
| WHO III | |||
| Anaplastic astrocytoma | 2 | 6 | 23.1 |
| Anaplastic oligodendroglioma | 2 | ||
| Anaplastic meningioma | 1 | ||
| Anaplastic hemangiopericytoma | 1 | ||
| WHO IV | |||
| Glioblastoma | 7 | 8 | 30.8 |
| Gliosarcoma | 1 | ||
| Surgery | |||
| GTR/STR | 22 | 84.6 | |
| Bx only/No surgery | 4 | 15.4 | |
| Concurrent Chemotherapy | 8 | 30.8 | |
| Location | |||
| Frontal | 5 | 19.2 | |
| Parietal | 5 | 19.2 | |
| Temporal | 6 | 23.1 | |
| Ocippital | 1 | 3.8 | |
| Temporo-parietal | 2 | 7.7 | |
| Fronto-temporal | 1 | 3.8 | |
| Fronto-Prietal | 1 | 3.8 | |
| Central | 5 | 19.2 | |
| Planning Target Volume (PTV) | |||
| Vol(cc) | 173.1 cm3 (30.3-493.6) | ||
| Prescribed dose | 60 Gy (40-60) | ||
GTR gross total resection; STR, subtotal resection
Dosimetric Analysis
The median doses to 100% of the structure (D100%) and Dmax of the contralateral hippocampus were 7.2 Gy (range 0.6–11.7) and 16.4 Gy (range 3.5–63.4), respectively. The median Dmean expressed in EQD2/2 to the contralateral hippocampus was 7.4 Gy2 (range 0.7–13.1). The ipsilateral hippocampus received a higher dose. In addition, the median Dmax and Dmean (EQD2/2) of the ipsilateral hippocampus were 40.9 Gy (range 5.7–64.3) and 10.3 Gy2 (range 1.0–62.3), respectively. The median values of the maximal doses to the brain stem and optic chiasm were 43.3 Gy (range 0.2–61.5) and 42.5 Gy (range 1.0–57.8), respectively. The other organs at risk could be effectively spared (Table 2).
Table 2.
Dosimetric analysis
| Dosimetric parameters | Median (range) |
|---|---|
| Hippocampus | |
| Contralateral | |
| Vol(cc) | 1.8 cm3 (0.9-2.4) |
| D100% | 7.2Gy (0.6-11.7) |
| Dmax | 16.4 Gy (3.5-63.4) |
| Dmean | 12.3 Gy (1.3-19.7) |
| Dmean (EQD2/2) | 7.4 Gy2 (0.7-13.1) |
| Ipsilateral | |
| Vol(cc) | 1.7 cm3 (0.6-2.3) |
| D100% | 8.4 Gy (0.7-60.0) |
| Dmax | 40.9 Gy (5.7-64.3) |
| Dmean | 15.9 Gy (2.0-60.3) |
| Dmean (EQD2/2) | 10.3 Gy2 (1.0-63.4) |
| Bilateral | |
| Dmean | 13.4 (1.8-38.3) |
| Dmean (EQD2/2) | 8.3 Gy2 (0.9-31.3) |
| Optic nerve | |
| Dmax | 31.7 Gy (0.5-58.5) |
| Optic chiasm | |
| Dmax | 42.5 Gy (1.0-57.8) |
| Brain stem | |
| Dmax | 43.3 Gy (0.2-61.5) |
| Eyeballs | |
| Dmax | 13.2Gy (0.5-38.1) |
| Lenses | |
| Dmax | 3.5Gy (0.4-14.0) |
D100% dose to 100% volume of the structure, Dmax maximum dose, Dmean mean dose, EQD2/2 equivalent 2 Gy dose with α/β = 2
NCF Test Results
Of the 26 patients who underwent neurocognitive testing at 7 months, two patients diagnosed with gliosarcoma and glioblastoma had progressive disease before 7 months. The other 24 patients presented with stable disease at 7 months. At the median follow-up of 13.9 months (range 7.0–25.6), the median PFS and overall survival were not reached. At the last follow-up, eight patients had progressed and one patient had died.
The NCF test results at baseline and at 7 months for the 26 patients are listed in Table 3 and Fig. 1. The mean relative change of SVLT-TR, SVLT-DR, and SVLT-Recognition at 7 months compared to the baseline were − 7.7% (95% confidence interval [CI], − 19.6% to 4.2%), − 9.2% (95% CI, − 25.4% to 7.0%, after excluding one patient with 0 at baseline), and − 3.4% (− 12.7% to 5.8%), respectively. The patients with deterioration in the tests included two (7.7%) in the SVLT-TR and SVLT-DR and three (11.5%) in the SVLT-Recognition. In regard to the RCFT, 24%, 8%, 8%, and 12% of the patients showed deterioration, respectively.
Table 3.
Change of neurocognitive function (NCF) test at seven months from baseline
| Mean change from Baseline (%)a | 95% CI | Probability of deteriorationb(%) | |
|---|---|---|---|
| MMSE | − 1.2 | − 9.8 to 7.4 | 3.8 |
| SVLT-Total recall | −7.7 | −19.6 to 4.2 | 7.7 |
| SVLT-Delayed recall | −9.2c | − 25.4 to 7.0c | 7.7 |
| SVLT-Recognition | −3.4 | −12.7 to 5.8 | 11.5 |
| RCFT-COPY | 1.8 | −7.4 to 11.1 | 24 |
| RCFT-Immediate recall | −8.1 | −36.2 to 20.0 | 8 |
| RCFT-Delayed recall | −25.2d | − 52.8 to 2.5d | 8 |
| RCFT-Recognition | −3.8 | −11.2 to 4.0 | 12 |
MMSE Mini-Mental State Examination, SVLT Seoul Verbal Learning Test, RCFT Rey Complex Figure Test and Recognition Trial
aΔNCF = (NCFB-NCFF)/NCFB, Where B = baseline and F = follow-up, (Minus change indicate improved NCF)
bDeterioration in NCF test from baseline defined as drop of z-score 1.5 (drop of 1.5 standard deviation)
cExclusion of one patient with 0 test result at baseline
dExclusion of one patient who could not be assessed at baseline
Fig. 1.
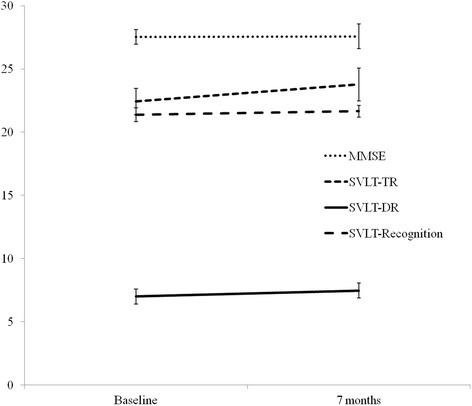
Mini-Mental State Examination (MMSE) and Seoul Verbal Learning Test (SVLT) score at baseline and at seven months
Hippocampal Dose and Neurocognitive Impairment
We compared the hippocampal dose of the patients with varying NCF test results (Table 4 and Additional file 1: Table S1). We compared the right, left, contralateral, ipsilateral, and bilateral hippocampi mean doses (EQD2/2), respectively. The mean doses of the left hippocampus and bilateral hippocampi were significantly higher in patients with deterioration of SVLT-TR and SVLT-Recognition than in those without deterioration. The bilateral hippocampal mean dose was significantly higher in patients with impaired RCFT-Recognition test results (p = 0.042).
Table 4.
Association between hippocampus dose and neurocognitive test deterioration
| Mean dose (EQD2/2) | ||||||
|---|---|---|---|---|---|---|
| Bilateral hippocampi | P value | Right hippocampus | P value | Left hippocampus | P value | |
| SVLT-Total recall | 0.033a | 0.398 | 0.013a | |||
| No Deterioration (n = 23) | 10.6 ± 6.5 | 15.7 ± 16.8 | 11.8 ± 14.1 | |||
| Deterioration (n = 3) | 20.3 ± 11.5 | 7.2 ± 1.7 | 37.7 ± 27.6 | |||
| SVLT-Delayed recall | 0.115 | 0.558 | 0.074 | |||
| No Deterioration (n = 24) | 11.0 ± 6.7 | 15.2 ± 16.6 | 13.1 ± 15.0 | |||
| Deterioration (n = 2) | 19.8 ± 16.3 | 8.2 ± 0.1 | 36.0 ± 38.9 | |||
| SVLT-Recognition | 0.003a | 0.427 | 0.001a | |||
| No Deterioration (n = 23) | 10.2 ± 6.0 | 13.8 ± 15.8 | 11.1 ± 11.7 | |||
| Deterioration (n = 3) | 23.3 ± 9.4 | 21.8 ± 19.5 | 43.1 ± 30.9 | |||
| RCFT-COPY | 0.469 | 0.211 | 0.261 | |||
| No Deterioration (n = 20) | 11.1 ± 6.6 | 16.9 ± 17.7 | 12.7 ± 14.9 | |||
| Deterioration (n = 6) | 13.7 ± 10.7 | 7.5 ± 3.7 | 22.0 ± 24.6 | |||
| RCFT-Immediate recall | 0.156 | 0.513 | 0.081 | |||
| No Deterioration (n = 24) | 11.1 ± 6.6 | 15.3 ± 16.5 | 13.1 ± 15.0 | |||
| Deterioration (n = 2) | 19.0 ± 17.4 | 7.4 ± 1.2 | 36.0 ± 39.4 | |||
| RCFT-Delayed recall | 0.156 | 0.513 | 0.081 | |||
| No Deterioration (n = 24) | 11.1 ± 6.6 | 15.3 ± 16.5 | 13.1 ± 15.0 | |||
| Deterioration (n = 2) | 19.0 ± 17.4 | 7.4 ± 1.2 | 35.6 ± 39.4 | |||
| RCFT-Recognition | 0.042a | 0.406 | 0.257 | |||
| No Deterioration (n = 23) | 10.6 ± 6.5 | 13.8 ± 16.0 | 13.4 ± 15.2 | |||
| Deterioration (n = 3) | 19.9 ± 11.5 | 22.1 ± 20.9 | 25.8 ± 32.7 | |||
Numbers are represented as mean ± SD
SVLT Seoul Verbal Learning Test, RCFT Rey Complex Figure Test and Recognition Trial
aindicate statistical significance by student’s t-test
Discussion
Numerous studies have assessed the association between the radiation dose to the hippocampus and memory function in patients [24, 25]. Furthermore, the NCF decline in patients treated with WBRT is associated with the hippocampal radiation dose [26, 27]. The recent development of radiotherapy techniques has made hippocampal-sparing radiotherapy possible, which was shown to be efficient in the WBRT in a recent clinical trial [6].
However, there are several considerations when applying the hippocampal-sparing strategy to primary brain tumors. First, compromising the target volume for hippocampal-sparing is not recommended. When treating brain metastases, hippocampal-sparing WBRT has an acceptable risk. Ghia et al. reviewed 100 patients with brain metastasis, reporting that 8% had metastases within 5 mm of the hippocampus [28]. The modest increase in the risk of recurrence could be balanced with salvage stereotactic radiosurgery. However, in primary brain tumor, the safety of compromising the target volume for the hippocampus has not been validated. In high-grade glioma, recurrences are most often located within 2 cm of the original tumor [29]. Moreover, the report that patients with glioblastoma involving the subventricular zone have decreased overall survival and PFS remains controversial [30, 31]. The recently published American Society for Radiation Oncology (ASTRO) guidelines for glioblastoma noted that given the absence of published data for the hippocampal-sparing in glioblastoma patients, the panel does not recommend compromising the target coverage for hippocampus protection [32].
Second, the hippocampi have a bilateral structure. In case the ipsilateral hippocampus is close to the target volume, we could at least spare the contralateral hippocampus by using the IMRT technique [8]. However, it is uncertain if this strategy could be beneficial for the preservation of the memory function. Lesion studies indicate that the left and right temporomesial structures are essential for verbal and visuospatial memory, respectively [33, 34]. Patients with left lobe-origin complex partial seizures have abnormalities in verbal memory [35], while those with nondominant foci may have deficits in visuospatial memory, even though this is less established [34]. Jalali et al. reported that radiotherapy doses to the left temporal lobe are predictors of neurocognitive decline [24]. In the current study, we could spare the contralateral hippocampus to the median value of Dmean (EQD2/2) to 7.4 Gy2. Moreover, the left hippocampal dose was significantly associated with SVLT, whereas the right hippocampal dose was not. In regard to the preservation of the verbal memory function, sparing the contralateral hippocampus with the right lobe lesion could be effective. In the current study, the patients had undergone RCFT, which evaluates visuospatial memory. However, we did not observe an association between the deterioration of RCFT results and the radiation dose to the right hippocampus. Further investigation to identify the association between the visuospatial memory function impairment and the radiation dose to the right hippocampus is required.
Third, unlike the WBRT, the target region differs among patients undergoing radiotherapy of the primary brain tumor. Therefore, comparisons of the hippocampal dosimetric profile and NCF toxicity are difficult. Several studies reported consistent results with those of our study regarding the dosimetric profile of the hippocampus when applying the hippocampal-sparing strategy using various IMRT techniques for the radiotherapy of the primary brain tumor [2, 7–17]. Pinkham et al. reported the dosimetric feasibility of hippocampal-sparing IMRT in grade II and grade III gliomas. They reported a median mean dose to the contralateral hippocampus of 24.9 Gy (range 5.1–58 Gy) [9]. Marsh and colleagues achieved mean doses of 15.8 Gy and 12 Gy for patients with high-grade and low-grade gliomas, respectively [13]. In regard to other critical structures, we achieved acceptable radiation doses for all vital organs.
The memory function deterioration is reportedly 30%–60% eight to 18 months after cranial irradiation for primary brain tumor [36–39]. In the RTOG 0933 trial, the probability of deterioration of the Hopkins Verbal Learning Test-Revised Delayed Recall score of patients who underwent hippocampal sparing radiotherapy was 17.2% at 6 months [6]. In the current study, the deterioration in the SVLT-DR test was 7.7%. However, direct comparison of this result with those of other studies has limitations. We only analyzed patients who underwent neurocognitive function tests at 7 months; the compliance with this test at 7 months was 38%, whereas the compliance of the NCF test at 6 months in the RTOG 0933 trial was 54%. Second, this study included patients with heterogeneous histology. Rapid progression of WHO IV disease might affect the neurocognitive function test. Of the two patients who progressed before the NCF test at 7 months, one patient with a left hippocampus dose as high as 63.4 Gy EQD2/2 exhibited an NCF test decline. Meanwhile, in patients with less aggressive histology, hippocampus-sparing radiotherapy may be more beneficial. However, the association between the integral dose to normal brain tissue and long-term neurocognitive changes should be carefully investigated in low-grade tumors especially in young patients. Further prospective studies with homogenous disease would clarify the benefit of hippocampal-sparing partial brain irradiation.
Conclusion
We used VMAT to apply hippocampal-sparing radiotherapy to primary brain tumors. The hippocampus could be reasonably spared and NCF tests performed 7 months after radiotherapy showed promising results in the preservation of verbal memory function. The left hippocampal mean dose was associated with the deterioration of the memory function, while the right hippocampal mean dose was not. Further investigation is needed in order to select patients who will most benefit from hippocampal-sparing radiotherapy of the primary brain tumor.
Additional file
Table S1. Association between hippocampus dose and neurocognitive test deterioration. (DOCX 20 kb)
Acknowledgements
Kyung Su Kim currently works at the Department of Radiation Oncology at the Dongnam Institute of Radiological and Medical Sciences.
Funding
This work was supported by a Cancer Control Program grant (#0820010) from the Korean Ministry of Health & Welfare.
Availability of data and materials
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CT V
Clinical target volume
- IMRT
Intensity modulated radiotherapy
- MMSE
Mini-Mental State Examination
- NCF
Neurocognitive function
- PFS
Progression-free survival
- PTV
Planning target volume
- RCFT
Rey Complex Figure Test, and Recognition Trial
- RTOG
Radiation Therapy Oncology Group
- SVLT-TR,-DR-Recognition
Seoul Verbal Learning Test - total recall, delayed recall, recognition
- VMAT
Volumetric modulated arc therapy
- WBRT
Whole brain radiotherapy
Authors’ contributions
Author contributions to the study and manuscript preparation include the following. Conception and design: IAK. Data acquisition: all authors. Data analysis and interpretation: KKS and IAK. Drafting of the article: KKS. Critical revision of the article: KKS, KE, JK, and IAK. Review of the final version of the manuscript and approval for submission: all authors. Statistical analysis: KKS. Study supervision: IAK.
Ethics approval and consent to participate
All studies on humans described in the present manuscript were carried out with the approval of the institutional review board and in accordance with national law and the current, revised form of the 1975 Declaration of Helsinki. Our institutional review board waived the need to obtain written informed consent from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13014-018-0975-4) contains supplementary material, which is available to authorized users.
Contributor Information
Kyung Su Kim, Email: happyend67@gmail.com.
Chan Woo Wee, Email: wcw0108@hanmail.net.
Jin-Yong Seok, Email: sjy515@hanmail.net.
Joo Wan Hong, Email: wani9387@gmail.com.
Jin-Beom Chung, Email: 35004@snubh.org.
Keun-Yong Eom, Email: 978sarang@hanmail.net.
Jae-Sung Kim, Email: jskim@snubh.org.
Chae-Yong Kim, Email: chaeyong@snu.ac.kr.
Young Ho Park, Email: kumimesy@gmail.com.
Yu Jung Kim, Email: cong1005@gmail.com.
In Ah Kim, Phone: +82-31-787-7651, Email: inah228@snu.ac.kr.
References
- 1.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazda T, Jancalek R, Pospisil P, Sevela O, Prochazka T, Vrzal M, et al. Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol. 2014;9:139. doi: 10.1186/1748-717X-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 4.Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh JC, Godbole RH, Herskovic AM, Gielda BT, Turian JV. Sparing of the neural stem cell compartment during whole-brain radiation therapy: a dosimetric study using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2010;78:946–954. doi: 10.1016/j.ijrobp.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of Memory With Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali AN, Ogunleye T, Hardy CW, Shu HK, Curran WJ, Crocker IR. Improved hippocampal dose with reduced margin radiotherapy for glioblastoma multiforme. Radiat Oncol. 2014;9:20. doi: 10.1186/1748-717X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodensohn R, Sohn M, Ganswindt U, Schupp G, Nachbichler SB, Schnell O, et al. Hippocampal EUD in primarily irradiated glioblastoma patients. Radiat Oncol. 2014;9:276. doi: 10.1186/s13014-014-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkham MB, Bertrand KC, Olson S, Zarate D, Oram J, Pullar A, et al. Hippocampal-sparing radiotherapy: the new standard of care for World Health Organization grade II and III gliomas? J Clin Neurosci. 2014;21:86–90. doi: 10.1016/j.jocn.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Canyilmaz E, Uslu GD, Colak F, Hazeral B, Haciislamoglu E, Zengin AY, et al. Comparison of dose distributions hippocampus in high grade gliomas irradiation with linac-based imrt and volumetric arc therapy: a dosimetric study. SpringerPlus. 2015;4:114. doi: 10.1186/s40064-015-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeberg S, Harrabi SB, Bougatf N, Bernhardt D, Rieber J, Koerber SA, et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma: A dosimetric comparison. Strahlenther Onkol. 2016;11:770–9. [DOI] [PubMed]
- 12.Smyth G, Evans PM, Bamber JC, Mandeville HC, Welsh LC, Saran FH, et al. Non-coplanar trajectories to improve organ at risk sparing in volumetric modulated arc therapy for primary brain tumors. Radiother Oncol. 2016;1:124–31. [DOI] [PubMed]
- 13.Marsh JC, Godbole R, Diaz AZ, Gielda BT, Turian JV. Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. Journal of medical imaging and radiation oncology. 2011;55:442–449. doi: 10.1111/j.1754-9485.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 14.Marsh J, Godbole R, Diaz A, Herskovic A, Turian J. Feasibility of cognitive sparing approaches in children with intracranial tumors requiring partial brain radiotherapy: A dosimetric study using tomotherapy. journal of cancer therapeutics and research. 2012;1:1. doi: 10.7243/2049-7962-1-1. [DOI] [Google Scholar]
- 15.Panet-Raymond V, Ansbacher W, Zavgorodni S, Bendorffe B, Nichol A, Truong PT, et al. Coplanar versus noncoplanar intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) treatment planning for fronto-temporal high-grade glioma. J Appl Clin Med Phys. 2012;13:3826. doi: 10.1120/jacmp.v13i4.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh JC, Ziel GE, Diaz AZ, Wendt JA, Gobole R, Turian JV. Integral dose delivered to normal brain with conventional intensity-modulated radiotherapy (IMRT) and helical tomotherapy IMRT during partial brain radiotherapy for high-grade gliomas with and without selective sparing of the hippocampus, limbic circuit and neural stem cell compartment. Journal of medical imaging and radiation oncology. 2013;57:378–383. doi: 10.1111/1754-9485.12048. [DOI] [PubMed] [Google Scholar]
- 17.Oehler J, Brachwitz T, Wendt TG, Banz N, Walther M, Wiezorek T. Neural stem cell sparing by linac based intensity modulated stereotactic radiotherapy in intracranial tumors. Radiat Oncol. 2013;8:187. doi: 10.1186/1748-717X-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KS, Seo SJ, Lee J, Seok JY, Hong JW, Chung JB, et al. Inclined head position improves dose distribution during hippocampal-sparing whole brain radiotherapy using VMAT. Strahlenther Onkol. 2016;192:473–480. doi: 10.1007/s00066-016-0973-0. [DOI] [PubMed] [Google Scholar]
- 19.Hippocampal contouring: a contouring Atlas for RTOG 0933. http://www.rtog.org/LinkClick.aspx?fileticket=59vaU8vfgQc%3d&tabid=338. Accessed 14 Feb 2018.
- 20.Ahn H-J, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25:1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang HR, Choi SH, Yoon DH, Yoon BN, Suh YJ, Lee D, et al. The effect of cognitive training in patients with mild cognitive impairment and early Alzheimer's disease: a preliminary study. J Clin Neurol. 2012;8:190–197. doi: 10.3988/jcn.2012.8.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek MJ, Kim HJ, Kim S. Comparison between the story recall test and the word-list learning test in Korean patients with mild cognitive impairment and early stage of Alzheimer's disease. J Clin Exp Neuropsychol. 2012;34:396–404. doi: 10.1080/13803395.2011.645020. [DOI] [PubMed] [Google Scholar]
- 23.Meyers JE, Bayless JD, Meyers KR. Rey complex figure: memory error patterns and functional abilities. Appl Neuropsychol. 1996;3:89–92. doi: 10.1207/s15324826an0302_8. [DOI] [PubMed] [Google Scholar]
- 24.Jalali R, Mallick I, Dutta D, Goswami S, Gupta T, Munshi A, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:974–979. doi: 10.1016/j.ijrobp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83:e487–e493. doi: 10.1016/j.ijrobp.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 27.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The lancet oncology. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 28.Ghia A, Tome WA, Thomas S, Cannon G, Khuntia D, Kuo JS, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–977. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 30.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-Oncology. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbs IC, Haas-Kogan D, Terezakis S, Kavanagh BD. The subventricular zone neural progenitor cell hypothesis in glioblastoma: epiphany, Trojan Horse, or Cheshire fact? Int J Radiat Oncol Biol Phys. 2013;86:606–608. doi: 10.1016/j.ijrobp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera AR, Kirkpatrick JP, Fiveash JB, Shih HA, Koay EJ, Lutz S, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Practical radiation oncology. 2016;6:217–225. doi: 10.1016/j.prro.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learn Mem. 2002;9:99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalton MA, Hornberger M, Piguet O. Material specific lateralization of medial temporal lobe function: An fMRI investigation. Hum Brain Mapp. 2016;37:933–941. doi: 10.1002/hbm.23077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann BP, Wyler AR, Richey ET, Rea JM. Memory function and verbal learning ability in patients with complex partial seizures of temporal lobe origin. Epilepsia. 1987;28:547–554. doi: 10.1111/j.1528-1157.1987.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 36.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 38.Groves MD, Maor MH, Meyers C, Kyritsis AP, Jaeckle KA, Yung WA, et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine, and vincristine for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1999;45:127–135. doi: 10.1016/S0360-3016(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 39.Levin V, Yung W, Bruner J, Kyritsis A, Leeds N, Gleason M, et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53:58–66. doi: 10.1016/S0360-3016(01)02819-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association between hippocampus dose and neurocognitive test deterioration. (DOCX 20 kb)
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.


