Abstract
Here, we report the case of a patient, diagnosed with BRAFV600E-mutated metastatic malignant melanoma M1a, who achieved a complete metabolic response after 7 months of treatment with the combination of dabrafenib and trametinib. After 31 months, the treatment was interrupted for patient’s decision. To date October 2017, 18 months after the interruption of the treatment with the combination of dabrafenib and trametinib, follow-up Positron Emission Tomography (PET) scans are still documenting complete metabolic response.
Keywords: case report, complete remission, dabrafenib, drug combination, melanoma, neoplasm metastasis, vemurafenib
Introduction
Several studies have identified different genetic profiles in patients affected by malignant melanoma [1–3]. Approximately 50% of cutaneous melanomas harbor activating BRAF mutations and the most frequent is BRAFV600E [4,5]. BRAF is a member of the RAF kinase family, with a role in the ERK/MAP kinase pathway, a signaling cascade that regulates cell proliferation, differentiation and survival [6].
Although BRAF inhibitors, such as dabrafenib and vemurafenib, have shown a promising activity in melanoma management [7], all patients eventually develop a drug resistance at some time after treatment start [5,8,9]. The addition of a MEK inhibitor, such as trametinib or cobimetinib, to BRAF inhibitors mitigates one pathway of resistance, increasing response rates with improved overall survival (OS) without relevant cumulative toxicity [10,11]. In a recent exploratory analysis of survival data from selected clinical trials in metastatic melanoma with a long-term follow-up [12], mean survival curves, obtained by weighted averaging, revealed that the combination treatment with BRAF plus MEK inhibitors is clearly superior to BRAF inhibition alone in first-line treatment as well as in second line or higher line. The superiority of the combination of BRAF plus MEK inhibitors remained consistent over time in both progression-free survival (PFS) and OS with follow-up times of up to 28 months. On the other hand, MEK monotherapy resulted to have only a limited efficacy (similar to chemotherapy as second line or beyond). The same analysis showed a superiority of the combination of BRAF plus MEK inhibitors within the first 6 months after treatment onset. After 6 months, a clear superiority of PD-1 blockers alone or in combination with CTLA-4 blockers was found. These findings are of high importance and reflect the clinical phenomena of acquired resistance – which is common in MAP kinase inhibition – and account for two phenomena: (a) the strong decline of the respective mean survival curves at 6 months of treatment; (b) primary resistance, which is common in immune checkpoint inhibition and accounts for the steep decline of the respective mean survival curves directly after therapy onset. These results indicate the usefulness of therapeutic approaches providing an intended switch from MAP kinase inhibition to immune checkpoint blockade to achieve the highest benefit from both therapeutic strategies. For this reason, data from the daily clinical practice by combining BRAF and MEK inhibitors may be useful to improve our knowledge in this disease setting.
We describe the case of one patient with BRAFV600E-mutated melanoma treated with the combination of dabrafenib and trametinib and achieving a long-term complete response (CR). Written informed consent was obtained from the patient for publication of this case report.
Case report
In August 2002, a 57-year-old Caucasian man was referred to our institution for excision of a cutaneous lesion on the left parasternal region, which was diagnosed as a superficial spreading melanoma with invasion of the papillary dermis to a depth of 0.3 mm, Clark level III, 1 mitosis/mmq, Stage IB pT1b,N0,M0 [13], with active chronic inflammation, capillary neogenesis and pigmented histiocytes in the dermis.
From 2003, the patient suffered from a controlled type 2 diabetes. In 2008, he underwent transurethral resection of the prostate for benign prostatic hyperplasia.
The patient was kept under regular follow-up every 6 months with clinical evaluation and complete skin examinations, blood tests (complete blood count [CBC], liver function tests [LFT], lipid profile and lactate dehydrogenase [LDH] level) ultrasound of regional lymph node and of the abdomen and optional X-ray diagnostics. Until 2012, no relevant abnormalities were found at follow-up visits excepting for an intermittent neutropenia with lymphocitosis.
At the beginning of 2013, owing to recurrent lymphadenitis, he was referred to a rheumatologist to exclude common rheumatic diseases. No alterations in the level of inflammatory biomarkers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were found; serum LDH levels were normal. In November 2013, ultrasonography of the left axillary region demonstrated three hypoechoic lymphnodes with abnormal vascularity on color Doppler and sharp border. Cytological examination confirmed the presence of melanoma metastases. The evaluation of tumor-infiltrating lymphocytes TILs revealed augmentation of CD4+ helper with an increased CD4/CD8 ratio. An exon 15 BRAFV600E mutation was detected.
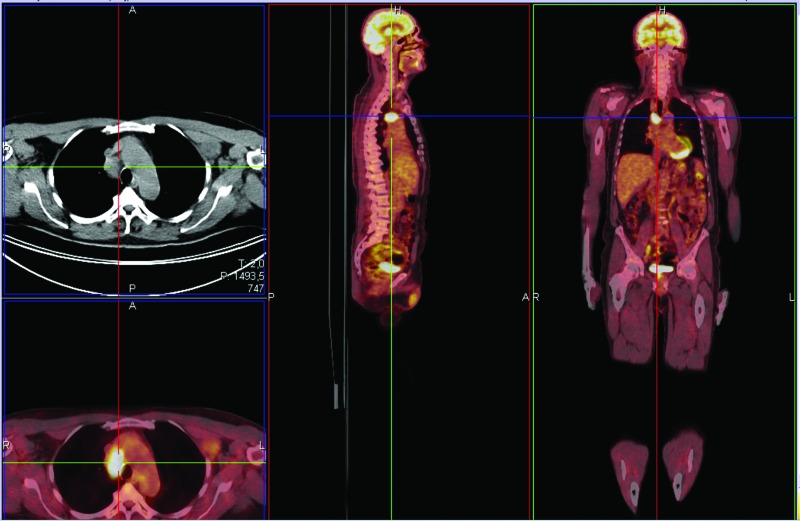
Staging with positron emission tomography Positron Emission Tomography (PET) indicated melanoma metastatic to right paratracheal lymph nodes and left axillary region (Figure 1). CT scan of the brain showed no metastases and LDH levels were normal M1a [13].
Figure 1.
PET staging 2013 indicating melanoma metastatic M1a.
According to the BRAFV600E mutation evidence, at the end of January 2014, we proposed to the patient a treatment with oral vemurafenib 1920 mg daily. The drug administration was stopped after three days owing to an intense cutaneous (allergic reaction/immune system disorders G2 CTC AE 4.03) and respiratory hypersensitivity reaction, treated with steroids and anti-histamines. After this hypersensitivity episode was resolved, we tried to restart vemurafenib at the reduced dose of 1440 mg daily, but palatal edema and swelling with pain was observed (allergic reaction/immune system disorders G2 CTC AE 4.03). After hypersensitivity resolution, vemurafenib was restarted at the dose of 960 mg daily, but a new allergic reaction led to a definitive treatment stop.
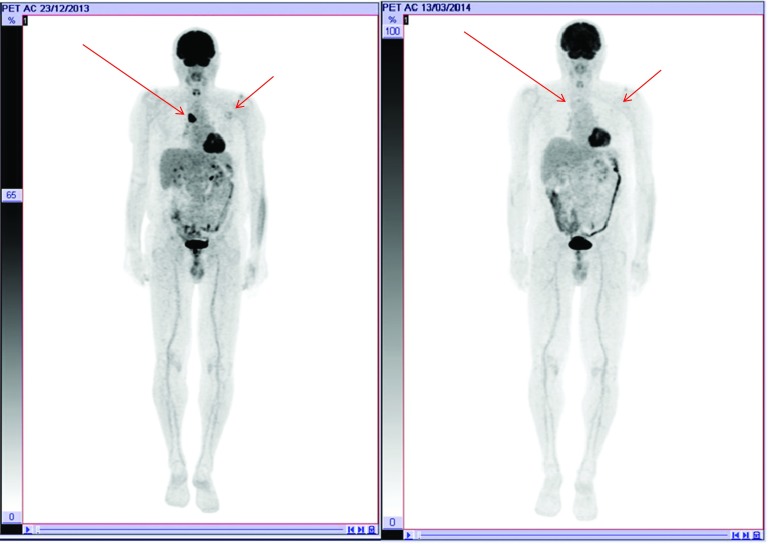
In March 2014, after the patient received overall only seven doses of vemurafenib, PET scan revealed a partial response (PR) (Figure 2). Considering the good response to the treatment with vemurafenib, we assumed a high efficacy of BRAF inhibitors.
Figure 2.
PET at follow-up 2014 indicating a partial response to vemurafenib treatment.
For this reason, we proposed to the patient a combination with dabrafenib and trametinib. In May 2014, the new treatment with dabrafenib 150 mg PO twice daily plus trametinib 2 mg PO daily was initiated. In July 2014, the treatment was suspended for a few days following a modest cutaneous allergic reaction (allergic reaction/immune system disorders G1 CTC AE 4.03) on the forearms. In December 2014, a new localized cutaneous reaction occurred (allergic reaction/immune system disorders G1 CTC AE 4.03). For this reason, a reduced dose of dabrafenib 100 mg PO twice daily and trametinib 1.5 mg daily of the combination treatment was indicated. In August 2014, PET scan showed a complete metabolic remission.
In April 2016, the treatment was interrupted for patient’s decision. Since then, there have been regular follow-up every 3 months with clinical evaluation and PET scans.
At the time of manuscript preparation in October 2017, the clinical condition of the patient remains good with performance status=0. To date, PET scans have showed a complete metabolic remission and blood test results, including LDH levels, are normal.
Conclusions
This report presents the case of a patient affected by melanoma, metastatic to the lymph nodes, which showed a long-term CR to dabrafenib plus trametinib despite treatment interruption.
According to a recent landmark analysis at the 5-years landmark point [14], 5-year OS in patients with BRAFV600-mutant metastatic melanoma treated with dabrafenib plus trametinib was 28% (PFS rate was 13%) with a response rate of 76%. CR was observed in 17% of patients. In detail, in patients with CR, PFS rate at 3 years was 67%, at 5 year was 40%; whereas, the median PFS was 39.6 months. Currently, we have no indications on the duration of treatment of patients with BRAFV600E-mutated metastatic malignant melanoma that shows CR to treatment with MEK/BRAF inhibitors [15]. Particularly, it is unknown whether patients who achieve CR can safely discontinue treatment [16]. Data from retrospective studies indicate that approximately 50% of patients achieving CR with dabrafenib plus trametinib relapse after treatment discontinuation [16,17]. Furthermore, the duration of treatment with MEK/BRAF inhibitors does not influence the rate of relapse following treatment cessation [16].
Higher percentages of relapses are observed in patients treated with MEK/BRAF inhibitors compared to patients treated with immunotherapy that discontinued the treatment following a CR [18]. This finding may indicate the importance of immune-mediated mechanisms of action.
In the absence of evidence-based clinical guidance on treatment duration, it is also important to consider individual preferences of patients with complete remission that integrates survival advantage as well as disease-associated and adverse-event-associated symptoms when making a decision on whether or not to halt the treatment with MEK/BRAF inhibitors. Our patient decided to discontinue the treatment following modest grade 1–2 cutaneous and mucosal side effects. Although combined use of BRAF and MEK inhibitors is well tolerated by many patients, it is not devoid of side effects. Several clinical trials reported that diarrhea, anorexia, nausea, and vomiting are common adverse events frequently associated with the use of a combination of BRAF and MEK inhibitors in daily clinical practice, thus requiring early and appropriate managements to avoid unnecessary dose reductions and transitory or definitive treatment discontinuations [19]. Therefore, there is a need to master the characteristic features, incidence, and relative risk (RR) of significant adverse events to take adequate prevention and intervention as early as possible [20].
In conclusion, we present the case of a patient with prolonged CR to treatment with dabrafenib plus trametinib despite treatment interruption. Our findings confirm similar long-term results of clinical trials indicating that that durable survival is achievable with dabrafenib plus trametinib in patients with BRAFV600-mutant metastatic melanoma [21]. However, case reports and case series may offer ‘real-life’ information on how to treat the selected population of long-term survivors with metastatic melanoma.
Acknowledgements
Medical writing was performed by Luca Giacomelli and Lilia Biscaglia on behalf of Content Ed Net.
Footnotes
Disclosure and potential conflicts of interest: The authors declare no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2018/01/dic.212515-COI.pdf
Funding declaration: Editorial assistance for this paper was supported by Novartis (Switzerland).
Correct attribution: Copyright © 2018 Brugnara S, Sicher M, Bonandini EM, Donner D, Chierichetti F, Barbareschi M, Girardelli CR, Caffo O. https://doi.org/10.7573/dic.212515. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 15 December 2017
References
- 1.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;35320:2135–47. doi: 10.1056/NEJMoa050092. http://doi.org/10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 2.Sekulic A, Haluska P, Jr, Miller AJ, Genebriera De Lamo J, Ejadi S, Pulido JS, Salomao DR, Thorland EC, Vile RG, Swanson DL, Pockaj BA, Laman SD, Pittelkow MR, Markovic SN Melanoma Study Group of Mayo Clinic Cancer Center. Malignant melanoma in the 21st century: the emerging molecular landscape. Mayo Clinic Proc. 2008;837:825–46. doi: 10.4065/83.7.825. http://doi.org/10.4065/83.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JM, Fisher DE. A new era: melanoma genetics and therapeutics. J Pathol. 2011;2232:241–50. doi: 10.1002/path.2804. http://doi.org/10.1002/path.2804. [DOI] [PubMed] [Google Scholar]
- 4.Huang T, Zhuge J, Zhang WW. Sensitive detection of BRAF V600E mutation by Amplification Refractory Mutation System ARMS-PCR. Biomark Res. 2013;1:3. doi: 10.1186/2050-7771-1-3. http://doi.org/10.1186/2050-7771-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N Engl J Med. 2011;3648:772–4. doi: 10.1056/NEJMcibr1013704. http://doi.org/10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 6.Wellbrock C, Hurlstone A. BRAF as therapeutic target in melanoma. Biochem Pharmacol. 2010;805:561–7. doi: 10.1016/j.bcp.2010.03.019. http://doi.org/10.1016/j.bcp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, Martín-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, Phase 3 randomized controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. http://doi.org/10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 8.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, Chodon T, Guo R, Johnson DB, Dahlman Kimberly B, Kelley MC, Kefford RF, Chmielowski B, Glaspy JA, Sosman JA, van Baren N, Long GV, Ribas A, Lo RS. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. http://doi.org/10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Allen EM, Wagle N, Sucker A, Treacy D, Johannessen C, Goetz EM, Place CS, Taylor-Weiner A, Whittaker S, Kryukov G, Hodis E, Rosenberg M, McKenna A, Cibulskis K, Farlow D, Zimmer L, Hillen U, Gutzmer R, Goldinger SM, Ugurel S, Gogas HJ, Egberts F, Berking C, Trefzer U, Loquai C, Weide B, Hassel JC, Gabriel SB, Carter SL, Getz G, Garraway LA, Schadendorf D. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. http://doi.org/10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spain L, Julve M, Larkin J. Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations. Expert Opin Pharmacother. 2016;177:1031–8. doi: 10.1517/14656566.2016.1168805. http://doi.org/10.1517/14656566.2016.1168805. [DOI] [PubMed] [Google Scholar]
- 11.Zia Y, Chen L, Daud A. Future of combination therapy with dabrafenib and trametinib in metastatic melanoma. Expert Opin Pharmacother. 2015;1614:2257–63. doi: 10.1517/14656566.2015.1085509. http://doi.org/10.1517/14656566.2015.1085509. [DOI] [PubMed] [Google Scholar]
- 12.Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA, Ribas A, Robert C, Schadendorf D, Garbe C. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer. 2017;83:247–57. doi: 10.1016/j.ejca.2017.06.028. http://doi.org/10.1016/j.ejca.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;2736:6199–206. doi: 10.1200/JCO.2009.23.4799. http://doi.org/10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long GV, Eroglu Z, Infante J, Patel S, Daud A, Patel S, Daud A, Johnson DB, Gonzalez R, Kefford R, Hamid O, Schuchter L, Cebon J, Sharfman W, McWilliams R, Sznol M, Redhu S, Gasal E, Mookerjee B, Weber J, Flaherty KT. Long-term outcomes in patients with BRAF V600–mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol. 2017:JCO2017741025. doi: 10.1200/JCO.2017.74.1025. http://doi.org/10.1200/JCO.2017.74.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, McQuade JL, Panka DJ, Hudgens CW, Amin-Mansour A, Mu XJ. Clinical, molecular, and immune analysis of dabrafenib-trametinib combination treatment for BRAF inhibitor-refractory metastatic melanoma: a Phase 2 clinical trial. JAMA Oncol. 2016;28:1056–64. doi: 10.1001/jamaoncol.2016.0509. http://doi.org/10.1001/jamaoncol.2016.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlino MS, Vanella V, Girgis C, Giannarelli D, Guminski A, Festino L, Kefford RF, Menzies AM, Long GV, Ascierto P. Cessation of targeted therapy after a complete response in BRAF-mutant advanced melanoma: a case series. Br J Cancer. 2016;11511:1280–4. doi: 10.1038/bjc.2016.321. http://doi.org/10.1038/bjc.2016.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolk H, Satzger I, Mohr P, Zimmer L, Weide B, Schäd S, Gutzmer R. Complete remission of metastatic melanoma upon BRAF inhibitor treatment – what happens after discontinuation? Melanoma Res. 2015;254:362–6. doi: 10.1097/CMR.0000000000000169. http://doi.org/10.1097/CMR.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;3721:30–9. doi: 10.1056/NEJMoa1412690. http://doi.org/10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 19.Cebollero A, Puértolas T, Pajares I, Calera L, Antón A. Comparative safety of BRAF and MEK inhibitors (vemurafenib, dabrafenib and trametinib) in first-line therapy for BRAF-mutated metastatic melanoma. Mol Clin Oncol. 2016;5(4):458–62. doi: 10.3892/mco.2016.978. http://doi.org/10.3892/mco.2016.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queirolo P, Picasso V, Spagnolo F. Combined BRAF and MEK inhibition for the treatment of BRAF-mutated metastatic melanoma. Cancer Treat Rev. 2015;41(6):519–26. doi: 10.1016/j.ctrv.2015.04.010. http://doi.org/10.1016/j.ctrv.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Chiarion-Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, Davies MA, Lane SR29, Legos JJ, Mookerjee B, Grob JJ. Dabrafenib plus trametinib compared with dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a Phase 3 study. Ann Oncol. 2017;28(7):1631–9. doi: 10.1093/annonc/mdx176. http://doi.org/10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]