Abstract
Background
The range of outcomes for young adults with Autism Spectrum Disorders (ASD) and the early childhood factors associated with this diversity have implications for clinicians and scientists.
Methods
This prospective study provided a unique opportunity to predict outcome 17 years later for a relatively large sample of children diagnosed with ASD at 2-years-old. Diagnostic and psychometric instruments were administered between 2 and 19 with data from 2, 3, and 19 included in this paper. Clinicians administered tests without knowledge of previous assessments whenever possible. Caregivers provided additional information through questionnaires.
Results
Significant intellectual disabilities at 19 were predicted by age 2 about 85% of the time from VIQ and NVIQ scores together, though prediction of young adult outcome for youths with average or higher intelligence was more complex. By 19, 9% of participants had largely overcome core difficulties associated with ASD and no longer retained a diagnosis. These youths with Very Positive Outcomes were more likely to have participated in treatment and had a greater reduction in repetitive behaviors between age 2 and 3 compared to other Cognitively Able youths (VIQ ≥70) with ASD. Very Positive Outcome youths did not differ phenotypically from Cognitively Able ASD individuals at 2 but both groups differed from Cognitively Less Able individuals (VIQ < 70).
Conclusion
Those most at risk for intellectual disabilities and ASD can be reliably identified at an early age to receive comprehensive treatment. Findings also suggest that some cognitively able children with ASD who participate in early intervention have very positive outcomes, although replication with randomized, larger samples is needed. In order to improve understanding of very positive outcomes in ASD, future research will need to identify how variations in child characteristics and environmental factors contribute to the nature and timing of growth across individuals and areas of development.
Keywords: autism, ASD, Very Positive Outcome, adult outcome, longitudinal
Introduction
While autism spectrum disorder (ASD) is generally considered a lifelong condition, recent attention has focused on diverse outcomes from severe disability to near normal functioning. Various investigations have reported 1%-16% of ASD individuals improve enough by adolescence or adulthood to no longer meet diagnostic thresholds (Billstedt, Gillberg, & Gillberg, 2005; Farley et al., 2009; McGovern & Sigman, 2005). However, the extent to which residual impairments persist and the validity of the original diagnosis have not been specifically addressed, with one exception. A landmark, retrospective study recently described children and adults with a history of ASD who no longer met diagnostic criteria and whose social and cognitive functioning across various measures was comparable to age-matched, typically-developing subjects (Fein et al., 2013). Yet prospective longitudinal data are necessary to shed light on: 1) the extent to which adult outcome can be identified in early childhood; 2) the proportion of children who eventually attain very positive outcomes; and 3) when attainment of typical- or near typical-functioning occurs across areas of development such as cognition, social competence, adaptive skills, and core symptoms.
Because intellectual disabilities in adults with autism are strongly associated with limited independence (Billstedt et al., 2005; Howlin, Goode, Hutton, & Rutter, 2004), studies of prognosis in ASD often begin with IQ as a measure of later outcome. It is encouraging that some follow-up and treatment studies have documented striking improvements between the preschool and school years in cognitive and language skills for a significant minority of children with ASD (Eaves & Ho, 2004; Sallows & Graupner, 2005; Sutera et al., 2007). Nevertheless, a higher IQ and more complex language in adulthood are not sufficient for independence or the remediation of marked social deficits in ASD (Farley et al., 2009; Howlin, Goode, Hutton, & Rutter, 2004; Fountain, Winter, & Bearman, 2005; Gillberg & Steffenburg, 1987; Mawhood, Howlin, & Rutter, 2000; Seltzer, Shattuck, Abbeduto, & Greenberg, 2004). Recent retrospective and short-term longitudinal studies suggest that children and adolescents with a history of ASD who have extremely positive outcomes had milder symptoms and received more treatment as young children (Fein et al., 2013; Pelicano, 2012); however, long-term prospective studies beginning in early childhood can provide unique information about the course of changes in intellectual and social functioning.
Methods
Participants
Participants were consecutive referrals of children under 37-months-old: 192 referred for possible autism and 21 with nonASD developmental delays, recruited from sources that referred to autism clinics. The autism referral group was composed of children from four North Carolina-based, state-funded autism centers (n=113) and an autism clinic in Chicago within a university hospital (n=79). Three-quarters of the 213 original participants received ASD diagnoses at age 2 (Anderson, Lord, Risi, DiLavore, Shulman, & Thurm, 2007).
Of the original 213 participants, five were lost to follow up after the initial assessment and the remainder was lost due to geographical relocation, unreachable status, or refusal to participate at later ages. By age 19 years, 142 youths from the original sample and their families continued to participate at least in part. At that time, 120 of the 142 families were revisited in person. Although African American families with less education were lost to the study at a higher rate than Caucasian families and families with more education, attrition was not related to diagnosis, gender, or IQ at the initial assessment.
The current study includes all 85 youths who were diagnosed with an ASD in early childhood and seen at age 19. The average ages at the first and last assessments were 2-years, 5-months (S.D.=0·43) and 19-years, 1-month (S.D.=1·08) respectively. Ethnic minorities, most of whom were African American, accounted for 24% of the largely male sample (92%), with a mix of children from rural and urban areas (North Carolina=49%; Chicago=51%). Nineteen percent of the sample had ever had a seizure. Over half had mothers who were married (67%) or had college degrees (62%). Excluded from the current analyses were participants with nonspectrum childhood diagnoses and profound cognitive delays (nonverbal IQ < 25).
Procedures
A battery of diagnostic and psychometric instruments was administered in person when the children were ages 2-, 3-, 5- (only North Carolina participants at age 5), 9- and 19-years, free of charge. The focus here is on data from age 2, 3, and 19. Clinicians administered the test batteries without knowledge of previous assessments whenever possible. Across the course of the study, logs of educational and intervention treatments, including medication usage and seizures were obtained through regular telephone interviews, mailed questionnaires, and diaries completed by caregivers. Informed consent was attained for all participating families. This research was approved by the appropriate IRBs.
At each assessment, with the exception of age 3, an overall best estimate consensus diagnosis of autism spectrum disorder, other nonspectrum disability or psychiatric disorder, or typical development was based on all available information, including psychometric and diagnostic and algorithm scores (ADI-R, ADOS, Vineland Adaptive Behavior Scales, various cognitive ability tests), behavioral measures of irritability, hyperactivity, and depression (Aberrant Behavior Checklist, Child Depression Rating Scale), videotapes of the direct observation of the child, and group consensus among the clinicians and principal investigator. At the age 19 assessment, a ‘typical’ diagnosis required overall global functioning in the normal range in terms of: 1) social adjustment; 2) restricted and repetitive behaviors; 3) independence (e.g., no extra support in the classroom; if not in school, then working); 4) psychometric measures; and 5) no comorbid conditions. The measures are described below.
Measures
Diagnostic Instruments
The Autism Diagnostic Instrument-Revised (ADI-R) is a comprehensive, standardized parent interview designed to distinguish children with ASD from non-ASD and other developmentally delayed populations (Lord, Rutter, & LeCouter, 1994). A toddler version of the ADI-R, which includes a number of additional items specific to the first two years of life, was administered when the children were 2 and 3 years-old. ADI-R algorithm scores are totaled for each of three domains: social behaviors, communication, and repetitive interests. In addition to the algorithm totals, current scores for the ‘Overactivity at home and elsewhere’ item on the Toddler ADI-R at age 3 (scores at age 2 were not reliable) were used as a measure of early childhood hyperactivity (coded as 0=none and 1 to 3=some).
The Autism Diagnostic Observation Schedule (ADOS; Lord, Risi, Lambrecht et al., 2000) and one of its predecessors, the Pre-Linguistic Autism Diagnostic Observation Schedule (PL-ADOS; DiLavore, Lord, & Rutter, 1995), acquire diagnostic information through direct observation of the child by a trained clinician. A revised algorithm calculates ADOS summary scores for the social and repetitive domains (Gotham, Risi, Pickles, & Lord, 2007; Gotham et al., 2008). For the purposes of this paper, the algorithm scores were slightly modified to include the same items across modules for comparability. Children in the current study were given the PL-ADOS at ages 2 and 3, which was scored using the algorithm for the Module 1 ADOS (for children without phrase speech).
Psychometric Instruments
Age 2 and 3 IQ scores were obtained from the Mullen Scales of Early Learning (MSEL; Mullen, 1985; Mullen, 1989). The most recent verbal IQ (VIQ) and nonverbal IQ (NVIQ) were taken from the assessment at age 19. Cognitive test selection followed a standard hierarchy designed for use when the youths could not achieve a basal score or achieved ceiling scores: Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999); Differential Abilities Scale (DAS; Elliot, 1990); Mullen Scales of Early Learning (Mullen, 1995). Ratio IQs were calculated when raw scores fell outside the ranges for deviation scores. For those who were cognitively able, academic achievement, including reading, sentence comprehension, and arithmetic, was assessed at age 19 with the Wide Range Achievement Test IV (WRAT IV; Wilkinson & Robertson, 2006).
Adaptive daily living skills were assessed using the Vineland Adaptive Behavior Scales II (Sparrow, Balla, & Cicchetti, 1984; Sparrow, Cicchetti, & Balla, 2005), a standardized, semi-structured, parent interview which yields domain scores in the areas of communication, daily living skills, and social skills, as well as an adaptive behavior composite score.
Mood and Behavior Instruments
The Aberrant Behavior Checklist (ABC; Aman, 1994) is a 58-item checklist designed for use with children and adults with developmental delays. Beginning when the youths were 13 years-old, caregivers were asked every four months to rate their children's maladaptive behaviors in the past 4 weeks on a 4-point scale, ranging from not at all a problem (0) to the problem is severe in degree (3). The current study used totals from the Irritability (15 items) and Hyperactivity (16 items) subscales. For cases missing up to two items on a subscale, the individual items were weighted accordingly to allow for comparability in scores across subjects (i.e., the number of total items divided by the number of non-missing items). In addition to the standard ABC items, a question about current medications was also administered.
The Childhood Depression Rating Scale (CDRS-R; Poznanski & Mokros, 1996) is a brief rating scale of depressed mood based on a semi-structured interview with the caregiver. It assists clinicians in rating 17 symptom areas, including Difficulty Having Fun, Sleep Disturbance, Excessive Fatigue, Irritability, Morbid Ideas, and Suicidal Ideas. Although the scale was originally intended for children, we selected this measure over others based on results from our pilot testing which found the questions to be meaningful to the caregivers of the young adults across ability levels (Gotham, Bishop, Brunwasser, & Lord, submitted). Questions for children were modified slightly (e.g., references to ‘the child’ replaced with ‘your teenager’) to be appropriate for the age 19 assessment. Raw scores were used as symptom counts to supplement clinical diagnoses.
Treatment
Parents completed diaries and then were interviewed about all educational and specific treatments received by their child. Two raters established reliability and coded the data. Findings from previous analyses with this sample indicate that very few children had intensive intervention prior to age 3 (Anderson, Oti, Lord, & Welch, 2009) and most began treatment around age 2 ½-years, soon after the diagnosis. (See online Table S6 for the types of treatment received before age 3). The distribution of treatment hours from 2 to 3 was skewed, with a significant minority of children receiving very little or no treatment and another group receiving more extensive hours (20 hours reflected weekly therapy from about the time of diagnosis to age 3; many of these families gradually added more treatment in the ensuing months). Hence, for the purposes of this paper, we found it most useful to collapse total hours of individual treatment through age 3 (Cognitively Less Able ASD Median=67, S.D.=137; Cognitively More Able Median=34, S.D.=138) into two categories: 0) ‘Minimal to None’ i.e., less than 20 hours; and 1) ‘Some’ i.e., 20 hours or more. Although this metric cannot be used as a measure of the quality of treatment, it does provide an indication of the family's participation in intervention.
Analyses
The sample was first divided into two IQ groups based on whether cognitive impairment was present at age 19: those with verbal IQ less than 70 (Cognitively Less Able: VIQ < 70; n=53) and verbal IQ equal to or greater than 70 (Cognitively Able: VIQ ≥ 70; n=32). In subsequent exploratory analyses, the VIQ ≥ 70 group was divided into two subgroups according to diagnosis at 19. The Cognitively Able ASD group (VIQ ≥ 70-ASD; n=24) retained an ASD diagnosis; the Very Positive Outcome (VPO) group (n=8) was considered ‘typical.’
We hypothesized that the VIQ < 70 group would show: 1) greater impairment at age 2 (i.e, more ASD symptoms; greater delays in cognitive and adaptive abilities) or age 3 (hyperactivity, for which reliable scores were only available at 3); and 2) less positive change in these characteristics between age 2 and 3. We left as exploratory the question of whether early childhood factors, including treatment, could distinguish among VIQ ≥ 70 youths with very positive vs. less optimal outcomes.
Univariate group differences were assessed in terms of effect size and level of significance. For differences in group means, we ran ANOVAs in SPSS 20.0 to generate Eta squared (η2), or the proportion of variance in the predictor variable accounted for by the categorical groupings. We used Cohen's rules of thumb (Cohen, 1988) to assess the magnitude of an effect (0.01=small; 0.06=medium; 0.14=large). For group differences in proportions, the Phi coefficient served as the measure of effect size (+/-0.00 to 0.19=negligible or weak; +/-0.20 to 0.39=moderate; +/-0.40 to 0.59=relatively strong; +/-0.60 and above=very strong)(Rea & Parker, 1992). For cells under five cases, Fisher's exact p value was used as the significance criterion. Adjustments for multiple comparisons were carried out via a false discovery rate procedure (Benjamini & Hochberg, 1995) with a significance criterion of .05.
Binary and multicategorical logistic regression models predicted categorical groupings at 19-years from early childhood characteristics. Odds ratios were used to assess the magnitude of an effect (1.5=small; 3.5=medium; 9.0=large)(Cohen, 1988). Although verbal IQ group at 19, and diagnosis within the cognitively able subgroups, were used as the primary measures of categorical outcome, we also examined other age 19 characteristics associated with the respective groupings.
There were few missing data points. However, three participants were missing Vineland standard scores at one time point—two at age 2 and one at age 3. A fourth individual was missing an age 2 ADOS social algorithm score. (Note that the eight Very Positive Outcome subjects were not among the missing cases). For these cases, we fit individual regression lines through the existing Vineland or ADOS data points at ages 2 or 3, and 5, 9, and 19 to provide an estimate for the missing one. We ran analyses with and without these cases; results were the same regardless.
Results
Cognivtively Able vs. Cognitively Less Able Youths
Current functioning
Table 1 highlights current functioning for the VIQ < 70 and VIQ ≥ 70 groups. Differences in cognitive abilities (the criteria for group selection), adaptive skills, and ASD symptoms at 19 were significant and pronounced in the expected direction. Also, the adaptive skills composite score was notably higher than verbal and performance IQ for those with intellectual disabilities while this was not the case for those in the VIQ ≥ 70 group. Demographic differences were not significant (see Table S1).
Table 1. Indicators of Current Functioning for Cognitively Less and More Able Young Adults.
| Sample Characteristics | VIQ < 70 N=53 |
VIQ ≥ 70 N=32 |
95% Confidence Interval | Effect Size | (Unadjustd) P value1 |
|---|---|---|---|---|---|
| Current (Age 19) | |||||
| M (S.D.) | M (S.D.) | (VIQ < 70/VIQ > 70) | Eta2 | ||
| Verbal IQ | 22 (15.33) | 105 (7.07) | (18, 26/ 98, 111) | .86 | < .001* |
| Nonverbal IQ | 34 (22.51) | 105 (16.72) | (28, 41/ 99, 111) | .74 | < .001* |
| Adaptive Skills | 49 (13.67) | 83 (16.00) | (45, 53/ 78, 89) | .57 | < .001* |
| ASD Core Features | |||||
| Social Deficits Total (ADOS) | 15 (3.61) | 6 (4.23) | (15, 16/ 5, 8) | .58 | < .001* |
| Social Deficits Total (ADI-R) | 18 (6.31) | 7 (5.68) | (16, 20/ 5, 9) | .45 | < .001* |
| RRBs Total (ADOS) | 4.1 (2.43) | 1.3 (1.77) | (3.4, 4.8/ .71, 2.0) | .27 | < .001* |
| RRBs Total (ADI-R) | 4.0 (2.09) | 2.7 (2.53) | (3.4, 4.6/ 1.8, 3.6) | .08 | .01* |
| Behavior Problems (Aberrant Behr. Checklist) | |||||
| Irritability | 7.1 (8.56) | 4.3 (6.15) | (4.7, 9.4/ 2.3, 7.2) | .03 | .13 |
| Hyperactivity | 10.0 (9.72) | 4.9 (7.10) | (7.4, 12.7/2.6, 7.8) | .07 | .01* |
| % Yes | % Yes | Phi | |||
| % Psychotropic Medication | 68 | 28 | -.39 | .001* | |
| Early Childhood | |||||
| M (S.D.) | M (S.D.) | Eta2 | |||
| Verbal IQ | |||||
| Age 2 | 26 (12.58) | 50 (24.91) | (23, 30/ 41, 59) | .30 | < .001* |
| Change 2 to 3 | 5 (11.11) | 23 (18.08) | (2, 8/ 16, 29) | .27 | < .001* |
| Nonverbal IQ | |||||
| Age 2 | 61 (17.23) | 83 (15.49) | (56, 66/ 77, 88) | .28 | < .001* |
| Change 2 to 3 | -4.02 (10.09) | 2.00 (13.84) | (-7, -1/ -3, 7)) | .06 | .02 |
| Adaptive Skills Composite (Vineland) | |||||
| Age 2 | 60(6.31) | 65 (8.12) | (58, 61/ 63, 68) | .09 | .01* |
| Change 2 to 3 | -5 (4.59) | 3 (12.33) | (-6, -4/ -2, 7) | .10 | .003* |
| Social Deficits (ADOS)3 | |||||
| Age 2 | 17 (3.67) | 13 (4.66) | (16, 18/ 12, 15) | .18 | < .001* |
| Change 2 to 3 | -1 (3.65) | -4 (4.92) | (-2, 0/ -6, -2) | .24 | < .001* |
| Repetitive Behaviors (ADOS) | |||||
| Age 2 | 4.6 (2.37) | 2.7 (2.10) | (3.9, 5.2/ 1.9, 3.5) | .14 | < .001* |
| Change 2 to 3 | -0.01 (2.72) | .21 (2.54) | (-.8, .7/ -7, 1.1) | < .01 | .71 |
| % Yes | % Yes | Phi | |||
| Mother's w/College Degree | 66 | 56 | -.10 | .25 | |
| Some Treatment from 2-3 | 93 | 66 | -.34 | .002* | |
| Hyperactivity | |||||
| Age 2 | 38 | 41 | .03 | .82 | |
| Age 3 | 45 | 38 | -.08 | .32 |
Asterisks indicate that alpha remained significant at p < .05 after adjustment for multiple comparisons.
Age 2-3 predictors
Results in Table 1 support the hypothesis that lower cognitive and adaptive abilities, along with more ASD-related symptoms at 2, predict membership in the VIQ < 70 group 17 years later. (Age 3 means are provided in Table S2). As expected, VIQ ≥ 70 probands showed greater improvements in verbal and performance IQ, adaptive skills, and social skills than VIQ < 70 youths from age 2 to 3 (Figure 1; Table 1). Average verbal IQ scores for the VIQ ≥ 70 youths rose nearly 25 points to within the low-normal range by age 3. Despite improvements in social deficits and adaptive skills for the VIQ ≥ 70 group, age 3 scores still indicated impairment consistent with an ASD diagnosis.
Figure 1. Change from Age 2 to 3 between Verbal IQ Groups.
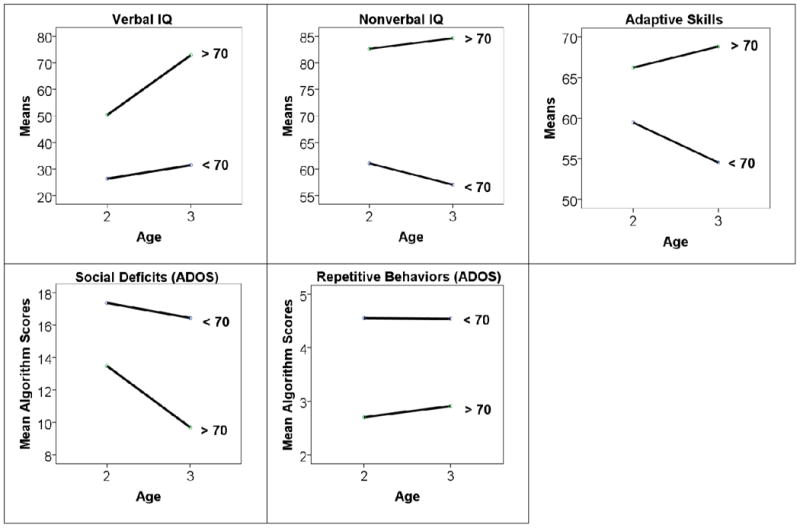
Logistic regressions were used to determine which predictors made a unique contribution to outcome at 19, first at age 2 and then at age 3 (Table S3). Childhood IQ emerged as the best predictors of age 19 IQ group at both time points. The models were not significantly improved with the addition of the other covariates in Table 1. At age 2, nonverbal and verbal IQ together provided the greatest predictive accuracy (χ2=37.73, df=3, p< .001). The rate of concordance between predicted and actual age 19 categorical groupings was very high for VIQ < 70 youths (85%) but lower for VIQ ≥ 70 youths (66%). By age 3, verbal IQ alone accurately predicted outcome at 19 for an even larger majority, with a concordance rate of 91% for VIQ < 70 youths and 82% for VIQ ≥ 70 individuals (χ2=47.01; df=2, p< .001). For every standard deviation increase in verbal IQ at age 3 (26 points), the odds of being in the VIQ ≥ 70 group at 19 increased 21-fold (OR=21.21; p< .001).
Cognitively Able ASD vs. Very Positive Outcome Youths
Current functioning
Of the 32 VIQ ≥ 70 youths, eight no longer retained a clinical diagnosis of ASD at age 19 (i.e., VPO youths). Table 2 compares the ASD and VPO youths within VIQ ≥ 70 at age 19 across various indicators of functioning. Not surprisingly, because knowledge about these variables likely contributed to the clinical diagnosis, greater adaptive behavior skills and fewer social deficits most clearly distinguished the Very Positive from the VIQ ≥ 70-ASD group. Adaptive skills for VPO youths were well within the normal range of functioning, more than a standard deviation higher than the mean score for the VIQ ≥ 70-ASD group which fell into the low-normal range. On the individual level, clear social strengths for all eight VPO youths were noted by clinicians and parents. The effect sizes for adaptive skills (Vineland) and social deficits (ADI-R) were very large, with group outcome accounting for 40% and 51% of the variance respectively. Notably, both groups had mean IQ (verbal and nonverbal) and academic achievement scores in the average to above average range and differences were not significant.
Table 2. Indicators of Current Functioning for Youths with IQ ≥70.
| Sample Characteristics | VIQ ≥ 70-ASD N=24 |
Very Positive N=8 |
95% Confidence Interval | Effect Size | (Unadjusted) P value1 |
|---|---|---|---|---|---|
| M (S.D.) | M (S.D.) | (VIQ > 70-ASD/VP) | Eta2 | ||
| ASD Core Features | |||||
| Social Deficits Total (ADOS) | 7.3 (4.07) | 3.0 (2.98) | (5.7, 9.0/ .51, 5.5) | .20 | .01 |
| Social Deficits Total (ADI-R) | 8.5 (5.33) | 1.1 (1.89) | (6.2, 10.7/ -.45, 2.7) | .51 | < .001 |
| RRB Total (ADOS) | 1.7 (1.90) | 0.25 (0.46) | (.91, 2.5/ -.14, .64) | .13 | .04 |
| RRB Total (ADI-R) | 3.4 (2.47) | 0.50 (1.07) | (2.4, 4.5/ -.4, 1.3) | .19 | < .001 |
| Adaptive Behavior Composite (Vineland) | 78 (12.23) (range 52-104) |
101 (13.94) (range 79=119) |
(73, 83/ 89, 112) | .40 | < .001 |
| Verbal IQ | 103 (17.69) (range 72-131) |
111(14.40) (range 84-132) |
(95, 110/ 99, 123) | .04 | .26 |
| Academic Achievement (WRAT- IV) | |||||
| Word Reading | 98 (13.99)1 | 106 (11.91) | (92, 104/ 96, 116) | .07 | .18 |
| Spelling | 105 (18.89) | 114 (16.29) | (97, 114/ 100, 127) | .04 | .29 |
| Arithmetic | 102 (20.96) | 114 (19.73) | (92, 111/ 98, 130) | .07 | .16 |
| Behavior/Mood Problems | |||||
| Irritability2 | 6.2 (7.26) | 0.25 (0.71) | (3.1, 9.3/ -.34, .84) | .15 | .03 |
| Hyperactivity2 | 6.8 (7.59) | 0.38 (0.52) | (3.6, 10.0/ -.06, .81) | .16 | .02 |
| Depressive Symptoms3 | 26 (8.95) | 16 (2.03) | (23, 30/ 14, 18) | .25 | .004 |
| Phi | |||||
| % Living Away from Family | 13 | 63 | .50 | .01 | |
| % in College | 71 | 88 | .35 | .33 | |
| % Employed | 25 | 63 | .34 | .07 | |
| % Psychotropic Medication | 38 | 0 | .19 | .19 |
Note that two youths are missing scores.
Scores are from the Aberrant Behavior Checklist.
Scores are from the Child Depression Rating Scale.
The effect size for depressive symptoms was substantial. Twenty-nine percent (n=7) of the VIQ ≥ 70-ASD youths had scores suggesting possible depression while none of the VPO youths had elevated scores. Of the seven VIQ ≥ 70-ASD youths with elevated depressive symptoms, two had irritability and hyperactivity scores that would meet cutoffs commonly used for inclusion into clinical trial studies (Aman et al., 2010; Woodard, Groden, Goodwin, & Bodfish, 2007). Those with Very Positive Outcome also had significantly fewer problems with repetitive behaviors, irritability, and hyperactivity by comparison (none had clinically elevated scores) and were more than four times as likely to be living independently from their families. There was a strong trend toward higher rates of employment among VPO youths though a majority of young people in both groups were also attending college. More than a third of the VIQ ≥ 70-ASD youths were taking psychotropic medications compared to none with a Very Positive Outcome. The groups did not differ with respect to demographic characteristics (i.e., gender, race, age, mothers' education, marital status, and site).
Age 2-3 predictors
Exploratory hypotheses addressed a potential relationship between early childhood characteristics (including those used to define groups at age 19) and group outcome at 19 among the 32 VIQ ≥ 70 youths. As shown in Table 3, the VPO youths were no less impaired at 2 than those in the VIQ ≥ 70-ASD group with respect to repetitive behaviors, social delays, or adaptive skills (for additional covariates, see Table S4). In contrast, by age 3 several group differences were observed (Table 3; also see Table S5), all with relatively large effect sizes. The VPO group showed a greater reduction in repetitive behavior symptoms from 2 to 3 than the VIQ ≥ 70-ASD. By age 3, fewer repetitive behaviors were observed among participants in the VPO group. Moreover, all eight VPO youths had received at least some individual treatment by age 3 compared to little more than half of the VIQ ≥ 70-ASD group. (Of those who received some treatment between age 2 and 3, the median number of total treatment hours was similar between the two groups--i.e., Medianvpo=59, S.D.=111.54; M edianVIQ ≥ 70-asd=67, S.D.=165.92; see Table S6 for the breakdown of the specific types of treatment received). Additionally, while 50% of parents reported the presence of some hyperactivity in their VIQ ≥ 70-ASD children at 3, none of the VPO parents had done so.
Table 3. Group Differences in Early Childhood for Cognitively More Able Youths.
| Measure | VIQ ≥ 70-ASD N=24 |
Very Positive N=8 |
95% Confidence Interval | Effect Size | (Unadjusted) P value1 | |
|---|---|---|---|---|---|---|
|
|
||||||
| M (S.D.) | M (S.D.) | (VIQ > 70-ASD/VP) | Eta2 | |||
| RRB Total (ADOS) | ||||||
| Age 2 | 2.5 (1.72) | 3.3 (3.02) | (1.8, 3.2/ .80, 5.8) | .03 | .34 | |
| Change 2 to 3 | 0.83 (2.19) | -1.7 (2.73) | (-.10, 1.8/ -3.9, .62) | .19 | .01* | |
| Social Deficits Total (ADOS) | ||||||
| Age 2 | 14 (4.94) | 12 (3.67) | (12, 16/ 9, 15) | .03 | .37 | |
| Change 2 to 3 | -3.6 (5.22) | -4.4 (4.16) | (-5.8, -1.4/ -8.0, -.95) | .01 | .68 | |
| Adaptive Skills Vineland) | ||||||
| Age 2 | 67 (9.77) | 65 (6.39) | (63, 71/ 59, 70) | .01 | .54 | |
| Change 2 to 3 | 2.2 (13.29) | 3.9 (9.54) | (-3.4, 7.8/ -4.1, 11.8) | < .01 | .74 | |
| % Yes | % Yes | Phi | ||||
| Mother's w/College Degree2 | 54 | 62 | .07 | .50 | ||
| Some Treatment | ||||||
| From 2 to 3 | 54 | 100 | .42 | .02* | ||
| Hyperactivity (ADI-R item) | ||||||
| Age 2 | 45 | 25 | -.18 | .42 | ||
| Age 3 | 50 | 0 | -.45 | .01* | ||
Asterisks indicate that alpha remained significant at p < .05 after adjustment for multiple comparisons.
Measured at baseline.
In the multivariate model predicting all three categorical groupings at 19 (Table 4), age 3 verbal IQ was the strongest predictor of being VIQ < 70 vs. VPO but did not distinguish between the VPO and VIQ ≥ 70-ASD groups. The effect of change in repetitive behaviors from 2 to 3 remained substantial. With each standard deviation increase in RRB change, the odds of being VIQ ≥ 70-ASD vs. VPO increased 13-fold controlling for baseline age and repetitive behaviors at 2. Differences in hyperactivity and hours of treatment in early childhood were reduced to nonsignificance. Overall model fit was very good with a correct classification rate of 84%. Nevertheless, Very Positive Outcome was predicted with the least accuracy (38%).
Table 4. Predicting 3 Outcome Groups at Age 19 from Early Childhood Characteristics.
| Predictors by Group | B (S.E.) | 95% Confidence Interval | Odds Ratio 1 S.D.1 | % Correct Classification | Fit Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|
| (Lower, Upper) | VIQ < 70 | VIQ ≥ 70-ASD | VP | Overall | Chi Sq | df | |||
| VIQ < 70 | |||||||||
| Age | .10 (.11) | (-.12, .32) | 1.65 | 94 | 75 | 38 | 84 | 77.34*** | 8 |
| Age 2 RRB total | .38 (.40) | (-.41, 1.16) | 2.50 | ||||||
| RRB Change from 2-3 | .55 (.43) | (-.29, 1.40) | 4.32 | ||||||
| Age 3 Verbal IQ | -.13 (.04)*** | (-.20, -.06) | .03 | ||||||
| constant | 7.90 | ||||||||
| VIQ > 70-ASD | |||||||||
| Age | .18 (.11) | (-.03, .39) | 2.53 | ||||||
| Age 2 RRB total | .55 (.40) | (-.23, 1.32) | 3.78 | ||||||
| RRB Change from 2-3 | .98 (.43)* | (.14, 1.82) | 13.31 | ||||||
| Age 3 Verbal IQ | -.01 (.03) | (-.07, .05) | .76 | ||||||
| constant | -.08 | ||||||||
Note: Very Positive Outcome Group is the reference category (n=8).
Odds ratio for 1 standard deviation change in the predictor variable.
The standard deviation is 26 points.
p < .05
p < .01
p < .001
Discussion
To our knowledge, this is the first prospective study of ASD to examine adult outcome in a relatively large inception cohort of toddlers. Intellectual disability at 19 was accurately predicted by age 2 about 85% of the time from IQ scores alone. Cognitively Less Able youths were characterized as much by early cognitive delays as by core ASD symptoms. The finding that these individuals can be identified so young has clinical implications, particularly in providing parents and professionals with a head start toward obtaining appropriate interventions. Despite significant cognitive impairment, adaptive skills were a relative strength for this group, beyond what their IQ scores would suggest--an encouraging finding which replicates several more recent studies with large ASD samples (e.g., Kanne et al., 2011; Perry et al., 2009). How these findings are related to early intervention, which a majority of these children received from age 2 on, is a question for further, more controlled studies.
The dramatic improvements in IQ, and to a lesser extent, RRBs, between ages 2 and 3 among participants without intellectual disabilities, replicates earlier longitudinal research (Eaves & Ho, 2004; Sallows & Graupner, 2005) and brings to mind the possibility of greater initial neuroplasticity and receptivity to environmental stimuli, potentially accelerating cognitive growth and behavioral improvements over time (Mundy & Neale, 2001). In the current study, Cognitively Able youths who eventually achieved Very Positive Outcome were more likely to have participated in a minimum of early treatment (in univariate analyses) defined as at least 20 hours (once per week parent-training for six months) as well as a greater reduction in repetitive behaviors between age 2 and 3 compared to Cognitively Able youths with less favorable outcomes at 19. Yet at 2, Very Positive Outcome youths did not differ phenotypically from the Cognitively Able ASD group. These findings, while speculative given the small sample size, invite professionals and caregivers to consider the importance of early family participation in intervention for intellectually able children with ASD despite their milder degree of impairment. Because this was not a clinical trial, we cannot discriminate between pre- and post-treatment differences between families who sought out and stayed with early intervention vs. those who did not, for many different possible reasons. Such long-term associations with what appears to be an environmental factor in early years (e.g., participating in intervention) are rare, and offer an impetus for further research and replication.
Although group differences in repetitive behaviors and the proportion receiving treatment were observed by age 3, prediction of adult outcome among Cognitively Able youths was less straightforward than for those with limited intellectual abilities. There are several possible explanations. First, as in typical development, higher intellectual abilities create the potential for a range of accomplishments but does not guarantee positive outcome. Our finding that IQ in particular is necessary but not sufficient for very positive outcome concurs with those of other studies (Howlin et al., 2004). Second, our statistical power and, therefore, predictive ability was limited by the relatively small number of youths with very positive outcomes. Although studies of recent cohorts report more favorable outcomes for young adults with ASD (Farley et al., 2009), the reality is that those with very positive outcomes still comprise a significant minority.
Third, the timing of progress seems to vary across aspects of development, making prediction from early childhood complex. Despite the enormous cognitive as well as behavioral gains that our intellectually more able youths showed as toddlers, the attainment of typical or near typical social functioning (i.e., overcoming core social deficits) occurred much later. In an earlier longitudinal analysis of the current sample of diagnostic change to age 9 (Lord, Risi, DiLavore, Shulman, Thurm, & Pickles, 2006) only two of our Very Positive Outcome youths presented with no clinically significant social delays on any parent report (Vineland Adaptive Behavior Scale, ADI-R) or direct observation (ADOS) measures at the 9-year-old assessment, and one of these had other significant behavior problems. The other six lost their ASD diagnoses during adolescence, a finding that highlights the necessity of following changes into adulthood.
That, in a prospective study begun 20 years ago, we could identify a group of individuals with a childhood diagnosis of ASD who are now doing quite well in young adulthood is exciting and builds on findings from other recent studies (Fein et al., 2013). At 19, 9% of our participants had largely overcome core difficulties associated with ASD and no longer had a clinical diagnosis. They had achieved a high level of functioning across various developmental areas and were fully participating in the social world. This finding differs from other recent adult outcome studies which, despite relatively good social outcomes for a substantial minority, found that few (1 of 37) or none (0 of 60) were free of core ASD symptoms or other mental health difficulties in adulthood (Farley et al., 2009; Howlin et al., 2013 respectively). This may be due in part to the current study's larger overall sample allowing for greater variability in outcomes as well as an earlier age of first diagnosis. Identification at age two may have both provided opportunities for support that affected outcomes in some of the children with ASD without intellectual disabilities, and also allowed identification of children who might have been missed in studies sampling from clinic populations recruited at older ages. Though all but two of the eight young men in the Very Positive Outcome group received independent ASD diagnoses at age 9 (and one of these received other diagnoses), a number of these families were no longer actively receiving services specific to autism and so might not have been included in other samples.
An additional 28% of our sample (the Cognitively Able ASD group) retained features of ASD but were doing very well in several areas, particularly cognitive and academic functioning. Their adaptive skills averaged in the low-normal range—lower than those with very positive outcomes—and their social deficits remained consistent with an ASD diagnosis. As found by other investigators (Gillespie-Lynch et al., 2012; Tonge & Einfeld, 2003; White, Ollendick, & Bray, 2011), symptoms of depression and anxiety were common among these youths. However, this group had also improved immensely since childhood. Many were quite independent within their social contexts. We are hopeful that growth will continue into the adult years. We plan to address this issue as well as the stability of very positive outcomes in future research.
One of the inherent limitations of longitudinal research is that cohorts of consecutive referrals for ASD 20 years ago are likely not representative of similar cohorts identified today. We also do not know if the early treatment for the Very Positive Outcome adults was a possible cause or mainly a marker of other factors (e.g., parental acceptance of the diagnosis, accessibility of resources), nor do we have information about the fidelity with which the interventions were administered. Nonetheless, professionals and families wondering whether initiating early treatment is appropriate when ASD symptoms are mild, and government and health officials debating if resources are needed for all children with ASD, may want to consider these findings. Further research is needed, however, to better understand the ways in which variations in neuroplasticity, treatment interventions, and other environmental factors contribute to the nature and timing of growth across individuals and areas of development many years later.
Supplementary Material
Appendix S1: Additional methods and measures
Appendix S2: Additional results
Table S1: Demographics by IQ group
Table S2: Age 3 means by IQ Group
Table S3: Predicting outcome group at age 19 from early childhood characteristics
Table S4: Age 2 means for additional covariates among cognitively able youths
Table S5: Other age 3 means among cognitively able youths
Table S6: Type of individual treatment from age 2 to 3-years
Key Points.
To our knowledge, this is the first prospective study of ASD to examine adult outcome in a relatively large inception cohort of toddlers.
Poorer outcome at 19 in terms of cognitive skills was accurately predicted by age 2 about 85% of the time from IQ scores alone.
By 19, 9% of the youths had largely overcome core difficulties associated with ASD and no longer had a clinical diagnosis. An additional 28% retained features of ASD but, nonetheless, had fewer social impairments than previously and were performing at age level with respect to cognitive and academic functioning.
Prediction of adult outcome among youths without intellectual disabilities was less straightforward than for those with limited intellectual abilities. At 2, youths with Very Positive Outcomes did not differ phenotypically from the Cognitively Able ASD group.
The findings that several characteristics by age 3 discriminated between those with Very Positive Outcomes and cognitively able youths with ASD (decreases in repetitive behaviors from 2 to 3, less hyperactivity, and a greater proportion participating in intervention before 3) were, nonetheless, intriguing.
Further research is needed to better understand the ways in which variations in child characteristics and environmental factors contribute to the nature and timing of growth across individuals and areas of development later in life.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH081873), the National Institute of Child Health and Human Development (U 19 HD 035482), and Autism Speaks (dated January 11, 2008) to Catherine Lord. The funding sources played no role in the writing of the manuscript or the decision to submit it for publication, including study design, recruitment of the sample, or the collection, analysis and interpretation of the data. The authors were not paid by a pharmaceutical company to write this article. The authors had full access to all of the data in the study as well as the final responsibility for the decision to submit for publication.
The author, D.A receives honoraria of less than $5000 per year from Western Psychological Services for the editing and approval of back-translations of the ADOS-2 from languages other than English. C.L receives royalties from two of the measures that were used in the current study (ADI-R, ADOS-2). J.L.W has declared she has no potential or competing conflicts of interest.
The authors would like to thank all of the participating families in this study. Special thanks to Pamela DiLavore, Audrey Thurm, Cory Shulman, and Susan Risi for their critical roles as project managers and clinicians. We thank Kathy Welch for statistical consultation and Shanping Qiu for technical assistance with the data. In addition, we thank all of the study participants who made this research possible.
Footnotes
Conflicts of interest statement: See acknowledgements for disclosures.
Supporting Information: Additional Supporting Information is provided along with the online version of this article.
Publisher's Disclaimer: Please note: Wiley-Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors (although this material was peer reviewed by JCPP referees and Editors along with the main article). Any queries (other than missing material) should be directed to the corresponding author for the article
References
- Aman M. Instruments for assessing treatment effects in developmentally disabled populations. Assessment in Rehabilitation and Exceptionality. 1994;1:1–20. [Google Scholar]
- Aman MG, Kasper W, Manos G, Mathew S, Marcus R, Owen R, Mankoski R. Line-item analysis of the Aberrant Behavior Checklist: Results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. Journal of Child and Adolescent Psychopharmacology. 2010;20:415–422. doi: 10.1089/cap.2009.0120. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Welch K, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75:594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson D, Oti R, Lord C, Welch K. Patterns of growth in adaptive social abilities among children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2009;37:1019–1034. doi: 10.1007/s10802-009-9326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Billstedt E, Gillberg C, Gillberg C. Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. Journal of Autism and Developmental Disorders. 2005;35(3):351–360. doi: 10.1007/s10803-005-3302-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- DiLavore P, Lord C, Rutter M. The Pre-Linguistic Autism Diagnostic Observation Schedule (PL-ADOS) Journal of Autism and Developmental Disorders. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Abilities Scale (DAS) San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Eaves L, Ho H. Young adult outcome of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:739–747. doi: 10.1007/s10803-007-0441-x. [DOI] [PubMed] [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, Jenson WR, Miller J, Gardner M, Coon H. Twenty-year outcome for individuals with autism and average or near average cognitive abilities. Autism Research. 2009;2:109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Fein D, Barton M, Eigsti I, Kelley E, Naigles L, Schultz R, Stevens M, Helt M, Orinstein A, Rosenthal M, Troyb E, Tyson K. Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry. 2013;54(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain C, Fountain, Winter A, Bearman P. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129(5):1112–1120. doi: 10.1542/peds.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, Rutter M. Social outcomes in mid- to later- adulthood among individuals diagnosed with Autism and average nonverbal IQ as children. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(6):572–581. doi: 10.1016/j.jaac.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Steffenburg S. Outcome and prognostic factors in infantile autism and similar conditions: A population-based study of 46 cases followed through puberty. Journal of Autism and Developmental Disorders. 1987;17:273–287. doi: 10.1007/BF01495061. [DOI] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Sepeta L, Wang Y, Marshall S, Gomez L, Sigman M, Hutman T. Early Childhood Predictors of the Social Competence of Adults with Autism. Journal of Autism and Developmental Disorders. 2012;42:161–174. doi: 10.1007/s10803-011-1222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Bishop S, Brunwasser S, Lord C. Psychosocial correlates of depressive symptoms in adolescents and adults with Autism Spectrum Disorders. Research in Autism Spectrum Disorders submitted. [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The autism diagnostic observation schedule: Revised algorithms for improved diagnostic reliability. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, Hepburn S, Mcmahon W, Rodier P, Hyman Susan, Sigman M, Rogers S, Landa R, Spence M, Osann K, Flodman P, Volkmar F, Hollander E, Buxbaum J, Pickles A, Lord C. A replication of the autism diagnostic observation schedule (ADOS) revised algorithms. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne S, Gerber A, Quirmbach L, Sparrow S, Cicchetti D, Saulnier C. The role of adaptive behavior in Autism Spectrum Disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders. 2011;41:1007–1018. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, Rutter M. The ADOS-G (Autism Diagnostic Observation Schedule—Generic): A standard measure of social and communication deficits associated with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mawhood L, Howlin P, Rutter M. Autism and developmental receptive language disorder—A comparative follow-up in early adult life. I: Cognitive and language outcomes. Journal of Child Psychology and Psychiatry. 2000;41:547–559. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- McGovern CW, Sigman M. Continuity and change from early childhood to adolescence in autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:401–408. doi: 10.1111/j.1469-7610.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Manual for the Infant Mullen Scales of Early Learning. Cranston, Rhode Island: T. O. T. A. L Child; 1985. [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1989. [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Mundy P, Neal R. Neural plasticity, joint attention, and a transactional social-orienting model of autism. In: Glidden L, editor. International review of research in mental retardation: Autism. Vol. 23. New York: Academic Press; 2001. pp. 139–169. [Google Scholar]
- Pellicano E. Do Autistic Symptoms Persist Across Time? Evidence of Substantial Change in Symptomatology Over a 3-year Period in Cognitively Able Children With Autism. American Journal on Intellectual and Developmental Disabilities. 2012;117(2):156–166. doi: 10.1352/1944-7558-117.2.156. [DOI] [PubMed] [Google Scholar]
- Perry A, Flanagan H, Geier J, Freeman N. Brief report: The Vineland Adaptive Behavior Scales in young children with Autism Spectrum Disorders at different cognitive levels. Journal of Autism and Developmental Disorders. 2009;39:1066–1078. doi: 10.1007/s10803-009-0783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB. Children's Depression Rating Scale Revised (CDRS-R) Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Rea LM, Parker RA. Designing and conducting survey research. San Francisco: Jossey-Bass; 1992. [Google Scholar]
- Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: Four-year outcome and predictors. American Journal on Mental Retardation. 2005;110:417–438. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism, research reviews. Mental Retardation and Developmental Disabilities. 2004;10:234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla D, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla D. Vineland Adaptive Behavior Scales. 2nd. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Sutera S, Pandey J, Esser E, Rosenthal M, Wilson L, Barton M, Green J, Hodgson S, Robins D, Dumont-Mathieu T, Fein D. Predictors of Optimal Outcome in Toddlers Diagnosed with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37:98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Tonge BJ, Einfeld SL. Psychopathology and intellectual disability: The Australian child to adult longitudinal study. International Review of Research in Mental Retardation. 2003;26:61–91. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- White S, Ollendick T, Bray B. College students on the autism spectrum: Prevalence and associated problems. Autism. 2011;15(6):683–701. doi: 10.1177/1362361310393363. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- Woodard C, Groden J, Goodwin M, Bodfish J. A placebo double-blind pilot study of dextromethorphan for problematic behaviors in children with autism. Autism. 2007;11:29–41. doi: 10.1177/1362361307070989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Additional methods and measures
Appendix S2: Additional results
Table S1: Demographics by IQ group
Table S2: Age 3 means by IQ Group
Table S3: Predicting outcome group at age 19 from early childhood characteristics
Table S4: Age 2 means for additional covariates among cognitively able youths
Table S5: Other age 3 means among cognitively able youths
Table S6: Type of individual treatment from age 2 to 3-years


