Abstract
Autoimmune connective tissue diseases are clinically variable, making biomarkers desirable for assessing future disease risk, supporting early and accurate diagnosis, monitoring disease activity and progression, selecting therapeutics, and assessing treatment response. Because of their correlations with specific clinical characteristics and often with disease progression, autoantibodies and other soluble mediators are considered potential biomarkers. Additional biomarkers may reflect downstream pathological processes or may appear due to ongoing inflammation and damage. Because of overlap between diseases, some biomarkers have limited specificity for a single autoimmune connective tissue disease. This review describes select current biomarkers that aid in diagnosis and treatment of several major systemic autoimmune connective tissue disorders: systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and ANCA-associated vasculitides. Newly proposed biomarkers that target various stages in disease onset or progression are also discussed. Newer approaches to overcome the diversity observed in these diseases and to facilitate personalized disease monitoring and treatment are also addressed.
Keywords: Connective tissue diseases, systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, vasculitis, biomarkers
Introduction
Autoimmune connective tissue disorders are a heterogenous group of diseases that affect connective tissue in various organs resulting from poorly controlled autoimmune responses, complement activation, interferon dysregulation, and associated inflammation. Although their clinical presentations vary, these diseases share significant genetic risk factors as demonstrated by cross-analysis of genome-wide association studies1 and common regulatory mechanisms of autoimmune diseases.2 Environmental and female-associated factors also play critical roles in development of autoimmune diseases.3–7 In nearly all systemic autoimmune rheumatic diseases evaluated to date, autoantibody production and immune dysregulation precede clinical onset,8–15 although a significant amount of this information is not yet integrated to standard clinical care. Ongoing research is focused on improving biomarkers for diagnosis, prognosis, treatment selection and optimized therapy. This review describes current and new emerging biomarkers for major connective tissue diseases: systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and ANCA-associated vasculitides.
Systemic lupus erythematosus (SLE)
SLE is a systemic autoimmune disease characterized by production of anti-nuclear autoantibodies (ANA). Early, accurate diagnosis and disease monitoring are hindered by its heterogenous presentation and clinical course. Serological and urinary biomarkers are either in use or are emerging as potential biomarkers for SLE. These autoantibodies, complement products, and cytokines/chemokines/soluble mediators have the potential to facilitate diagnosis, identify individuals at greater risk for developing SLE, and monitor disease activity or specific organ involvement.
Autoantibodies
ANA are a hallmark of SLE. Nearly all SLE patients exhibit ANA at diagnosis, with a 1:80 immunofluorescent titer showing up to 98% sensitivity but 75% specificity for SLE classification.16 ANA are also found in individuals with many other autoimmune diseases, malignancies, hepatic diseases, unaffected family members of lupus patients, and even up to 14% of healthy individuals,17 especially with increasing age. Therefore, a positive ANA serves as a necessary but insufficient criterion for SLE classification or diagnosis, but not as a definitive test.18 Individuals with a negative ANA are extremely unlikely to have any lupus-specific autoantibodies. Therefore, through the Choosing Wisely campaign, the American College of Rheumatology (ACR) recommends testing for specific autoantibodies only when a positive ANA and clinical suspicion are present.19 Repeat testing is not indicated in ANA-positive individuals, as changes in ANA titer alone show no clinical correlation with increased disease activity or worsening prognosis. Testing of ANA and other autoantibodies in preclinical disease states or to identify individuals for potential preventive interventions will require additional studies and guidelines.20
Anti-double stranded DNA (dsDNA) antibody responses have high specificity (92–96%) and moderate sensitivity (57–67%) for SLE.21 They constitute a criterion for SLE classification by ACR criteria (requiring 4 of 11 criteria for classification) and by the Systemic Lupus International Collaborating Clinics (SLICC) criteria (requiring 4 of 17 criteria, or dsDNA plus biopsy-proven lupus nephritis [LN]).22–24 Anti-dsDNA forms immune complexes with nucleosomes observed in SLE patients, leading to immune complex deposition in the kidney.25 Furthermore, anti-dsDNA antibodies show cross-reactivity to alpha actinin and can bind to mesangial cells in the kidney.26 Immune complexes formed by anti-dsDNA antibodies in the kidney can activate the complement cascade, leading to damage in glomerulonephritis.27 Patients with proliferative LN have elevated anti-dsDNA as early as four years before diagnosis, and an increase of >1 IU/mL/year was specific for LN.28 Anti-dsDNA with low complement also associates with mucocutaneous, renal, and hematological flare within one year.29 In clinically stable SLE patients with rising anti-dsDNA (≥25%) and rising C3a, the free released product of complement activation, (≥50%), treatment with moderate prednisone may avert severe clinical flares.30
Although less common (sensitivity 26–31%) antibodies against the Sm antigen are highly specific (95–99%) for SLE and may associate with early mortality.31 About 30–70% of SLE patients have anti-Ro/SSA (Sjögren’s syndrome type A antigen), and Ro/SSA is associated with subacute lupus erythematosus, sicca symptoms and secondary Sjögren’s. Anti-Ro/SSA antibodies may bind to either of two antigenic proteins, 52 kD Ro and 60 kD Ro. Antibodies to 60 kD Ro/SSA are more frequently observed in SLE, and correlate with photosensitivity, cutaneous vasculitis, and hematological disorders.32 Antibodies to a related antigen, La/SSB, are present in ~10% of SLE patients and are associated with lower prevalence of renal disease.32 Anti-ribosomal P antibodies, similar to anti-Sm, are very specific for SLE, but occur in only ~20% of Caucasian SLE patients. Anti-ribosomal P is enriched in neuropsychiatric33 and pediatric-onset disease.34 A number of other autoreactivities have been reported in SLE.21 Of interest are anti-nucleosome responses, which correlate with disease activity in clinically quiescent patients,35 and anti-cardiolipin responses, which are implicated in thrombosis and recurrent fetal loss and are associated with a complex clinical outcome, with patients meeting a higher number of ACR criteria.10
Since no single autospecificity is sufficient for SLE diagnosis, recent efforts have focused on detecting autoantibody signatures encompassing combinations of autoreactivities. One autoantigen array covers antigens related to eight distinct autoimmune diseases.36 Another microarray-based test has a reported sensitivity of 94% and specificity of 75%. The test is validated as a clinical test to exclude a diagnosis of SLE if no compelling clinical evidence exists or to support a low likelihood of SLE.37
Autoantibodies typically are detectable before diagnosis; 63 to 88% of individuals have autoantibodies before disease classification (0.1 to 9 years).9,8 Anti-Ro/SSA is among the earliest detectable specificities, while anti-ds DNA antibodies appear closer to classification (~ 3 years before).8 Therefore, autoantibodies may serve as a biomarker of disease risk prior to SLE onset. In individuals meeting <4 ACR classification criteria for SLE, those who later reached SLE classification showed higher baseline ANA and increased IgG autoreactivity in IgG profiling of over 80 autoantigens.38 In a follow-up study of previously unaffected relatives of SLE patients, 89% of individuals who later reached SLE classification were ANA-positive at baseline, compared to 48% of those who remained unaffected.13 Therefore ANA positivity alone is not a definitive marker for increased risk of future disease classification, and additional predictive markers are needed.
Complement
Levels of complement C3 and C4 are used to monitor SLE disease activity. Reduced C3 and C4 levels are associated with more severe disease, and reduced C1q, C3, and C4 levels can precede disease flare.39 Complement is activated in SLE by immune complex deposition. Therefore, cell bound complement C4 activation products (CBCAP) on erythrocytes are increased in SLE and have 22% higher sensitivity for SLE than reduced C3/C4. ANA positivity, anti-MCV negativity, anti-dsDNA positivity, elevated erythrocyte C4d and elevated B cell C4d demonstrated 80% sensitivity for SLE and 87% specificity against other rheumatic diseases.40 These findings have been confirmed and refined to a commercially available, weighted SLE risk score with a two tier design.41
Emerging Biomarkers
Soluble mediators
Recent studies have suggested specific soluble mediators as potential biomarkers for SLE onset and SLE disease flare. In a survey of nearly 30 immune or inflammation-based soluble mediators, several soluble mediators were elevated >3.5 years before SLE classification, including interleukin (IL)-5, IL-6, and IFNγ. Some cytokines (BLyS, APRIL) became elevated closer to diagnosis. In this preclinical period, a combination of IFNγ, IL-4, IL-6, ANA, and anti-Ro distinguished patients from controls with 84% accuracy, compared to 58% accuracy with ANA positivity alone. Thus, evaluating immune pathway dysregulation in conjunction with ANA positivity may help identify individuals at higher risk for SLE.11
Flares are a significant risk factor for end organ damage in SLE, and soluble mediators are promising biomarkers of imminent SLE flare. Levels of BLyS in SLE patients are associated with anti-dsDNA levels and disease activity.42 Baseline BLyS concentration ≥2ng/mL predicted SLE flare at week 52 in a combined analysis of data from phase II, world-wide clinical trials.43 In a longitudinal study of SLE patients, reduced levels of regulatory cytokines such as IL1 receptor antagonist, tumor growth factor (TGF)-β and IL10, preceded disease flares. A combined soluble mediator score incorporating 52 analytes was increased in patients with impending flare compared to either matched stable patients or the same patients during a clinically stable period.44, 45 This score accurately predicted flare in both European American and African American study groups. Accurate prediction of SLE flare may allow early treatment or prevention of flares.
Urinary biomarkers
Glomerulonephritis, a major cause of morbidity and mortality in SLE, currently requires renal biopsy for definitive diagnosis. Urinary biomarkers would be useful for identifying LN patients at the highest risk of end stage renal disease or distinguishing between LN and other forms of renal disease in lupus patients. Urinary levels of TNF-like weak inducer of apoptosis (TWEAK) correlated with disease activity in a large multicenter longitudinal study.46 Other possible urinary biomarkers include monocyte chemoattractant protein (MCP-1), high mobility group box-1 protein (HMGB-1), vascular cell adhesion molecule (VCAM-1), and angiostatin.47–50 The levels of these markers correlate with histological changes in the renal tissue and can distinguish between active and inactive LN.
Interferons
Interferons (IFN) are consistently associated with SLE. Serum IFNα activity associates with autoantibodies as well as BLyS.51, 52 IFNα levels correlated with the number of autoantibody specificities in preclinical samples obtained from individuals later classified as SLE, suggesting a role for IFNα in autoantibody accrual.12 Interestingly, IFNγ activity was increased before autoantibody positivity, and autoantibody positivity preceded increases in IFNα. IFNγ regulates both IFNα and B cell differentiation and possibly drives B cell maturation and class switching in early stages of SLE pathogenesis. However, only a subset of adult SLE patients show IFN activity in serum.51
IFN response is most often measured indirectly as an ‘IFN signature’ defined by upregulation of sets of IFN-regulated genes. In a large, longitudinal monitoring study, 84.8% of pediatric lupus patients demonstrated an IFN signature.53 A similar IFN signature has been reported in about half of adult SLE patients, and IFN activity levels have been associated with autoantibody production and disease activity.54–56 Longitudinal monitoring of whole blood gene expression profiles and parallel disease activity in a large pediatric cohort demonstrates seven different clusters of reactivity and association with various gene expression modules.53 Although these data associate IFNs and IFN-associated gene regulation with SLE, some aspects of the IFN signature may be relatively stable over the course of the disease, based on paired analyses of longitudinal data.57, 58 Indeed, high serum IFNα activity appears to be a complex heritable trait.51 Therefore, IFN may influence SLE predisposition, while together with dysregulation of other immune pathways, such as BLyS, T helper, and inflammatory mediators, may influence disease pathogenesis.
Due to the heterogeneity among SLE patients, personalized treatment/monitoring based on molecular mechanisms involved may be beneficial. Using an unbiased approach with modular gene expression panels that include IFN signature genes, Bancheareau et al. stratified pediatric SLE patients into separate groups that were supported by patient genotypes.53 Such patient stratification may enable studying effectiveness of biomarkers for disease activity or treatment response in a particular subset of patients with a relevant molecular mechanism of disease pathogenesis.
Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA), a systemic autoimmune rheumatic disease afflicting up to 0.8% of the population, is characterized by synovitis leading to irreversible joint destruction. Effective management of RA requires initiation of therapy with disease modifying anti-rheumatic drugs (DMARDs) within months following disease onset to maximize outcomes.59 Even a brief delay can have a significant impact on disease progression. New autoantibody specificities can develop over time, so periodic monitoring may be warranted, especially in arthralgia-positive patients who do not yet have an RA diagnosis. However, once autoantibodies are present in clinical RA serial monitoring is not necessary, as fluctuations in titers over time are not associated with disease activity or further prognosis.
Autoantibodies
Two of the most common autoantibodies in RA are rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA).
Rheumatoid factor
Rheumatoid factor is directed against the Fc portion of IgG class of antibodies. Although RF occurs in 70–80% of RA patients, it also occurs in patients with SLE, Sjögren’s syndrome, or systemic infections, as well as ~10% of healthy individuals.60 For RA disease classification, RF has a sensitivity of 69% and specificity of 85%.61 Higher levels of RF are associated with more severe disease marked by disease progression, rheumatoid nodules, and various extra-articular manifestations.62 Based upon the higher sensitivity and clinical utility of ACPA, RF has become more of a historical test and is usually now only tested in combination with anti-CCP.
Antibodies against anti-citrullinated protein antigens (ACPAs)
ACPAs target proteins or peptides where arginine residues have been converted to citrulline. HLA-DRB1 alleles are the strongest genetic association for seropositive RA. HLA-DRB1 interacts with cigarette smoking to increase the risk of ACPA-positive RA, but not seronegative RA63, and IgA ACPA responses have been found in the sputum of preclinical or early clinical RA patients.64 ACPAs also appear before the onset of clinical disease, making them valuable markers for diagnosis and prognosis. Please see65 for a more extensive review of ACPA pathogenesis in RA.
Clinically, antibodies against cyclic citrullinated peptides (CCP) are used in RA diagnosis. In a meta-analysis, anti CCP had a pooled sensitivity of 67% and 95% specificity for RA, and anti-CCP positivity was associated with increased risk of radiographic progression.61
Anti-CCP positivity predicted progression to RA in a 3-year follow-up study of undifferentiated arthritis patients.66 Anti-CCP and RF were strongly associated with extra-articular manifestations.62 Anti-CCP positivity and initial DAS28 were associated with EULAR response to abatacept in analyses to predict factors of efficacy using data from the Orencia and Rheumatoid Arthritis registry.67 High anti-CCP titer was an independent predictor of decrease in DAS28 and EULAR good response to rituximab.68 In recent onset RA, IgA anti-CCP was observed in 29% of patients, along with IgG. Patients positive for IgA anti-CCP had a more severe disease course over 3 years compared with IgA anti-CCP-negative cases.69 A higher number of anti-CCP isotypes was associated with significantly more radiographic damage during the disease course over 10 years of followup.70 Even though anti-CCP isotypes may not provide significant improvement in diagnosis compared to anti-CCP IgG, the isotypes may have possible prognostic implications.
Anti-perinuclear factor (APF) and anti-keratin antibodies (AKA) antibodies recognize citrullinated epitopes on the same autoantigen, filaggrin or pro-filaggrin, and may serve as early diagnostic markers. Anti-Sa recognizes citrullinated vimentin71 and shows high specificity of 92–100% and a moderate sensitivity of 32–43%.72 Several other citrullinated antigens have been identified in RA including fibronectin, filaggrin, fibrinogen, alpha enolase, and collagen.
Autoantibodies with other specificities
Antibodies against carbamylated proteins (anti-CarP) are detected in 45% of RA patients, including anti-CCP–negative patients. The targets of anti-CarP in RA include vimentin, fibrinogen, and albumin.73 Anti-CarP responses have been associated with mortality in seropositive RA, and specifically with respiratory causes of death in a Spanish cohort.74 Anti-A2/anti-RA33 antibodies occur in >60% of RA patients, and are also seen in SLE. If diagnoses of SLE or mixed connective tissue disease can be excluded, the specificity of anti-A2/anti-RA33 antibodies for RA can be as high as 96%. The specificity of anti-BiP antibodies for RA has been reported as 96%, making these antibodies promising additional candidates for the classification or diagnosis of RA.
Acute phase reactants
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in RA patients compared to controls. ESR and swollen joint count were associated with radiographic disease progression in a systemic literature review including 57 studies with disease activity measurements in 13 to 1433 patients.75 ESR and CRP levels are also measured as components of RA disease activity indices, such as the DAS28, which are used in trials for clinical disease monitoring.
Cytokines
Many cytokines and chemokines are active in RA patient joints, and these cytokines are critical in inflammation, joint damage and RA-associated comorbidities.76 Indeed, a number of different cytokines, such as TNFα, IL6, and IL1RA, are successfully targeted in RA treatment as are small molecule inhibitors, such as JAK inhibitors that regulate cytokine secretion pathways. In addition, in serial samples preceding RA classification in a military cohort, the number of elevated cytokines and chemokines predicted time to RA diagnosis/classification.77 A commercially available blood test monitors RA disease activity with a score calculated from the concentrations of 12 serum biomarkers: VCAM-1, epidermal growth factor (EGF), vascular endothelial growth factor-A (VEGF-A), IL-6, TNF-receptor type 1 (TNF-RI), matrix metalloproteinase (MMP)-1, MMP-3, cartilage glycoprotein 39 (YKL-40), leptin, resistin, serum amyloid A, and CRP. Changes in the score correlate with changes in other indicators of RA disease activity, including the DAS28 index, ESR and CRP. In addition, this score declined significantly in patients who responded to TNF inhibitors by EULAR criteria, while patients with a higher score showed greater radiographic progression over 52 weeks of TNF inhibitor treatment.78
Emerging biomarkers
Biologics such as TNF inhibitors, anti-IL-6 receptor antibodies, anti-CD20 antibodies, and CTLA-4-Ig have shown efficacy in RA. Several new biomarkers have been proposed to identify patients who may respond to these therapies. Response to rituximab is associated with RF positivity and with normal levels of CD19+ B cells together with increased CD19+ CD27− IgD− B cells.79 Treatment with infliximab leads to decreases in the chemokines CXCL10/IP-10, CCL2/MCP-1, and CCL4/MIP-1β.80 Serum concentrations of myeloid related protein (MRP) 8/14 protein complex at baseline were proposed predictors of response to biological therapy (adalimumab, infliximab or rituximab)81 and methotrexate82 in active RA patients, and may be useful for monitoring response to treatment across different mechanisms of action. Additional expanded biomarker studies are needed to help select the ideal therapy at the ideal time and dose for an individual patient.
Systemic Sclerosis (SSc, Scleroderma)
SSc is a systemic autoimmune disease characterized by extensive fibrosis in skin and internal organs. Patients with limited cutaneous SSc (lcSSc) show restricted distal skin sclerosis, long history of Raynaud’s phenomenon, and better prognosis. Diffuse cutaneous SSc (dcSSc) has much more extensive skin involvement, and earlier and more severe organ manifestations.83 Major complications of SSc include skin and musculoskeletal complications, pulmonary arterial hypertension (PAH), interstitial lung disease (ILD), digital vasculopathy, renal crisis, and cardiac and gastrointestinal manifestations. Autoantibodies and other serologic markers, when present, can be very useful in ascertaining potential organ involvement, monitoring needs, or overall prognosis. However, as with other systemic autoimmune rheumatic diseases, monitoring levels once detected is usually unwarranted.
Autoantibodies
SSc is diagnosed based on clinical features, which are supplemented by the ANA profile. Anti-centromere antibodies (ACA) occur in ~20–42% pts in North America, mostly in lcSSc.84, 85 Anti-centromere is 97% specific for lcSSc against other connective tissue diseases, with a positive predictive value of 89.5%.85 The most likely severe complication in ACA positive patients is PAH, while digital ulcers, myocardial and kidney involvement are rare. Anti-topoisomerase I (Scl70) antibodies are also highly specific (99.5%) and predictive (98%) for SSc.85 They occur in 14–42% of patients in North America 85, with the vast majority having dcSSc. These autoantibodies associate with progressive pulmonary fibrosis, digital ulcers and hand disability. Anti-RNA polymerase III (RNAP III) has 98–100% specificity for SSc and occurs in 16–20% of patients, mostly in the dcSSc subset.84, 85 Anti-RNAP III is associated with hand disability and renal involvement, and rarely with pulmonary fibrosis.84 Similar to Scl70, anti-RNAP III is associated with higher rates of SSc-related mortality. Patients with anti-RNAP antibodies are about twice as likely as ACA positive and four-fold as likely at anti-Topo1 positive individuals to develop cancer within 3 years of SSc onset. 86
Anti-Th/To and U3-RNP antibodies target nucleolar antigens. Th/To autoantibodies are directed against subunits of RNase P and RNase MRP. They occur in 2–5 % of patients with SSc, 8.4% with lcSSc, and 0.6% with dcSSC. Anti-Th/To may be a marker for PAH.87 Anti-U3 RNP antibodies target fibrillarin and are found in 18.5% of African American patients. These patients had a younger age of onset, higher frequency of digital ulcers and pericarditis, but lower lung severity scores and no difference in survival.88 Anti-U3 RNP is most frequent in males and African Americans with SSc and is associated with muscle involvement and increased risk of PAH.84 Anti-U11/U12 RNP antibodies have been reported in 3.2% of SSc patients who had no other SSc-associated autoantibodies. The presence of anti-U11/U12 RNP antibodies was associated with pulmonary fibrosis (79% of antibody positive vs 37% of antibody negative) and a 2.25 greater risk of death.89 In a recent study, anti-U11/U12 RNP antibodies were associated with myopathy as well as severe gastrointestinal disease and severe Raynaud’s phenomenon, in patients with SSc and cancer.90
Emerging biomarkers
Because the major complications of SSc include pulmonary dysfunction and ILD, lung proteins have been studied as potential biomarkers for SSc. Serum levels of both Krebs von den Lungen protein [KL-6] and surfactant protein-D are elevated in SSc and associate with decreased forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO). Elevated levels are more frequent in Scl70 positive patients than ACA positive patients.91 One of the more extensively studied biomarkers in SSc is the N-terminal prohormone of brain natriuretic peptide (NT-proBNP). Higher circulating levels of NT-proBNP are associated with more severe PAH and greater risk of mortality.92 However, NT-proBNP is not specific to PAH and may result from cardiac dysfunction.
Fibrosis is a major player in SSc pathogenesis. TGFβ is a key regulator of fibrosis, but its utility as a biomarker is limited by technical difficulties in measuring its free, circulating form. Mediators regulated by or related to the TGFβ family have been studied in SSc. TGFβ regulates cartilage oligomeric matrix protein (COMP; also known as thrombospondin-5), and sera from SSc patients have increased COMP levels, which correlate with the extent of skin involvement.93 Growth differentiation factor 15 (GDF-15), a distant member of the TGFβ family, is elevated in sera from SSc patients. GDF-15 levels strongly correlate with a modified Rodnan severity score for skin involvement and negatively correlate with FVC and DLCO.94, 95 Levels of GDF-15 are increased in SSc patients with ILD compared to other SSc patients, and higher levels of GDF-15 at baseline were predictive of worse lung disease severity scores at 30 months of follow-up. Therefore, GDF-15 may be a prognostic marker for lung function. Interestingly, GDF-15 levels correlate with NT-proBNP and identified PAH with higher specificity and sensitivity compared to NT-proBNP.96
Along with TGF family proteins, certain pro-inflammatory proteins are increased in SSc and associated with fibrosis. The pro-inflammatory cytokine IL-6 is elevated in sera of SSc patients with anti-Scl70 or anti-RNAP III, but not in patients with anti-centromere.97 Elevated IL-6 is associated with skin fibrosis, lung fibrosis, and increased mortality,98, 99 but has not been conclusively correlated to disease activity. Levels of CXCL4, a pro-inflammatory chemokine that regulates several immune and non-immune cells, were higher in SSc patients compared to controls. Elevated CXCL4 associated with faster progression of skin fibrosis, PAH, and faster decline of DLCO.100 S100A8 and a dimer of S100A8/A9 are calcium binding proteins involved in inflammatory processes. Their levels are higher in sera and bronchoalveolar lavage fluid from SSc patients.101, 102 An independent study found increased levels in lcSSc patients with lung fibrosis and Scl70 positivity, but observed no correlation to pulmonary function tests.103 Further studies are required to identify and validate specific biomarkers and their roles in pathogenesis, and to enable early prediction of patients who will need a lung transplant.
ANCA Associated Vasculitides (AAV)
ANCA associated vasculitides (AAV) are a group of autoimmune diseases characterized by the presence of anti-neutrophil cytoplasmic antibodies (ANCA). AAV include eosinophilic granulomatosis with polyangiitis (EGPA, Churg-Strauss Syndrome), microscopic polyangiitis (MPA), and granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis).
Eosinophilic Granulomatosis with Polyangiitis (EGPA, Churg-Strauss Syndrome)
EGPA is an AAV distinguished by a history of allergic disease in the majority of patients, as well as the presence of eosinophilic infiltration in extravascular granulomas. EGPA often starts as chronic rhinitis followed by eosinophilia, which progresses to small vessel vasculitis with associated symptoms. EGPA shares several features with asthma and hypereosinophilic syndrome (HES), making early diagnosis challenging. Currently, EGPA is often a diagnosis of exclusion and based upon associated organ damage. Therefore, identifying biomarkers for early diagnosis is important. ANCA are detected in a third of patients (mostly p-ANCA directed against myeloperoxidase [MPO]). ANCA-positive patients are less likely to develop heart and non-hemorrhagic lung involvement compared to ANCA-negative patients.
EGPA is characterized by increased numbers of circulating eosinophils (>1500 cells/μL). Glucocorticoid treatment drastically lowers eosinophil numbers, and eosinophilia is usually not an adequate biomarker for EGPA once a patient has started treatment.104 Chemokine eotaxin-3 (CCL26) is one of the most widely studied biomarkers of pathological significance in EGPA. Eotaxin-3 is secreted by epithelial cells and acts as a chemoattractant for eosinophils. Higher serum levels of eotaxin-3 were associated with active EGPA, although the levels dropped significantly upon treatment.105 Interestingly, eotaxin-3 levels were lower in hypereosinophilic syndrome, thereby making it potentially useful in diagnosis.105 At a cut-off of 80 pg/mL, eotaxin-3 has 87.5% sensitivity and 98.6% specificity for EGPA,105 suggesting that eotaxin-3 may be a highly sensitive and specific marker for distinguishing active EGPA from eosinophilic (asthma, HES), rheumatic (SLE, SSc) and other AAV diseases. Serum IgG4 levels are also increased in active EGPA, and correlate with the number of organ manifestations and Birmingham vasculitis activity score.106 CCL17/TARC is secreted by peripheral blood mononuclear cells and is a chemoattractant for Th-2 type cells that are important in EGPA pathogenesis. Levels of CCL17 were elevated in patients with active EGPA compared to controls and to patients with inactive disease.107 CCL17 levels decreased drastically after initiation of glucocorticoid therapy, but increased prior to clinical relapse.107 A study of eicosanoid levels in excreted breath condensate found increased levels of arachidonic acid metabolite 12-hydroxy-eicosatetraenoic acid (12-HETE) in active EGPA compared to inactive EGPA, HES, asthmatic, and healthy controls.108 Although progress is being made, EGPA management would benefit from biomarkers to aid with initial, early diagnosis and longitudinal information about associations of emerging biomarkers with disease outcomes.
Microscopic Polyangiitis (MPA)
MPA is another AAV affecting the arterioles, capillaries, and venules, and thereby involving the skin, nerves, gastrointestinal tract, lungs, kidneys and joints. ANCA in MPA are often directed against myeloperoxidase.109 However, MPO-ANCA are not specific for MPA, as they occur in EGPA, necrotizing crescentic glomerulonephritis, sarcoidosis, IgA nephropathy, and infections.110 MPA flares are often accompanied by increases in anti-MPO titers, elevated ESR and/or CRP. A C-terminal fragment of apolipoprotein A1, AC-13, was elevated in MPA patients compared to EGPA, GPA, and RA patients and healthy individuals, with reduced levels following treatment. Therefore, AC-13 may serve as a specific marker for MPA disease activity.111 Necrotizing glomerulonephritis is common in MPA patients, and anti-MPO associated glomerulonephritis is less responsive to standard of care treatments and has worse renal survival.112 A serum creatinine level above 4.6 mg/dL at initial MPA diagnosis was shown to be a good predictive factor for development of end stage renal failure, with 92.3% sensitivity and 84.6%specificity.113 A recent study identified differential expression of genes associated with toll-like receptor signaling in peripheral neutrophils in MPA, however, evaluating the diagnostic potential of these patterns requires further research. 114
Granulomatosis with Polyangiitis (GPA, Wegener’s granulomatosis)
The hallmarks of GPA include necrotizing granulomatous inflammation in the respiratory tract and pauci-immune vasculitis, primarily in lung and kidneys. ANCA in GPA are directed mostly against proteinase-3 (PR3), and much less frequently against MPO.115 PR3-ANCA has a high sensitivity and specificity for the diagnosis of active GPA (>90%), although ANCA-negative cases do occur.
Even with improved therapies, up to 50% of GPA patients may face relapse within 5 years. Although an increase in ANCA is generally believed to precede relapse, many ANCA rises are not followed by a clinical relapse, and relapses may occur without a preceding ANCA rise.116 However, ANCA rises correlated with clinical relapses in patients with renal involvement, and the avidity of anti-PR3 antibodies increased during the period preceding clinical relapse, but not during the preceding ANCA rise.117 Patients with severe GPA exhibit lower levels of Fc glycosylation in PR3-ANCA,118 and the glycosylation profile of total IgG at the time of an ANCA rise predicted a clinical relapse in patients with severe disease.119
Several cytokines have been proposed to contribute to the systemic inflammation in GPA and could potentially serve as biomarkers of disease activity or upcoming flare. Serum levels of S100A8/A9 are elevated in patients with active AAV compared to those in remission or to healthy controls, and serum S100A8/A9 may identify PR3-positive patients at risk of relapse.120 Serum levels of high mobility group box-1 protein (HMGB1) are significantly higher in patients with GPA than controls.121 Recently, a reduction in the number of Tregs was reported during disease flare in GPA, whereas expansion of both Treg and Th2 compartments was observed during remission.122 The utility of immunophenotyping in assisting disease monitoring needs further evaluation.
Summary/conclusions
Autoimmune connective tissue diseases are complex disorders driven by environmental, genetic, and immunological mechanisms. Antibodies are used clinically for diagnosis, but fall short due to low specificity and limited understanding of their role in pathogenicity. Moreover, autoantibodies alone are often not sufficient to identify individuals at risk of developing disease. Implementing preventive measures will require biomarkers that can predict disease progression within a specific time frame or in patients with milder disease. Newer studies have proposed several new biomarkers that could serve this purpose, but several areas still need extensive research. Despite overlapping mechanisms of pathogenesis between autoimmune connective tissue diseases, these diseases are diverse, with varied clinical presentations and therapeutic efficacies. Therefore, the underlying immunological mechanisms may vary, and it is unlikely that a single biomarker will be suitable in all diseases. Indeed, even within a single disease, multiple biomarkers may be required to account for mechanistic and clinical heterogeneity between patients. A multivariate approach accounting for several aspects of the disease, as has been developed for SLE and RA, may prove useful to support diagnosis, monitor disease progression/prognosis, and to select appropriate therapy for individual patients. Confirming the clinical validity of these approaches will require longitudinal analyses with sufficient power on well characterized clinical cohorts.
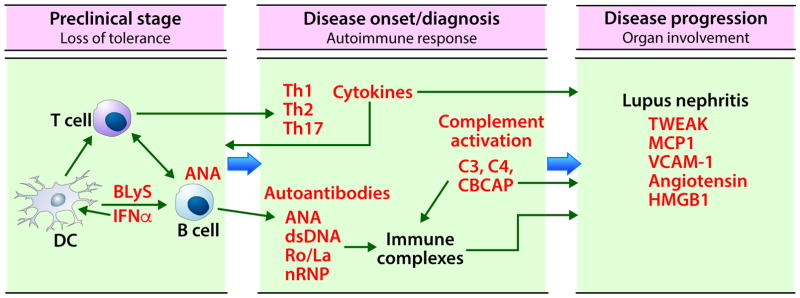
Figure 1. Biomarkers in SLE.
The initial stage in SLE pathogenesis is loss of tolerance to self-antigens. Genetic predisposition and environmental factors such as viral infections can lead to anti-nuclear antibody generation by epitope spreading. Disease onset or diagnosis occurs following the amplification of autoimmune response through the interactions between innate and adaptive immune cells (dendritic cells, T cells, and B cells), differentiation of T cells into Th1, Th2, Th17 subsets, and maturation and class switching of B cells to secrete autoantibodies. These autoantibodies form immune complexes, fix complement, and activate both classical and non-classical complement. This results in decreased complement C4 and C3, and an increase in cell bound C4d (CBCAP). The increased levels of cytokines, autoantibodies, and CBCAP, and decreased levels of C3 and C4 in circulation are the most useful biomarkers at this stage. Passive deposition of immune complexes or in situ immune complex formation in end organs such as kidneys, together with complement activation and high levels of secreted pro-inflammatory cytokines causes further organ damage. Urinary biomarkers are a convenient and effective way to monitor renal disease progression in SLE. DC; dendritic cells, BLyS: B lymphocyte stimulator, IFN: interferon, Th: T helper, ANA: antinuclear antibodies, dsDNA; anti double stranded DNA antibodies, nRNP; nuclear ribonuclear protein, TWEAK: TNF like weak inducer of apoptosis, MCP1: monocyte chemoattractant protein 1, VCAM-1: vascular adhesion molecule 1, HMGB1: high mobility group box 1 protein.
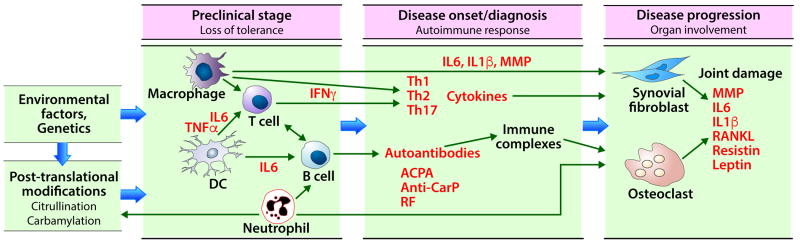
Figure 2. Biomarkers in RA.
The initial stage in RA pathogenesis is loss of tolerance to self-antigens. Genetic predisposition and environmental factors such as viral infections can lead to loss of tolerance and autoantibody generation by post-translational modification of self-proteins. Disease onset or diagnosis occurs following the amplification of autoimmune response through the interactions between innate and adaptive immune cells (dendritic cells, T cells, and B cells), leading to secretion of pro-inflammatory cytokines (IL6, TNFα) that activate macrophages and neutrophils. Both macrophages and neutrophils contribute to the adaptive immune response. Antigen-exposed T cells differentiate into Th1, Th2, Th17 subsets and maturation and class switching of B cells leads to secretion of autoantibodies. The increased levels of cytokines and autoantibodies are the most useful markers at this stage. The inflammatory cytokines secreted by macrophages, T cells and reactive oxygen species secreted by neutrophils activate synovial fibroblasts and induce osteoclast maturation from pro-osteoclasts. The ensuing inflammation causes joint damage. The soluble mediators secreted by macrophages, T cells, synovial fibroblasts and osteoclasts are the major biomarkers at this stage. TNF: tumor necrosis factor, IL6: interleukin 6, IFN: interferon, DC: dendritic cells, Th: T helper, ACPA: anti-citrullinated protein antibodies, Anti-CarP: anti-carbamylated protein antibodies, RF: rheumatoid factor, MMP: matrix metalloproteinase, RANKL: receptor activator of nuclear factor-kappa B ligand.
Table 1.
Autoantibody specificities in connective tissue diseases and their association with disease phenotype
| Antibody | Associated disease phenotype | Other associated diseases | Reference |
|---|---|---|---|
| Systemic lupus erythematosus | |||
| Anti-dsDNA | Lupus nephritis, Neuropsychiatric lupus erythematosus | 21 | |
| Anti-Sm | Lupus nephritis | 31 | |
| Anti-Ro/SSA (Ro60) | Lupus nephritis Neonatal lupus erythematosus Subacute cutaneous lupus erythematosus |
Sjögren’s syndrome | 32 |
| Anti-La/SSB | Lupus nephritis Neonatal lupus erythematosus Subacute cutaneous lupus erythematosus |
Sjögren’s syndrome | 32 |
| Anti- Ribosomal P | Lupus nephritis Neuropsychiatric lupus erythematosus Pediatric-onset SLE |
33,34 | |
| Anti-RNP | SLE | Systemic sclerosis | 21,31 |
| Anti- Cardiolipin | SLE, Anti-phospholipid syndrome | 10 | |
| Rheumatoid arthritis | |||
| RF | Rheumatoid arthritis | Systemic lupus erythematosus, Sjögren’s syndrome | 60,61 |
| ACPA | Rheumatoid arthritis | 61,65 | |
| Anti-CarP | Rheumatoid arthritis | 73,74 | |
| RA33 | Rheumatoid arthritis | 60 | |
| Systemic sclerosis | |||
| Anti- centromere | Limited cutaneous systemic sclerosis, Pulmonary arterial hypertension | 83,84,85 | |
| Scl70 | Diffuse cutaneous systemic sclerosis, Pulmonary fibrosis, renal crisis | 83,84,85 | |
| Anti-RNAP III | Diffuse cutaneous systemic sclerosis, renal crisis | 83,86 | |
| Anti-Th/To | Limited cutaneous systemic sclerosis, Pulmonary arterial hypertension, Pulmonary fibrosis | 87 | |
| ANCA associated vasculitis | |||
| Anti-PR3 ANCA |
Granulomatosis with polyangiitis | Microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis | 109,110 |
| Anti-MPO ANCA |
Microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis | Granulomatosis with polyangiitis | 109,110 |
ANCA: anti-neutrophil cytoplasmic antibodies, dsDNA: double stranded DNA, RNP: ribonuclear protein, RF: rheumatoid factor, ACPA: anti-citrullinated protein antibodies, anti-CarP: anti-carbamylated protein antibodies, anti-RNAP III: anti-RNA Polymerase III antibodies, ANCA: anti-neutrophil cytoplasmic antibodies, PR3: Proteinase 3, MPO: Myeloperoxidase.
What do we know?
The spectrum of autoimmune connective tissue disorders show a large degree of overlap in disease pathogenesis.
Autoantibodies are one of most useful biomarkers to support diagnosis, and in some disease states can be used to monitor progression or response to treatment.
Antibodies to dsDNA are more specific for SLE, and along with several soluble mediators may assist in identifying individuals at increased risk of developing SLE and in predicting disease flares.
ACPA are more specific for RA and precede disease onset. A circulating soluble mediator score has been proved to be useful in monitoring disease progression.
ACA, Scl70, RNAP III, and Th/To autoantibodies are observed in SSc and although their role in pathogenic mechanisms are unknown, they may allow patients to be divided into specific subsets based on clinical features.
Patients with EGPA show eosinophilia, but this cannot distinguish between EGPA and other rheumatic or eosinophilic pathologies once treatment is initiated. A third of patients with EGPA have MPO-ANCA.
GPA is characterized by presence of PR3-ANCA. ANCA levels may be used to monitor disease progression in patients with renal involvement.
A multivariate approach that encompasses different measures of pathologic processes, such as autoantibodies and soluble mediators, may prove to be most informative for early accurate diagnosis, monitoring and predicting disease progress.
What is still unknown?
A precise understanding of disease pathogenesis, and which mechanisms are disease-specific or shared among autoimmune rheumatic diseases.
Which cytokines, chemokines and soluble mediators will be the most informative in predicting flares in SLE, RA and AAV patients.
Which biomarkers can monitor response to treatment across diseases or within a specific autoimmune connective tissue disease
Which biomarkers can be used to monitor disease progression in SLE, SSc, GPA, and EGPA
Biomarkers to aid early EGPA diagnosis
Whether biomarkers can be used for early diagnosis of EGPA or to identify high-risk AAV patients for prevention trials
Acknowledgments
Funding: This work was supported by the National Institutes of Health (U54GM104938, U01AI101934, U19AI082714, P30GM103510, P30AR053483).
The authors thank Rebecka L. Bourn, PhD for editorial assistance and Emily McEwen for bibliographic assistance.
Abbreviations
- AAV
ANCA associated vasculitides
- ACA
Anti-centromere antibodies
- ACPA
anti-citrullinated protein antibodies
- ACR
American College of Rheumatology
- ANA
anti-nuclear autoantibodies
- ANCA
anti-neutrophil cytoplasmic antibodies
- anti-CarP
antibodies against carbamylated proteins
- CBCAP
cell bound complement C4 activation products
- CCP
cyclic citrullinated peptides
- COMP
cartilage oligomeric matrix protein also known as thrombospondin-5
- CRP
C-reactive protein
- dcSSc
diffuse cutaneous systemic sclerosis
- DLCO
diffusing capacity of the lungs for carbon monoxide
- DMARDs
disease modifying anti-rheumatic drugs
- EGF
epidermal growth factor
- ESR
Erythrocyte sedimentation rate
- FVC
forced vital capacity
- GDF-15
Growth differentiation factor 15
- GPA
granulomatosis with polyangiitis also known as Wegener’s granulomatosis
- HMGB-1
high mobility group box-1 protein
- IFN
interferons
- IL
interleukin
- ILD
interstitial lung disease
- lcSSc
limited cutaneous systemic sclerosis
- LN
proliferative lupus nephritis
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- MPA
microscopic polyangiitis
- MPO
myeloperoxidase
- MRP
myeloid related protein
- NT-proBNP
brain natriuretic peptide
- PAH
pulmonary arterial hypertension
- PR3
proteinase-3
- RA
Rheumatoid arthritis
- RF
rheumatoid factor
- RNAP III
Anti-RNA polymerase III
- SSA
Sjögren’s syndrome type A antigen
- SLE
systemic lupus erythematosus
- SLICC
Systemic Lupus International Collaborating Clinics
- SSc
systemic sclerosis
- TGF
tumor growth factor
- TNF-RI
TNF-receptor type 1
- TWEAK
TNF-like weak inducer of apoptosis
- VCAM-1
vascular cell adhesion molecule
- VEGF-A
vascular endothelial growth factor-A
- YKL-40
cartilage glycoprotein 39.
Footnotes
Conflict of interest: No direct conflict. However, OMRF holds a patent for work done by Dr. James regarding soluble mediators and SLE disease onset and SLE disease flare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–43. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shooshtari P, Huang H, Cotsapas C. Integrative Genetic and Epigenetic Analysis Uncovers Regulatory Mechanisms of Autoimmune Disease. Am J Hum Genet. 2017;101:75–86. doi: 10.1016/j.ajhg.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol. 2014;10:212–28. doi: 10.1038/nrrheum.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niklas K, Niklas AA, Majewski D, Puszczewicz M. Rheumatic diseases induced by drugs and environmental factors: the state-of-the-art - part one. Reumatologia. 2016;54:122–7. doi: 10.5114/reum.2016.61212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niklas K, Niklas AA, Majewski D, Puszczewicz MJ. Rheumatic diseases induced by drugs and environmental factors: the state-of-the-art - part two. Reumatologia. 2016;54:165–9. doi: 10.5114/reum.2016.62470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Harris VM, Cavett J, Kurien BT, Liu K, Koelsch KA, et al. Rare X chromosome abnormalities in systemic lupus erythematosus and Sjogren’s syndrome. Arthritis Rheumatol. 2017 doi: 10.1002/art.40207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver JE, Silman AJ. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res Ther. 2009;11:252. doi: 10.1186/ar2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapaa-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther. 2011;13:R30. doi: 10.1186/ar3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClain MT, Arbuckle MR, Heinlen LD, Dennis GJ, Roebuck J, Rubertone MV, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50:1226–32. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 11.Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun. 2016;74:182–93. doi: 10.1016/j.jaut.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016;75:2014–21. doi: 10.1136/annrheumdis-2015-208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munroe ME, Young KA, Kamen DL, Guthridge JM, Niewold TB, Costenbader KH, et al. Discerning Risk of Disease Transition in Relatives of Systemic Lupus Erythematosus Patients Utilizing Soluble Mediators and Clinical Features. Arthritis Rheumatol. 2017;69:630–42. doi: 10.1002/art.40004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma WT, Chang C, Gershwin ME, Lian ZX. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Paul BJ, Kandy HI, Krishnan V. Pre-rheumatoid arthritis and its prevention. Eur J Rheumatol. 2017;4:161–5. doi: 10.5152/eurjrheum.2017.16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuchten N, Hoyer A, Brinks R, Schoels M, Schneider M, Smolen J, et al. Performance of Anti-nuclear Antibodies for Classifying Systemic Lupus Erythematosus: a Systematic Literature Review and Meta-regression of Diagnostic Data. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23292. [DOI] [PubMed] [Google Scholar]
- 17.Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64:2319–27. doi: 10.1002/art.34380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedeschi SK, Johnson SR, Boumpas D, Daikh D, Dorner T, Jayne D, et al. Developing and Refining New Candidate Criteria for SLE Classification: An International Collaboration. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdany J, Schmajuk G, Robbins M, Daikh D, Beall A, Yelin E, et al. Choosing wisely: the American College of Rheumatology’s Top 5 list of things physicians and patients should question. Arthritis Care Res (Hoboken) 2013;65:329–39. doi: 10.1002/acr.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritzler MJ. Choosing wisely: Review and commentary on anti-nuclear antibody (ANA) testing. Autoimmun Rev. 2016;15:272–80. doi: 10.1016/j.autrev.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of Lupus Erythematosus: Correlation between Immunopathological Features and Clinical Aspects. Autoimmune Dis. 2014;2014:321359. doi: 10.1155/2014/321359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardana EJ, Jr, Harbeck RJ, Hoffman AA, Pirofsky B, Carr RI. The prognostic and therapeutic implications of DNA:anti-DNA immune complexes in systemic lupus erythematosus (SLE) Am J Med. 1975;59:515–22. doi: 10.1016/0002-9343(75)90259-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Weinstein E, Tuzova M, Davidson A, Mundel P, Marambio P, et al. Cross-reactivity of human lupus anti-DNA antibodies with alpha-actinin and nephritogenic potential. Arthritis Rheum. 2005;52:522–30. doi: 10.1002/art.20862. [DOI] [PubMed] [Google Scholar]
- 27.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 28.Olson SW, Lee JJ, Prince LK, Baker TP, Papadopoulos P, Edison J, et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol. 2013;8:1702–8. doi: 10.2215/CJN.01910213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petri M, Singh S, Tesfasyone H, Malik A. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol. 2009;36:2476–80. doi: 10.3899/jrheum.090019. [DOI] [PubMed] [Google Scholar]
- 30.Tseng CE, Buyon JP, Kim M, Belmont HM, Mackay M, Diamond B, et al. The effect of moderate-dose corticosteroids in preventing severe flares in patients with serologically active, but clinically stable, systemic lupus erythematosus: findings of a prospective, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:3623–32. doi: 10.1002/art.22198. [DOI] [PubMed] [Google Scholar]
- 31.Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47–54. doi: 10.1080/08916930400022715. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. 2012;2012:606195. doi: 10.1155/2012/606195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briani C, Lucchetta M, Ghirardello A, Toffanin E, Zampieri S, Ruggero S, et al. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. J Autoimmun. 2009;32:79–84. doi: 10.1016/j.jaut.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Valoes CC, Molinari BC, Pitta AC, Gormezano NW, Farhat SC, Kozu K, et al. Anti-ribosomal P antibody: a multicenter study in childhood-onset systemic lupus erythematosus patients. Lupus. 2017;26:484–9. doi: 10.1177/0961203316676386. [DOI] [PubMed] [Google Scholar]
- 35.Ng KP, Manson JJ, Rahman A, Isenberg DA. Association of antinucleosome antibodies with disease flare in serologically active clinically quiescent patients with systemic lupus erythematosus. Arthritis Rheum. 2006;55:900–4. doi: 10.1002/art.22356. [DOI] [PubMed] [Google Scholar]
- 36.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 37.Putterman C, Wu A, Reiner-Benaim A, Batty DS, Jr, Sanz I, Oates J, et al. SLE-key((R)) rule-out serologic test for excluding the diagnosis of systemic lupus erythematosus: Developing the ImmunArray iCHIP((R)) J Immunol Methods. 2016;429:1–6. doi: 10.1016/j.jim.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen NJ, Li QZ, Quan J, Wang L, Mutwally A, Karp DR. Autoantibody profiling to follow evolution of lupus syndromes. Arthritis Res Ther. 2012;14:R174. doi: 10.1186/ar3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaak AJ, Groenwold J, Bronsveld W. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis. 1986;45:359–66. doi: 10.1136/ard.45.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalunian KC, Chatham WW, Massarotti EM, Reyes-Thomas J, Harris C, Furie RA, et al. Measurement of cell-bound complement activation products enhances diagnostic performance in systemic lupus erythematosus. Arthritis Rheum. 2012;64:4040–7. doi: 10.1002/art.34669. [DOI] [PubMed] [Google Scholar]
- 41.Putterman C, Furie R, Ramsey-Goldman R, Askanase A, Buyon J, Kalunian K, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med. 2014;1:e000056. doi: 10.1136/lupus-2014-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–9. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 43.Petri MA, van Vollenhoven RF, Buyon J, Levy RA, Navarra SV, Cervera R, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum. 2013;65:2143–53. doi: 10.1002/art.37995. [DOI] [PubMed] [Google Scholar]
- 44.Munroe ME, Vista ES, Guthridge JM, Thompson LF, Merrill JT, James JA. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol. 2014;66:1888–99. doi: 10.1002/art.38573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munroe ME, Vista ES, Merrill JT, Guthridge JM, Roberts VC, James JA. Pathways of impending disease flare in African-American systemic lupus erythematosus patients. J Autoimmun. 2017;78:70–8. doi: 10.1016/j.jaut.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther. 2009;11:R143. doi: 10.1186/ar2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunner HI, Bennett MR, Mina R, Suzuki M, Petri M, Kiani AN, et al. Association of noninvasively measured renal protein biomarkers with histologic features of lupus nephritis. Arthritis Rheum. 2012;64:2687–97. doi: 10.1002/art.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S, Wu T, Xie C, Vanarsa K, Han J, Mahajan T, et al. Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res Ther. 2012;14:R164. doi: 10.1186/ar3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jog NR, Blanco I, Lee I, Putterman C, Caricchio R. Urinary high-mobility group box-1 associates specifically with lupus nephritis class V. Lupus. 2016;25:1551–7. doi: 10.1177/0961203316644331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu T, Du Y, Han J, Singh S, Xie C, Guo Y, et al. Urinary angiostatin--a novel putative marker of renal pathology chronicity in lupus nephritis. Mol Cell Proteomics. 2013;12:1170–9. doi: 10.1074/mcp.M112.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritterhouse LL, Crowe SR, Niewold TB, Merrill JT, Roberts VC, Dedeke AB, et al. B lymphocyte stimulator levels in systemic lupus erythematosus: higher circulating levels in African American patients and increased production after influenza vaccination in patients with low baseline levels. Arthritis Rheum. 2011;63:3931–41. doi: 10.1002/art.30598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell. 2016;165:551–65. doi: 10.1016/j.cell.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006;3:e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 57.Landolt-Marticorena C, Bonventi G, Lubovich A, Ferguson C, Unnithan T, Su J, et al. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2009;68:1440–6. doi: 10.1136/ard.2008.093146. [DOI] [PubMed] [Google Scholar]
- 58.Petri M, Singh S, Tesfasyone H, Dedrick R, Fry K, Lal P, et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus. 2009;18:980–9. doi: 10.1177/0961203309105529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46:283–5. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 60.Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002;4(Suppl 2):S1–5. doi: 10.1186/ar551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. doi: 10.7326/0003-4819-146-11-200706050-00008. [DOI] [PubMed] [Google Scholar]
- 62.Turesson C, Jacobsson LT, Sturfelt G, Matteson EL, Mathsson L, Ronnelid J. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:59–64. doi: 10.1136/ard.2006.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Too CL, Yahya A, Murad S, Dhaliwal JS, Larsson PT, Muhamad NA, et al. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA) Arthritis Res Ther. 2012;14:R89. doi: 10.1186/ar3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-Citrullinated Protein Antibodies Are Associated With Neutrophil Extracellular Traps in the Sputum in Relatives of Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2017;69:1165–75. doi: 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.England BR, Thiele GM, Mikuls TR. Anticitrullinated protein antibodies: origin and role in the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 2017;29:57–64. doi: 10.1097/BOR.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 66.van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum. 2004;50:709–15. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 67.Gottenberg JE, Ravaud P, Cantagrel A, Combe B, Flipo RM, Schaeverbeke T, et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis. 2012;71:1815–9. doi: 10.1136/annrheumdis-2011-201109. [DOI] [PubMed] [Google Scholar]
- 68.Gardette A, Ottaviani S, Tubach F, Roy C, Nicaise-Roland P, Palazzo E, et al. High anti-CCP antibody titres predict good response to rituximab in patients with active rheumatoid arthritis. Joint Bone Spine. 2014;81:416–20. doi: 10.1016/j.jbspin.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 69.Svard A, Kastbom A, Soderlin MK, Reckner-Olsson A, Skogh T. A comparison between IgG- and IgA-class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J Rheumatol. 2011;38:1265–72. doi: 10.3899/jrheum.101086. [DOI] [PubMed] [Google Scholar]
- 70.van der Woude D, Syversen SW, van der Voort EI, Verpoort KN, Goll GL, van der Linden MP, et al. The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1110–6. doi: 10.1136/ard.2009.116384. [DOI] [PubMed] [Google Scholar]
- 71.Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menard HA, Lapointe E, Rochdi MD, Zhou ZJ. Insights into rheumatoid arthritis derived from the Sa immune system. Arthritis Res. 2000;2:429–32. doi: 10.1186/ar122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gan RW, Trouw LA, Shi J, Toes RE, Huizinga TW, Demoruelle MK, et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. J Rheumatol. 2015;42:572–9. doi: 10.3899/jrheum.140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidal-Bralo L, Perez-Pampin E, Regueiro C, Montes A, Varela R, Boveda MD, et al. Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients. PLoS One. 2017;12:e0180144. doi: 10.1371/journal.pone.0180144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Navarro-Compan V, Gherghe AM, Smolen JS, Aletaha D, Landewe R, van der Heijde D. Relationship between disease activity indices and their individual components and radiographic progression in RA: a systematic literature review. Rheumatology (Oxford) 2015;54:994–1007. doi: 10.1093/rheumatology/keu413. [DOI] [PubMed] [Google Scholar]
- 76.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–72. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maijer KI, Li W, Sasso EH, Gerlag DM, Defranoux NA, Tak PP. Does the multi-biomarker disease activity score have diagnostic value in early rheumatoid arthritis and unclassified arthritis? Ann Rheum Dis. 2015;74:2097–9. doi: 10.1136/annrheumdis-2015-207911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tony HP, Roll P, Mei HE, Blumner E, Straka A, Gnuegge L, et al. Combination of B cell biomarkers as independent predictors of response in patients with rheumatoid arthritis treated with rituximab. Clin Exp Rheumatol. 2015;33:887–94. [PubMed] [Google Scholar]
- 80.Eriksson C, Rantapaa-Dahlqvist S, Sundqvist KG. Changes in chemokines and their receptors in blood during treatment with the TNF inhibitor infliximab in patients with rheumatoid arthritis. Scand J Rheumatol. 2013;42:260–5. doi: 10.3109/03009742.2012.754937. [DOI] [PubMed] [Google Scholar]
- 81.Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499–505. doi: 10.1136/annrheumdis-2013-203923. [DOI] [PubMed] [Google Scholar]
- 82.Patro PS, Singh A, Misra R, Aggarwal A. Myeloid-related Protein 8/14 Levels in Rheumatoid Arthritis: Marker of Disease Activity and Response to Methotrexate. J Rheumatol. 2016;43:731–7. doi: 10.3899/jrheum.150998. [DOI] [PubMed] [Google Scholar]
- 83.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017 doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 84.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 85.Koenig M, Dieude M, Senecal JL. Predictive value of antinuclear autoantibodies: the lessons of the systemic sclerosis autoantibodies. Autoimmun Rev. 2008;7:588–93. doi: 10.1016/j.autrev.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 86.Moinzadeh P, Fonseca C, Hellmich M, Shah AA, Chighizola C, Denton CP, et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther. 2014;16:R53. doi: 10.1186/ar4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okano Y, Medsger TA., Jr Autoantibody to Th ribonucleoprotein (nucleolar 7-2 RNA protein particle) in patients with systemic sclerosis. Arthritis Rheum. 1990;33:1822–8. doi: 10.1002/art.1780331210. [DOI] [PubMed] [Google Scholar]
- 88.Sharif R, Fritzler MJ, Mayes MD, Gonzalez EB, McNearney TA, Draeger H, et al. Anti-fibrillarin antibody in African American patients with systemic sclerosis: immunogenetics, clinical features, and survival analysis. J Rheumatol. 2011;38:1622–30. doi: 10.3899/jrheum.110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fertig N, Domsic RT, Rodriguez-Reyna T, Kuwana M, Lucas M, Medsger TA, Jr, et al. Anti-U11/U12 RNP antibodies in systemic sclerosis: a new serologic marker associated with pulmonary fibrosis. Arthritis Rheum. 2009;61:958–65. doi: 10.1002/art.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah AA, Xu G, Rosen A, Hummers LK, Wigley FM, Elledge SJ, et al. Brief Report: Anti-RNPC-3 Antibodies As a Marker of Cancer-Associated Scleroderma. Arthritis Rheumatol. 2017;69:1306–12. doi: 10.1002/art.40065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yanaba K, Hasegawa M, Takehara K, Sato S. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J Rheumatol. 2004;31:1112–20. [PubMed] [Google Scholar]
- 92.Williams MH, Handler CE, Akram R, Smith CJ, Das C, Smee J, et al. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27:1485–94. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 93.Hesselstrand R, Kassner A, Heinegard D, Saxne T. COMP: a candidate molecule in the pathogenesis of systemic sclerosis with a potential as a disease marker. Ann Rheum Dis. 2008;67:1242–8. doi: 10.1136/ard.2007.082099. [DOI] [PubMed] [Google Scholar]
- 94.Yanaba K, Asano Y, Tada Y, Sugaya M, Kadono T, Sato S. Clinical significance of serum growth differentiation factor-15 levels in systemic sclerosis: association with disease severity. Mod Rheumatol. 2012;22:668–75. doi: 10.1007/s10165-011-0568-7. [DOI] [PubMed] [Google Scholar]
- 95.Lambrecht S, Smith V, De Wilde K, Coudenys J, Decuman S, Deforce D, et al. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol. 2014;66:418–27. doi: 10.1002/art.38241. [DOI] [PubMed] [Google Scholar]
- 96.Meadows CA, Risbano MG, Zhang L, Geraci MW, Tuder RM, Collier DH, et al. Increased expression of growth differentiation factor-15 in systemic sclerosis-associated pulmonary arterial hypertension. Chest. 2011;139:994–1002. doi: 10.1378/chest.10-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, et al. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther. 2009;11:R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140–6. doi: 10.1016/s0923-1811(01)00128-1. [DOI] [PubMed] [Google Scholar]
- 99.De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40:435–46. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 100.van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370:433–43. doi: 10.1056/NEJMoa1114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu X, Wu WY, Tu WZ, Chu HY, Zhu XX, Liang MR, et al. Increased expression of S100A8 and S100A9 in patients with diffuse cutaneous systemic sclerosis. A correlation with organ involvement and immunological abnormalities. Clin Rheumatol. 2013;32:1501–10. doi: 10.1007/s10067-013-2305-4. [DOI] [PubMed] [Google Scholar]
- 102.Hesselstrand R, Wildt M, Bozovic G, Andersson-Sjoland A, Andreasson K, Scheja A, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respir Med. 2013;107:1079–86. doi: 10.1016/j.rmed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 103.van Bon L, Cossu M, Loof A, Gohar F, Wittkowski H, Vonk M, et al. Proteomic analysis of plasma identifies the Toll-like receptor agonists S100A8/A9 as a novel possible marker for systemic sclerosis phenotype. Ann Rheum Dis. 2014;73:1585–9. doi: 10.1136/annrheumdis-2013-205013. [DOI] [PubMed] [Google Scholar]
- 104.Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Ruoppolo G, et al. Churg-Strauss syndrome. Autoimmun Rev. 2015;14:341–8. doi: 10.1016/j.autrev.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Zwerina J, Bach C, Martorana D, Jatzwauk M, Hegasy G, Moosig F, et al. Eotaxin-3 in Churg-Strauss syndrome: a clinical and immunogenetic study. Rheumatology (Oxford) 2011;50:1823–7. doi: 10.1093/rheumatology/keq445. [DOI] [PubMed] [Google Scholar]
- 106.Vaglio A, Strehl JD, Manger B, Maritati F, Alberici F, Beyer C, et al. IgG4 immune response in Churg-Strauss syndrome. Ann Rheum Dis. 2012;71:390–3. doi: 10.1136/ard.2011.155382. [DOI] [PubMed] [Google Scholar]
- 107.Dallos T, Heiland GR, Strehl J, Karonitsch T, Gross WL, Moosig F, et al. CCL17/thymus and activation-related chemokine in Churg-Strauss syndrome. Arthritis Rheum. 2010;62:3496–503. doi: 10.1002/art.27678. [DOI] [PubMed] [Google Scholar]
- 108.Szczeklik W, Sanak M, Mastalerz L, Sokolowska BM, Gielicz A, Soja J, et al. 12-hydroxy-eicosatetraenoic acid (12-HETE): a biomarker of Churg-Strauss syndrome. Clin Exp Allergy. 2012;42:513–22. doi: 10.1111/j.1365-2222.2011.03943.x. [DOI] [PubMed] [Google Scholar]
- 109.Kallenberg CG. Key advances in the clinical approach to ANCA-associated vasculitis. Nat Rev Rheumatol. 2014;10:484–93. doi: 10.1038/nrrheum.2014.104. [DOI] [PubMed] [Google Scholar]
- 110.Franssen CF, Stegeman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 111.Takakuwa Y, Kurokawa MS, Ooka S, Sato T, Nagai K, Arito M, et al. AC13, a C-terminal fragment of apolipoprotein A-I, is a candidate biomarker for microscopic polyangiitis. Arthritis Rheum. 2011;63:3613–24. doi: 10.1002/art.30560. [DOI] [PubMed] [Google Scholar]
- 112.de Joode AA, Sanders JS, Stegeman CA. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol. 2013;8:1709–17. doi: 10.2215/CJN.01020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawai H, Banno S, Kikuchi S, Nishimura N, Nobata H, Kimura Y, et al. Retrospective analysis of factors predicting end-stage renal failure or death in patients with microscopic polyangiitis with mainly renal involvement. Clin Exp Nephrol. 2014;18:795–802. doi: 10.1007/s10157-013-0926-1. [DOI] [PubMed] [Google Scholar]
- 114.Lai Y, Xue C, Liao Y, Huang L, Peng Q, Huang B, et al. Differential Expression of Toll-Like Receptor Signaling Pathway Is Associated with Microscopic Polyangiitis in Peripheral Blood Neutrophils. Immunol Invest. 2017;46:375–84. doi: 10.1080/08820139.2017.1288236. [DOI] [PubMed] [Google Scholar]
- 115.Wilde B, van Paassen P, Witzke O, Tervaert JW. New pathophysiological insights and treatment of ANCA-associated vasculitis. Kidney Int. 2011;79:599–612. doi: 10.1038/ki.2010.472. [DOI] [PubMed] [Google Scholar]
- 116.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis--a meta-analysis. Rheumatology (Oxford) 2012;51:100–9. doi: 10.1093/rheumatology/ker280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kemna MJ, Schlumberger W, van Paassen P, Dahnrich C, Damoiseaux JG, Cohen Tervaert JW. The avidity of PR3-ANCA in patients with granulomatosis with polyangiitis during follow-up. Clin Exp Immunol. 2016;185:141–7. doi: 10.1111/cei.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wuhrer M, Stavenhagen K, Koeleman CA, Selman MH, Harper L, Jacobs BC, et al. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J Proteome Res. 2015;14:1657–65. doi: 10.1021/pr500780a. [DOI] [PubMed] [Google Scholar]
- 119.Kemna MJ, Plomp R, van Paassen P, Koeleman CA, Jansen BC, Damoiseaux JG, et al. Galactosylation and Sialylation Levels of IgG Predict Relapse in Patients With PR3-ANCA Associated Vasculitis. EBioMedicine. 2017;17:108–18. doi: 10.1016/j.ebiom.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pepper RJ, Draibe JB, Caplin B, Fervenza FC, Hoffman GS, Kallenberg CG, et al. Association of Serum Calprotectin (S100A8/A9) Level With Disease Relapse in Proteinase 3-Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2017;69:185–93. doi: 10.1002/art.39814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henes FO, Chen Y, Bley TA, Fabel M, Both M, Herrmann K, et al. Correlation of serum level of high mobility group box 1 with the burden of granulomatous inflammation in granulomatosis with polyangiitis (Wegener’s) Ann Rheum Dis. 2011;70:1926–9. doi: 10.1136/ard.2010.146456. [DOI] [PubMed] [Google Scholar]
- 122.Szczeklik W, Jakiela B, Wawrzycka-Adamczyk K, Sanak M, Hubalewska-Mazgaj M, Padjas A, et al. Skewing toward Treg and Th2 responses is a characteristic feature of sustained remission in ANCA-positive granulomatosis with polyangiitis. Eur J Immunol. 2017;47:724–33. doi: 10.1002/eji.201646810. [DOI] [PubMed] [Google Scholar]