Abstract
Cortical stimulation mapping (CSM) has provided important insights into the neuroanatomy of language, due to its high spatial and temporal resolution, and the causal relationships that can be inferred from transient disruption of specific functions. Almost all CSM studies to date have focused on word level processes such as naming, comprehension and repetition. In this study, we used CSM to identify sites where stimulation interfered selectively with syntactic encoding during sentence production. Fourteen patients undergoing left hemisphere neurosurgery participated in the study. In seven of the fourteen patients, we identified a total of nine sites where cortical stimulation interfered with syntactic encoding, but did not interfere with single word processing. All nine sites were localized to the inferior frontal gyrus, mostly to the pars triangularis and opercularis. Interference with syntactic encoding took several different forms, including misassignment of arguments to grammatical roles, misassignment of nouns to verb slots, omission of function words and inflectional morphology, and various paragrammatic constructions. Our findings suggest that the left inferior frontal gyrus plays an important role in the encoding of syntactic structure during sentence production.
1. Introduction
Cortical stimulation mapping (CSM) is widely used to identify critical language and motor sites prior to resective surgery, so that essential sites can be spared in order to minimize post-operative deficits (Duffau, Gatignol, Mandonnet, Capelle, & Taillandier, 2008; Haglund, Berger, Shamseldin, Lettich, & Ojemann, 1994; Ojemann, Ojemann, Lettich, & Berger, 1989; Penfield and Roberts, 1959). Due to its high spatial and temporal resolution, CSM has provided important insights into the cortical organization of motor (Krause, 1909), sensory (Cushing, 1909), and language (Duffau et al., 2003, 2005; Ojemann, 1983; Ojemann et al., 1989; Penfield and Roberts, 1959) functions. While the most commonly used language tasks are picture naming and automatic speech (e.g. counting), CSM studies using a range of other tasks such as phonemic perception (Boatman, 2004), comprehension of spoken and written words (Roux et al., 2015), and repetition of words and pseudowords (Leonard, Cai, Babiak, Ren, & Chang, 2016) have revealed discrete localization of many of the component processes of language. However, only a minority of CSM studies have investigated language processes beyond the level of single words (Rofes and Miceli, 2014).
In this study, we used CSM to investigate the neural substrates of syntactic encoding during sentence production. Syntactic encoding entails the mapping of lexical items (lemmas) onto thematic and grammatical roles, the generation of hierarchical, linearly sequenced, syntactic constituents, and the inflection of open and closed class words for grammatical categories such as tense and agreement (Ferreira and Engelhardt, 2006; Garrett, 1980). We applied direct electrocortical stimulation as patients undergoing awake craniotomy attempted to describe pictures of transitive actions using complete sentences. Three other tasks—counting, picture naming, and repetition—were also performed to determine the specificity of any disturbances of syntactic encoding. We reasoned that any brain regions where cortical stimulation interferes with sentence production, but not with single word or automatic speech tasks, must play an important role in syntactic encoding during sentence production. On the basis of prior lesion-deficit (Sapolsky et al., 2010; Wilson et al., 2010b) and functional neuroimaging (Haller, Radue, Erb, Grodd, & Kircher, 2005; Indefrey et al., 2001; Indefrey, Hellwig, Herzog, Seitz, & Hagoort, 2004) studies, we hypothesized that the left inferior frontal gyrus (IFG) is critically important for syntactic encoding.
2. Materials and methods
2.1. Participants
Fourteen patients undergoing left hemisphere awake craniotomy (8 men, 6 women, mean age = 46 years, age range = 21 to 70 years) took part in this study (Table 1). The inclusion criteria were (1) awake craniotomy involving electrocortical stimulation mapping to identify and preserve eloquent cortex; (2) first time brain surgery; (3) significant exposure of left frontal and temporal perisylvian cortex; (4) no significant pre-operative language deficits, per the Western Aphasia Battery (Kertesz, 1982) or the Quick Aphasia Battery (Wilson, Eriksson, Schneck, & Lucanie, submitted).
Table 1.
Patient demographic and etiological information
| Patient | Sex | Age | Handedness | Etiology | Site of resection |
|---|---|---|---|---|---|
| P1 | M | 66 | R | Epilepsy | Anterior temporal lobe, hippocampus |
| P2 | F | 27 | R | Epilepsy and mild gliosis | Anterior temporal lobe, hippocampus |
| P3 | M | 46 | A | Epilepsy | Hippocampus, amygdala |
| P4 | F | 21 | L | Epilepsy and gliosis | Anterior temporal lobe, hippocampus, amygdala |
| P5 | F | 40 | R | Epilepsy | Anterior temporal lobe, hippocampus |
| P6 | F | 48 | R | Epilepsy | Anterior temporal lobe, hippocampus |
| P7 | M | 27 | R | Grade III oligodendroglioma | Insula, frontal operculum |
| P8 | M | 58 | R | Grade IV glioblastoma multiforme | Insula |
| P9 | M | 61 | R | Grade IV glioblastoma multiforme | Anterior temporal lobe |
| P10 | M | 68 | R | Grade IV glioblastoma multiforme | Posterior superior temporal gyrus |
| P11 | F | 63 | R | Grade IV glioblastoma multiforme | Insula |
| P12 | M | 27 | R | Cavernous malformation | Inferior temporal lobe |
| P13 | F | 70 | R | Cavernous malformation | Ventral precentral gyrus |
| P14 | M | 30 | R | Cavernous malformation | Medial temporal lobe, head of hippocampus |
Etiology was epilepsy in 6 cases, glioma in 5 cases, and cavernous malformation in 3 cases. Resection sites are outlined in Table 1. Twelve of the patients were right-handed, one (P4) was left-handed with mixed language dominance per Wada testing, and one (P3) was ambidextrous with left-lateralized language dominance per Wada testing. Twelve of the patients were native speakers of English and the remaining two (P2, P13) were fluent in English. One patient (P8) underwent a second awake resection seven months later, and the data obtained during this second procedure were also included in the study.
The study was approved by the UCSF Human Research Protection Program. All CSM was carried out for clinical purposes, including the sentence production component. Participants gave written informed consent for the use of this data for research.
2.2. Cortical stimulation mapping
Patients underwent CSM to determine essential language and sensorimotor sites located in the exposed left hemisphere cortex. Electrocortical stimulation was carried out using an Ojemann Cortical Stimulator (Integra LifeSciences, Plainsboro, NJ) with typical settings (60 Hz, bipolar, biphasic, 1 ms pulse width). Stimulation threshold was determined on an individual basis, such that speech arrest could be elicited without causing after-discharges as determined by intraoperative electrocorticography. This threshold fell between 1.5 and 4.5 mA for all participants. The standard language tasks used were counting, picture naming, and repetition, all of which have been described previously (Corina et al., 2010; Haglund et al., 1994; Leonard et al., 2016; Ojemann, 1978; Ojemann et al., 1989). In some patients, additional tasks were used depending on the surgical site, for instance, naming to auditory definition, and reading. Language and sensorimotor sites were temporarily marked with sterile paper tags.
2.3. Sentence production task during electrocortical stimulation
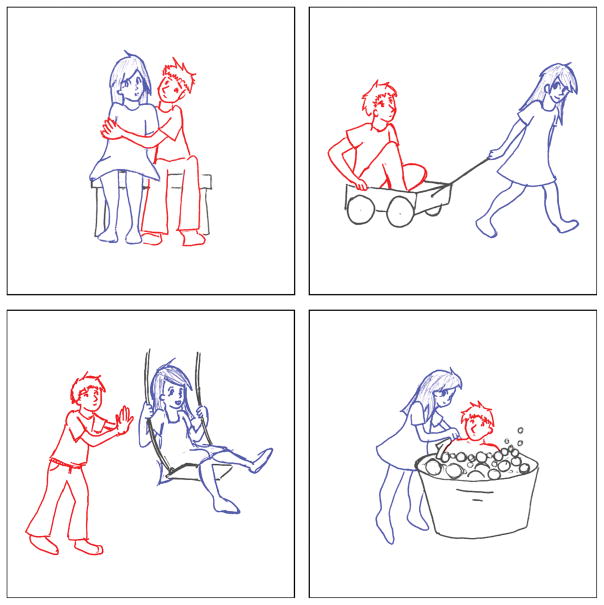
To elicit sentence production, patients were presented with pictures depicting a boy and a girl engaged in one of seven simple transitive actions (push, pull, hug, kiss, kick, chase, wash) (Wilson et al., 2010a) (Figure 1). Either the boy or the girl could be the agent, so there were 14 pictures in total. Patients were asked to describe each picture using a simple sentence (e.g. the boy is pushing the girl). The two nouns and seven verbs required to describe the pictures were all high in frequency and were used repeatedly throughout the procedure, minimizing demands on lexical access. The task was practiced prior to surgery, at which time all patients were readily able to describe the action pictures using appropriate syntactic constructions and lexical items.
Figure 1.
Examples of stimulus pictures.
During awake craniotomy, after completion of the other CSM tasks, action pictures were presented for description using a laptop computer, while stimulation was applied to a range of exposed frontal, temporal and parietal sites. Pictures were presented in random order, and were repeated as necessary since there were more trials than pictures. At the onset of each trial, a sound cued the surgeon to begin stimulation. The action picture was presented 1 s after the sound, corresponding to about 500 ms after the onset of stimulation. Stimulation continued until the patient had completed their response (except in the case of speech arrest). When sites were found where stimulation interfered with syntactic encoding, the same location was stimulated at least twice more nonconsecutively, to determine whether the disturbance was reproducible. Action pictures for sentence production were also presented periodically in the absence of cortical stimulation, to determine the baseline rate and nature of any errors.
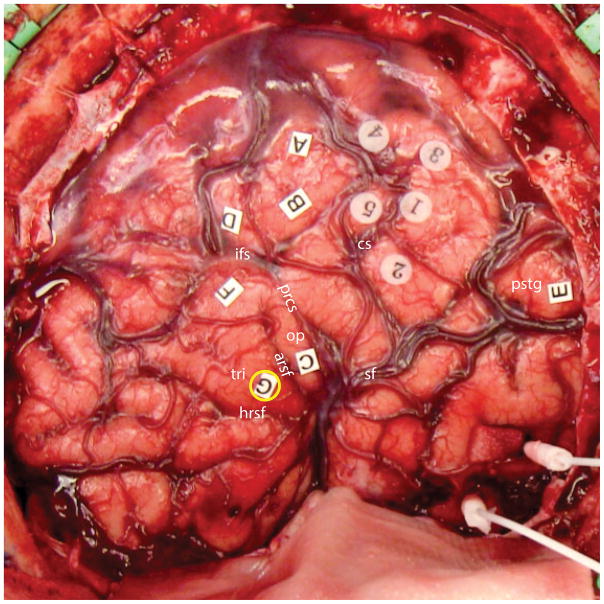
Once mapping was completed, a photograph of the completed map was taken and sites were registered using Brainlab stereotactic navigation system. An example of a completed map for one patient is shown in Figure 2.
Figure 2.
Final cortical stimulation map from a representative patient (P1). One syntactic encoding site was observed in this patient, localized to the pars triangularis of the inferior frontal gyrus (label G, yellow circle). Six other language sites were documented: stimulation of A, B, C, D and F resulted in speech arrest, while stimulation of site E interfered with comprehension. Sites 1 through 5 were sensorimotor sites. Abbreviations: cs = central sulcus; ifs = inferior frontal sulcus; prcs = precentral sulcus; arsf = ascending ramus of Sylvian fissure; hrsf = horizontal ramus of Sylvian fissure; op = pars opercularis of the inferior frontal gyrus; tri = pars triangularis of the inferior frontal gyrus; sf = Sylvian fissure; pstg = posterior superior temporal gyrus.
2.4. Analysis of sentence production data
Audiovisual recordings were made of all sessions, and every trial from every patient was transcribed and coded independently by two of the authors (GK and SMW), one of whom (SMW) was blinded to stimulus site. The two authors then discussed and resolved each discrepancy to arrive at a final set of transcriptions and codes reflecting mutual agreement.
Each trial was coded with one or more of nine possible error codes. (1) Syntactic errors were defined as utterances that were ungrammatical in any way, or where the arguments were mis-assigned to thematic/grammatical roles (e.g. the boy is pulling the girl in response to the picture of the girl pulling the boy). Missing determiners were not counted as syntactic errors, because we found that some patients colloquially omitted determiners on trials without stimulation, e.g. boy’s kissing a girl. (2) Semantic paraphasias were defined as real words that were not appropriate for describing the presented stimulus. (3) Phonemic paraphasias were defined as additions, deletions or substitutions of phonemes, or neologistic forms for which was the target was unclear. (4) Perseveration was coded when the action was described with the wrong verb, reflecting the action of a previous trial. No other types of perseveration were possible, given the experimental design. (5) Pauses could be filled (um, uh, etc.) or unfilled. (6) Retracings were defined as sequences of one or more complete words that were made redundant by subsequent repetitions or amendments. Virtually all retracings involved pauses, so if an utterance was coded as retraced, it was not also coded as containing a pause. (7) False starts were coded when words were abandoned after just one or two phonemes had been produced. (8) Abandoned utterances were coded when the patient did not complete the sentence. (9) Speech arrest was defined as the complete or near-complete interruption of speech production for the duration of stimulation. Trials with speech arrest were not coded for other error codes. Finally, 15 trials (6 with stimulation, 9 without) were excluded for a variety of reasons, the most common being late onset of stimulation, or significant after-discharge or seizure activity related to the previous trial.
While trials coded as syntactic errors were considered to provide the clearest evidence of disruption of syntactic encoding, several other error codes could potentially also reflect interference with this process. For instance, retracings may reflect attempts to amend and repair incorrectly produced sequences, while perseverations may reflect difficulties in linking appropriate lexical items to the open slots in the syntactic structure.
Sites where cortical stimulation reproducibly and selectively interfered with syntactic encoding will be referred to as syntactic encoding sites, and were defined as follows. Each syntactic encoding site (1) was stimulated at least three times; (2) yielded a syntactic error as defined above (that is, an unambiguously ungrammatical utterance) on at least one trial; (3) interfered with sentence production in a manner consistent with disruption of syntactic encoding on at least 50% of stimulations; these utterances included syntactic errors but also could include retracings, perseverations, or occasionally other kinds of errors that were consistent with interference with syntactic encoding and (4) was not identified as a language site through the standard CSM tasks of counting, picture naming or repetition. These criteria ensured that the sites identified were reliably and selectively associated with disruption of syntactic encoding.
3. Results
Across the 14 patients, electrocortical stimulation was applied on a total of 419 trials (233 IFG; 121 superior and middle temporal gyri; 30 middle frontal gyrus; 21 inferior parietal lobule; 14 precentral or postcentral gyri), and there were 85 control trials where no stimulation was applied (mean 6.1 per patient, range 2 to 13). There were 88 distinct sites stimulated in the IFG (mean 6.3 per patient, range 2 to 9), 62 in the superior and middle temporal gyri (mean 4.4 per patient, range 1 to 10), and 28 elsewhere in the middle frontal gyrus, inferior parietal lobule, precentral gyrus or postcentral gyrus (mean 2.0 per patient, range 0 to 8).
In 7 out of 14 patients, we found syntactic encoding sites where stimulation interfered with syntactic encoding in a reproducible manner, but did not disrupt word-level language function or automatic speech (Figure 3). In five of these patients, one syntactic site was identified, and in the other two patients, two sites were identified. All nine sites were localized to the IFG: five to the pars triangularis, three to the pars opercularis, and one to the pars orbitalis.
Figure 3.
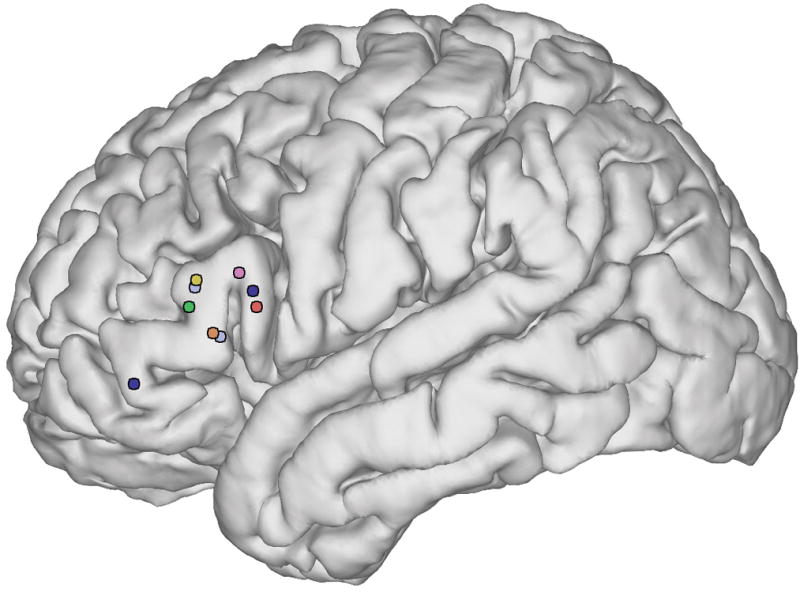
Syntactic encoding sites. Colors denote the seven patients in whom sites were observed: orange = P1; yellow = P2; light blue = P3; red = P7; dark blue = P8; green = P12; pink = P14.
Transcriptions and codes of all sentences produced during stimulation of these nine sites are shown in Table 2. Disruptions of syntactic encoding took several different forms, including misassignment of arguments to grammatical roles (e.g. the boy is chasing the boy, P12; the girl is being kissed by the girl, P7), misassignment of nouns to verb slots (e.g. the girl is boying (.) is uh (.) pushing the boy, P1), omission of function words and/or inflectional morphology (e.g. girl (.) kick boy, P8; girl is (..) kiss a (.) kiss a boy, P8), and various paragrammatic constructions (e.g. uh (.) the girl (.) is (.) is (..) um (.) it was bathed she bathed him, P8).
Table 2.
Transcriptions of all responses elicited at each sentence production site
| Patient | Site | Stimulus | Response | Error code(s) |
|---|---|---|---|---|
| P1 | tri | boy hugging girl | the boy (.) is kicking (.) the girl (...) I mean is hugging | per/ret |
| girl chasing boy | (..) the girl is boying (...) uh the girl is kicking, the girl is chasing the boy | syn/per/ret | ||
| boy washing girl | (...) the girl (.) is being tʃeɪ- er, being (.) scrubbed (.) by the boy | fs/pau/ret | ||
| girl pushing boy | (...) the girl is boying (.) is uh (.) pushing the boy | syn/ret | ||
| boy pushing girl | the boy is pushing the girl | none | ||
| P2 | tri | girl chasing boy | the girl is chasing the boy | none |
| boy washing girl | () the girl is (...) bathing (...) bathing the girl (.) the boy is bathing the girl | syn/ret | ||
| girl washing boy | the boy is pulling... | per/aban | ||
| boy hugging girl | (...) boy is hugging the girl | none | ||
| P3 | tri (ventral) | boy pushing girl | the girl is (..) uh let me try that again (.) the boy is pushing the girl | ret |
| girl kissing boy | the girl is (.) kicking the (..) is kɪ- moving uh holding the bʌg the boy | ret/fs/sem/phon | ||
| girl kicking boy | the () girl is kicking the boy | arr | ||
| girl kicking boy | the um girl is kicking the (...) the boy is kicking the girl | syn/ret | ||
| tri (dorsal) | girl washing boy | the (...) um bɔ- the girl is (...) washing her wrist | fs/ret | |
| boy kissing girl | um (.) the (.) I can’t (.) uh (.) jeez the [wəɹs] uh chasing a girl | arr | ||
| boy kissing girl | the girl is kissing the (.) the boy is kissing the the girl | syn/ret | ||
| girl washing boy | (...) uh the girl is (..) um (.) washing the boy | arr | ||
| P7 | op | girl kicking boy | the boy is being kissed (.) by the girl, kicked | per/ret |
| girl kissing boy | the girl is being kissed by the girl | syn | ||
| girl washing boy | he’s being washed by the girl | none | ||
| P8 | op | girl pulling boy | uh girl is pulling a boy | none |
| boy pushing girl | uh (..) well I (..) boy is pushing girl in a swing | arr | ||
| girl kissing boy | girl is (..) kiss a (.) kiss a boy | syn/ret | ||
| girl kicking boy | girl (.) kick boy | syn/pau | ||
| boy chasing girl* | um ... girl is is boy chasing girl | arr | ||
| boy kicking girl* | um (.) boy kɪ- kɪ- kicking kicking girl or | fs/ret | ||
| boy pushing girl* | um (..) sh- uh he’s p- p- uh s- sw- swinging him (.) on a little swing (.) he’s pushing her | syn/fs/ret | ||
| boy kissing girl* | the girl is kɪ- kɪ- kissing the boy ah is kiss- I mean the boy is kissing the girl | syn/fs/ret | ||
| boy kissing girl* | the girl’s (.) kiss- is kiss- kissing (.) the boy is kissing the girl | syn/ret | ||
| girl washing boy* | ah (.) the girl (.) is (.) is (..) um (.) it was bathed she bathed him | syn/ret | ||
| orb | boy kicking girl | (..) um (..) the (.) uh boy is uh um (..) uh um (.) kick girl | syn/pau | |
| girl washing boy | um (..) girl is chasing (.) a boy | pau | ||
| girl pushing boy | same thing um (..) boy a- are um (..) oh switcheroos ok xxx a girl (.) pushin’ a boy (.) in a swing | syn/fs/ret | ||
| boy chasing girl | um (..) girl (.) um (.) boy is chasing girl | ret | ||
| boy washing girl | boy is (..) is uh um (..) is uh uh (.) um (.) washing (.) uh (.) wash girl in the tub | syn/ret | ||
| P12 | tri | boy kissing girl | the boy is kissing the girl | none |
| girl chasing boy | the boy is chasing the boy ... er sorry did i say that wrong? the girl is chasing the boy | syn | ||
| boy pushing girl | the girl is (.) s- swinging the girl | syn/fs/pau | ||
| girl pulling boy | the girl is pulling the boy | none | ||
| P14 | op | girl chasing boy | um (..) girl is chasing (.) boy | pau |
| boy pushing girl | (...) boy is effective um (..) sorry ha, boy is pushing the girl’s swing | sem/pau | ||
| girl pulling boy | (..) um boy (.) has (..) turned um (.) trailer | sem/pau | ||
| boy pulling girl | (...) boy (.) i- is chase of girl in th- um (..) trailer | syn/per/fs/pau | ||
| boy washing girl | (...) boy has (.) enough um (...) boy... | sem/ret |
Sites: op = pars opercularis; tri = pars triangularis; orb = pars orbitalis. Error codes: syn = syntactic error (utterances in bold typeface); sem = semantic paraphasia; phon = phonemic paraphasia; per = perseveration; pau = pause; ret = retracing; fs = false start; aban = abandoned utterance; arr = speech arrest.
= Trails from second surgery.
Syntactic errors were also elicited by inferior frontal stimulation on a handful of trials from some of the seven patients for whom no sites were identified that met our definition of syntactic encoding sites. For example, P13 produced the boy is washing the boy when the pars triangularis was stimulated, and P6 produced the boy is pulling the girl (in response to the picture of the girl pulling the boy) when the pars opercularis was stimulated. Moreover, some of the seven patients in whom syntactic encoding sites were identified also produced some errors when other inferior frontal locations were stimulated; for instance, P14, who showed a reproducible site in the pars opercularis, also produced the agrammatic sentence girl is hug the boy when a different site in the pars opercularis was stimulated. In each of these three examples, the reproducibility of the syntactic disturbances was not demonstrated and therefore these sites did not meet our criteria for syntactic encoding sites. That said, repeated response effects after stimulation can sometimes be inconsistent, even in sensory and motor cortex, and such errors did not occur at all in the absence of stimulation.
In one patient (P10), a site was identified in the superior temporal gyrus where disruption of sentence production was reproducible. However, stimulation of this site also reliably disrupted the word-level tasks of naming and repetition, therefore it was not considered a syntactic encoding site. Syntactic errors elicited from stimulation of this site included (.) um (.) boy is (..) kicking the boy (.) đ- the girl and the uh (.) uh girl is m- g- (.) the boy.
As an alternative analytical approach that did not depend on our definition of syntactic encoding sites, we tabulated across all 14 patients the percentages of trials coded with each of the nine error types as a function of stimulation site (Table 3). This analysis showed that stimulation of the IFG was more likely than stimulation of temporal cortex to result in syntactic errors (p = 0.002), perseverations (p = 0.040) and retracings (p = 0.030), but not any of the other error types. Syntactic errors are likely to directly reflect disruption of syntactic encoding, while perseverations and retracings could also represent consequences of interference with syntactic encoding.
Table 3.
Percentages of error types arising from stimulation to each brain region
| Error code | IFG | STG/MTG | Other | No stimulation | p (F ≠ T) | p (F ≠ N) |
|---|---|---|---|---|---|---|
| Syntactic error (%) | 10 | 2 | 0 | 0 | 0.0022* | 0.0006* |
| Semantic paraphasia (%) | 2 | 0 | 2 | 0 | N/A | N/A |
| Phonemic paraphasia (%) | 1 | 2 | 2 | 1 | N/A | N/A |
| Perserveration (%) | 7 | 2 | 2 | 2 | 0.040* | 0.17 |
| Pause (%) | 21 | 21 | 12 | 16 | 0.89 | 0.35 |
| Retracing (%) | 16 | 7 | 9 | 6 | 0.030* | 0.024* |
| False start (%) | 7 | 5 | 5 | 5 | 0.50 | 0.61 |
| Abandoned utterance (%) | 1 | 2 | 0 | 0 | N/A | N/A |
| Speech arrest (%) | 10 | 4 | 8 | 0 | 0.064 | 0.0006* |
| Total number of trials | 233 | 121 | 65 | 85 |
IFG = inferior frontal gyrus; STG/MTG = superior temporal gyrus/middle temporal gyrus. Error frequencies were compared between trials with frontal (F) and temporal (T) stimulation, and between frontal (F) and no (N) stimulation, for error types that occurred at least 10 times, using a series of Fisher’s exact tests (2-tailed).
= Significant after correction for multiple comparisons using positive false discovery rate (Storey, 2002) as implemented in the MATLAB function mafdr.
4. Discussion
Our results indicate that the left IFG is critically important for syntactic encoding during sentence production. We observed clear evidence that direct electrocortical stimulation of the IFG resulted in reproducible and selective disturbances of sentence production. Syntactic encoding sites were documented in 7 out of 14 patients (50%), which is comparable to the frequency at which speech arrest sites are detected in the course of standard CSM (58.0%; Sanai, Mirzadeh, & Berger, 2008), and higher than the frequency at which sites associated with anomia have been identified (32.8%; Sanai et al., 2008). All but one of the syntactic encoding sites were localized to the pars triangularis or pars opercularis of the IFG, which together make up Broca’s area (Amunts et al., 1999). None of the syntactic encoding sites were identified during routine cortical stimulation mapping tasks of counting, naming, or repetition, suggesting that these sites are selectively involved in sentence production.
While few CSM studies have investigated language processes beyond the word level (Rofes and Miceli, 2014), there have been several prior studies that have used tasks incorporating aspects of syntactic encoding. Vidorreta, Garcia, Moritz-Gasser, and Duffau (2011) used CSM to investigate the production of determiners in French, which are marked for grammatical gender, a lexical-syntactic feature. In six out of nine patients, sites were found where cortical stimulation elicited reproducible disturbances of grammatical gender selection. In three patients, these sites were localized to the IFG, and in the other three, they were localized to the posterior middle temporal gyrus. Interestingly, there was no overlap between sites linked to grammatical gender processing and those where stimulation induced naming disturbances. The involvement of temporal as well as frontal sites in producing determiners marked for grammatical gender may reflect the fact that grammatical gender is a syntactic property of individual lexical items; that is, grammatical gender is largely arbitrary and so must be stored in the lexical entry for each noun.
Lubrano, Filleron, Démonet, and Roux (2014) compared object and action naming, but in the later case, the verb describing the action was required to be produced in a context requiring finite inflection (e.g. he runs). Naming errors were elicited from a range of frontal, temporal and parietal sites, with a tendency for frontal sites to be differentially associated with action naming disturbances. However the authors did not report that any (morpho-)syntactic disturbances were induced (e.g. he run). Gonen et al. (2017) reported that one out of a series of fifteen patients made syntactic errors when two sites were simultaneously stimulated—one in the IFG and one in the anterior superior temporal sulcus—but the nature of the syntactic errors was not described.
Syntactic errors induced by cortical stimulation have been described by Ojemann and Mateer (1979) and Ojemann (1983), e.g. If my son will getting late today, he’ll see the principal (Ojemann & Mateer, 1979, p. 1402). Errors of this nature were elicited through stimulation of several frontal, temporal and parietal sites, unlike in the present study where such errors were elicited only by inferior frontal stimulation. The tasks used in these studies involved completion of written sentences, or reading of written sentences. It is possible that the discrepancy between these findings and the present study may relate to the receptive syntactic component entailed by the sentence reading tasks. Several studies have used CSM to investigate sentence comprehension (Bello et al., 2007) and naming to auditory description, which depends on sentence comprehension (Hamberger, McClelland, McKhann, Williams, A. C., & Goodman, 2007; Hamberger, Seidel, Goodman, Perrine, & McKhann, 2003; Hamberger, Seidel, Mckhann, Perrine, & Goodman, 2005), and have uniformly reported that these tasks are disrupted by stimulation of posterior temporal and inferior parietal sites, rather than frontal sites. This suggests that the distributed set of regions reported to elicit syntactic errors in sentence reading and sentence completion tasks may reflect a combination of expressive disturbances (frontal sites) and receptive disturbances (posterior sites). Other studies using sentence reading have reported several kinds of disturbances induced by frontal, temporal and parietal stimulation, but syntactic disturbances were not included among the types of disruptions reported (Roux and Tremoulet, 2002; Roux et al., 2004).
Our finding that the left IFG is important for syntactic encoding during sentence production accords well with some findings from other methodologies. Using positron emission tomography, Indefrey et al. (2001, 2004) showed that caudal Broca’s area is modulated by the complexity of syntactic encoding during a restrictive scene description task. Another study using functional MRI showed that Broca’s area is recruited for re-ordering pseudo-randomly ordered sets of words into grammatical sentences, above and beyond its involvement in word or sentence reading (Haller et al., 2005). Anodal transcranial direct current stimulation of Broca’s area has been reported to improve sentence production in healthy participants (Nozari, Arnold, & Thompson-Schill, 2014) and individuals with aphasia (Marangolo et al., 2013).
Some lesion-deficit studies are also concordant with the present findings. In primary progressive aphasia, neurodegeneration of left inferior frontal regions is associated with syntactic errors, non-sentence utterances, and reduced use of embedded clauses (Wilson et al., 2010b), and with reductions on grammar and fluency measures (Sapolsky et al., 2010; Wilson et al., 2011). One study in an acute stroke patient showed that transient hypoperfusion of Broca’s area resulted in agrammatism, among other language symptoms, which resolved when perfusion was restored (Davis et al., 2008). Generally in vascular aphasia, frontal regions are more strongly associated with expressive agrammatism than temporal regions (Vanier and Caplan, 1990; Borovsky, Saygin, Bates, & Dronkers, 2007; Faroqi-Shah et al., 2014).
However, other lesion-deficit studies suggest that the IFG is not critical for syntactic encoding, or perhaps even for any language functions. In a seminal stroke study, Mohr (1976; Mohr et al., 1978) showed that when infarction is restricted to the IFG, agrammatism is rare, and any deficits are generally only transient. Surgical resection of the frontal operculum also does not often result in agrammatism or any significant persistent aphasia (Kral, Kurthen, Schramm, Urbach, & Meyer, 2006; Lubrano, Draper, & Roux, 2010; Penfield and Roberts, 1959; Plaza, Gatignol, Leroy, & Duffau, 2009; Rolston et al., 2015; Wilson et al., 2015). For example, Rolston and colleagues (2015) reported persistent language deficits in only 4 of 41 patients who were followed up after resection of tumors in the frontal operculum. A limitation of this study is that detailed language or neuropsychological testing was not carried out; subtle post-operative language deficits may be apparent only with quantitative linguistic analysis (McCarron et al., 2017). Resolving the discrepancies between these divergent results from different methodological approaches and patient populations will be an important goal for future research.
Our study had several noteworthy limitations. First, because all of the patients were undergoing surgery for epilepsy, gliomas, or cavernous malformations, it is possible that some reorganization of language regions may have already taken place (Lubrano et al., 2010). However, individual patterns of reorganization would presumably make the neural correlates of any given cognitive process less consistent rather than more consistent, so the tight clustering of syntactic encoding sites to the IFG suggests that there was minimal reorganization of this function in our patient cohort. Furthermore, most of the lesions in our study cohort were not directly adjacent to the IFG, reducing the likelihood of reorganizational processes. Also contributing to potential variability, one patient was left handed with mixed language dominance, another was ambidextrous, and two were not native speakers of English, which could have implications for language organization (Giussani, Roux, Lubrano, Gaini, & Bello, 2007; Roux and Tremoulet, 2002). However, any individual differences in language organization arising from these factors was not sufficient to obscure the consistent localization of syntactic encoding sites we observed.
Second, our study focused on the use of CSM for identifying critical sites that are localized to the surfaces of gyri. Because of this, we cannot determine whether there are additional syntactic encoding sites buried in sulci or underlying subcortical white matter tracts. It is possible that in some or all of the seven patients for whom no syntactic encoding sites were identified, syntactic encoding sites might exist in these regions that were not stimulated.
Third, not all of the sentence production disruptions that were elicited by stimulation of the syntactic encoding sites were unambiguously syntactic. Although each site was required to be associated with at least one syntactic error, other utterances were characterized by retracings or perseverations, the provenance of which is less clear. Note that in our study, perseverations were elicited more frequently by inferior frontal than temporal stimulation. In contrast, Leonard and colleagues (2016) showed that in a repetition task, perseverations were elicited by stimulation of the posterior superior temporal gyrus and supramarginal gyrus. This suggests that the perseveration in any given task reflects filling in after the neural substrates specific to the particular task are disrupted, which bolsters our assumption that perseveration in the sentence production task reflects breakdowns of syntactic encoding.
In sum, we showed that in many patients, direct electrocortical stimulation of the IFG resulted in reproducible and selective disturbances of sentence production, suggesting that this region plays an important role in syntactic encoding.
Acknowledgments
We thank the patients for their participation in this research. This work was supported by the National Institutes of Health [grant numbers NS065120, NS098971, DC013270, DC016080, DC012379], the New York Stem Cell Foundation, the Howard Hughes Medical Institute, the McKnight Foundation, the Shurl and Kay Curci Foundation, and the William K. Bowes Foundation. EFC is a New York Stem Cell Foundation Robertson Investigator.
References
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bello L, Gallucci M, Fava M, Carrabba G, Giussani C, Acerbi F, … Gaini SM. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67–82. doi: 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- Boatman D. Cortical bases of speech perception: evidence from functional lesion studies. Cognition. 2004;92:47–65. doi: 10.1016/j.cognition.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Borovsky A, Saygin AP, Bates E, Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45:2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain and Language. 2010;115:101–112. doi: 10.1016/j.bandl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing H. A note upon the faradic stimulation of the postcentral gyrus in conscious patients. Brain. 1909;32:44–53. [Google Scholar]
- Davis C, Kleinman JT, Newhart M, Gingis L, Pawlak M, Hillis AE. Speech and language functions that require a functioning Broca’s area. Brain and Language. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Lopes M, … Van Effenterre R. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. Journal of Neurology, Neurosurgery & Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109:461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Faroqi-Shah Y, Kling T, Solomon J, Liu S, Park G, Braun A. Lesion analysis of language production deficits in aphasia. Aphasiology. 2014;28:258–277. [Google Scholar]
- Ferreira F, Engelhardt PE. Syntax and production. In: Traxler MJ, Gernsbacher MA, editors. Handbook of psycholinguistics. 2. San Diego, CA: Academic Press; 2006. pp. 61–92. [Google Scholar]
- Garrett MF. Levels of processing in sentence production. In: Butterworth B, editor. Language production, Vol. 1, Speech and talk. London: Academic Press; 1980. pp. 177–220. [Google Scholar]
- Giussani C, Roux FE, Lubrano V, Gaini SM, Bello L. Review of language organisation in bilingual patients: what can we learn from direct brain mapping? Acta Neurochirurgica. 2007;149:1109–16. doi: 10.1007/s00701-007-1266-2. [DOI] [PubMed] [Google Scholar]
- Gonen T, Gazit T, Korn A, Kirschner A, Perry D, Hendler T, Ram Z. Intra-operative multi-site stimulation: Expanding methodology for cortical brain mapping of language functions. PLOS One. 2017;12(7):e0180740. doi: 10.1371/journal.pone.0180740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- Haller S, Radue EW, Erb M, Grodd W, Kircher T. Overt sentence production in event-related fMRI. Neuropsychologia. 2005;43:807–814. doi: 10.1016/j.neuropsychologia.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, McKhann GM, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48:531–538. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Perrine K, McKhann GM. Temporal lobe stimulation reveals anatomic distinction between auditory naming processes. Neurology. 2003;60:1478–1483. doi: 10.1212/01.wnl.0000061489.25675.3e. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742–2749. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Brown CM, Hellwig F, Amunts K, Herzog H, Seitz RJ, Hagoort P. A neural correlate of syntactic encoding during speech production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5933–5936. doi: 10.1073/pnas.101118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Hellwig F, Herzog H, Seitz RJ, Hagoort P. Neural responses to the production and comprehension of syntax in identical utterances. Brain and Language. 2004;89:312–319. doi: 10.1016/S0093-934X(03)00352-3. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: Grune and Stratton; 1982. [Google Scholar]
- Kral T, Kurthen M, Schramm J, Urbach H, Meyer B. Stimulation mapping via implanted grid electrodes prior to surgery for gliomas in highly eloquent cortex. Neurosurgery. 2006;58(1 Suppl):ONS36–43. doi: 10.1227/01.neu.0000193925.98348.f5. [DOI] [PubMed] [Google Scholar]
- Krause F. Die operative Behandlung der Epilepsie. Medizinische Klinik. 1909;5:1418–1422. [Google Scholar]
- Leonard MK, Cai R, Babiak MC, Ren A, Chang EF. The peri-sylvian cortical network underlying single word repetition revealed by electrocortical stimulation and direct neural recordings. Brain and Language. 2016 doi: 10.1016/j.bandl.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrano V, Draper L, Roux FE. What makes surgical tumor resection feasible in Broca’s area? Insights into intraoperative brain mapping. Neurosurgery. 2010;66:868–875. doi: 10.1227/01.NEU.0000368442.92290.04. [DOI] [PubMed] [Google Scholar]
- Lubrano V, Filleron T, Démonet JF, Roux FE. Anatomical correlates for category-specific naming of objects and actions: A brain stimulation mapping study. Human Brain Mapping. 2014;35:429–443. doi: 10.1002/hbm.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Calpagnano MA, Campana S, Razzano C, Caltagirone C, Marini A. tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience. 2013;7:539. doi: 10.3389/fnhum.2013.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron A, Chavez A, Babiak MC, Berger MS, Chang EF, Wilson SM. Connected speech in transient aphasias after left hemisphere resective surgery. Aphasiology. 2017 doi: 10.1080/02687038.2017.1278740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP. Broca’s area and Broca’s aphasia. In: Whitaker H, Whitaker H, editors. Studies in neurolinguistics. Vol. 1. New York: Academic Press; 1976. pp. 201–233. [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology. 1978;28:311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Nozari N, Arnold JE, Thompson-Schill SL. The effects of anodal stimulation of the left prefrontal cortex on sentence production. Brain Stimulation. 2014;7:784–792. doi: 10.1016/j.brs.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA. Organization of short-term verbal memory in language areas of human cortex: evidence from electrical stimulation. Brain and Language. 1978;5:331–340. doi: 10.1016/0093-934x(78)90030-5. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Brain organization for language from the perspective of electrical stimulation mapping. Behavioral and Brain Sciences. 1983;6:189–206. [Google Scholar]
- Ojemann G, Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401–1403. doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. Journal of Neurosurgery. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Plaza M, Gatignol P, Leroy M, Duffau H. Speaking without Broca’s area after tumor resection. Neurocase. 2009;15:294–310. doi: 10.1080/13554790902729473. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and Brain-Mechanisms. Princeton, NJ: Princeton University Press; 1959. [Google Scholar]
- Rofes A, Miceli G. Language mapping with verbs and sentences in awake surgery: a review. Neuropsychology Review. 2014;24:185–199. doi: 10.1007/s11065-014-9258-5. [DOI] [PubMed] [Google Scholar]
- Rolston JD, Englot DJ, Benet A, Li J, Cha S, Berger MS. Frontal operculum gliomas: language outcome following resection. Journal of Neurosurgery. 2015;122:725–734. doi: 10.3171/2014.11.JNS132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Lubrano V, Lauwers-Cances V, Trémoulet M, Mascott CR, Démonet JF. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004;127:1796–1810. doi: 10.1093/brain/awh204. [DOI] [PubMed] [Google Scholar]
- Roux FE, Minkin K, Durand JB, Sacko O, Réhault E, Tanova R, Démonet JF. Electrostimulation mapping of comprehension of auditory and visual words. Cortex. 2015;71:398–408. doi: 10.1016/j.cortex.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Roux FE, Trémoulet M. Organization of language areas in bilingual patients: a cortical stimulation study. Journal of Neurosurgery. 2002;97:857–864. doi: 10.3171/jns.2002.97.4.0857. [DOI] [PubMed] [Google Scholar]
- Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. New England Journal of Medicine. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, … Dickerson BC. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–366. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:479–498. [Google Scholar]
- Vanier M, Caplan D. CT-scan correlates of agrammatism. In: Menn L, Obler LK, editors. Agrammatic aphasia: A cross-language narrative sourcebook. Vol. 1. Amsterdam: John Benjamins; 1990. pp. 37–115. [Google Scholar]
- Vidorreta JG, Garcia R, Moritz-Gasser S, Duffau H. Double dissociation between syntactic gender and picture naming processing: a brain stimulation mapping study. Human Brain Mapping. 2011;32:331–340. doi: 10.1002/hbm.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Dronkers NF, Ogar JM, Jang J, Growdon ME, Agosta F, … Gorno-Tempini ML. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. Journal of Neuroscience. 2010a;30:16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Eriksson DK, Schneck SM, Lucanie JM. A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. doi: 10.1371/journal.pone.0192773. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, … Gorno-Tempini ML. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, … Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010b;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Lam D, Babiak MC, Perry DW, Shih T, Hess CP, … Chang EF. Transient aphasias after left hemisphere resective surgery. Journal of Neurosurgery. 2015;123:581–593. doi: 10.3171/2015.4.JNS141962. [DOI] [PMC free article] [PubMed] [Google Scholar]