Abstract
Background
Over the past 15 years, 300 out of 6000 breeds of all farm animal species identified by the Food and Agriculture Organization of the United Nations (FAO) have gone extinct. Among cattle, many Podolian breeds are seriously endangered in various European areas. Podolian cattle include a group of very ancient European breeds, phenotypically close to the aurochs ancestors (Bos primigenius). The aim of the present study was to assess the genetic diversity of Podolian breeds and to reconstruct their origin.
Methodology
The mitochondrial DNA (mtDNA) control-regions of 18 Podolian breeds have been phylogenetically assessed. Nine non-Podolian breeds have been also included for comparison.
Conclusion
The overall analysis clearly highlights some peculiarities in the mtDNA gene pool of some Podolian breeds. In particular, a principal component analysis point to a genetic proximity between five breeds (Chianina, Marchigiana, Maremmana, Podolica Italiana and Romagnola) reared in Central Italy and the Turkish Grey. We here propose the suggestive hypothesis of a dual ancestral contribution to the present gene pool of Podolian breeds, one deriving from Eastern European cattle; the other arising from the arrival of Middle Eastern cattle into Central Italy through a different route, perhaps by sea, ferried by Etruscan boats. The historical migration of Podolian cattle from North Eastern Europe towards Italy has not cancelled the mtDNA footprints of this previous ancient migration.
Introduction
Over the past 15 years, 300 out of 6000 livestock breeds identified by Food and Agriculture Organization of the United Nations (FAO) have gone extinct [1]. Risk factors for farm animal breeds are mainly: i) a reduction of genetic variability due to strict selection processes; ii) a strong economic pressure focused on specific traits, such as milk production, which leads to the replacement of local less productive breeds with highly productive industrial breeds; iii) an unrestricted and indiscriminate cross-breeding, especially in developing countries [2]. Bos taurus is one of the most economically important livestock species [3]. Both in historic and current societies it has fulfilled agricultural, economic, cultural, and even religious key roles, often paralleling human evolution [4]. Among cattle, many Podolian breeds are seriously endangered in various European countries [5]; [6]; [7]. Podolian cattle include a group of very ancient European breeds, with a grey coat colour and long horns, phenotypically close to the aurochs (Bos primigenius). According to many traditional notes the name Podolian refers to a common ancestral origin in Podolia (the modern western Ukraine). However place of origin and timing of spread out of the source area are both debated. Alternative hypotheses have been proposed: Podolian cattle might have spread from the eastern steppe southward into Anatolia and westward into the Balkans and Italy in historical times (3rd-5th century AD) along with East-European Barbarian people [8]; other authors suggest a more ancient migration (~3 kya BP) from the Near East to Central Italy through the Mediterranean Sea [9], together with a possible contribution from local wild aurochs through a secondary local domestication/introgression events[10]; [11].
Nowadays, some phenotypic distinctions stand out among Podolian cattle [12]. The noble aurochs-shaped ancient breeds with long horns (such as Hungarian Grey, Katerini, Podolsko, Slavonian Syrmian and Maremmana) are considered as the only true Podolian breeds by some scholars. However, some local breeds (i.e. Podolica Italiana, Ukrainian Grey, Turkish Grey and other Balkan breeds) do not necessarily show the long horns, but maintained some distinctive Podolian traits such as a red coat in calves and light grey in adults [13]. In general, a commercial trait shared by all Podolian cattle is that they are more suitable for beef production rather than for dairy. Because of that some improved beef breeds (Chianina, Marchigiana, Romagnola and Piemontese) are also considered within the Podolian group, although the inclusion of Chianina and Piemontese is still debated [14].
During the last decades, mitochondrial DNA (mtDNA) has been widely used as a molecular tool to investigate genetic origin, history and diversity of livestock species [15] [16] [17] [18] [19] [20]. Following this trend, the aim of the present study is to re-assess the mtDNA diversity of the major Podolian cattle breeds (ten of which classified as endangered or critical by FAO) to obtain additional information on their ancestral origin and ancient dispersal routes.
Results
We have analysed the mitochondrial DNA of 18 Podolian cattle breeds (Fig 1, Table 1, S1–S3 Tables).
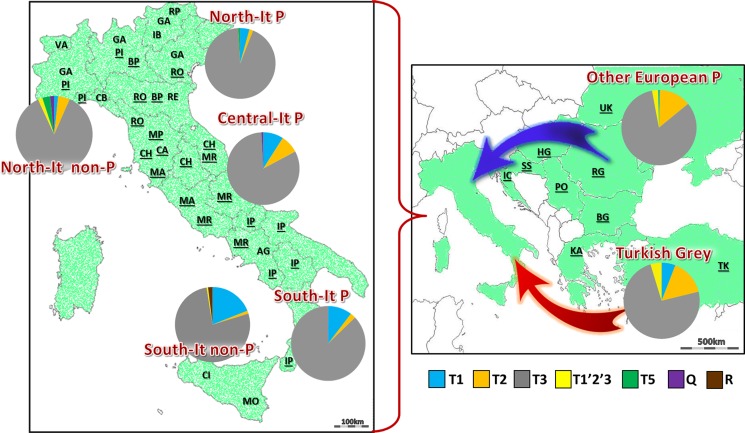
Fig 1. Prevalent locations and frequency distributions of mitochondrial haplogroups in 18 Podolian (P, underlined) and 9 non-Podolian (non-P) breeds analyzed in this study.
Breed codes as in Table 1 (see also Table 2 and S3 Table for details). Note that RP and IB are also widespread in Italy. Maps (www.histgeo.ac-aix-marseille.fr/ancien_site/carto/) were used for illustrative purposes only and largely modified by the authors.
Table 1. Estimates of genetic diversitya on the 27 breeds analyzed in this work.
| Breed | Country | Podolian/non-Podolian | ID name | N | π | Hd | N | π | Hd |
|---|---|---|---|---|---|---|---|---|---|
| Range: | 16042- | 16262 | Range: | 15823- | 215 | ||||
| Piemontese | Northern Italy | Podolian | PI | 72 | 0.008 | 0.833 | 72 | 0.005 | 0.970 |
| Bianca di Val Padana | Northern Italy | Podolian | BP | 45 | 0.006 | 0.630 | 45 | 0.003 | 0.834 |
| Romagnola | Central Italy | Podolian | RO | 225 | 0.014 | 0.890 | 225 | 0.007 | 0.965 |
| Mucco Pisano | Central Italy | Podolian | MP | 33 | 0.006 | 0.788 | 33 | 0.004 | 0.814 |
| Calvana | Central Italy | Podolian | CA | 35 | 0.008 | 0.662 | 26 | 0.004 | 0.889 |
| Chianina | Central Italy | Podolian | CH | 369 | 0.013 | 0.895 | 338 | 0.006 | 0.973 |
| Maremmana | Central Italy | Podolian | MA | 75 | 0.013 | 0.857 | 62 | 0.006 | 0.973 |
| Marchigiana | Central Italy | Podolian | MR | 146 | 0.013 | 0.903 | 146 | 0.006 | 0.967 |
| Italian Podolian | Southern Italy | Podolian | IP | 125 | 0.008 | 0.780 | 91 | 0.005 | 0.872 |
| Ukrainian Grey | Ukraine | Podolian | UK | 32 | 0.010 | 0.856 | 1 | ||
| Romanian Grey | Romania | Podolian | RG | 17 | 0.014 | 0.890 | - | ||
| Hungarian Grey | Hungary | Podolian | HG | 93 | 0.010 | 0.856 | 1 | ||
| Slavonian Syrmian Pod. | Croatia | Podolian | SS | 9 | 0.004 | 0.583 | - | ||
| Istrian Cattle | Croatia | Podolian | IC | 17 | 0.010 | 0.794 | - | ||
| Podolsko | Serbia | Podolian | PO | 11 | 0.005 | 0.709 | - | ||
| Bulgarian Grey | Bulgaria | Podolian | BG | 36 | 0.014 | 0.838 | 30 | 0.005 | 0.779 |
| Katerini | Greece | Podolian | KA | 12 | 0.019 | 0.879 | - | ||
| Turkish Grey | Turkey | Podolian | TK | 85 | 0.012 | 0.922 | 70 | 0.006 | 0.971 |
| Valdostana | Northern Italy | non-Podolian | VA | 54 | 0.011 | 0.934 | 54 | 0.006 | 0.941 |
| Grey Alpine | Northern Italy | non-Podolian | GA | 45 | 0.012 | 0.853 | 45 | 0.006 | 0.979 |
| Italian Brown | Northern Italy | non-Podolian | IB | 34 | 0.010 | 0.852 | 34 | 0.005 | 0.929 |
| Italian Red Pied | Northern Italy | non-Podolian | RP | 136 | 0.010 | 0.896 | 125 | 0.006 | 0.982 |
| Cabannina | Northern Italy | non-Podolian | CB | 55 | 0.011 | 0.882 | 43 | 0.005 | 0.928 |
| Reggiana | Northern Italy | non-Podolian | RE | 38 | 0.006 | 0.713 | 38 | 0.003 | 0.845 |
| Agerolese | Southern Italy | non-Podolian | AG | 36 | 0.014 | 0.913 | 36 | 0.006 | 0.956 |
| Cinisara | Southern Italy | non-Podolian | CI | 81 | 0.014 | 0.881 | 69 | 0.007 | 0.966 |
| Modicana | Southern Italy | non-Podolian | MO | 41 | 0.010 | 0.763 | 33 | 0.005 | 0.864 |
| All Podolian | 1437 | 0.010 | 0.837 | 1140 | 0.006 | 0.980 | |||
| Non-Podolian | 520 | 0.011 | 0.879 | 477 | 0.006 | 0.963 | |||
| All samples | 1957 | 0.010 | 0.845 | 1617 | 0.006 | 0.977 | |||
a N = number of sequences
π = nucleotide diversity, Hd = haplotype diversity.
The molecular analysis of 221 base pairs of the control region (from np 16042 to np 16262) on the entire dataset of 1,957 samples revealed a total of 247 distinct haplotypes (from four to 70 haplotypes per breed) and 91 polymorphic sites (S), all represented by single nucleotide polymorphisms (SNPs). The average nucleotide diversity (π) was comparable between Podolian and non-Podolian breeds (~0.010–0.011; Table 1), while haplotype diversity was significantly lower (P-value < 0.01) in Podolian (Hd = 0.837 ± 0.010) than in non-Podolian breeds (Hd = 0.879 ± 0.013). Both indices varied largely across breeds as already seen in previous mtDNA studies [11]; [21]; [22]; [23]. Among all Podolian, the highest Hd values (≥0.90) were identified in Chianina, Marchigiana and Turkish Grey, while the lowest values of Hd (<0.70), as well as of nucleotide diversity (≤0.008), were scored in Bianca di Val Padana, Calvana, and Slavonian Syrmian Podolian. As for 1,617 sequences, we were able to extend the analysis to a longer control-region fragment encompassing 731 bps (Table 1). The results largely confirmed the same trend, with the only notable exception of Piemontese, Romagnola and Maremmana showing a higher Hd (>0.960) on this extended fragment. It is also interesting that the highest Hd values were identified in Chianina, and Maremmana, which showed values (>0.970) comparable to the Turkish Grey (0.971).
All control-region haplotypes have been classified in haplogroups and sub-haplogroups through an accurate analysis of mutational motifs (Table 2 and S3 Table), according to previously published classification criteria [11]; [24]; [25]; [26]. Haplogroup T3 was the most common (83%) in all breeds, with the highest value in MP and PO (both 100%), followed by PI (96%) (acronyms are listed in Table 1). The second and third most common haplogroups (both 7%) were T1 and T2, which were missing in MP, PO and SS. The frequency of T2 is lower in non-Podolian (3.85%) than in Podolian breeds (8.35%) with extraordinary high peaks in three breeds, KA (42%), RG (24%) and BG (22%), followed by UK (16%) and MA and TK (both 15%). T1 haplogroup was predominantly found among breeds from Central and Southern Italy, both in Podolian (10%) and non-Podolian (19%) groups (Fig 1). Haplogroup T5 was found exclusively in non-Podolian breeds, and was restricted to IB, RP and VA except for one sequence found in SS and one in PI. Finally, haplogroups Q and R showed very low incidences restricted to Italian non-Podolian (Q = 1.15%, and R = 0.58%) and Podolian (Q = 0.77%, and R = 0.49%) breeds. Overall, the haplogroup distribution differed significantly between the Podolian and non-Podolian groups of breeds included in the current analysis (Table 2; chi-square P-value < 0.001) with the highest contribution given by the T2 haplogroup. This result was also verified by considering haplogroup frequencies based on different haplotypes (chi-square P-value < 0.001) in order to mitigate the effect of inbreeding and recent founder effects.
Table 2. Sources and haplogroup affiliation for the Podolian and non-Podolian mtDNA sequences.
Haplogroup frequencies (%) are in parentheses.
| Code | Group/Breed | T1 | T2 | T3 | T1'2'3 | T5 | Q | Q1 | Q2 | R | R1 | R2 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Podolian | 103(7.17) | 120(8.35) | 1184(82.39) | 10(0.70) | 2(0.14) | 1(0.07) | 5(0.35) | 5(0.35) | 0(0.00) | 5(0.35) | 2(0.14) | 1437 | |
| BP | Bianca di Val Padana | 5(11.11) | - | 40(88.89) | - | - | - | - | - | - | - | - | 45 |
| PI | Piemontese | - | 2(2.78) | 69(95.83) | - | 1(1.39) | - | - | - | - | - | - | 72 |
| CA | Calvana | 5(14.29) | - | 30(85.71) | - | - | - | - | - | - | - | - | 35 |
| CH | Chianina | 35(9.49) | 36(9.76) | 292(79.13) | - | - | 1(0.27) | 2(0.54) | 3(0.81) | - | - | - | 369 |
| MR | Marchigiana | 18(12.33) | 5(3.42) | 122(83.56) | - | - | - | - | - | - | - | 1(0.68) | 146 |
| MA | Maremmana | 9(9.33) | 11(14.67) | 55(73.33) | - | - | - | - | - | - | - | - | 75 |
| MP | Mucco Pisano | - | - | 33(100.00) | - | - | - | - | - | - | - | - | 33 |
| RO | Romagnola | 12(5.33) | 19(8.44) | 183(81.33) | - | - | - | 3(1.33) | 2(0.89) | - | 5(2.22) | 1(0.44) | 225 |
| IP | Podolica Italiana | 13(10.40) | 3(2.40) | 109(87.20) | - | - | - | - | - | - | - | - | 125 |
| HG | Hungarian Grey | 8(8.60) | 82(88.17) | 3(3.23) | - | - | - | - | - | - | - | 93 | |
| UK | Ukrainian Grey | - | 5(15.63) | 25(78.13) | 2(6.25) | - | - | - | - | - | - | - | 32 |
| BG | Bulgarian Grey | 1(2.78) | 8(22.22) | 27(75.00) | - | - | - | - | - | - | - | - | 36 |
| IC | Istrian Cattle | - | 1(5.88) | 15(88.24) | 1(5.88) | - | - | - | - | - | - | - | 17 |
| PO | Podolsko | - | - | 11(100.0) | - | - | - | - | - | - | - | - | 11 |
| RG | Romanian Grey | - | 4(23.53) | 13(76.47) | - | - | - | - | - | - | - | - | 17 |
| SS | Slavonian Syrmian Pod. | - | - | 8(88.89) | - | 1(11.11) | - | - | - | - | - | - | 9 |
| KA | Katerini | - | 5(41.67) | 7(58.33) | - | - | - | - | - | - | - | - | 12 |
| TK | Turkish Grey | 5(5.88) | 13(15.29) | 63(74.12) | 4(4.71) | - | - | - | - | - | - | - | 85 |
| Non-Podolian | 36(6.92) | 20(3.85) | 435(83.65) | 8(1.54) | 12(2.31) | 3(0.57) | 3(0.58) | - | 2(0.38) | 1(0.19) | - | 520 | |
| AG | Agerolese | 7(19.44) | - | 27(75.00) | 1(2.78) | - | - | - | - | - | 1(2.78) | - | 36 |
| CB | Cabannina | - | 5(9.09) | 40(72.73) | 7(12.73) | - | 3(5.45) | - | - | - | - | - | 55 |
| CI | Cinisara | 17(20.99) | 2(2.47) | 60(74.07) | - | - | - | - | - | 2(2.47) | - | - | 81 |
| GA | Grigio Alpina | - | 2(4.44) | 41(91.11) | - | - | - | 2(4.44) | - | - | - | - | 45 |
| IB | Bruna Italiana | 2(5.88) | - | 29(85.29) | - | 3(8.82) | - | - | - | - | - | - | 34 |
| RP | Pezzata Rossa Italiana | 2(1.47) | 10(7.35) | 122(89.71) | - | 1(0.74) | - | 1(0.74) | - | - | - | - | 136 |
| MO | Modicana | 6(14.63) | - | 35(85.37) | - | - | - | - | - | - | - | - | 41 |
| RE | Reggiana | 2(5.26) | - | 36(94.74) | - | - | - | - | - | - | - | - | 38 |
| VA | Valdostana | - | 1(1.85) | 45(83.33) | - | 8(14.81) | - | - | - | - | - | - | 54 |
| Total | 139(7.10) | 140(7.15) | 1619(82.73) | 18(0.92) | 14(0.712 | 4(0.20) | 8(0.41) | 5(0.26) | 2(0.10) | 6(0.31) | 2(0.10) | 1957 |
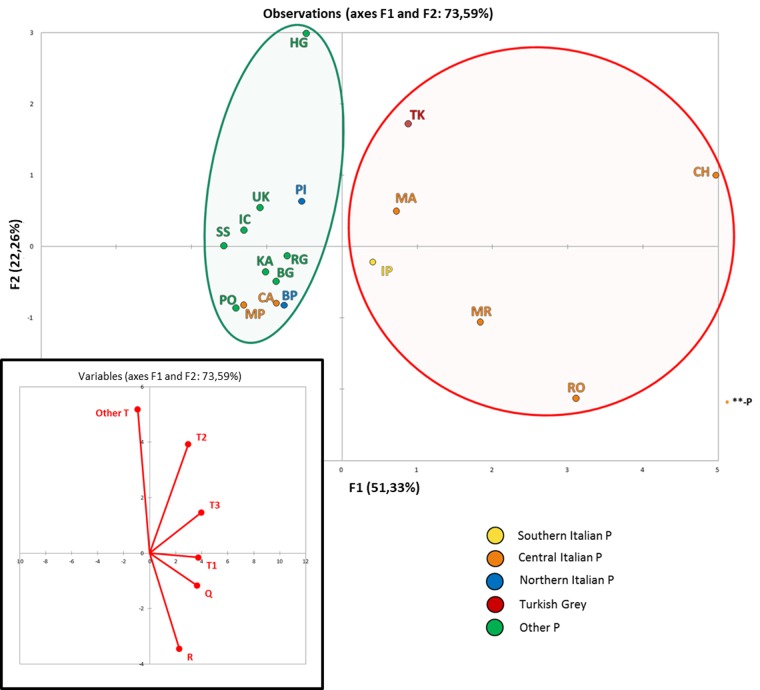
Thus, we performed a principal component analysis (PCA) to graphically display the different haplogroup distributions among the Podolian breeds. In order to consider as many populations as possible, the dataset based on the short fragment was included. After variables reduction to PCs, the coordinates of the observations for the 18 breeds were displayed in a two-dimensional plot representing the European Podolian genetic landscape (Fig 2). PC1 clearly separated the Turkish Grey and the five most important Central and Southern Italian beef cattle breeds (CH, RO, MR, MA and IP; S1 Fig) from all the remaining populations, while PC2 contributed to separate the Hungarian and the Turkish Grey.
Fig 2. Principal Component Analysis (PCA) of all Podolian breeds.
Below is the plot of the contribution of each haplogroup to the first and second PC (projections of the axes of the original variables).
Because of the peculiar position of some Italian breeds, we used an analysis of molecular variance (AMOVA) to investigate fixation indices in three (artificially created) population groups, one including the Italian Podolian breeds, the other encompassing the European Podolian breeds and the Turkish Grey, and the third group covering the Italian non-Podolian breeds. Most of the variance (about 98%) observed in the Italian Podolian populations explained differences among samples within breeds, while less than 2% represented differences between breeds (Table 3), a value three times lower than in the other Podolian breeds.
Table 3. Analysis of molecular variance (AMOVA).
| Group | Variation within breeds (%) | Variation among breeds (%) |
|---|---|---|
| Italian Podolian breeds | 98.08 | 1.92 |
| Non-Italian Podolian breed | 93.85 | 6.15 |
| Non-Podolian breeds | 97.21 | 2.79 |
Discussion
To date, only a limited number of studies have addressed the genetic composition of Podolian breeds. These investigations were generally limited to few breeds and focused on the nuclear genome [8]; [27]; [28]; [29]; [30]. Mitochondrial DNA data have been previously reported by Ivankovic et al. [6] and Ilie et al. [7] who analysed the control-region sequences of some Croatian and Romanian cattle breeds, respectively. The present study extended the analysis of the mitochondrial genetic variation to 18 Podolian breeds by evaluating their haplogroup distributions, which were eventually compared among them and to nine non-Podolian breeds. Genetic diversity considered in terms of number of haplotypes and nucleotide diversity revealed some peculiarities of several Podolian breeds. The low mtDNA diversities of Bianca di Val Padana, Calvana and Slavonian Syrmian Podolian could be due to a combination of factors, such as i) a sampling bias depending on the low consistence of current herds (S1 Table), ii) a bottleneck effect caused by the strong reduction in population size experienced by these breeds during the last decades, iii) genetic drift acting on small populations. On the contrary, the large diffusion of Piemontese, Marchigiana and Chianina cattle probably favoured the accumulation and maintenance of a high level of mtDNA variation, which is evident also in the Maremmana breed in spite of its lower consistency (S1 Table). In general, the most common European haplogroup T3 is predominant (83%) in our dataset. High frequencies of T1 in central and southern Italy might be due to the intensive migrations across the Mediterranean Sea, eased by the proximity to northern Africa, where T1 is prevalent [20]; [25]; [26]; [31] and to the Near East where T1 is also present [32, 33]. It is worth noting that the central-Italian Podolian breeds show higher frequencies of T2, while the presence of Q and R within the Podolian group is limited to three Italian breeds: Romagnola, Chianina and Marchigiana, the latter derived from crossbreeding between the first two in the early 20th century.
The significant differences between the haplogroup distributions of Podolian and non-Podolian and the low genetic differentiation among the Italian Podolian breeds (three times less than in other Podolian breeds or in the non-Podolian group) points to a common (and perhaps peculiar) origin. As a matter of fact, according to the first component of the PCA, five Italian beef cattle breeds formed a clearly separated group and were also closer to the Turkish Grey than to any other Podolian breeds. At first this peculiarity could be explained as the effect of a stronger beef-oriented selection carried out on these breeds compared to the other Podolian populations. However, another important Italian beef cattle, the Piemontese, is placed within the other Podolian cluster, which shares the common feature of a strong grey coat. Thus, an alternative explanation might assume a different ancestral origin for the two groups of Podolian breeds, as summarized in Fig 1: a first group, mostly consisting of breeds from East Europe and northern Italy that share a similar mitochondrial gene pool, may have originated from ancestors migrated through an inland route from Podolia across eastern Europe all the way into northern Italy, in accordance with the great wave of cattle migration occurred during the Barbarian invasions. Whereas, a second group, including the white Podolian cattle closely related to the Turkish Grey, may descend from ancestral bovines brought to Italy through a different and likely maritime route crossing the Mediterranean Sea. A previous study [34] suggested also a genetic link between the Turkish Grey and Bulgarian and Hungarian breeds, but our results do not support such hypothesis, highlight instead a stronger maternal relationship between the Turkish Grey and five central-southern Italian Podolian breeds. Those cattle are bred since the medieval time [29] in an area that largely overlaps with the ancient territory of Etruria. This finding further supports and extends another hypothesis, according to which at least part of the maternal ancestry of those breeds could be related to the Etruscan migration from Lydia, a region on the south-western coast of ancient Anatolia [9]; [35]; [36], [37]; [38]. It is worth noting that the five Podolian breeds are also the main Italian beef cattle together with the Piemontese, and that previous studies suggested a possible common genetic origin [39]. Our findings suggest that, in spite of a stronger beef-oriented selection, their mitochondrial gene pool still preserves genetic traces of a different maternal origin, confirming that the selection practices were mostly male-mediated and enforcing the importance of the mtDNA screening to reconstruct the ancestry and history of current breeds.
Material and methods
Ethics statement
All experimental procedures were reviewed and approved by the Animal Research Ethics Committee of the Universities of Perugia and Pavia in accordance with the European Union Directive 86/609.
Samples
The entire dataset analyzed in this study encompasses 1,957 mtDNA control-region sequences including 1,321 from our previous studies [11]; [24]; [40], 428 retrieved from GenBank, and 208 additional samples specifically collected for this study (Table 1, S1–S3 Tables). Piemontese (also called Piedmontese) (PI, n = 72), Romagnola (RO, n = 225), Marchigiana (MR, n = 146), Chianina (CH, n = 369), Maremmana (MA, n = 75), Podolica Italiana (also known as Italian Podolian) (IP, n = 125), Mucco Pisano (MP, n = 33), Calvana (CA, n = 35), Bianca di Val Padana (BP, n = 45), Hungarian Grey (HG, n = 93), Bulgarian Grey (BG, n = 36), Istrian cattle (IC, n = 17), Katerini (KA, n = 12), Romanian Grey (RG, n = 17), Slavonian Syrmian Podolian (SS, n = 9), Turkish Grey (TK, n = 85), Ukrainian Grey (UK, n = 32), Podolsko (PO, n = 11). Moreover, nine unrelated non-Podolian breeds from Italy, are included as a control group: Valdostana (VA, n = 54), Bruna Italiana (also known as Italian Brown) (IB, n = 34), Grigio Alpina (also called Grey Alpine) (GA, n = 45), Pezzata Rossa Italiana (also known as Italian Red Pied) (RP, n = 136), Modicana (MO, n = 41), Reggiana (RE, n = 38), Agerolese (AG, n = 36), Cinisara (CI, n = 81), Cabannina (CB, n = 55).
DNA extraction, amplification and sequencing
As for the 208 novel mtDNAs, blood samples were collected from the jugular vein of each animal in vacutainer tubes, containing EDTA as anticoagulant. These animals were chosen in different farms in order to avoid closely related individuals and gather a representative sample of the breeds. Whole blood was stored at -20°C until DNA extraction. DNA was isolated using the GenElute Blood Genomic DNA kit (Sigma Aldrich, St. Louis, MO, USA) and stored at -20°C until genotyping. PCR amplification of the control region was performed using forward and reverse primers (5’-CCTAAGACTCAAGGAAGAAACTGC-3’ and 3’-AACCTAGAGGGCATTCTCACTG-5’ respectively) specifically designed on the Bovine Reference Sequence (BRS; GenBank V00654). The 1138 bp PCR fragment encompassed the mtDNA control region from np 15718 to 517. Amplicons were first purified using exonuclease I and alkaline phosphatase (ExoSAP-IT® enzymatic system-USB Corporation, Cleveland, OH, USA), then sequenced with the primer 15757F (5’-CCCCAAAGCTGAAGTTCTAT-3’), as previously described [40]. A dataset of 1,321 sequences was already available in our laboratories. All data were recorded in GenBank with accession numbers MF474376-MF475904 (S3 Table) and compared to those retrieved from the database (S2 Table).
Data analyses
Sequences were aligned to the Bovine Reference Sequence (BRS; V00654) using the software SequencherTM 5.0. For a total of 1,617 samples, we were able to analyse a 731-bps fragment trimmed from np 15823 to np 215, while only a short fragment (221 bps, from np 16042 to np 16262) was considered in order to include the widest possible number of samples (N = 1,957). Haplotypes were classified in haplogroups and sub-haplogroups according to previously identified mutational motifs [24]. Indices of molecular variation were calculated using the DNAsp 5.1 software [41], while an analysis of molecular variance was computed using AMOVA program implemented in the ARLEQUIN 3.01 package [42]. Finally, principal component analyses (PCA) were performed using Excel software implemented by XLSTAT, as described elsewhere [43, 44]. The PCA is a widely used dimension-reduction method that summarizes the variance of multivariate data in a smaller number of variables (the principal components, PCs), which are linear functions of the original variables, here expressed as haplogroup and sub-haplogroup frequencies, The rarest haplogroups were phylogenetically grouped and frequencies were calculated by considering only different haplotypes within the same breed.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(PDF)
Data Availability
Sequences were recorded in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with accession numbers MF474376-MF475904. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This research received support from: the Italian Ministry of Education, University and Research: Progetti Futuro in Ricerca 2012 (RBFR126B8I) (to AA) and Progetti Ricerca Interesse Nazionale 2012 (2012JA4BTY) (to AA); the University of Pavia strategic theme MIGRATions: towards an INterdisciplinary Governance model" (MIGRAT.IN.G; to AA). We thank Dr. Giovanni Capuzzello and Dr. Gilberto Leonelli for their precious help in providing biological samples for Modicana and Bianca di Val Padana breeds respectively.
References
- 1.Scherf B. World Watch List for Domestic Animal Diversity. 3rd ed. Rome. 2000.
- 2.Soysal M, Tuna Y, Gurcan E, Ozkan E. Farms in Turkey: sustainable development in the preservation of animal genetic resources in Turkey and in the world. Trakia J Sci. 2004;2(3):47–53. [Google Scholar]
- 3.Cunningham E. Selected Issues in Livestock Industry Development. Washington, DC.1992.
- 4.Bradley DG, MacHugh DE, Cunningham P, Loftus RT. Mitochondrial diversity and the origins of African and European cattle. Proc Natl Acad Sci U S A. 1996;93(10):5131–5. ; PubMed Central PMCID: PMCPMC39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pariset L, Joost S, Marsan PA, Valentini A, Econogene Consortium E. Landscape genomics and biased FST approaches reveal single nucleotide polymorphisms under selection in goat breeds of North-East Mediterranean. BMC Genet. 2009;10:7 Epub 2009/02/19. doi: 10.1186/1471-2156-10-7 ; PubMed Central PMCID: PMCPMC2663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivankovic A, Paprika S, Ramljak J, Dovc P, M. K. Mitochondrial DNA-based genetic evaluation of autochthonous cattle breeds in Croatia. Czech J Anim Sci 2014;59(11):519–28. [Google Scholar]
- 7.Ilie DE, Cean A, Cziszter LT, Gavojdian D, Ivan A, Kusza S. Microsatellite and Mitochondrial DNA Study of Native Eastern European Cattle Populations: The Case of the Romanian Grey. PLoS One. 2015;10(9):e0138736 Epub 2015/09/23. doi: 10.1371/journal.pone.0138736 ; PubMed Central PMCID: PMCPMC4580412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maretto F, Ramljak J, Sbarra F, Penasa M, Mantovani R, Ivankovic A, et al. Genetic relationship among Italian and Croatian Podolian cattle breeds assessed by microsatellite markers. Livest Sci. 2012;September 13;150 (1–3):256–64. [Google Scholar]
- 9.Pellecchia M, Negrini R, Colli L, Patrini M, Milanesi E, Achilli A, et al. The mystery of Etruscan origins: novel clues from Bos taurus mitochondrial DNA. Proc Biol Sci. 2007;274(1614):1175–9. doi: 10.1098/rspb.2006.0258 ; PubMed Central PMCID: PMCPMC2189563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beja-Pereira A, Caramelli D, Lalueza-Fox C, Vernesi C, Ferrand N, Casoli A, et al. The origin of European cattle: evidence from modern and ancient DNA. Proc Natl Acad Sci U S A. 2006;103(21):8113–8. doi: 10.1073/pnas.0509210103 ; PubMed Central PMCID: PMCPMC1472438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonfiglio S, Achilli A, Olivieri A, Negrini R, Colli L, Liotta L, et al. The enigmatic origin of bovine mtDNA haplogroup R: sporadic interbreeding or an independent event of Bos primigenius domestication in Italy? PLoS One. 2010;5(12):e15760 Epub 2011/01/07. doi: 10.1371/journal.pone.0015760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodò I, Gera I, Koppàny G. The Hungarian Grey cattle breed Budapest: Association of the hungarian grey cattle breeders; 2004. 128 p p. [Google Scholar]
- 13.Bonadonna T. Etnologia zootecnica Torino: UTET. [Google Scholar]
- 14.Bartosiewicz L. Hungarian Grey cattle in search of origins. Hungarian Agricultural research 1996;(3):13–20. [Google Scholar]
- 15.Groeneveld LF, Lenstra JA, Eding H, Toro MA, Scherf B, Pilling D, et al. Genetic diversity in farm animals—a review. Anim Genet. 2010;41 Suppl 1:6–31. doi: 10.1111/j.1365-2052.2010.02038.x . [DOI] [PubMed] [Google Scholar]
- 16.Achilli A, Olivieri A, Soares P, Lancioni H, Hooshiar Kashani B, Perego UA, et al. Mitochondrial genomes from modern horses reveal the major haplogroups that underwent domestication. Proc Natl Acad Sci U S A. 2012;109(7):2449–54. doi: 10.1073/pnas.1111637109 ; PubMed Central PMCID: PMCPMC3289334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenstra JA, Groeneveld LF, Eding H, Kantanen J, Williams JL, Taberlet P, et al. Molecular tools and analytical approaches for the characterization of farm animal genetic diversity. Anim Genet. 2012;43(5):483–502. doi: 10.1111/j.1365-2052.2011.02309.x . [DOI] [PubMed] [Google Scholar]
- 18.Colli L, Lancioni H, Cardinali I, Olivieri A, Capodiferro MR, Pellecchia M, et al. Whole mitochondrial genomes unveil the impact of domestication on goat matrilineal variability. BMC Genomics. 2015;16(1):1115 doi: 10.1186/s12864-015-2342-2 ; PubMed Central PMCID: PMCPMC4696231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheu A, Powell A, Bollongino R, Vigne JD, Tresset A, Çakırlar C, et al. The genetic prehistory of domesticated cattle from their origin to the spread across Europe. BMC Genet. 2015;16:54 doi: 10.1186/s12863-015-0203-2 ; PubMed Central PMCID: PMCPMC4445560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Lorenzo P, Lancioni H, Ceccobelli S, Curcio L, Panella F, et al. Uniparental genetic systems: a male and a female perspective in the domestic cattle origin and evolution. Electronic Journal of Biotechnology. 2016;September;23:69–78. [Google Scholar]
- 21.Lai SJ, Liu YP, Liu YX, Li XW, Yao YG. Genetic diversity and origin of Chinese cattle revealed by mtDNA D-loop sequence variation. Mol Phylogenet Evol. 2006;38(1):146–54. Epub 2005/07/28. doi: 10.1016/j.ympev.2005.06.013 . [DOI] [PubMed] [Google Scholar]
- 22.Dadi H, Tibbo M, Takahashi Y, Nomura K, Hanada H, Amano T. Variation in mitochondrial DNA and maternal genetic ancestry of Ethiopian cattle populations. Anim Genet. 2009;40(4):556–9. Epub 2009/04/03. doi: 10.1111/j.1365-2052.2009.01866.x . [DOI] [PubMed] [Google Scholar]
- 23.Hristov P, Spassov N, Iliev N, Radoslavov G. An independent event of Neolithic cattle domestication on the South-eastern Balkans: evidence from prehistoric aurochs and cattle populations. Mitochondrial DNA A DNA Mapp Seq Anal. 2017;28(3):383–91. Epub 2015/12/29. doi: 10.3109/19401736.2015.1127361 . [DOI] [PubMed] [Google Scholar]
- 24.Achilli A, Bonfiglio S, Olivieri A, Malusà A, Pala M, Hooshiar Kashani B, et al. The multifaceted origin of taurine cattle reflected by the mitochondrial genome. PLoS One. 2009;4(6):e5753 doi: 10.1371/journal.pone.0005753 ; PubMed Central PMCID: PMCPMC2684589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonfiglio S, Ginja C, De Gaetano A, Achilli A, Olivieri A, Colli L, et al. Origin and spread of Bos taurus: new clues from mitochondrial genomes belonging to haplogroup T1. PLoS One. 2012;7(6):e38601 doi: 10.1371/journal.pone.0038601 ; PubMed Central PMCID: PMCPMC3369859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivieri A, Gandini F, Achilli A, Fichera A, Rizzi E, Bonfiglio S, et al. Mitogenomes from Egyptian Cattle Breeds: New Clues on the Origin of Haplogroup Q and the Early Spread of Bos taurus from the Near East. PLoS One. 2015;10(10):e0141170 doi: 10.1371/journal.pone.0141170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moioli B, Napolitano F, Catillo G. Genetic diversity between Piedmontese, Maremmana, and Podolica cattle breeds. J Hered. 2004;95(3):250–6. . [DOI] [PubMed] [Google Scholar]
- 28.Pariset L, Mariotti M, Nardone A, Soysal MI, Ozkan E, Williams JL, et al. Relationships between Podolic cattle breeds assessed by single nucleotide polymorphisms (SNPs) genotyping. J Anim Breed Genet. 2010;127(6):481–8. Epub 2010/10/28. doi: 10.1111/j.1439-0388.2010.00868.x . [DOI] [PubMed] [Google Scholar]
- 29.Gargani M, Pariset L, Lenstra JA, De Minicis E, Valentini A, Consortium ECGD. Microsatellite genotyping of medieval cattle from central Italy suggests an old origin of Chianina and Romagnola cattle. Front Genet. 2015;6:68 doi: 10.3389/fgene.2015.00068 ; PubMed Central PMCID: PMCPMC4349168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhyay MR, Chen W, Lenstra JA, Goderie CR, MacHugh DE, Park SD, et al. Genetic origin, admixture and population history of aurochs (Bos primigenius) and primitive European cattle. Heredity (Edinb). 2017;118(2):169–76. Epub 2016/09/28. doi: 10.1038/hdy.2016.79 ; PubMed Central PMCID: PMCPMC5234481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenstra J, Ajmone, Marsan P, Beja Pereira A, Bollongino R, Bradley DG, et al. Meta-Analysis of Mitochondrial DNA Reveals Several Population Bottlenecks during Worldwide Migrations of Cattle. Diversity. 2014;6 178–87. [Google Scholar]
- 32.Cerezo M, Achilli A, Olivieri A, Perego UA, Gómez-Carballa A, Brisighelli F, et al. Reconstructing ancient mitochondrial DNA links between Africa and Europe. Genome Res. 2012;22(5):821–6. doi: 10.1101/gr.134452.111 ; PubMed Central PMCID: PMCPMC3337428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascunce S, Kitchen A, Schmidt P, Miyamoto M, Mulligan C. An Unusual Pattern of Ancient Mitochondrial DNA Haplogroups in Northern African Cattle. Zool Stud. 2007;46(1):123–5. [Google Scholar]
- 34.Mason I. A world dictionary of livestock breeds, types and varieties. 4th ed. 1996.
- 35.Gómez-Carballa A, Pardo-Seco J, Amigo J, Martinón-Torres F, Salas A. Mitogenomes from The 1000 Genome Project reveal new Near Eastern features in present-day Tuscans. PLoS One. 2015;10(3):e0119242 Epub 2015/03/18. doi: 10.1371/journal.pone.0119242 ; PubMed Central PMCID: PMCPMC4365045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achilli A, Olivieri A, Pala M, Metspalu E, Fornarino S, Battaglia V, et al. Mitochondrial DNA variation of modern Tuscans supports the near eastern origin of Etruscans. Am J Hum Genet. 2007;80(4):759–68. Epub 2007/02/06. doi: 10.1086/512822 ; PubMed Central PMCID: PMCPMC1852723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brisighelli F, Capelli C, Alvarez-Iglesias V, Onofri V, Paoli G, Tofanelli S, et al. The Etruscan timeline: a recent Anatolian connection. Eur J Hum Genet. 2009;17(5):693–6. Epub 2008/12/03. doi: 10.1038/ejhg.2008.224 ; PubMed Central PMCID: PMCPMC2986270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardo-Seco J, Gómez-Carballa A, Amigo J, Martinón-Torres F, Salas A. A genome-wide study of modern-day Tuscans: revisiting Herodotus's theory on the origin of the Etruscans. PLoS One. 2014;9(9):e105920 Epub 2014/09/17. doi: 10.1371/journal.pone.0105920 ; PubMed Central PMCID: PMCPMC4167696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rognoni G, Pagnacco G. Atlante Etnografico delle popolazioni bovine allevate in Italia Rome: Consiglio Nazionale delle Ricerche; 1983. [Google Scholar]
- 40.Achilli A, Olivieri A, Pellecchia M, Uboldi C, Colli L, Al-Zahery N, et al. Mitochondrial genomes of extinct aurochs survive in domestic cattle. Curr Biol. 2008;18(4):R157–8. doi: 10.1016/j.cub.2008.01.019 . [DOI] [PubMed] [Google Scholar]
- 41.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–2. Epub 2009/04/03. doi: 10.1093/bioinformatics/btp187 . [DOI] [PubMed] [Google Scholar]
- 42.Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online. 2007;1:47–50. Epub 2007/02/23. ; PubMed Central PMCID: PMCPMC2658868. [PMC free article] [PubMed] [Google Scholar]
- 43.Lindgren G, Backström N, Swinburne J, Hellborg L, Einarsson A, Sandberg K, et al. Limited number of patrilines in horse domestication. Nat Genet. 2004;36(4):335–6. doi: 10.1038/ng1326 . [DOI] [PubMed] [Google Scholar]
- 44.Cardinali I, Lancioni H, Giontella A, Capodiferro MR, Capomaccio S, Buttazzoni L, et al. An Overview of Ten Italian Horse Breeds through Mitochondrial DNA. PLoS One. 2016;11(4):e0153004 Epub 2016/04/07. doi: 10.1371/journal.pone.0153004 ; PubMed Central PMCID: PMCPMC4824442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(PDF)
Data Availability Statement
Sequences were recorded in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with accession numbers MF474376-MF475904. All other relevant data are within the paper and its Supporting Information files.