Abstract
The renal function is a key-issue in HIV/HCV co-infected patients, nevertheless, it has not established so far whether HCV treatment with new direct acting agents could impact on estimated glomerular filtration rate (eGFR) variations. In the present work, we examined the real-life data on renal function that have been prospectively collected in the SIMIT compassionate-use program of ombitasvir/paritaprevir/ritonavir plus dasabuvir (OBV/PTV/r + DSV) in 144 HIV/HCV genotype 1 co-infected patients. The population was 74% male, 30.5% in CDC stage C, with median age of 52 years (48.0–56.5) and median liver stiffness of 7.8 kPa (6.7–9.2). Median baseline eGFR was 102.0 (90.8–108.1), changing to 99.8 (83.5–104.8) at the end of treatment (EoT), and 100.0 (87.3–105.6) 12 weeks after the EoT (FU12), p<0.0001. No patient had grade 3–4 increase of creatinine. At EoT 60/144 (41.7%) patients had ≥ 5% reduction in their eGFR, confirmed at FU12 in 39/60 (65.0%) cases. Longer duration of HCV infection (cut-off 12.9 years), lower HCV-RNA viral load (cut-off 1,970,160 IU/ml) and lower platelet count (cut-off 167,000 x106/L) were significantly associated with eGFR decline at logistic analysis (adjOR 2.9, 95%CI 1.0–8.8, p = 0.05; adjOR 3.5, 95%CI 1.2–10.4, p = 0.02; adjOR 2.8, 95%CI 1.1–6.8, p = 0.03, respectively). After repeating the analysis throughout a mixed model, a higher eGFR decline was highlighted in patients concomitantly treated with tenofovir (p = 0.0001), ribavirin (p = 0.0001), or integrase inhibitors (p <0.0001), with longer duration of HIV (p = 0.0002) and HCV infection (p = 0.035), lower baseline HCV RNA (p <0.0001), previous HCV treatment (p<0.0001), and older age (p<0.0001). In conclusion, our study confirms a good renal safety profile of OBV/PTV/r + DSV treatment in HIV/HCV patients, and the median decline of 2 ml/min in eGFR, albeit statistically significant, is of doubtful clinical significance. The role of aging, concomitant therapies and duration of HIV/HCV infection needs to be further investigated.
Introduction
New direct-acting antiviral (DAA) agents have radically changed the therapeutic scenario of chronic HCV infection in both mono-infected and HIV co-infected patients [1, 2]. The 3-DAA regimen of ombitasvir, paritaprevir plus ritonavir, and dasabuvir (OBV/PTV/r + DSV) has showed high efficacy in clinical trials in HIV/HCV co-infected patients [3, 4] and recent data on compassionate-use program for OBV/PTV/r + DSV, coordinated by the Italian Society of Infectious and Tropical Diseases (SIMIT), have confirmed high efficacy in real-life setting in HCV genotype 1 infected patients [5]. Moreover, good tolerability without major adverse events attributable to the study drugs has been reported in the same context [5], but an analysis focalized on the trend of the renal function has not been performed yet. In patients co-infected with HIV/HCV the renal safety is an issue of primary interest, as HCV and HIV both constitute risk factors for renal disease. HIV infection may be linked to HIV-associated nephropathy (HIVAN) or favour renal thrombotic microangiopathy, focal segmental glomerulosclerosis and immunecomplexes deposition in the glomerulus [6]. Moreover, some of the drugs used in combined antiretroviral therapy (cART) have possible renal side effects or long-term toxicity [7]. On the same time, HCV infection is linked to a series of immune-mediated glomerulopathies as well as to possible cryoglobulinemia-linked renal vasculitis [8] and hepatorenal syndromes in more advanced stages of liver disease [9]. Nevertheless, little is known on the trend of estimated glomerular filtration rate (eGFR) in patients who clear HCV infection during and after treatment with new DAAs, especially in real life settings [10, 11]. A worsening of renal function after DAA treatment has been reported in patients with cirrhosis, and in those treated with OBV/PTV/r + DSV [10]. Nevertheless, it has not established so far whether HCV clearance is related or not with an improvement in GFR, or if, on the contrary, the combination of antiretroviral drugs and DAAs can even cause a reduction of GFR, throughout a direct mechanism or indirectly, for drug-drug interactions (DDI).
With the aim of analysing the creatinine and eGFR trends in a specific population of HIV/HCV genotype 1 co-infected individuals treated with the same DAA regimen, we examined the data on renal function of the population treated in the SIMIT compassionate-use program of OBV/PTV/r + DSV [5].
Materials and methods
The SIMIT compassionate-use program provided access to treatment for patients co-infected with HIV and HCV genotype 1 or 4, with or without compensated cirrhosis.
The program enrolled 213 patients at 26 sites. Patients were treated for 12 or 24 weeks based on the absence or presence of cirrhosis. Ribavirin (RBV) was administered twice daily according to body weight and label recommendations [12]. Patients receiving a ritonavir-boosted ART regimen discontinued the ritonavir component of their ART regimen as recommended by the local OBV/PTV/r + DSV label [13, 14].
In this sub-study, the inclusion criteria were: a) Patient is at least 18 years of age, infected with HIV and with HCV genotype 1 (subgenotype 1a, 1b, other) or 4; b) Patient has compensated cirrhosis (Child Pugh score A) OR liver fibrosis ≤F3 (by Metavir or equivalent assessment including those by an alternative technology such as FibroScan®) and a condition which contraindicates an IFN based treatment, or clinically significant extra-hepatic manifestation(s) of HCV infection, or rapidly progressive fibrosis OR Patient has a liver transplant and HCV Genotype 1 infection and is at least 12 months post liver transplantation, has had a recent (within 6 months) assessment of liver fibrosis as ≤F2, does not have a diagnosis of fibrosing cholestatic hepatitis; c) Patients has creatinine levels available at baseline (BL), at the end of treatment (EoT) and 12 weeks after the end of treatment (FU12). Duration of HIV and HCV infection was estimated assuming that infection was contracted at June 15th of the year in which the first positive serologic test was reported. Laboratory data were prospectively collected at BL, EoT, FU12 and 24 weeks after the end of treatment (FU24). GFR was calculated according to the CKD-EPI equation [15], while the stage of kidney disease was classified according to KDIGO (Kidney Disease: Improving Global Outcomes) definition [16]. Creatinine increase was classified according to tables for grading the severity of adult and pediatric adverse events of the division of AIDS (DAIDS) [17]. Fibrosis scores were assigned on the basis of the liver stiffness measured by Fibroscan®, F1 (<7.1 kPa), F2 (7.1–9.6 kPa), F3 (> 9.6–12.5 kPa), F4 (>12.5 kPa) [5]. The complete dataset used to perform the statistical analysis is available in S1 Table. Quantitative data were presented as medians (1st and 3rd quartile) and categorical data as absolute numbers and percentages. Some variables (i.e. age, HIV and HCV duration, BL HCV-RNA) were dichotomized according to the best threshold obtained from the receiver operating characteristic (ROC) curve analysis [18]. Dichotomization of explanatory variables has the advantage of providing clinically meaningful odds ratios (ORs) with 95% confidence intervals (95% CIs). A logistic regression model was used to assess the impact of baseline characteristics of patients (age, gender, BMI, CDC stage, grade of liver fibrosis, BL HCV-RNA, years of HIV and HCV infection, BL CD4+ T-lymphocytes and nadir, CKD stage), lifestyle habits (smoke, alcohol use), comorbidities (diabetes, hypertension, symptomatic cryoglobulinemia) concomitant treatments in course of OBV/PTV/r + DSV (tenofovir [TDF] and ribavirin [RBV] use), and previous treatment for HCV, on the reduction of at least 5% in eGFR at both EoT and FU12. The same variables were also studied in a logistic regression model in the post-hoc analyses, to investigate if they could influence a ≥5% improvement of eGFR in a subgroup of patients. Factors significant at univariate analysis were added in a multivariate model after stepwise procedure. A two-way repeated measures mixed model ANOVA analysis with Tuckey post-test was run to establish if there was a relationship between eGFR at follow-up and age, hypertension, stage of fibrosis, TDF, integrase strand transfer inhibitors (INSTI), protease inhibitors (PI) and RBV use, previous HCV treatment, years of HCV and HIV infection, CD4+T-lymphocyte nadir and BL HCV-RNA.
The compassionate use of the combination of OBV/PTV/r + DSV was approved on individual base for each patient (AbbVie® named-Patient Program). The full list of Ethical Committees that approved the program is available in S1 File. All patients in the study provided written informed consent. The study was conducted in accordance with the International Conference on Harmonization guidelines, applicable regulations, and the principles of the Declaration of Helsinki.
Results
Among patients included in the SIMIT compassionate-use program, 144 HIV/HCV co-infected people satisfied inclusion criteria. The population was 74% male (107/144 patients), with median age of 52 years (48.0–56.5), and median CD4+T-cell count of 658 cells /μl (480–929).The median CD4+T cell nadir was 190 cells /μl (74–314). All but three (2.1%) patients had HIV-RNA load <50 copies/ml. According to Centers for Disease Control and Prevention (CDC) definition, 66 (45.8%) patients were stage A, 44 (30.5%) stage B and 29 (30.5%) stage C. All patients had documented HCV genotype 1 infection (1a in 94, 1b in 44, 1a/b in five and 1 not further characterized in one). Median liver stiffness was 7.8 kPa (6.7–9.2) at baseline. Regarding the route of HIV/HCV transmission, the majority of patients (n = 107, 74.3%) reported previous intravenous drug use, 26 (18.1%) unprotected sexual intercourse (5.6% homo/bi-sexual and 12.5% eterosexual), five (3.4%) transfusions of blood components, while in the remaining seven (4.9%) it was unknown. Three patients had more than one risk factor: two patients with previous intravenous drug use and one with previous transfusions of blood components, that also reported unprotected sexual intercourse. Demographic and clinic characteristics of the study population are summarized in Table 1.
Table 1. General characteristics of the study population at baseline (BL).
Data are expressed as medians (1st - 3rd quartiles) or absolute frequencies (percentage).
| Variable | N | Median (q1-q3) | ||
|---|---|---|---|---|
| Age | 144 | 52 (48.0–56.05) | ||
| Years of HCV | 119 | 17.9 (10.9–20.9) | ||
| Years of HIV | 142 | 23.9 (16.9–27.9) | ||
| BL CD4 | 143 | 658 (480–929) | ||
| CD4 nadir | 133 | 190 (74–314) | ||
| BL HCV-RNA (IU/ml) | 144 | 1,137,573 (517,764.5–3,041,840) | ||
| BL HIV-RNA (copies/ml) | 144 | 0.0 (0.0–0.0) | ||
| BL AST (IU/L) | 142 | 48.5 (34–77.5) | ||
| BL ALT (IU/L) | 143 | 64 (41–99.5) | ||
| BL PLT (x106/L) | 143 | 179,000 (145,000–266,500) | ||
| Variable | N | n (%) | ||
| Female | 144 | 37 (25.69) | ||
| Caucasian | 144 | 144 (100.00) | ||
| Experienced to HCV treatment | 142 | 65 (45.77) | ||
| BMI | 134 | |||
| <20 | 16 (11.94) | |||
| 20–25 | 75 (55.97) | |||
| >25 | 43 (32.09) | |||
| CDC stage | 139 | |||
| A | 66 (47.48) | |||
| B | 44 (31.65) | |||
| C | 29 (20.86) | |||
| CKD stage | 144 | |||
| 1 | 111 (77.08) | |||
| 2 | 30 (20.83) | |||
| 3 | 3 (2.08) | |||
| HCV Genotype | 144 | |||
| 1a | 94 (65.28) | |||
| 1b | 44 (30.55) | |||
| 1 a/b | 5 (3.47) | |||
| 1 (unknown) | 1 (0.69) | |||
| RISK FACTOR | 144 | |||
| IVDU | 106 (73.6) | |||
| Homosexual/ bisexual | 8 (5.56) | |||
| Heterosexual | 18 (12.5) | |||
| Hemotransfusion | 5 (3.47) | |||
| Other/unknown | 7 (4.86) | |||
| HABITS AND COMORBIDITIES | ||||
| Smoking | 132 | 76 (57.58) | ||
| Alcool use | 140 | 48 (34.29) | ||
| Diabetes | 144 | 14 (9.72) | ||
| Hypertension | 144 | 34 (23.61) | ||
| Symptomatic Cryoglobulinemia | 144 | 11 (7.64) | ||
| CONCOMITANT THERAPIES | 144 | |||
| cART | 143 (99.31) | |||
| RBV | 115 (79.86) | |||
| TDF | 111 (77.08) | |||
| INSTI | 110 (76.39) | |||
| PI | 38 (26.39) | |||
N = number of patients for which data was available; n = absolute number of patients with each variable; % = percentage of patients with the specific variable, calculated as n/N; q1 = first quartile; q3 = third quartile; AST = aspartate aminotransferase; ALT = alanine aminotransferase; PLT = platelets; IVDU = intravenous drug use; BMI = body mass index; CKD chronic kidney disease; cART = combined antiretroviral therapy; RBV = ribavirin; TDF = tenofovir; INSTI = integrase inhibitors; PI = protease inhibitors.
The eGFR was available for all 144 patients at BL, at the EoT and at FU12, according to inclusion criteria, while 73/144 (50.7%) patients also had an available follow-up at FU24. Median BL eGFR was 102.0 (90.8–108.1), changing to 99.8 (83.5–104.8) at the EoT, and 100.0 (87.3–105.6) at FU12 (p<0.0001). No patient had grade 3–4 increase of creatinine. The maximum increase of creatinine levels during treatment was 0.35 mg/dl. At the EoT 60/144 (41.7%) patients had ≥ 5% reduction in their GFR, confirmed at FU12 in 39/60 (65.0%) cases. FU24 was available for 30/60 (50%). Of them, 15/30 (50%) had confirmed eGFR decline ≥ 5% also at FU24.
For 39 patients with confirmed eGFR decline both at the EoT and FU12, the possible association among renal function impairment and other comorbidities (hypertension, diabetes), TDF, PI, INSTI or RBV use, as well as baseline characteristics of the patients (age, sex, duration of HIV and HCV infection, HIV stage, baseline HCV-RNA and platelet count, CD4+ T-cell nadir and BL CD4+T-cell count, liver stiffness and previous HCV treatment) was investigated (Table 2).
Table 2. Univariate and multivariate predictors of event (eGFR decline > 5%) in the study population (144 patients).
|
OR (95% CI) univariate |
p-value | adjOR (95%CI) multivariate | p-value | |
|---|---|---|---|---|
| Age >45 years* | 3.0 (0.8–10.7) | 0.09 | ||
| Gender (Male) | 1.2 (0.5–2.9) | 0.66 | ||
| Smoke | 0.7 (0.3–1.6) | 0.46 | ||
| Alcohol | 1.2 (0.6–2.7) | 0.59 | ||
| Diabetes | 1.6 (0.5–5.0) | 0.45 | ||
| Hypertension | 1.4 (0.6–3.2) | 0.43 | ||
| Symptomatic cryoglobulinemia | 1.0 (0.2–4.0) | 0.99 | ||
| CDC stage B vs. A | 1.4 (0.6–3.1) | 0.44 | ||
| CDC stage C vs. A | 0.7 (0.2–2.0) | 0.50 | ||
| Grade of liver fibrosis F2 vs. F1 | 0.5 (0.1–2.2) | 0.33 | ||
| Grade of liver fibrosis F3 vs. F1 | 0.8 (0.2–4.4) | 0.83 | ||
| RBV use | 1.6 (0.6–3.7) | 0.32 | ||
| TDF use | 1 (0.4–2.4) | 0.98 | ||
| PI use | 0.4 (0.2–1.1) | 0.07 | ||
| INSTI use | 2.6 (0.9–7.3) | 0.07 | ||
| Previous HCV treatment | 0.6 (0.3–1.2) | 0.12 | ||
| BMI (<20) vs. (20–25) | 0.7 (0.2–2.4) | 0.58 | ||
| BMI 2 (>25) vs. (20–25) | 0.5 (0.2–1.2) | 0.12 | ||
| Years of HCV > 12.9* | 3.1 (1.1–8.8) | 0.03 | 2.9 (1.0–8.8) | 0.05 |
| Years of HIV > 16.9* | 2.3 (0.9–5.9) | 0.10 | ||
| CKD stage 2 vs. 1 | 0.8 (0.3–2.0) | 0.61 | ||
| CKD stage 3 vs. 1 | 1.3 (0.1–14.7) | 0.84 | ||
| Baseline CD4 < = 350* | 0.2 (0.3–1.8) | 0.16 | ||
| CD4 Nadir < 100 cells/μl | 1.3 (0.6–2.9) | 0.48 | ||
| BL HCV RNA < = 1,970,160* | 3.4 (1.3–8.8) | 0.01 | 3.5 (1.2–10.4) | 0.02 |
| PLT < = 167,000* | 4.1 (1.9–8.8) | 0.0004 | 2.8 (1.1–6.8) | 0.03 |
TDF: tenofovir; RBV: ribavirin; PI: protease inhibitors; INSTI: integrase strand transfer inhibitors; PLT: platelets; OR: odds ratio; adjOR: adjusted odds ratio; CI: confidence interval.
*Dichotomized as per ROC
In a multivariate model including duration of HCV infection (cut-off 12.9 years, dichotomized as per ROC), BL HCV-RNA viral load (cut-off level 1,970,160 IU/ml, dichotomized as per ROC), and BL platelet count (cut-off level 167,000 platelets x106/L, dichotomized as per ROC), longer HCV infection and lower HCV-RNA levels were significantly associated with eGFR decline > 5% (adjOR 2.9, 95%CI 1.0–8.8, p = 0.05, and adjOR 3.5, 95%CI 1.2–10.4, p = 0.02, respectively), as well as lower platelet count (adjOR 2.8, 95%CI 1.1–6.8, p = 0.03).
The results obtained without the dichotomizations as per ROC of continue variables (age, BL HCV-RNA, platelet count, transaminases and years of HCV and HIV infection) were also studied and are presented in S2 Table.
To account for possible differences into restricted subgroups of patients, differences among eGFR values were also estimated at all available time-points, including FU24, for all 144 patients throughout a mixed model (Table 3).
Table 3. Number of patients tested throughout mixed models and correlation with eGFR decline at different time-points.
| Variable | n | correlation with GFR decline (adjusted p) | |||
|---|---|---|---|---|---|
| from BL toEoT |
from BL toFU12 |
from BL to FU24 |
|||
| TDF | Y | 111 | 0.0001 | 0.119 | 0.078 |
| N | 33 | 0.408 | 0.686 | 0.970 | |
| RBV | Y | 115 | 0.0005 | 0.151 | 0.067 |
| N | 29 | 0.117 | 0.536 | 0.998 | |
| PI | Y | 38 | 0.994 | 0.994 | 1.000 |
| N | 106 | <0.0001 | 0.024 | 0.016 | |
| INSTI | Y | 110 | <0.0001 | 0.031 | 0.046 |
| N | 34 | 0.918 | 0.991 | 0.999 | |
| Age >45 years* | Y | 120 | 0.0001 | 0.032 | 0.1096 |
| N | 24 | 0.4645 | 0.9969 | 0.9854 | |
| Hypertension | Y | 34 | 0.9961 | 0.9126 | 0.9564 |
| N | 110 | <0.0001 | 0.0595 | 0.0984 | |
| Metavir stage of fibrosis | F1 | 117 | 0.0028 | 0.0407 | 0.4627 |
| F2 | 17 | 0.6405 | 1.0000 | 0.9996 | |
| F3 | 8 | 0.2521 | 0.9996 | 0.1427 | |
| Experienced to HCV treatment | Y | 65 | <0.0001 | 0.2631 | 0.0739 |
| N | 77 | 0.2198 | 0.343 | 0.8492 | |
| HCV since >12.9 years* | Y | 80 | 0.0035 | 0.094 | 0.1861 |
| N | 39 | 0.3615 | 1.0000 | 0.9961 | |
| HIV since >16.9 years* | Y | 106 | 0.0002 | 0.0034 | 0.0319 |
| N | 36 | 0,3547 | 1.0000 | 0.9998 | |
| Nadir CD4>100 cells/μL | Y | 90 | 0.0035 | 0.2951 | 0.5598 |
| N | 43 | 0.0082 | 0.0813 | 0.2733 | |
| BL HCV-RNA< = 1,970,160* IU/ml | Y | 98 | <0.0001 | 0.001 | 0.0879 |
| N | 46 | 0.9723 | 1.0000 | 0.9739 | |
GFR: glomerular filtration rate; n: number of patients; Y: yes; N: no; BL: baseline; EoT: end of treatment; FU12: 12 weeks after the end of treatment; FU24: 24 weeks after the end of treatment; TDF: tenofovir; RBV: ribavirin.
*Dichotomized as per ROC. Metavir stage of fibrosis is given according to stiffness evaluation by Fibroscan®
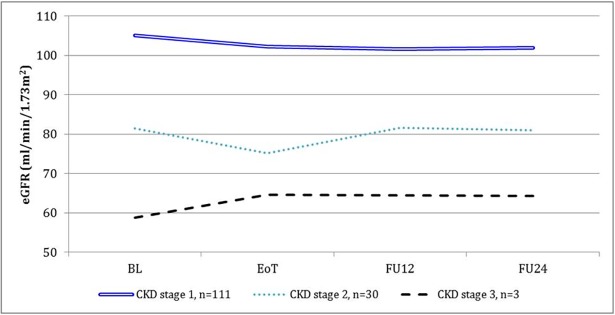
In particular, patients on concomitant treatment with TDF or RBV were at risk for significant eGFR decline compared to people who were not (p = 0.0001 and p = 0.0001, respectively) in the time between BL and EoT, but the difference was not confirmed when comparing BL to FU12 and FU24, nor between values of these time-points compared in pairs. A significant eGFR decline between BL and EoT was also found in patients with at least one of the following characteristics: age > 45 years (p<0.0001), previous HCV treatment (p<0.0001), longer duration of HIV and HCV infections (p = 0.0002 for more than 16.9 years of HIV infection and p = 0.035 for more than 12.9 years of HCV infection), lower baseline HCV-RNA (p <0.0001 for HCVRNA values ≤ 1,970,160 IU/ml), treatment with an INSTI (<0.0001) and not on a PI-containing cART (<0.0001). A longer duration of HIV infection (p = 0.0034), an older age (p = 0.0324), a lower baseline HCV RNA (p = 0.001), INSTI use (p = 0.031) and not being on PI-based cART (p = 0.024) were associated with a significant decrease in eGFR levels in the time between BL and FU12. A longer duration of HIV infection and INSTI use also maintained correlation with a decrease of the eGFR at FU24 (Table 3). The trend of eGFR according to baseline CKD stage is shown in Fig 1. In order to clarify if there was a sub-group of patients who improved their eGFR, a post-hoc analysis was also performed. Overall, 14 patients had confirmed eGFR improvement of ≥ 5% at both EoT and FU12. The logistic analysis revealed that the improvement was more probable in CKD stage 2 and 3 when compared to CKD stage 1 (Odds Ratio, OR, 9.7, 95%CI 2.7–35.2, p = 0.0005 and OR 53.5, 95%CI 4.0–720.1, p = 0.003, respectively), while no significant correlation was found with other factors.
Fig 1. Trend of estimated glomerular filtration rate (eGFR) in the global population, according to GITMO renal stage at baseline (BL).
n = number of patients in each chronic kidney disease (CKD) stage. EoT: End of treatment; FU12: follow-up 12 weeks after the EoT; FU24: follow-up 24 weeks after the EoT.
Discussion
This study analyses the eGFR trend in a large and homogeneous cohort of patients with HIV/HCV genotype 1 co-infection and mild fibrosis prospectively treated with OBV/PTV/r + DSV.
The main findings is that no patient experienced grade 3–4 increase of creatinine levels throughout HCV treatment and FU, and the finding of a median decline of 2 ml/min in eGFR, albeit statistically significant, was of doubtful clinical significance.
Previous trials evidenced the good safety profile and efficacy (higher than 90%) of OBV/PTV/r + DSV among patients with stage IV or V CKD [19]. A very few cases (2/179 patients) of creatinine clearance decrease to <50 ml/min (in a single determination) have been reported in PEARL II study, (3/419 patients, all in treatment with ribavirine) in PEARL III and (3/305 patients, 1 who was in treatment with RBV, and 2 who were not) in PEARL IV [20–22]. Mehta et al. [23] have recently analysed the eGFR trend in HCV mono-infected patients treated with OBV/PTV/r + DSV in phase 3 trials SAPPHIRE I/ II, TOPAZ I /II, and RUBY I /II. In this comprehensive analysis, they found a change in eGFR of + 0.39 ml/min in patients enrolled in SAPPHIRE I/II trials (n = 776 patients), with significant improvement in the subgroup of patients with CKD stage 2 and 3. On the other hand, in TOPAZ I/II (n = 2206 patients) and in RUBY I/II trials (n = 78 patients), the overall eGFR decreased, with a significant negative trend (p<0.001) in patients in CKD stage 1 enrolled in TOPAZ I/II trials. Similarly, in our study, we found HIV/HCV co-infected patients had a significant decrease of the eGFR, driven by the high prevalence of CKD stage 1, while, in patients in CKD stages 2 and 3, the eGFR improved. In other trials and registration studies of other DAAs, on-treatment assessments included the evaluations of renal function, but specific analyses on the trend of creatinine clearance have not been performed [22–34]. Some studies had arisen more specifically the issue of renal safety using different criteria for CKD classification and progression as, at present, the evaluation of renal function in course of DAA therapy has not been standardized so far. As a consequence, some studies have reported the numbers of abnormal creatinine rises of any grade [35], while others only those of grade 3–4 [36–38], using different scaling systems. Additionally, the number of patients with an increase in creatinine levels of 0.4 mg/dl or more during treatment has also been used [36,37], or the change in mean creatinine clearance through week 12 [37, 39]. In the general population, the age-related decline in eGFR is generally believed to be less than 1 ml/min each year, i.e. about 1% of eGFR, assuming a filtration rate of 100 ml/min [40], while, in HIV infected patients, a rapid eGFR decline is usually defined by a reduction in eGFR ≥5 ml/min/year in patients with baseline eGFR > 90 ml/min [41]. This corresponds to about 5% decline in patients with normal eGFR (CKD stage 1), while, in patients with baseline eGFR<90 ml/min, a 5% decline permits to take into account also lower absolute changes in eGFR.
We chose to use a decline of eGFR of ≥5% within 12 weeks, i.e. 5-fold higher than what expected in one year period, as a marker of abnormal renal function decline.
Of note, we did not found any creatinine rise of grade >2 or of ≥0.4 ml/min and the reported median decline of 2 ml/min in eGFR, even if statistically significant, has doubtful clinical significance. In fact, current guidelines recommend to refer to a nephrologist in case of eGFR decline by > 25% or to a level < 60 ml/min [42]. Moreover, we cannot exclude that this slight decline could be followed by a late improvement, as previously found in liver transplant recipients with mild chronic kidney disease that achieved SVR after interferon-based regimens [43].
Another remarkable finding of our study is that a ≥5% eGFR decline was related to a longer history of HCV infection and to lower baseline HCV-RNA and platelet count. The correlation with duration of HCV infection could be expected, as, even if the linkage between HCV and CKD is controversial, many evidences underline a possible correlation. In the veteran cohort, HCV infection was found not to have a relation with progressive CKD [44], but, in other large cohorts, both HCV viremic and HCV aviremic individuals were found at increased risk for moderate and advanced CKD compared with HIV-infected subjects who were HCV seronegative [45] and HCV co-infection was found as an independent risk factor for CKD in HIV [46]. On the contrary, the higher probability of eGFR decline in patients with lower BL HCV-RNA was unexpected, and is in contrast with the evidence of higher Odds Ratios for CKD that have been found in patients with higher HCV-RNA [46, 47]. A previous paper on 1,676 HIV/HCV co-infected patients treated for HCV, revealed an incidence of CKD of 5.32 per 1000 person/years of follow-up, without significant differences between patients who achieved sustained virologic response (SVR) and those who not, suggesting a possible minor role for the current viremia on the development of renal injury [48]. On the other hand, higher risks of CKD have been found in patients with detectable HCV RNA than in those who cleared the infection [49] and a higher HCV-RNA has been demonstrated an independent predictor of CKD [50]. We think that these evidences, all together, underline a major role of the global burden of the HCV disease, that is correlated to high HCV-RNA and to years of HCV infection. In the same direction also goes the association between eGFR decrease and lower platelet count, that might suggest a higher probability of renal injury in more advanced stages of HCV disease. Indeed, HCV-RNA is fluctuating and the punctual value at the time of study entry might not represent the real burden of HCV disease in the study population. Another unexpected finding of our study was that the classical risk factors for kidney disease, such as increasing age, male gender, history of smoking, diabetes, and hypertension, had no correlation with impairment of renal function, although it is possible that the effect of such factors could not be appreciable in a relatively young population with low prevalence of diabetes and hypertension.
We also tested eGFR trend within the groups for each measure period, through a mixed model for multiple comparisons that permitted to consider also the interaction between the variables and the time. The results confirmed a role of the longer duration of HCV infection and lower BL HCV-RNA, but also revealed other potential risk factors linked to significant eGFR decline, i.e. TDF, INSTI or RBV use, age > 45 years, previous HCV treatment (mainly IFN-based), and longer duration of HIV infection. Indeed, TDF and IFN are drugs with recognized potential nephrotoxicity, which has also been hypothesized for RBV [51–53] while the effect of INSTI on possible creatinine increase, driven by both dolutegravir and, to a lesser extent, raltegravir [54–55], might have had a role in patients who switched to INSTI to avoid drug interactions during DAA treatment. Moreover, HIV infection and ageing have potential influence on renal function [56]. However, although these results may be of great interest, they must be interpreted with caution, because a significant trend toward reduction was seen in the general population in correlation with time (and in particular between BL and EoT), and it is possible that it is confirmed in any group big enough to allow a reliable analysis. For the same reason, the lack of significance in eGFR decline in patients treated with PI and in those with a history of hypertension do not allow to interpret these factors as protective, but should be rather interpreted as a non-significance, probably due to small number of patients.
This study has several limitations. The lack of a control group constitutes a major limit of this study, as we could not compare the eGFR trend of the study population with that of HIV/HCV co-infected people not undergoing HCV treatment, nor with that of patients treated with other DAA regimens, including those that are now the preferred first-line agents for HCV treatment [57–58]. Moreover, we had no available urinalysis for the study population, and it limits the accuracy of a precise diagnosis of renal injury. Also, we did not have complete data about potentially nephrotoxic concomitant therapies, other than the cART, that could also have played a role, and FU24 was not available for a large part of the study population, so that the possibility of a recovery 24 weeks after the EoT was not adequately investigated. Lastly, we dichotomized the continues variables included in the regression model, as binary covariates offer a simple risk classification into high versus low and, in the regression setting, the interpretation of the impact of a binary covariate on outcome is easier than that for a change of 1 unit in a continuous covariate [59]. However, this approach could have influenced the significance of some results, as it can sometimes lead to biased estimates in regression settings. When categorizing continuous covariates, there is always the possibility of loss of information, possible loss of power to detect actual significance, and possibility of false positive results as well, and using two groups conceals any non-linearity in the relation between the variable and outcome [60].
Despite these limitations, we found that the eGFR decline was significant during HCV treatment, but the same was not found prolonging observation to FU12 in the majority of groups examined through the mixed models. This could lead us to speculate that a temporary nephrotoxicity is possible in course of therapy, but, at least in part, reversible. Low baseline HCV-RNA seems not to be protective in our series, while duration of HCV infection and low platelet count are risk factors for >5% eGFR decline, independently from chronological age. On the contrary, patients with poor renal function at the beginning of treatment had higher probability of renal improvement, even if numbers are too low to draw conclusions.
In summary, our study confirms a good renal safety profile of OBV/PTV/r + DSV treatment in a large homogeneous cohort of HIV/HCV patients. The role of aging, concomitant therapy with TDF, INSTI or RBV, previous HCV treatment and duration of HIV infection need to be further investigated, and studies on larger populations and with longer follow-up are desirable to better clarify the dynamics of renal function in the contest of DAA treatment.
Supporting information
(XLSX)
(DOCX)
(DOCX)
Acknowledgments
This study was coordinated by the Italian Society of Infectious and Tropical Diseases (SIMIT). SIMIT Scientific Director: Professor Massimo Andreoni, simit@promoleader.com.
AbbVie® provided study drugs at no charge which were distributed through a competitive enrollment program. No additional financial support was provided for this study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
AbbVie provided study drugs at no charge which were distributed through a competitive enrollment program. No additional financial support was provided for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Milazzo L, Lai A, Calvi E, Ronzi P, Micheli V, Binda F, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med. 2017;18:284–291. doi: 10.1111/hiv.12429 [DOI] [PubMed] [Google Scholar]
- 2.Hawkins C, Grant J, Ammerman LR, Palella F, Mclaughlin M, Green R, et al. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: a real-world perspective. J Antimicrob Chemother. 2016;71:2642–5. doi: 10.1093/jac/dkw203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyles D, Saag M, Viani RM, Lalezari J, Adeyemi O, Bhatti L, et al. TURQUOISE-I Part 1b: Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir with Ribavirin for Hepatitis C Virus Infection in HIV-1 Coinfected Patients on Darunavir. J Infect Dis. 2017;215:599–605 doi: 10.1093/infdis/jiw597 [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313:1223–31. doi: 10.1001/jama.2015.1328 [DOI] [PubMed] [Google Scholar]
- 5.Andreoni M, Teti E, Antinori A, Milazzo L, Sollima S, Rizzardini G, et al. Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir Combination Treatment in Patients with HIV/HCV Co-Infection: Results of an Italian Compassionate Use Program. Clin Infect Dis. 2017;64:680–683. doi: 10.1093/cid/ciw846 [DOI] [PubMed] [Google Scholar]
- 6.Ellis CL. HIV associated kidney diseases: Clarifying concordance between renal failure in HIV infection and histopathologic manifestations at kidney biopsy. Semin Diagn Pathol. 2017;34:377–383. doi: 10.1053/j.semdp.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 7.Campos P, Ortiz A, Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J. 2016;9:772–781. doi: 10.1093/ckj/sfw104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20:7544–54. doi: 10.3748/wjg.v20.i24.7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Butt AA, Ren Y, Marks K, Shaikh OS, Sherman KE; ERCHIVES study. Do directly acting antiviral agents for HCV increase the risk of hepatic decompensation and decline in renal function? Results from ERCHIVES. Aliment Pharmacol Ther. 2017;45:150–159. doi: 10.1111/apt.13837 [DOI] [PubMed] [Google Scholar]
- 11.Taramasso L, Ricci E, Celesia BM, Bonfanti P, Quirino T, Squillace N, et al. Co-administration of tenofovir plus protease inhibitor based antiretroviral therapy during sofosbuvir/ledipasvir treatment for HCV infection: Much Ado About Nothing? Clin Res Hepatol Gastroenterol. 2017; 41: e76–e79 doi: 10.1016/j.clinre.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Electronic Medicines Compendium (eMC). Copegus EU summary of product characteristics. Available from: https://medicines.org.uk/emc/medicine/11755/SPC/Copegus+200mg+Film-coated+Tablets/.
- 13.European Medicines Agency. Exviera summary of product characteristics. Mar 2016 [cited 2018 Jan 24]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003837/WC500182236.pdf.
- 14.European Medicines Agency. Viekirax summary of product characteristics. Mar 2016 [cited 2018 Jan 24]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003839/WC500184000.pdf.
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health, US Department of Health and Human Services. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Nov 2014; Version 2.0 [cited 2018 Jan 24]. Available from: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf.
- 18.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98. [DOI] [PubMed] [Google Scholar]
- 19.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. Efficacy of Direct-Acting Antiviral Combination for Patients With Hepatitis C Virus Genotype 1 Infection and Severe Renal Impairment or End-Stage Renal Disease. Gastroenterology. 2016;150:1590–8. doi: 10.1053/j.gastro.2016.02.078 [DOI] [PubMed] [Google Scholar]
- 20.Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365.e1 doi: 10.1053/j.gastro.2014.04.045 [DOI] [PubMed] [Google Scholar]
- 21.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C,et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338 [DOI] [PubMed] [Google Scholar]
- 22.Hézode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet. 2015;385:2502–9. doi: 10.1016/S0140-6736(15)60159-3 [DOI] [PubMed] [Google Scholar]
- 23.Mehta DA, Cohen E, Charafeddine M, Cohen DE, Bao Y, Sanchez Gonzalez Y, et al. Effect of Hepatitis C Treatment with Ombitasvir/Paritaprevir/R + Dasabuvir on Renal, Cardiovascular and Metabolic Extrahepatic Manifestations: A Post-Hoc Analysis of Phase 3 Clinical Trials. Infect Dis Ther. 2017; doi: 10.1007/s40121-017-0171-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dore GJ, Conway B, Luo Y, Janczewska E, Knysz B, Liu Y, et al. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: The MALACHITE-I/II trials. J Hepatol. 2016;64:19–28. doi: 10.1016/j.jhep.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 25.Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82. doi: 10.1056/NEJMoa1402869 [DOI] [PubMed] [Google Scholar]
- 26.Feld JJ, Moreno C, Trinh R, Tam E, Bourgeois S, Horsmans Y, et al. Sustained virologic response of 100% in HCV genotype 1b patients with cirrhosis receiving ombitasvir/paritaprevir/r and dasabuvir for 12weeks. J Hepatol. 2016;64:301–7. doi: 10.1016/j.jhep.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145 [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski MS, Naggie S, Lalezari J, Fessel WJ, Mounzer K, Shuhart M, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353–61. doi: 10.1001/jama.2014.7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385:1098–106. doi: 10.1016/S0140-6736(14)62483-1 [DOI] [PubMed] [Google Scholar]
- 30.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401–8. doi: 10.1016/S1473-3099(13)70033-1 [DOI] [PubMed] [Google Scholar]
- 31.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naïve patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–7. doi: 10.1016/S0140-6736(13)60247-0 [DOI] [PubMed] [Google Scholar]
- 32.Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015; 61:1127–35. doi: 10.1002/hep.27726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buti M, Gordon SC, Zuckerman E, Lawitz E, Calleja JL, Hofer H, et al. Grazoprevir, Elbasvir, and Ribavirin for Chronic Hepatitis C Virus Genotype 1 Infection After Failure of Pegylated Interferon and Ribavirin With an Earlier-Generation Protease Inhibitor: Final 24-Week Results From C-SALVAGE. Clin Infect Dis. 2016;62:32–6. doi: 10.1093/cid/civ722 [DOI] [PubMed] [Google Scholar]
- 34.Lawitz E, Gane E, Pearlman B, Tam E, Ghesquiere W, Guyader D, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1075–86. doi: 10.1016/S0140-6736(14)61795-5 [DOI] [PubMed] [Google Scholar]
- 35.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–23. doi: 10.1016/S0140-6736(13)62121-2 [DOI] [PubMed] [Google Scholar]
- 36.Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705–13. doi: 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luetkemeyer AF, McDonald C, Ramgopal M, Noviello S, Bhore R, Ackerman P. 12 Weeks of Daclatasvir in Combination With Sofosbuvir for HIV-HCV Coinfection (ALLY-2 Study): Efficacy and Safety by HIV Combination Antiretroviral Regimens. Clin Infect Dis. 2016;62:1489–96. doi: 10.1093/cid/ciw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–45. doi: 10.1016/S0140-6736(15)00349-9 [DOI] [PubMed] [Google Scholar]
- 39.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493–505. doi: 10.1002/hep.28446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85. [DOI] [PubMed] [Google Scholar]
- 41.Kamara DA, Ryom L, Ross M, Kirk O, Reiss P, Morlat P, et al. Development of a definition for Rapid Progression (RP) of renal function in HIV-positive persons: the D:A:D study. BMC Nephrol. 2014;15:51 doi: 10.1186/1471-2369-15-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e96–138. doi: 10.1093/cid/ciu617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blé M, Aguilera V, Rubín A, García-Eliz M, Vinaixa C, Prieto M, et al. Improved renal function in liver transplant recipients treated for hepatitis C virus with a sustained virological response and mild chronic kidney disease. Liver Transpl. 2014; 20:25–34. doi: 10.1002/lt.23756 [DOI] [PubMed] [Google Scholar]
- 44.Rogal SS, Yan P, Rimland D, Lo Re V 3rd, Al-Rowais H, Fried L, et al. Electronically Retrieved Cohort of HCV Infected Veterans Study Group. Incidence and Progression of Chronic Kidney Disease After Hepatitis C Seroconversion: Results from ERCHIVES. Dig Dis Sci. 2016;61:930–6. doi: 10.1007/s10620-015-3918-z [DOI] [PubMed] [Google Scholar]
- 45.Lucas GM, Jing Y, Sulkowski M, Abraham AG, Estrella MM, Atta MG, et al. Hepatitis C viremia and the risk of chronic kidney disease in HIV-infected individuals. J Infect Dis. 2013;208:1240–9. doi: 10.1093/infdis/jit373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mocroft A, Neuhaus J, Peters L, Ryom L, Bickel M, Grint D, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS One. 2012;7:e40245 doi: 10.1371/journal.pone.0040245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, et al. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease. Kidney Int. 2017;92:703–709. doi: 10.1016/j.kint.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 48.Leone S, Prosperi M, Costarelli S, Nasta P, Maggiolo F, Di Giambenedetto S, et al. Incidence and predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV-coinfected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis. 2016;35:1511–20. doi: 10.1007/s10096-016-2692-y [DOI] [PubMed] [Google Scholar]
- 49.Peters L, Grint D, Lundgren JD, Rockstroh JK, Soriano V, Reiss P, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS. 2012; 26:1917–26. doi: 10.1097/QAD.0b013e3283574e71 [DOI] [PubMed] [Google Scholar]
- 50.Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2012; 6:369–78. doi: 10.1007/s12072-011-9284-9 [DOI] [PubMed] [Google Scholar]
- 51.Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971–5. doi: 10.1097/QAD.0b013e32832c96e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3:e23–32. doi: 10.1016/S2352-3018(15)00211-8 [DOI] [PubMed] [Google Scholar]
- 53.Carrier P, Essig M, Debette-Gratien M, Sautereau D, Rousseau A, Marquet P, et al. Anti-hepatitis C virus drugs and kidney. World J Hepatol. 2016;8:1343–1353. doi: 10.4254/wjh.v8.i32.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382:700–8. doi: 10.1016/S0140-6736(13)61221-0 [DOI] [PubMed] [Google Scholar]
- 55.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381:735–43. doi: 10.1016/S0140-6736(12)61853-4 [DOI] [PubMed] [Google Scholar]
- 56.Kopp JB. Chronic Kidney Disease in the Aging Human Immunodeficiency Virus-Infected Population. J Infect Dis. 2017; 216:619–621. doi: 10.1093/infdis/jix205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017; 66:153–194. doi: 10.1016/j.jhep.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 58.The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Last Updated: September 21, 2017 [cited 2018 Jan 24]. Available from: https://www.hcvguidelines.org/sites/default/files/full-guidance-pdf/HCVGuidance_September_21_2017_f.pdf.
- 59.Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Mayo Foundation. Jun 2006 [cited 2018 Jan 24]. Technical Report Series #79. Available from: http://www.mayo.edu/research/documents/biostat-79pdf/doc-10027230.
- 60.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080 doi: 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.