Abstract
Background
Omega-3 fatty acids are central to brain-development of children. Evidence from clinical trials and systematic reviews demonstrates the potential of long-chain Omega-3 supplementation for learning and behavior. However, findings are inconclusive and in need of robust replication studies since such work is lacking.
Objectives
Replication of the 2012 DOLAB 1 study findings that a dietary supplementation with the long-chain omega-3 docosahexaenoic acid (DHA) had beneficial effects on the reading, working memory, and behavior of healthy schoolchildren.
Design
Parallel group, fixed-dose, randomized (minimization, 30% random element), double-blind, placebo-controlled trial (RCT).
Setting
Mainstream primary schools (n = 84) from five counties in the UK in 2012–2015.
Participants
Healthy children aged 7–9 underperforming in reading (<20th centile). 1230 invited, 376 met study criteria.
Intervention
600 mg/day DHA (from algal oil), placebo: taste/color matched corn/soybean oil; for 16 weeks.
Main outcome measures
Age-standardized measures of reading, working memory, and behavior, parent-rated and as secondary outcome teacher-rated.
Results
376 children were randomized. Reading, working memory, and behavior change scores showed no consistent differences between intervention and placebo group. Some behavioral subscales showed minor group differences.
Conclusions
This RCT did not replicate results of the earlier DOLAB 1 study on the effectiveness of nutritional supplementation with DHA for learning and behavior. Possible reasons are discussed, particularly regarding the replication of complex interventions.
Trial registration and protocol
www.controlled-trials.com (ISRCTN48803273) and protocols.io (https://dx.doi.org/10.17504/protocols.io.k8kczuw)
Introduction
Some high-quality evidence demonstrates that increasing children’s dietary intake of the long-chain omega-3 fatty acids may improve concentration, reduce disruptive behavior and leads to better reading and spelling [1,2]. Biochemical and neuroscientific research has long demonstrated the important role of longer-chain omega-3 fatty acids–docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)- for brain development [3,4].
Influential evidence for the potential benefits from DHA omega-3 supplementation in children stems from the DOLAB (DHA Oxford Learning and Behavior) I study [5]. This randomized, controlled trial (RCT) found that a 16-week dietary intervention with 600mg/day of algal-source DHA led to significant improvement over placebo for behavior and learning among healthy but under-performing children, aged 7–9 years, from mainstream UK schools.
Prior to DOLAB I, most studies of omega-3 supplementation for learning and behavior had involved child populations with specific developmental conditions such as attention deficit hyperactivity disorder (ADHD) [6,7], dyslexia and developmental coordination disorder (DCD) [8]. Those studies were small and their generalizability was limited by differences between the populations being studied, the treatment formulations that were used, and the outcomes assessed [9]. By contrast the DOLAB I study provided the first good evidence for the benefits of DHA omega-3 in a large sample of healthy pupils with particularly poor reading but otherwise without any behavioral or learning diagnosis.
Since the publication of the original study and the observation of heterogeneous evidence regarding learning and behaviour outcomes several trials have been published. Notably these usually focus on population with diagnosed learning or behavioural problems. A recent systematic review of polyunsaturated fatty acid (PUFA) supplementation for learning disorders found insufficient evidence of benefits in children with ADHD [10]. Notably, this review also pointed to the lack of comparable studies reporting reading as an outcome. Since then a few smaller trials found no effects for ADHD [11] however positive effects on spelling [12] and comprehensive assessments of reading ability in mainstream Scandinavian children [13,14] have been found, while other trials obtained insufficient evidence in these domains [15]. However, these studies test combinations of PUFAs with e.g. iron, and often recruited very different samples of school age children.
Three recent systematic reviews find small improvements in ADHD-type behavioral outcomes [16–18]. At the same time two Cochrane reviews [10,19] and a recent review of reviews [20] conclude that current evidence for a positive effect of polyunsaturated fatty acid supplementation for ADHD is insufficient. Interestingly, Gillies et al. [19] comment on the contradicting results to Bloch & Qawasmi [1],partly suggest that such results are due to differing combinations of parent- and teacher-rated behavior and different sets of inclusion criteria.
Of the aforementioned reviews, two included the original DOLAB I study. Whilst Tan et al.’s [10] inclusion criteria excluded DOLAB I, and Gillies et al. [19] was written prior to the publication of the original trial paper. The DOLAB I study was part of meta-analyses in Hawkey & Nigg [18] and notably in Cooper et al. [17]. For example, the latter study’s findings are strongly influenced by the results from the DOLAB 1 study, with meta-regression weights >40%.
The inconclusiveness of the current evidence on PUFA supplementation for learning and behavior in young children, particularly due to the lack of comparable studies, and the potential impact of the original DOLAB I study in past systematic reviews, highlights the need for the replication of the trial.
Importantly for the current state of evidence, Gillies et al. recommend that “future research [should] address[…] current weaknesses in this area, which include small sample sizes, variability of selection criteria, variability of the type and dosage of supplementation, short follow-up times and other methodological weaknesses.” [19]. This recommendation relates to ADHD studies, and should apply even more to studies in more general populations that are less common. The DOLAB II trial was a well-designed and well-powered study, with the same selection criteria, dosage and intervention period as the initial trial, thus providing the most rigorous direct test of the original findings. To the authors’ knowledge it is the first trial to assess the effects of DHA omega-3 on children’s learning and behavior in a replication RCT.
Objectives
To replicate the beneficial effects of dietary supplementation with the long-chain omega-3 docosahexaenoic acid (DHA) on the reading, working memory, and behavior of healthy schoolchildren as originally found in the DHA-Oxford-Learning-and-Behaviour (DOLAB I) study.
Methods
This was a parallel group, fixed-dose, randomized, double-blind placebo-controlled trial (RCT). The protocol for this trial and CONSORT checklist are available as supporting information; see Protocol S1 File (and at https://dx.doi.org/10.17504/protocols.io.k8kczuw) and Checklist S2 File and the study was registered at www.controlled-trials.com (ISRCTN48803273).
Participants and setting
The study was open to healthy children attending mainstream UK primary schools in Oxfordshire, Northamptonshire, Buckinghamshire, Milton Keynes and Swindon who were aged 7–9 years.
Inclusion
Included children had to be below the 20th centile on a standardized word reading test, “The British Ability Scales” (BAS II) [21] but with no other significant special educational needs.
However, during the first wave of recruitment it was found that due to recent changes in the teaching of literacy, children’s ability to decode words had considerably improved. Consequently this study used a recalibrated version of the BAS II (New BAS II) and for comparison the new BAS 3 [22], to appropriately measure children’s reading ability. In order to meet the planned sample size, it was decided to recruit children who fell below the 20th centile on either the recalibrated new BAS II or the BAS 3 word reading tests and the protocol was modified accordingly.
Exclusion
Children with specific medical disorders (e.g. visual or hearing impairment), or who were taking medications expected to affect behavior and learning, were excluded from the study, as were those whose first language at home was not English. Schools were also asked to exclude any children whose social/family circumstances would have made inclusion into the study inappropriate (e.g. serious illness in the family). Children who, according to their parents, ate oily fish twice or more a week or took omega-3 supplements were also excluded.
Local authorities in Oxfordshire, Buckinghamshire and Northamptonshire and the Unitary Authorities in Swindon and Milton Keynes were partners in the research, providing information on children’s performance on national attainment tests conducted at age 7 (Key Stage 1)–further details on the recruitment can be found in the supporting information. (Recruitment S3 File)
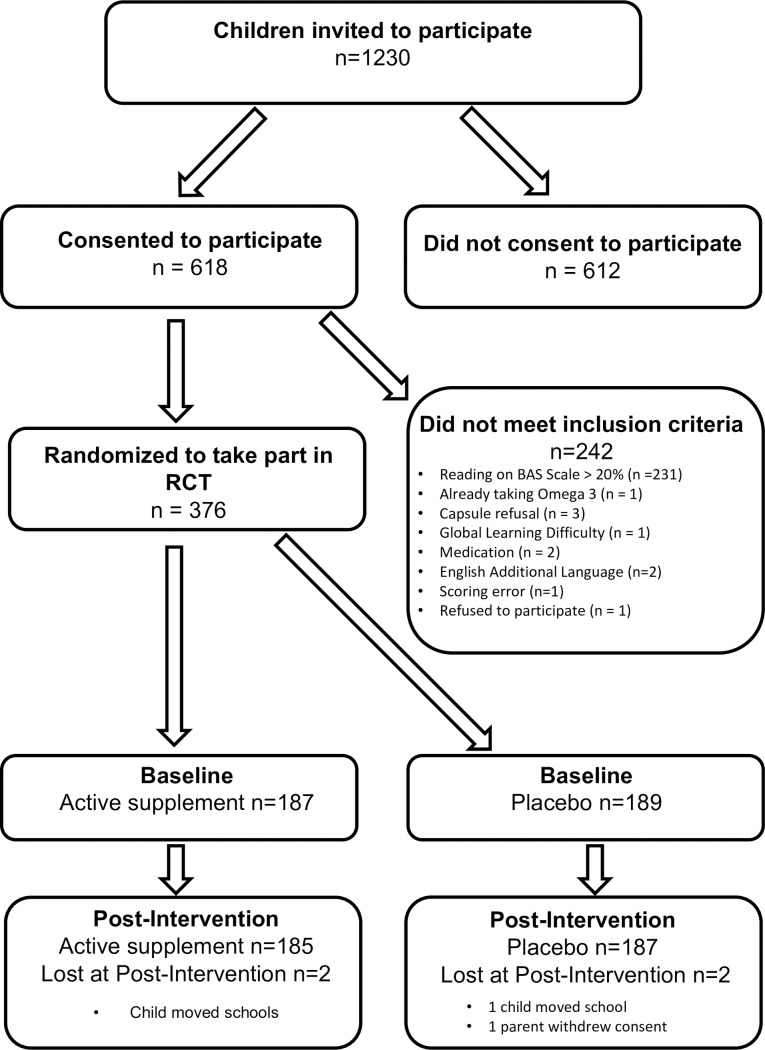
Having been informed about inclusion and exclusion criteria for the study, teachers at participating primary schools and academies created lists of those children whose current reading performance suggested they may benefit from inclusion to the study and on this basis, letters of invitation were sent to parents (see Fig 1).
Fig 1. Flow of participants from invitation to randomization (CONSORT flowchart).
Ethics
Written informed consent was gained from parents, and verbal assent from the children, prior to the initial screening assessments. Ethical consent was gained from the Oxford B NHS Ethics Board, 15/10/2012, ref:12/SC/0465. Data was stored and processed anonymously.
Intervention
Active treatment consisted of a fixed dose of 600 mg DHA (from algal oil), delivered in three 500 mg capsules per day, each providing 200 mg DHA. The placebo treatment consisted of three, taste- and color-matched 500 mg capsules per day containing corn/soybean oil. Both treatments were provided by DSM Nutritional Products, for full details see Supporting Information Capsule Content S4 File.
Schools were given a 16-week supply of capsules (labelled with each participating child’s name) and asked to dispense 3 capsules daily at lunch time during school terms. Likewise, parents were given a 16-week capsule supply for weekends, school holidays and at any other time pupils were absent from school.
To ensure implementation fidelity schools and parents were given detailed instructions for dispensing capsules. To increase compliance parents further received a sticker diary to record capsule consumption. To log any health issues and/or problems with capsule consumption, schools and parents received fortnightly phone calls during the course of the intervention, which were also used to encourage continued compliance.
Due to issues with the colorant and key ingredient (non-vegetarian gelatine) of the capsule shells these were changed in January 2014 and the protocol amended (for more information see Protocol Amendment S5 File).
Outcomes
Primary outcomes assessed at baseline and at 16-week follow-up were:
a) Reading
Assessment through both the Word Reading Achievement sub-tests from the British Ability Scales (New BAS II and BAS 3 [21,22]). These are a widely used age-standardized, single word reading test, normed on UK children, and sensitive enough to show significant change over four months. Standardized scores have a mean of 100 and a standard deviation of 15, with higher scores indicating better reading.
b) Working memory
Assessment via the recall of digits forward and recall of digits backward sub-tests from the BAS II. Again, these measures are age standardized, but use T-scores, with a mean of 50 and a standard deviation of 10, with higher scores indicating better working memory.
c) Behavior
Assessment by parents using the long version of the Conners’ Rating Scale (CPRS-L) [23,24]. This is an age-standardized, highly valid and reliable instrument, measuring child behavioral problems over several domains, expressed as T-scores (mean = 50, sd = 10). Reductions in these scores represent an improvement of child behavior.
For many years these scales have been routinely used in medication trials for children with behavior problems such as ADHD; they have also been successfully used in several previous trials of fatty acid supplementation. The secondary outcome of behavior in school was measured with the teacher version of the Conners’ Rating Scale (CTRS-L)[25,26].
Other measures
i) Demographic information
Information on eligibility for free school meals (FSM) was gained from local authority data and used as a proxy for Social Economic Status (SES). Local authority data were also used to report gender and age. Where such information was unavailable, parent reported data was used instead.
ii) Health information
At baseline information was collected from parents/guardians on each child’s current health status (including items from the side effects scale, see below). Information was also collected on possible diagnoses of ADHD and Dyslexia. Height and weight were assessed by the researchers at each child’s baseline assessment and BMI percentiles were calculated using Center for Disease Control and Prevention (CDC) guidelines [27].
iii) Medication
Medication information along with supplement use and fish consumption were collected from parents using a checklist. This latter information was used to confirm eligibility for the study.
iv) Compliance
Compliance was assessed by counting the capsules returned and by way of analyses of fingerstick blood tests pre- and post-intervention (for technical details see Supporting Information Blood fatty acid data S6 File). Schools and parents were also provided with a ‘calendar’ and stickers to encourage children’s compliance and to help keep track of each day’s capsule consumption. Fortnightly health-check calls also provided an opportunity for researchers to encourage compliance.
v) Side effects
Side effects were recorded using the Barkley Side Effects Rating Scale (SERS) [28], a commonly-used instrument assessing the frequency and severity of 17 common side effects which may occur as the result of taking medication or supplements. Each symptom is rated on a 10-point scale from absent to severe.
vi) Attendance
Parental consent was gained for schools to disclose each child’s attendance at school during the 16-week intervention, and this was recorded and collected at post-intervention measuring each half day’s absenteeism due to illness. Parents were also asked to report the number of days off school due to ill-health in the past school term at baseline and during the course of the intervention at the end of the study.
Description of procedures
Baseline
Baseline assessments took place in schools during normal school hours in a quiet room by two trained researchers. Each child was assessed individually on reading. Only those children who met our inclusion criteria (< 20th centile on the New BAS II or BAS 3), were included into the study and assessed on their working memory. Behavior questionnaires were sent out to parents with our letter of invitation whilst teachers of all those included in the study were given these questionnaires at the end of this assessment.
Post-intervention
Children were re-assessed at school 16 weeks post-intervention, when all primary outcome measures were repeated. On completion of the study, all participants were given a three months’ supply of the active supplement, as well as a £5 gift token.
Sample size
Power calculations were based on change scores of reading ability from DOLAB I. In children with initial reading performance below the 20th percentile these were mean = 2.0 (SD 4.2) for the active group and mean = 0.9 (SD 3.9) for the placebo group, giving an effectsize of d = 0.28. Sample sizes were calculated with GPOWER, v3.15 [29] for a t-test. These indicated that approximately 200 participants per group would provide 80% power with an α of 5%.
Randomization
A statistician at Sealed Envelope Ltd. independently performed the randomization with minimization via a 1:1 allocation ratio. The program’s minimization algorithm ensured balanced allocation of participants between the treatment groups for each school (to allow for any sociodemographic/school differences) and sex of the child (a potentially important factor [30]) but also included a 30% random allocation element. It was performed after eligibility was assured and was independently concealed until after the initial two-group analyses were complete. All processes are in line with CONSORT 2010 Explanation and Elaboration procedures [31] (For technical specifications see Supporting Information–Randomisation S7 File).
Blinding
Investigators, participants and those assessing outcomes were all blind to treatment allocation. Post-intervention, both teachers and parents of participants were asked whether they thought their child had been allocated to Active treatment or Placebo, and these estimates were used to assess the maintenance of blinding.
Imputation
Item-missing values in the Conner’s Rating Scales were imputed using treatment group median values, which provide some robustness against outliers, whilst not relying on an uncertain MAR assumption needed for multiple imputations. Observations lost to follow-up were also imputed using treatment group median values. Appropriate checks were made that participants with missing data did not differ significantly on any demographic variables. The methods replicated those used in DOLAB 1.
Statistical methods
The assessment of blinding (i.e. treatment group guess) was examined using χ2–test by treatment group, whilst differences in side-effects scores were tested using Wilcoxon-rank sum tests.
Group comparisons on primary outcomes were carried out using change scores (i.e. the post-intervention score minus baseline score), in line with previous studies including DOLAB I. Main analyses were conducted using t-tests for mean differences of changes (in line with the original study) on an intention-to-treat principle (ITT): thus, all children were included according to treatment allocation, irrespective of continued participation in the trial after randomization.
For all primary outcomes, pre-planned group comparisons were carried out on the whole sample of children who were recruited into the study. Subgroup comparisons were also carried out on those children whose baseline reading scores were ≤10th centile (to evaluate any possible trends related to the severity of initial reading problems).
To assess potential biases due to missingness additional per-protocol analyses were conducted on any measure with >15% missing values. Furthermore, post-hoc multivariate (OLS) regressions were undertaken to assess whether the statistically inefficient use of change-scores (in line with original paper) might affected the results. A second set of models further accounted for the minimization factors (school and gender) and assessed the consistency of the results based on the group comparisons (for details see Supporting Information–Multivariate Analyses S1 Table). These robustness checks are briefly discussed.
All analyses were undertaken using Stata 15.0 (StataCorp, College Station TX). Analysis syntax and an anonymised dataset are available for replication through the Open Science Framework: https://osf.io/9ynjf.
Results
Recruitment
Recruitment was carried out in 84 primary schools and academies in five local and unitary authorities proximate to Oxfordshire, beginning in January 2013 and finishing in March 2015. Post-intervention assessments (16 weeks after enrolment) were completed in July 2015. Of the 1230 children who were invited, 618 of their parents/guardians gave consent and their children were assessed. Of these, 376 met study inclusion criteria and were randomized. The most common reason for exclusion was that their reading exceeded the 20thcentile (n = 231); other reasons for exclusion are described in the flowchart of participants (n = 11) detailed in Fig 1. The achieved sample size is 24 short of the planned N reflecting resource constraints.
Follow-up
Of the 376 children randomized, 372 were assessed again after the 16-week intervention (185 Active, 187 Placebo). Lost participants were equally balanced between groups.
Baseline data
The two treatment groups did not differ on any of the core demographic variables, nor on any of the primary outcome measures at baseline with the exception of working memory (Digits Forward). Demographic information is provided in Table 1. The mean age of the sample was 8 years 7 months, 62.5% were male, 84% white, and around 20% were eligible for free school meals. Baseline data on the primary outcomes are shown in Table 2. With respect to these, mean reading performance of the children randomized was 1.3 sd (20.4 points) below the normative value (score = 100), equating to a reading performance around 27 months below chronological age. Working memory scores were around 0.8 sd (8 points, digits forward) and 0.7 sd (7 points, digits backward) below population norms (score = 50). On the behavior measures, both teacher and parent ratings were all within the normative range, with the exception of the ‘cognitive problems’ sub-scale (assessing attentional and related difficulties), where these children scored 1 (parent rated, approx. 10 points) to 1.5 (teacher rated, approx. 15 points) sd above population means, as well as parent rated DSM-IV Inattentive, +1.2sd. All other behavioral measures were slightly elevated (> +0.5 sd), with the exception of ‘perfectionism’ (parent rated) and ‘oppositional’, ‘global emotional lability’, as well as ‘DSM-IV Hyperactive Impulsive’.
Table 1. Demographic information.
| Whole sample | (SD) | Active | (SD) | Placebo | (SD) | ||
|---|---|---|---|---|---|---|---|
| N = 376 | N = 187 | N = 189 | |||||
| Age | (in months) | 105.5 | 10.1 | 105.6 | 10.2 | 105.3 | 10.1 |
| Sex | (%) | (%) | (%) | ||||
| Male | 235 | 62.5 | 120 | 64.2 | 115 | 63.2 | |
| Female | 141 | 37.5 | 67 | 35.8 | 74 | 40.7 | |
| Ethnicity | |||||||
| White | 315 | 83.8 | 155 | 82.9 | 160 | 87.9 | |
| Mixed | 5 | 1.3 | 3 | 1.6 | 2 | 1.1 | |
| Asian | 9 | 2.4 | 5 | 2.7 | 4 | 2.2 | |
| Black | 8 | 2.1 | 5 | 2.7 | 3 | 1.6 | |
| Other | 2 | 0.5 | 2 | 1.1 | 0 | 0 | |
| Unknown | 37 | 9.8 | 17 | 9.1 | 20 | 11.0 | |
| Eligibility for Free School Meals (FSM) | |||||||
| Not entitled | 214 | 56.9 | 109 | 58.3 | 105 | 57.7 | |
| Entitled to FSM | 78 | 20.7 | 33 | 17.6 | 45 | 24.7 | |
| Unknown | 84 | 22.3 | 45 | 24.1 | 39 | 21.4 | |
Table 2. Primary outcomes at baseline, mean (sd).
| Whole Sample (n = 376) | (SD) | N | Active (n = 187) | (SD) | N | Placebo (n = 189) | (SD) | N | |
|---|---|---|---|---|---|---|---|---|---|
| READING* | |||||||||
| Word Reading–Standard Score (sd) § | 79.6 | (6.5) | 376 | 80 | (6.4) | 187 | 79.2 | (6.5) | 189 |
| Reading age, months (sd) | 78.4 | (8) | 376 | 79.1 | (7.8) | 187 | 77.7 | (8.1) | 189 |
| Working memory* | 42 | (8.6) | 376 | 42.9 | (8.6) | 187 | 41.1 | (8.5) | 189 |
| Digits Forward–T-Scores†† (sd) | 42 | (8.6) | 376 | 42.9 | (8.6) | 187 | 41.1 | (8.5) | 189 |
| Digits Backward–T-Scores†† (sd) | 42.8 | (8.4) | 374 | 43.2 | (8.1) | 187 | 42.5 | (8.8) | 187 |
| BEHAVIOR: | |||||||||
| Parent rated‡ | |||||||||
| Sub-scales (T-scores)† | |||||||||
| Oppositional | 55.5 | (12.6) | 303 | 55.3 | (12.5) | 147 | 55.7 | (12.7) | 156 |
| Cognitive Problems | 60.5 | (11.6) | 307 | 60.6 | (11.7) | 150 | 60.5 | (11.5) | 157 |
| Hyperactivity | 57.1 | (13.1) | 306 | 57.7 | (12.6) | 148 | 56.6 | (13.5) | 158 |
| Anxiety | 52.7 | (11.8) | 307 | 51.6 | (11.1) | 148 | 53.8 | (12.4) | 159 |
| Perfectionism | 50.2 | (11.4) | 307 | 50.8 | (11.7) | 148 | 49.7 | (11.1) | 159 |
| Social Problems | 55.9 | (13.3) | 307 | 55.2 | (13.2) | 148 | 56.4 | (13.4) | 159 |
| Psychosomatic | 54.9 | (13.3) | 307 | 55 | (14) | 148 | 54.8 | (12.7) | 159 |
| Global scales (T-scores) †† | |||||||||
| ADHD Index | 58.4 | (11.7) | 305 | 58.5 | (11.7) | 146 | 58.3 | (11.8) | 159 |
| Global Restless-Impulsive | 57.5 | (12.7) | 302 | 58 | (12.8) | 144 | 57.1 | (12.6) | 158 |
| Global Emotional Lability | 55.2 | (12) | 303 | 54.9 | (12) | 146 | 55.5 | (12) | 157 |
| Global Index Total | 57.4 | (12.5) | 302 | 57.6 | (12.6) | 144 | 57.2 | (12.5) | 158 |
| DSM-IV Inattentive | 57.5 | (11.8) | 305 | 57.5 | (11.9) | 148 | 57.6 | (11.8) | 157 |
| DSM-IV Hyperactive-Impulsive | 58.1 | (13.1) | 303 | 58.7 | (12.8) | 145 | 57.6 | (13.4) | 158 |
| DSM-IV Total | 58.1 | (12.6) | 306 | 58.5 | (12.6) | 148 | 57.8 | (12.6) | 158 |
| Teacher rated‡‡ | |||||||||
| Sub-scales (T-scores) †† | |||||||||
| Oppositional | 54.7 | (12.2) | 269 | 55.4 | (12.9) | 136 | 54 | (11.5) | 133 |
| Cognitive Problems | 66.8 | (8.4) | 266 | 66.7 | (8.1) | 134 | 67 | (8.6) | 132 |
| Hyperactivity | 55.2 | (11.2) | 268 | 55.8 | (11.2) | 135 | 54.6 | (11.1) | 133 |
| Anxiety | 59.2 | (13) | 269 | 59 | (12.9) | 136 | 59.4 | (13.1) | 133 |
| Perfectionism | 47.7 | (7.5) | 267 | 48 | (8.4) | 135 | 47.4 | (6.5) | 132 |
| Social Problems | 55 | (12) | 268 | 54.8 | (11.5) | 135 | 55.3 | (12.6) | 133 |
| Global scales (T-scores)†† | |||||||||
| ADHD Index | 59.3 | (10.8) | 267 | 59.7 | (10.5) | 135 | 58.9 | (11.2) | 132 |
| Global Restless-Impulsive | 58.7 | (11) | 269 | 59.4 | (10.5) | 136 | 57.9 | (11.4) | 133 |
| Global Emotional Lability | 54.6 | (12.4) | 267 | 55.5 | (13.1) | 135 | 53.6 | (11.7) | 132 |
| Global Index Total | 58.2 | (11.5) | 268 | 59.1 | (11.2) | 135 | 57.4 | (11.8) | 133 |
| DSM-IV Inattentive | 62.4 | (9.8) | 267 | 62.3 | (9.6) | 135 | 62.5 | (10) | 132 |
| DSM-IV Hyperactive-Impulsive | 54 | (11.4) | 267 | 54.6 | (11.3) | 135 | 53.4 | (11.5) | 132 |
| DSM-IV Total | 59.5 | (10.1) | 267 | 59.7 | (10) | 135 | 59.2 | (10.4) | 132 |
*Obtained from the British Ability Scales II.
‡Obtained from Conners' Parent Rating Scale (CPRS-P).
‡‡Obtained from Conners' Teacher Rating Scale (CTRS-L).
†Standard Scores have a mean of 100 (sd = 15).
††Standard Scores have a mean of 50 (sd = 10).
Did blinding work?
Parent and teacher estimates of group allocation at post-intervention were used to assess the maintenance of blinding. Group comparisons carried out on these estimates showed there were no significant differences between groups (parents’ estimate: chi2(df) = 1.327(2); teachers’ estimate: chi2(df) = 0.818(2), as shown in Table 3.
Table 3. Maintenance of blinding for parents and teachers, n (%) returned questionnaires.
| Actual Treatment Allocation | ||||
|---|---|---|---|---|
| Parents | Teachers | |||
| "Guessed" treatment allocation | Active (n = 99) | Placebo (n = 110) | Active (n = 124) | Placebo (n = 122) |
| Active | 39 (39.4%) | 46 (41.8%) | 49 (39.5%) | 43 (35.2%) |
| Placebo | 58 (58.5%) | 61 (55.5%) | 69 (55.7%) | 71 (58.2%) |
| Don’t know | 2 (2.1%) | 3 (2.7%) | 6 (4.8%) | 8 (6.6%) |
| Missing (questionnaires not returned) | 88 | 79 | 63 | 67 |
| Test (Allocation vs. Guess) | Parents: chi2 (df) | Pvalue | Teachers: chi2 (df) | Pvalue |
| 1.327 (2) | 0.723 | 0.818 (2) | 0.845 | |
Numbers analysed
Intention-to-treat analyses were carried out on the whole sample randomized (n = 376). Analyses were also carried out on the pre-planned sub-group defined by baseline reading of below the 10th centile (n = 213) in line with the protocol. Behavior ratings were the only measures with >15% of the data missing (change scores n = 196 for teachers (52%), and n = 187 for parents (50%)), so additional per-protocol analyses were conducted on these measures.
Outcomes
a) Reading
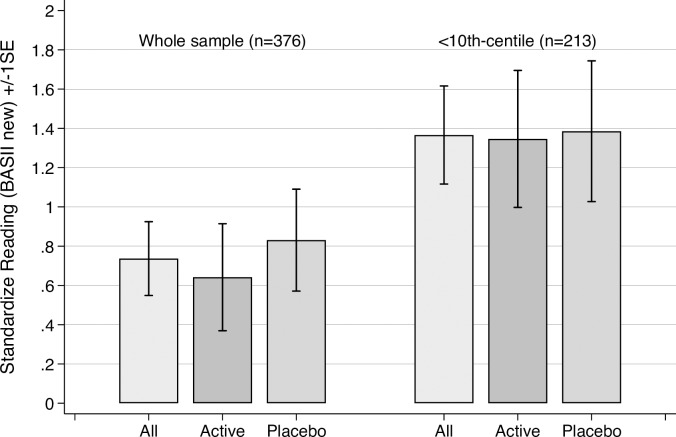
Standardized reading score data are shown in Table 4, and changes on this measure, which were the primary outcome, are illustrated in Fig 2. The same data expressed as ‘reading ages’ are shown in Table 5.
Table 4. Standardized* reading measures†, means (sd).
| Baseline | Post-Intervention | Change Scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | p | t | Active | Placebo | p | t | Active | Placebo | p | t | |
| All randomized (n = 376) | 80.0 (6.4) | 79.2. (6.5) | 0.240 | 1.177 | 80.6 (6.7) | 80.0 (6.5) | 0.385 | 0.870 | .64 (3.7) | .83 (3.6) | 0.616 | -0.502 |
| Reading ≤ 10th Centile (n = 213) | 75.4 (4.5) | 74.8 (4.8) | 0.332 | 0.972 | 76.7 (5.5) | 76.2 (5.1) | 0.432 | 0.786 | 1.4 (3.6) | 1.4 (3.7) | 0.938 | -0.078 |
* Obtained from the British Ability Scales II.
†Standardized scores have a mean of 100 (sd = 15).
Fig 2. Change in standardized reading scores† by treatment group for all children randomised and for sub-groups with initial reading of ≤10th centiles (± 1 SE).
Note: †Obtained from the British Ability Scales II new calibration. Standardized scores have a mean of 100 (sd = 15).
Table 5. Standardized* reading age (in months), means (sd).
| Baseline | Post-Intervention | Change Scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | p | t | Active | Placebo | p | t | Active | Placebo | p | t | |
| All randomized (n = 376) | 79.1 (7.9) | 77.7 (8.2) | 0.091 | 1.695 | 82.1 (9.1) | 81.4 (8.1) | 0.437 | 0.778 | 3.1 (4.4) | 3.7 (4.9) | 0.143 | -1.467 |
| Reading ≤ 10th Centile (n = 213) | 75.6 (5.7) | 74.5 (5.9) | 0.172 | 1.369 | 78.4 (6) | 78 (6.5) | 0.672 | 0.424 | 2.7 (3.5) | 3.5 (4.3) | 0.179 | -1.348 |
* Obtained from the British Ability Scales II.
After the 16-week treatment period no statistically significant differences were found between treatment groups post-intervention.
The whole group randomized (n = 376), showed no statistically significant reading gain differences by treatment group above those that would be expected over this time period (Active change(sd) = 0.64(3.7); placebo change(sd) 0.83(3.6), p(t) = 0.616(-0.502). This is further illustrated by the fact that children’s reading age increased by 3.1 months (active) and 3.7 months (placebo) respectively over the 4 months of the intervention (Table 5).
The same result was obtained for the pre-planned sub-group whose baseline reading was at or below the 10th centile (n = 213). In this subgroup, no statistically significant group differences in change-scores were observed (Active change(sd) = 1.4(3.6); Placebo change(sd) = 1.4(3.7); p(t) = 0.938(-0.078)).
Finally, Table 6 reports the group mean differences and 95% confidence intervals, in the main sample the differences is -0.594 (95% CI: -1.937, 0.749) in the subgroup -0.576 (-2.019, 0.867) points on the BASII reading scores. This further shows that the treatment group differences are not substantially meaningful.
Table 6. Post-intervention mean differences (95% CI) for standardized† reading and reading age (in months) *.
| All randomized (n = 376) | Reading ≤ 10th Centile (n = 213): | |||||
|---|---|---|---|---|---|---|
| Mean Difference | 95% | CI | Mean Difference | 95% | CI | |
| Reading | -0.594 | -1.937 | 0.749 | -0.576 | -2.019 | 0.867 |
| Reading Age | -0.689 | -2.431 | 1.052 | -0.365 | -2.06 | 1.331 |
* Obtained from the British Ability Scales II.
†Standardized scores have a mean of 100 (sd = 15).
b) Working memory
At baseline (Table 7), digits forward scores differed statistically significantly between the treatment groups in both the whole sample and the subgroup of children in the <10-centile of the (normative BAS) reading distribution. At post-intervention, group means differed significantly for digits forward (Whole sample: active mean(sd) = 43.8, (9.1), placebo mean(sd) = 42.0(9.3), p(t) = 0.059(1.982); 10th centile subgroup: Active mean(sd) = 43.7(8.1), placebo mean (sd) = 41.9(9.0), p(t) = 0.047(1.994)). In line with these differences the change scores are both small and not statistically significant for digits forward (active change(sd) = 0.91, (7.7), placebo change(sd) = 1.72(7.9), p(t) = 0.826(-0.22)). Table 8 reports the group mean differences and 95% confidence intervals, in the main sample the differences is -1.797 (95% CI: -3.665, 0.071) in the subgroup -0.576 (95% CI; -5.304, -0.031) points, neither is close to the 10-point, one standard deviation measure indicating a clinically relevant difference.
Table 7. Standardized* working memory (recall of digits forward)†, means (sd).
| Baseline T Score (sd) | Post-Intervention T Score (sd) | Change Scores, T Score (sd) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | p | t | Active | Placebo | p | t | Active | Placebo | p | t | |
| All randomized (n = 376) | 42.9 (8.6) | 41.1 (8.5) | 0.048 | 1.980 | 43.8 (9.1) | 42.0 (9.3) | 0.059 | 1.892 | .95 (7.4) | .91 (7.7) | 0.957 | 0.054 |
| Reading ≤ 10th Centile (n = 213) | 42.9 (8.8) | 40.0 (8.3) | 0.014 | 2.475 | 44.4 (9.8) | 41.7 (7.8) | 0.047 | 1.994 | 1.48 (7.7) | 1.72 (7.9) | 0.826 | -0.220 |
* Obtained from the British Ability Scales II.
†Standardized scores have a mean of 50 (sd = 10).
Table 8. Post-intervention mean differences (95% CI) for working memory (recall of digits forward and backward) *†.
| All randomized (n = 376) | Reading ≤ 10th Centile (n = 213): | |||||
|---|---|---|---|---|---|---|
| Mean Difference | 95% | CI | Mean Difference | 95% | CI | |
| Digits Forward | -1.797 | -3.665 | 0.071 | -2.667 | -5.304 | -0.031 |
| Digits Backward | -1.774 | -3.503 | -0.045 | -3.061 | -5.597 | -0.526 |
* Obtained from the British Ability Scales II.
†Standardized scores have a mean of 50 (sd = 10).
Digits Backwards (Table 9) only differed statistically significantly at post-intervention (Whole sample: Active mean(sd) = 43.7(8.1), placebo mean(sd) = 41.9(9.0), p(t) = 0.044(2.018); and for the 10% Subgroup: Active mean(sd) = 43.5(9.3), placebo mean(sd) = 40.5(9.5), p(t) = 0.018(2.38)). Again, we find the change scores for digits backwards (active change(sd) = -0.4, (9.8), placebo change(sd) = -0.4(9.8), p(t) = 0.356(0.925)), do not differ in a statistically significant way.
Table 9. Standardized* working memory (recall of digits backward)†, means (sd).
| Baseline T Score (sd) | Post-Intervention T Score (sd) | Change Scores, T Score (sd) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | Placebo | p | t | Active | Placebo | p | t | Active | Placebo | p | t | |
| All randomized (n = 376) | 43.2 (8.1) | 42.5 (8.8) | 0.427 | 0.795 | 43.7 (8.1) | 41.9 (9.0) | 0.044 | 2.018 | 0.4 (9.3) | -0.4 (9.8) | 0.356 | 0.925 |
| Reading ≤ 10th Centile (n = 213) | 42.3 (7.8) | 41.9 (9.8) | 0.742 | 0.330 | 43.5 (9.3) | 40.5 (9.5) | 0.018 | 2.380 | 1.1 (10.1) | -1.3 (10.7) | 0.075 | 1.788 |
* Obtained from the British Ability Scales II.
†Standardized scores have a mean of 50 (sd = 10).
The group mean differences (Table 8) for digits backwards are, in the main sample -1.774 (95% CI: -3.503, -0.045) and in the subgroup -3.061 (95% CI; -5.597–0.526) points.
c) Behavior
Across both treatment groups, behavior ratings from parents showed small changes (ranging from -1 to -3.8 points) over the 16-week treatment period, as shown in Table 10 (ITT) and Table 11 (per-protocol). These reductions of behavioral problems at post-intervention occur across both treatment groups, and no statistically significant group differences are found. Table 12 further highlights this point due to the small group mean differences and corresponding 95% confidence intervals including zero.
Table 10. Standardized* behavior measures—Parent rated† (ITT), means (sd).
| Baseline | Post-intervention | Change scores | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | |
| Oppositional | 54.6 | (11.1) | 55.4 | (11.5) | 0.487 | -0.696 | 53.6 | (8.8) | 53.3 | (8.7) | 0.782 | 0.276 | -1.0 | (9.4) | -2.1 | (10.5) | 0.302 | 1.034 |
| Cognitive Problems | 60.3 | (10.5) | 60.4 | (10.5) | 0.874 | -0.159 | 59.2 | (8.3) | 57.5 | (8.2) | 0.059 | 1.895 | -1.1 | (9.4) | -2.9 | (9.5) | 0.068 | 1.833 |
| Hyperactivity | 57.2 | (11.3) | 55.9 | (12.5) | 0.262 | 1.123 | 54.3 | (8.9) | 53.1 | (9.2) | 0.195 | 1.298 | -2.9 | (10.1) | -2.7 | (9.7) | 0.872 | -0.161 |
| Anxiety | 50.9 | (10.) | 53.2 | (11.4) | 0.036 | -2.108 | 49.9 | (7.9) | 49.4 | (7.1) | 0.526 | 0.634 | -1.0 | (7.9) | -3.8 | (9.6) | 0.002 | 3.123 |
| Perfectionism | 49.9 | (10.6) | 49.0 | (10.3) | 0.358 | 0.92 | 47.6 | (7.1) | 47.4 | (8.) | 0.773 | 0.288 | -2.3 | (9.6) | -1.5 | (9.) | 0.423 | -0.802 |
| Social Problems | 54.1 | (12.) | 55.6 | (12.5) | 0.261 | -1.125 | 52.6 | (9.3) | 53.1 | (8.9) | 0.645 | -0.461 | -1.5 | (10.3) | -2.5 | (11.3) | 0.377 | 0.884 |
| Psychosomatic | 53.5 | (12.7) | 54.2 | (11.7) | 0.604 | -0.52 | 51.3 | (11.) | 51.4 | (9.8) | 0.928 | -0.09 | -2.2 | (14.1) | -2.8 | (12.2) | 0.681 | 0.412 |
| ADHD Index | 58.1 | (10.4) | 58.1 | (10.8) | 0.949 | 0.064 | 56.3 | (8.2) | 55.3 | (8.2) | 0.242 | 1.172 | -1.9 | (8.1) | -2.8 | (9.6) | 0.318 | 0.999 |
| Global Restless-Impulsive | 57.3 | (11.3) | 56.8 | (11.5) | 0.658 | 0.442 | 55.0 | (8.4) | 53.7 | (8.5) | 0.145 | 1.461 | -2.3 | (9.5) | -3.1 | (9.9) | 0.455 | 0.747 |
| Global Emotional Lability | 54.3 | (10.7) | 55.2 | (10.9) | 0.409 | -0.827 | 53.4 | (9.5) | 52.0 | (8.3) | 0.119 | 1.562 | -0.9 | (9.6) | -3.2 | (9.8) | 0.019 | 2.357 |
| Global Index Total | 57.6 | (12.6) | 57.2 | (12.5) | 0.76 | 0.306 | 56.0 | 11.7 | 54.5 | (10.5) | 0.334 | 0.969 | -1.7 | (8.4) | -3.2 | 7.9 | 0.22 | 1.231 |
| DSM-IV Inattention | 57.5 | (11.9) | 57.6 | (11.8) | 0.926 | -0.093 | 57.3 | 11.2 | 55.1 | (12.) | 0.189 | 1.318 | -1.7 | (8.) | -2.9 | 10.5 | 0.368 | 0.902 |
| DSM-IV Hyperactive-Impulsive | 58.7 | (12.8) | 57.6 | (13.4) | 0.468 | 0.726 | 56.5 | 11.6 | 55.6 | (11.6) | 0.604 | 0.519 | -2.8 | (8.1) | -2.2 | 8.1 | 0.615 | -0.504 |
| DSM-IV Total ADHD | 58.5 | (12.6) | 57.8 | (12.6) | 0.588 | 0.543 | 57.0 | 11.5 | 55.7 | (10.9) | 0.382 | 0.877 | -2.6 | (8.) | -2.6 | 8.5 | 0.99 | 0.013 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Parent Rating Scale (CPRS).
Table 11. Standardized* behavior measures—Parent rated† (per-protocol), means (sd).
| Baseline | Post-intervention | Change scores | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | sd | N | Placebo | sd | N | P | t | Active | sd | N | Placebo | sd | N | P | t | Active | sd | N (listwise) | Placebo | sd | N (listwise) | P | t | |
| Oppositional | 55.3 | (12.5) | 147 | 55.7 | (12.7) | 156 | 0.779 | -0.281 | 54.9 | 11.8 | 101 | 54.2 | (11.3) | 111 | 0.68 | 0.414 | -0.8 | (9.3) | 92 | -1.1 | 9.9 | 95 | 0.789 | 0.268 |
| Cognitive Problems | 60.6 | (11.7) | 150 | 60.5 | (11.5) | 157 | 0.969 | 0.039 | 60.1 | 11.2 | 101 | 57.9 | (10.8) | 111 | 0.143 | 1.471 | -2.1 | (8.4) | 92 | -3.2 | 9.7 | 96 | 0.429 | 0.793 |
| Hyperactivity | 57.7 | (12.6) | 148 | 56.6 | (13.5) | 158 | 0.4735 | 0.718 | 55.5 | 12.1 | 101 | 54.6 | (11.7) | 111 | 0.602 | 0.523 | -2.8 | (9.7) | 92 | -2.1 | 8.0 | 97 | 0.616 | -0.502 |
| Anxiety | 51.6 | (11.1) | 148 | 53.8 | (12.4) | 159 | 0.106 | -1.621 | 51.5 | 10.5 | 100 | 50.4 | (9.2) | 111 | 0.398 | 0.847 | -0.8 | (7.3) | 91 | -4.1 | 9.2 | 98 | 0.007 | 2.717 |
| Perfectionism | 50.8 | (11.7) | 148 | 49.7 | (11.1) | 159 | 0.377 | 0.885 | 49.1 | 9.4 | 100 | 49.1 | (10.1) | 111 | 0.971 | -0.036 | -0.7 | (10.3) | 91 | -0.7 | 8.0 | 98 | 0.957 | -0.055 |
| Social Problems | 55.2 | (13.2) | 148 | 56.4 | (13.4) | 159 | 0.437 | -0.778 | 54.9 | 12.3 | 100 | 55.2 | (11.2) | 111 | 0.854 | -0.185 | -0.5 | (8.9) | 91 | -2.2 | 11.8 | 98 | 0.285 | 1.073 |
| Psychosomatic | 55.0 | (14.) | 148 | 54.8 | (12.7) | 159 | 0.895 | 0.132 | 54.2 | 14.5 | 100 | 53.8 | (12.2) | 111 | 0.831 | 0.214 | -0.9 | (15.9) | 91 | -2.0 | 12.6 | 98 | 0.598 | 0.528 |
| ADHD Index | 58.5 | (11.7) | 146 | 58.3 | (11.8) | 159 | 0.889 | 0.140 | 57.3 | 11.0 | 101 | 56.2 | (10.6) | 110 | 0.444 | 0.767 | -2.3 | (7.1) | 92 | -2.9 | 9.1 | 97 | 0.64 | 0.469 |
| Global Restless-Impulsive | 58.0 | (12.8) | 144 | 57.1 | (12.6) | 158 | 0.558 | 0.587 | 55.8 | 11.4 | 100 | 54.9 | (10.9) | 111 | 0.55 | 0.599 | -2.4 | (8.) | 91 | -2.9 | 8.2 | 97 | 0.677 | 0.417 |
| Global Emotional Lability | 54.9 | (12.) | 146 | 55.5 | (12.) | 157 | 0.704 | -0.381 | 54.7 | 12.9 | 100 | 52.7 | (10.8) | 110 | 0.232 | 1.198 | -0.2 | (9.7) | 91 | -2.7 | 9.2 | 95 | 0.071 | 1.814 |
| Global Index Total | 57.6 | (12.6) | 144 | 57.2 | (12.5) | 158 | 0.76 | 0.306 | 56.0 | 11.7 | 100 | 54.5 | (10.5) | 110 | 0.334 | 0.969 | -1.7 | (8.4) | 91 | -3.2 | 7.9 | 96 | 0.22 | 1.231 |
| DSM-IV Inattention | 57.5 | (11.9) | 148 | 57.6 | (11.8) | 157 | 0.926 | -0.093 | 57.3 | 11.2 | 100 | 55.1 | (12.) | 111 | 0.189 | 1.318 | -1.7 | (8.) | 91 | -2.9 | 10.5 | 96 | 0.368 | 0.902 |
| DSM-IV Hyperactive-Impulsive | 58.7 | (12.8) | 145 | 57.6 | (13.4) | 158 | 0.468 | 0.726 | 56.5 | 11.6 | 101 | 55.6 | (11.6) | 110 | 0.604 | 0.519 | -2.8 | (8.1) | 92 | -2.2 | 8.1 | 96 | 0.615 | -0.504 |
| DSM-IV Total ADHD | 58.5 | (12.6) | 148 | 57.8 | (12.6) | 158 | 0.588 | 0.543 | 57.0 | 11.5 | 100 | 55.7 | (10.9) | 111 | 0.382 | 0.877 | -2.6 | (8.) | 91 | -2.6 | 8.5 | 97 | 0.99 | 0.013 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Parent Rating Scale (CPRS)
Table 12. Post-intervention mean differences (95% CI) for standardized* behavior measures—Parent rated† (ITT).
| All randomized (n = 376) | Reading ≤ 10th Centile (n = 213): | |||||
|---|---|---|---|---|---|---|
| Mean Difference | 95% | CI | Mean Difference | 95% | CI | |
| Oppositional | -0.249 | -2.024 | 1.525 | -1.639 | -4.068 | 0.79 |
| Cognitive Problems | -1.615 | -3.292 | 0.061 | -3.222 | -5.523 | -0.92 |
| Hyperactivity | -1.21 | -3.043 | 0.623 | -2.826 | -5.315 | -0.338 |
| Anxiety | -0.491 | -2.012 | 1.03 | -0.887 | -3.077 | 1.303 |
| Perfectionism | -0.224 | -1.748 | 1.301 | -0.84 | -2.963 | 1.282 |
| Social Problems | 0.433 | -1.414 | 2.279 | -1.016 | -3.578 | 1.546 |
| Psychosomatic | 0.097 | -2.011 | 2.204 | -0.94 | -3.603 | 1.724 |
| ADHD Index | -0.987 | -2.642 | 0.669 | -3.293 | -5.6 | -0.985 |
| Global Restless-Impulsive | -1.27 | -2.978 | 0.439 | -3.178 | -5.604 | -0.753 |
| Global Emotional Lability | -1.433 | -3.237 | 0.371 | -3.082 | -5.676 | -0.487 |
| Global Index Total | -1.144 | -2.845 | 0.558 | -3.266 | -5.682 | -0.85 |
| DSM-IV Inattention | -1.949 | -3.727 | -0.171 | -3.991 | -6.527 | -1.454 |
| DSM-IV Hyperactive-Impulsive | -0.797 | -2.571 | 0.977 | -2.559 | -4.919 | -0.199 |
| DSM-IV Total ADHD | -0.636 | -2.354 | 1.081 | -2.553 | -4.923 | -0.182 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Parent Rating Scale (CPRS).
1) Parent-ratings:
The ITT analyses showed a significant difference in favor of the Placebo group change scores for the Anxiety sub-scale (Active mean(sd) = -1.0(7.9), Placebo mean(sd) = -3.8(9.6), p(t)<0.01(3.123)) and for the Global change scores for Emotional Lability (Active mean(sd) = 0.9(9.6), Placebo mean(sd) = -2.8(9.8), p(t)<0.05(2.357) and DSM IV Inattention (Active mean(sd) = -1.0(8.9), Placebo mean(sd) = -3.2(10.2), p(t)<0.02(2.417).
In the per-protocol analyses (n = 187–8), no group differences were significant with the exception of a trend in favor of the Placebo group on the Anxiety sub-scale (Active mean(sd) = -0.8(7.3), Placebo mean(sd) = -4.1(9.3), p(t)<0.007(2.717)).
2) Teacher-ratings:
The ITT analyses (Table 13) showed that behavioral improvements (that is lower scores) as rated by teachers were greater for the Placebo group over Active treatment on the Anxiety sub-scale (Active mean = -0.5(10.8), Placebo mean(sd) = -3.7(11.0); p(t)<0.01(2.847)).
Table 13. Standardized* behavior measures—Teacher rated† (ITT), means (sd).
| Baseline | Post-intervention | Change scores | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | Active (n = 187) | sd | Placebo (n = 189 | sd | P | t | |
| Oppositional | 53.4 | (11.5) | 52.2 | (10.) | 0.291 | 1.057 | 54.2 | (10.9) | 53.8 | (10.1) | 0.694 | 0.393 | 0.8 | (10.3) | 1.5 | (9.7) | 0.467 | -0.729 |
| Cognitive Problems | 66.6 | (6.9) | 66.8 | (7.2) | 0.795 | -0.26 | 63.3 | (7.7) | 64.9 | (7.9) | 0.063 | -1.868 | -3.3 | (7.3) | -2.0 | (7.9) | 0.093 | -1.685 |
| Hyperactivity | 55.3 | (9.5) | 53.3 | (9.6) | 0.036 | 2.104 | 54.1 | (8.5) | 52.8 | (8.6) | 0.131 | 1.514 | -1.2 | (8.1) | -0.5 | (8.4) | 0.387 | -0.865 |
| Anxiety | 58.7 | (11.) | 59.0 | (11.) | 0.799 | -0.254 | 58.3 | (10.5) | 55.3 | (10.) | 0.006 | 2.752 | -0.5 | (10.8) | -3.7 | (11.) | 0.005 | 2.847 |
| Perfectionism | 46.9 | (7.3) | 46.4 | (5.6) | 0.477 | 0.713 | 48.2 | (7.7) | 46.7 | (5.9) | 0.038 | 2.079 | 1.3 | (6.9) | 0.3 | (4.9) | 0.109 | 1.605 |
| Social Problems | 53.4 | (10.) | 53.7 | (10.8) | 0.794 | -0.262 | 53.8 | (10.4) | 52.8 | (10.) | 0.374 | 0.889 | 0.3 | (9.5) | -0.9 | (9.4) | 0.211 | 1.252 |
| ADHD Index | 59.5 | (8.9) | 58.6 | (9.4) | 0.364 | 0.91 | 57.9 | (8.8) | 56.6 | (9.4) | 0.166 | 1.388 | -1.6 | (8.6) | -2.0 | (9.1) | 0.623 | 0.492 |
| Global Restless-Impulsive | 59.3 | (8.9) | 57.1 | (9.7) | 0.019 | 2.362 | 57.8 | (8.8) | 56.4 | (9.2) | 0.124 | 1.544 | -1.5 | (8.5) | -0.6 | (9.1) | 0.357 | -0.922 |
| Global Emotional Lability | 54.0 | (11.4) | 52.5 | (9.9) | 0.186 | 1.326 | 54.2 | (11.2) | 51.8 | (8.8) | 0.021 | 2.325 | 0.2 | (10.7) | -0.8 | (8.6) | 0.342 | 0.951 |
| Global Index Total | 58.8 | (9.5) | 56.7 | (9.9) | 0.036 | 2.11 | 57.8 | (9.2) | 55.5 | (9.3) | 0.014 | 2.481 | -1.0 | (8.7) | -1.2 | (8.8) | 0.773 | 0.288 |
| DSM-IV Inattention | 62.2 | (8.2) | 62.3 | (8.4) | 0.889 | -0.14 | 59.6 | (8.4) | 59.4 | (8.3) | 0.772 | 0.289 | -2.6 | (7.9) | -3.0 | (8.4) | 0.66 | 0.441 |
| DSM-IV Hyperactive-Impulsive | 53.6 | (9.7) | 52.1 | (9.8) | 0.126 | 1.534 | 53.1 | (8.9) | 51.1 | (9.2) | 0.029 | 2.189 | -0.5 | (8.9) | -1.0 | (8.7) | 0.584 | 0.548 |
| DSM-IV Total ADHD | 59.2 | (8.5) | 58.6 | (8.7) | 0.469 | 0.724 | 57.9 | (8.5) | 57.0 | (8.4) | 0.312 | 1.013 | -1.3 | (8.1) | -1.5 | (8.4) | 0.779 | 0.281 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Teacher Rating Scale (CTRS).
However, these were not consistent across sub- and global scales and the per-protocol analyses (n = 196, Table 14), no significant effects of treatment were found. Table 15 further highlights this point due to the small group mean differences and corresponding 95% confidence intervals including zero.
Table 14. Standardized* behavior measures—Teacher rated† (per-protocol), means (sd).
| Baseline | Post-intervention | Change scores | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active | sd | N | Placebo | sd | N | P | t | Active | sd | N | Placebo | sd | N | P | t | Active | sd | N (listwise) | Placebo | sd | N (listwise) | P | t | |
| Oppositional | 55.4 | (12.9) | 136 | 54.0 | (11.5) | 133 | 0.342 | 0.952 | 55.6 | 12.8 | 130 | 55.1 | (12.1) | 127 | 0.76 | 0.305 | -0.3 | (9.) | 101 | 0.4 | 9.5 | 95 | 0.594 | -0.534 |
| Cognitive Problems | 66.7 | (8.1) | 134 | 67.0 | (8.6) | 132 | 0.789 | 0.268 | 63.9 | 9.3 | 129 | 64.8 | (9.7) | 127 | 0.482 | 0.704 | -3.1 | (7.5) | 101 | -2.2 | 8.4 | 95 | 0.397 | -0.849 |
| Hyperactivity | 55.8 | (11.2) | 135 | 54.6 | (11.1) | 133 | 0.374 | 0.890 | 54.8 | 10.1 | 130 | 53.6 | (10.4) | 127 | 0.359 | 0.919 | -1.7 | (9.) | 101 | -1.9 | 9.1 | 95 | 0.887 | 0.142 |
| Anxiety | 59.0 | (12.9) | 136 | 59.4 | (13.1) | 133 | 0.78 | 0.280 | 58.8 | 12.6 | 130 | 56.0 | (12.2) | 127 | 0.07 | 1.818 | -1.4 | (11.) | 101 | -2.7 | 11.2 | 95 | 0.405 | 0.834 |
| Perfectionism | 48.0 | (8.4) | 135 | 47.4 | (6.5) | 132 | 0.547 | 0.604 | 49.4 | 9.0 | 130 | 47.6 | (7.) | 127 | 0.074 | 1.792 | 0.5 | (6.1) | 101 | 0.1 | 5.2 | 95 | 0.64 | 0.469 |
| Social Problems | 54.8 | (11.5) | 135 | 55.3 | (12.6) | 133 | 0.723 | 0.355 | 55.4 | 12.1 | 130 | 54.2 | (11.9) | 127 | 0.423 | 0.802 | -0.1 | (8.9) | 101 | -0.8 | 9.7 | 95 | 0.619 | 0.497 |
| ADHD Index | 59.7 | (10.5) | 135 | 58.9 | (11.2) | 132 | 0.561 | 0.583 | 58.3 | 10.6 | 129 | 57.4 | (11.4) | 127 | 0.499 | 0.677 | -2.0 | (8.6) | 101 | -2.6 | 9.3 | 95 | 0.644 | 0.462 |
| Global Restless-Impulsive | 59.4 | (10.5) | 136 | 57.9 | (11.4) | 133 | 0.255 | 1.140 | 58.2 | 10.5 | 130 | 57.1 | (11.2) | 127 | 0.412 | 0.822 | -1.9 | (8.6) | 101 | -1.5 | 9.9 | 95 | 0.765 | -0.299 |
| Global Emotional Lability | 55.5 | (13.1) | 135 | 53.6 | (11.7) | 132 | 0.212 | 1.252 | 55.6 | 13.2 | 129 | 53.1 | (10.5) | 127 | 0.097 | 1.666 | -1.6 | (10.1) | 101 | -0.8 | 9.7 | 95 | 0.585 | -0.547 |
| Global Index Total | 59.1 | (11.2) | 135 | 57.4 | (11.8) | 133 | 0.223 | 1.222 | 58.2 | 11.1 | 129 | 56.7 | (11.2) | 127 | 0.268 | 1.111 | -1.9 | (8.9) | 101 | -1.4 | 9.5 | 95 | 0.703 | -0.382 |
| DSM-IV Inattention | 62.3 | (9.6) | 135 | 62.5 | (10.) | 132 | 0.88 | 0.151 | 59.9 | 10.1 | 130 | 60.0 | (10.1) | 127 | 0.907 | 0.116 | -2.8 | (8.4) | 101 | -2.9 | 8.7 | 95 | 0.9 | 0.126 |
| DSM-IV Hyperactive-Impulsive | 54.6 | (11.3) | 135 | 53.4 | (11.5) | 132 | 0.379 | 0.880 | 54.1 | 10.6 | 129 | 52.6 | (10.9) | 127 | 0.267 | 1.112 | -1.4 | (9.5) | 101 | -1.8 | 9.0 | 95 | 0.756 | 0.311 |
| DSM-IV Total ADHD | 59.7 | (10.) | 135 | 59.2 | (10.4) | 132 | 0.729 | 0.347 | 58.3 | 10.2 | 129 | 57.5 | (10.2) | 127 | 0.537 | 0.619 | -1.9 | (8.6) | 101 | -2.5 | 9.1 | 95 | 0.68 | 0.414 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Teacher Rating Scale (CTRS).
Table 15. Post-intervention mean differences (95% CI) for standardized* behavior measures—Teacher rated† (ITT).
| All randomized (n = 376) | Reading ≤ 10th Centile (n = 213): | |||||
|---|---|---|---|---|---|---|
| Mean Difference | 95% | CI | Mean Difference | 95% | CI | |
| Oppositional | -0.425 | -2.551 | 1.701 | -0.704 | -3.744 | 2.336 |
| Cognitive Problems | 1.51 | -0.079 | 3.099 | 0.695 | -1.442 | 2.832 |
| Hyperactivity | -1.337 | -3.073 | 0.399 | -1.395 | -4.042 | 1.252 |
| Anxiety | -2.913 | -4.994 | -0.832 | -3.834 | -6.534 | -1.133 |
| Perfectionism | -1.47 | -2.861 | -0.08 | -2.021 | -4.024 | -0.018 |
| Social Problems | -0.934 | -2.998 | 1.131 | -1.898 | -4.747 | 0.951 |
| Psychosomatic | -1.306 | -3.156 | 0.544 | -1.476 | -4.028 | 1.075 |
| ADHD Index | -1.432 | -3.257 | 0.392 | -1.368 | -3.956 | 1.221 |
| Global Restless-Impulsive | -2.409 | -4.447 | -0.371 | -3.021 | -6.056 | 0.014 |
| Global Emotional Lability | -2.374 | -4.255 | -0.493 | -2.63 | -5.395 | 0.136 |
| Global Index Total | -0.25 | -1.948 | 1.448 | -0.719 | -3.1 | 1.662 |
| DSM-IV Inattention | -2.044 | -3.88 | -0.208 | -2.121 | -4.914 | 0.672 |
| DSM-IV Hyperactive-Impulsive | -0.883 | -2.595 | 0.83 | -1.203 | -3.693 | 1.286 |
| DSM-IV Total ADHD | -0.425 | -2.551 | 1.701 | -0.704 | -3.744 | 2.336 |
*T-scores have a mean of 50 (sd = 10).
†Behaviour measures are derived from the Conners’ Parent Rating Scale (CPRS).
One systematic finding was the consistent reduction in the teacher ratings across both treatment groups.
Multivariate robustness checks
The above results were check for robustness given the statistically inefficient use of change-scores as well as for the influence of the minimization factors gender and school. Multivariate (OLS) regressions resulted in the same overall conclusions and are reported in Supporting Materials—Multivariate Analyses S1 Table.
Other measures
Adverse events
The DHA supplement provided is generally regarded as safe (G.R.A.S.) [32] and so no stopping guidelines were put in place except in the case of severe adverse events. As expected, there were none in the course of this trial. The parents of one child in each group reported episodes of diarrhoea and one child in the placebo group was diagnosed with Asperger’s and prescribed Ritalin during the course of the intervention. In addition, one school reported a negative behavior change in 9 children (4 in the Active and 5 in the Placebo group) and another school reported the onset of severe nose bleeds in a child in the Active group.
Health information and attendance
No group differences were found post-intervention either on child’s health status reported in the health questionnaire. No differences were found in school-reported “half-day absences for illness” between groups at post-intervention assessment. Those in the active group (n = 169) reported 4.9 (sd = 5.3) half day’s absence as compared to those in the placebo group (n = 170) who had 5.4 (sd = 6.2) half day’s absence, p = 0.63 (Wilcoxon-z = -0.31).
Reported side effects
No group differences were found for potential side effects assessed by the Barkley scale (Table 16 and Table 17).
Table 16. Scores for Barkley’s side effects questionnaire I (for all returned).
| Counts: | Test for association | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Absent: 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Serious: 9 | Z(Wilcoxon) | Pvalue | |
| Insomnia: Placebo | 97 | 59 | 8 | 7 | 5 | 3 | 3 | 1 | 5 | 1 | 5 | 0.252 | 0.401 |
| Active | 106 | 63 | 14 | 7 | 9 | 3 | 2 | 2 | 3 | 1 | 2 | . | . |
| Nightmares: Placebo | 98 | 75 | 5 | 6 | 3 | 4 | 2 | 1 | 1 | 1 | 0.170 | 0.432 | |
| Active | 107 | 81 | 16 | 2 | 1 | 2 | 3 | 0 | 1 | 1 | . | . | |
| Day Dreams: Placebo | 98 | 51 | 6 | 15 | 9 | 3 | 4 | 1 | 3 | 2 | 4 | 0.801 | 0.212 |
| Active | 106 | 58 | 15 | 12 | 5 | 2 | 3 | 2 | 3 | 3 | 3 | . | . |
| Talks Less: Placebo | 98 | 82 | 5 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | -0.193 | 0.577 | |
| Active | 106 | 87 | 7 | 4 | 1 | 4 | 2 | 1 | 0 | 0 | . | . | |
| Uninterested: Placebo | 98 | 79 | 5 | 5 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | -0.511 | 0.695 |
| Active | 107 | 82 | 11 | 4 | 3 | 3 | 2 | 0 | 1 | 1 | 0 | . | . |
| Decreased Appetite: Placebo | 97 | 78 | 3 | 6 | 4 | 2 | 2 | 1 | 1 | 0.115 | 0.454 | ||
| Active | 106 | 86 | 3 | 7 | 2 | 2 | 5 | 1 | 0 | . | . | ||
| Irritability: Placebo | 98 | 39 | 11 | 12 | 6 | 10 | 10 | 4 | 2 | 4 | 0.876 | 0.190 | |
| Active | 106 | 46 | 11 | 13 | 13 | 10 | 5 | 5 | 1 | 2 | . | . | |
| Stomach: Placebo | 98 | 66 | 11 | 7 | 2 | 3 | 5 | 2 | 2 | -0.412 | 0.660 | ||
| Active | 107 | 69 | 11 | 10 | 5 | 3 | 7 | 0 | 2 | . | |||
| Headache: Placebo | 98 | 70 | 10 | 6 | 2 | 3 | 4 | 2 | 1 | 0 | -0.118 | 0.547 | |
| Active | 107 | 76 | 10 | 4 | 6 | 6 | 1 | 1 | 2 | 1 | . | . | |
Table 17. Scores for barkley’s side effects questionnaire II (for all returned).
| Counts: | Test for association | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Absent: 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Serious: 9 | Z(Wilcoxon) | Pvalue | |
| Drowsiness: Placebo | 98 | 83 | 4 | 3 | 2 | 3 | 2 | 1 | 0.780 | 0.218 | |||
| Active | 107 | 94 | 6 | 3 | 2 | 2 | 0 | 0 | . | . | |||
| Sad: Placebo | 98 | 57 | 6 | 9 | 8 | 4 | 5 | 2 | 5 | 2 | -0.067 | 0.527 | |
| Active | 107 | 54 | 20 | 13 | 8 | 4 | 4 | 1 | 3 | 0 | . | . | |
| Crying: Placebo | 98 | 55 | 12 | 8 | 7 | 1 | 5 | 1 | 3 | 3 | 3 | 0.193 | 0.423 |
| Active | 107 | 59 | 13 | 15 | 7 | 3 | 4 | 3 | 1 | 0 | 2 | . | . |
| Anxious: Placebo | 99 | 57 | 9 | 9 | 5 | 4 | 3 | 4 | 3 | 2 | 3 | 0.864 | 0.194 |
| Active | 109 | 66 | 13 | 11 | 4 | 6 | 3 | 2 | 4 | 0 | 0 | . | . |
| Bites Nails: Placebo | 97 | 57 | 8 | 3 | 4 | 4 | 3 | 1 | 5 | 1 | 11 | 1.383 | 0.083 |
| Active | 107 | 73 | 4 | 3 | 3 | 7 | 4 | 2 | 3 | 2 | 6 | . | . |
| Euphoric: Placebo | 98 | 70 | 5 | 6 | 4 | 6 | 4 | 1 | 1 | 1 | 0.633 | 0.263 | |
| Active | 107 | 80 | 8 | 4 | 5 | 3 | 4 | 0 | 2 | 1 | . | . | |
| Dizziness: Placebo | 98 | 92 | 2 | 1 | 1 | 1 | 1 | 0 | 0.162 | 0.436 | |||
| Active | 107 | 101 | 3 | 1 | 0 | 0 | 1 | 1 | . | . | |||
| Tics: Placebo | 98 | 85 | 1 | 3 | 1 | 3 | 1 | 2 | 0 | 1 | 1 | -0.247 | 0.598 |
| Active | 107 | 91 | 5 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | . | . |
Compliance
Counts of capsules returned by schools indicated mean compliance of approximately 75% and this did not significantly differ between Active (capsules were returned from n = 108 participants) and Placebo groups (capsules were returned from n = 104 participants). From 200 capsules allocated to schools for each child, quantities returned were: Active mean(sd) = 42.5(43.8) and Placebo mean(sd) = 48.9(48.8) (p(t)<0.317(-1.1)). Of the 142 capsules allocated to parents for non-school days, more than 50% of data were missing and so these are not reported.
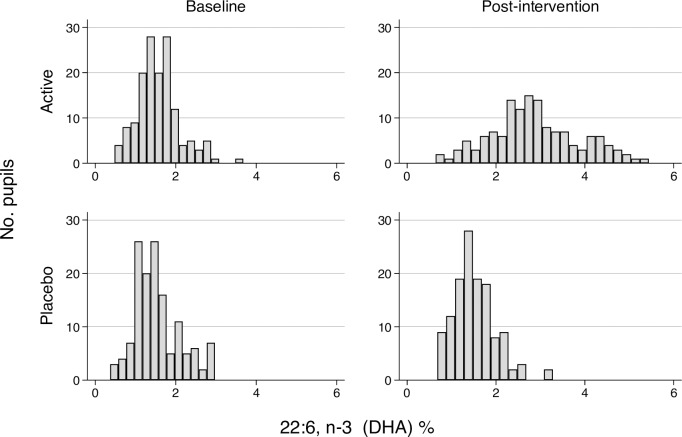
Objective data from fingerstick tests show that children in the active group had DHA levels of 2.9% (n = 140) compared to 1.5% in the placebo group (n = 129) (p(z)<0.001(11.3)) at post-intervention. Change scores indicate the active group increase their blood DHA from 1.6% to 2.9%, while the placebo group showed no such changes (p<0.001(10.54)). The baseline and post-intervention distribution of blood DHA levels by treatment group are illustrated in Fig 3. below.
Fig 3. Blood DHA omega-3 (22:6) distributions by treatment group before and after intervention.
Discussion
With this randomized, control trial, we made every attempt to rigorously replicate our previous findings of an improvement in reading and behavior following a dietary supplementation with the omega-3 fatty acid DHA amongst school children aged 7–9 whose reading was initially below the 20th-centile of pupils. In line with the original DOLAB I study, our primary outcomes were changes in reading, working memory and behavior (ADHD-type symptoms, parent-rated). In summary, this study did not replicate the original findings of significant, positive effects of omega-3 DHA on either learning or behavior. No systematic adverse effects from the supplementation were observed. As such the study does not provide supporting evidence for the benefits of this safe nutritional intervention.
Why did the DOLAB studies not replicate?
The results of the DOLAB II replication RCT and DOLAB I are clearly at odds. It is not entirely surprising that this study did not replicate the earlier one as has been found in many trials recently [33,34]. A number of substantive and necessary differences between the initial and the replication study might have contributed to these findings, despite the similar design of the two studies a combination of recruitment, measurement and uptake differences will have introduced considerable between-study heterogeneity.
First, the UK national curriculum relating to reading was changed in 2011 with a re-introduction of the phonic teaching approach. To address this change, a recalibrated version of the BAS II reading measure was used, which may, perhaps, have been less sensitive to detecting reading changes than its uncalibrated version.
Second, whilst the trial design of the DOLAB II replication RCT was identical to the initial study, we focused from the onset on the poorest reader amongst the pupils. Arguably this should have provided a higher power for detecting statistically significant intervention effects. However, the more restrictive inclusion criteria made recruitment more difficult. Compared to DOLAB I, pupils were recruited from five counties rather than one and the recruitment period was extended to 29 instead of 23 months. The larger recruitment area prevented the research team from repeated follow-up data collection visits, and consequently was identified as one source of the substantive missing teacher- and parent- self-report data.
Third, an additional recruitment challenge arose from the change of local authority run primary schools to self-governing academies, which had to be individually approached to gain school consent.
Fourthly, the recruitment issues further meant that a well-powered sample size of n = 400 was not quite fully achieved, and thus anticipated power gains by focusing on the subgroup of the 20th-centile readers were not fully realized. For illustrative purposes only, had we taken the observed effect size (d = 0.05) on the primary outcome–reading–the achieved power (α = 0.05) of this study would be 0.08 (8%), correspondingly to achieve 80% power given this effect size a sample of more than 11500 participants would have been necessary.
Finally, there appears to have been a lower omega 3 DHA uptake than in the previous trial, with DHA levels post-intervention being 2.9% as opposed to 3.8% in DOLAB I. However, changes in blood DHA levels bear no clear relationship in changes with primary outcomes when considering those with higher increases in DHA levels compared with those with lower increases or no changes (see Supporting Information S8 File).
Contrasting with common challenges to replication
This study is a good example of the replication problems outlined in the literature [33], we will discuss key issues following from John Ioannidis seminal paper. Protocol power calculations indicated a sample size of n = 400 would be required and in the event n = 376 participants were recruited. Our achieved power calculations underscoring this point even further. Several potential sources of bias may have affected the results, however our preregistered protocol (Protocol S1 File) and CONSORT-compliant (Checklist S2 File) reporting attends to most of these and provides transparency through the study. For example, clear hypotheses and a preselected (and reported) outcomes are provided therein. Both implementers and assessors were blinded to treatment group. Further, data and analysis syntax (Stata dofile) are available without restriction through the Open Science Framework: https://osf.io/9ynjf.
For additional analyses. Systematic reviews and other studies of this question provide inconsistent results, as they include heterogeneous groups of participants, interventions, comparators and outcomes [10,11,16–20]. Furthermore, there are implementation differences in dose, delivery, uptake and context both generally [35], specifically to this field [36], and with regard to this trial as discussed (see above). Consequently, the ratio of true to no relationships in the area of fatty-acid supplementation is problematic, and this is partly due to the large number of small studies finding small effects which are known to provide a poor basis for replication. This is arguably a complex intervention to evaluate [37], with multiple modes of delivery and outcome (child, parent, school), long causal pathway (bio-psycho-social mechanism for a behavioral change), where proximal (16-week) outcomes may not indicate distal change. This study was conducted without direct influence of its funder by way of a robust contract, there may remain researcher biases (self-serving, consistency and allegiance [38]) but again, transparent reporting guidelines aim to address these matters.
Finally, the reporting of these null-results illustrates our commitment to avoid publication biases, and our conviction that these add to the knowledge base on nutritional interventions. At a minimum, these studies contribute to the increased power of systematic reviews and meta-analyses.
Implications for research and practice
This study serves as an example for the need for robust, comparable trials for replication. Standardization of populations, interventions in terms of dose, composition and delivery would help evaluate the evidence base for this safe intervention. Currently trials use a range of placebos making comparisons difficult and result in mixed and vague outcomes. This poses a particular challenge to systematic reviews and meta-analysis trying to establish the best available evidence. The development of a core outcome set for similar trials on nutrition, learning, and behavior would be helpful [39]. Secular changes, such as reading curricula updates, may make replication challenging. And thus, even if the design and setting of studies are comparable non-replication will occur as this study demonstrated.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank:
The many participating children, parents, teachers and schools as well as supportive local authorities.
Gina Sandham, Charlotte van Nus for their assistance with the data collection.
Tony Brady from Sealed Envelope Ltd (http://www.sealedenvelope.com) for randomization services and consultation.
Eileen Bailey Hall and Gloria Chung at DSM Analytical Science for blood fatty acid analyses.
Sian Lawrence for editing and proof reading.
Finally, the authors would like to thank the editor and two anonymous reviewers for two very quick and thorough rounds of reviews and their numerous recommendations that considerably improved this paper.
Data Availability
All anonymized data and syntax files are available from the Open Science Framework project page: https://osf.io/9ynjf.
Funding Statement
The study was funded by DSM Nutritional Products (http://www.lifesdha.com/). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. Elsevier Inc.; 2011;50: 991–1000. doi: 10.1016/j.jaac.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition. Elsevier Inc.; 2012;28: 670–677. doi: 10.1016/j.nut.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 3.Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken J-W. Nutrients for Cognitive Development in School-aged Children. Nutr Rev. 2004;62: 295–306. doi: 10.1301/nr.2004.aug.295-306 [DOI] [PubMed] [Google Scholar]
- 4.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fat Acids. 2006;75: 329–349. doi: 10.1016/j.plefa.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 5.Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic Acid for Reading, Cognition and Behavior in Children Aged 7–9 Years: A Randomized, Controlled Trial (The DOLAB Study). PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. Springer-Verlag; 2003;38: 1007–1021. doi: 10.1007/s11745-006-1155-0 [DOI] [PubMed] [Google Scholar]
- 7.Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuro-Psychopharmacology Biol Psychiatry. 2002;26: 233–239. doi: 10.1016/S0278-5846(01)00254-8 [DOI] [PubMed] [Google Scholar]
- 8.Richardson AJ, Montgomery P. The Oxford-Durham Study: A Randomized, Controlled Trial of Dietary Supplementation With Fatty Acids in Children With Developmental Coordination Disorder. Pediatrics. 2005;115: 1360–1366. doi: 10.1542/peds.2004-2164 [DOI] [PubMed] [Google Scholar]
- 9.Richardson AJ. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int Rev Psychiatry. 2006;18: 155–172. doi: 10.1080/09540260600583031 [DOI] [PubMed] [Google Scholar]
- 10.Tan ML, Ho JJ, Teh KH. Polyunsaturated fatty acids (PUFAs) for children with specific learning disorders. Cochrane Database Syst Rev. 2012; doi: 10.1002/14651858.CD009398.pub2 [DOI] [PubMed] [Google Scholar]
- 11.Cornu C, Mercier C, Ginhoux T, Masson S, Mouchet J, Nony P, et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur Child Adolesc Psychiatry. Springer Berlin Heidelberg; 2017;electronic. doi: 10.1007/s00787-017-1058-z [DOI] [PubMed] [Google Scholar]
- 12.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Increased Erythrocyte Eicosapentaenoic Acid and Docosahexaenoic Acid Are Associated With Improved Attention and Behavior in Children With ADHD in a Randomized Controlled Three-Way Crossover Trial. J Atten Disord. 2015;19: 954–964. doi: 10.1177/1087054713510562 [DOI] [PubMed] [Google Scholar]
- 13.Johnson M, Fransson G, Östlund S, Areskoug B, Gillberg C. Omega 3/6 fatty acids for reading in children: a randomized, double-blind, placebo-controlled trial in 9-year-old mainstream schoolchildren in Sweden. J Child Psychol Psychiatry Allied Discip. 2017;58: 83–93. doi: 10.1111/jcpp.12614 [DOI] [PubMed] [Google Scholar]
- 14.Sørensen LB, Damsgaard CT, Dalskov S-M, Petersen RA, Egelund N, Dyssegaard CB, et al. Diet-induced changes in iron and n-3 fatty acid status and associations with cognitive performance in 8–11-year-old Danish children: secondary analyses of the Optimal Well-Being, Development and Health for Danish Children through a Healthy New Nordic Diet. Br J Nutr. 2015;114: 1623–1637. doi: 10.1017/S0007114515003323 [DOI] [PubMed] [Google Scholar]
- 15.Parletta N, Cooper P, Gent DN, Petkov J, O’Dea K. Effects of fish oil supplementation on learning and behaviour of children from Australian Indigenous remote community schools: A randomised controlled trial. Prostaglandins Leukot Essent Fat Acids. Elsevier; 2013;89: 71–79. doi: 10.1016/j.plefa.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 16.Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological Interventions for ADHD: Systematic Review and Meta-Analyses of Randomized Controlled Trials of Dietary and Psychological Treatments. Am J Psychiatry. 2013;170: 275–289. doi: 10.1176/appi.ajp.2012.12070991 [DOI] [PubMed] [Google Scholar]
- 17.Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: A systematic review and meta-analysis. J Affect Disord. Elsevier; 2016;190: 474–482. doi: 10.1016/j.jad.2015.09.053 [DOI] [PubMed] [Google Scholar]
- 18.Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: Blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. Elsevier Ltd; 2014;34: 496–505. doi: 10.1016/j.cpr.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies D, Sinn J, Lad S, Leach M, Ross M. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents (Review). Cochrane Libr. 2012; 1–75. doi: 10.1002/14651858.CD010274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelsser LM, Frankena K, Toorman J, Pereira RR. Diet and ADHD, reviewing the evidence: A systematic review of meta-analyses of double-blind placebo-controlled trials evaluating the efficacy of diet interventions on the behavior of children with ADHD. PLoS One. 2017;12: 1–25. doi: 10.1371/journal.pone.0169277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott CD, Smith P, McCulloch K. British Ability Scales second edition (BAS II): administration and scoring manual. London: NFER-Nelson; 1996. [Google Scholar]
- 22.Elliott CD, Smith P, McCulloch K. British Ability Scales: Third Edition (BAS 3). London: GL Assessment Ltd.; 2011. [Google Scholar]
- 23.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The Revised Conners’ Parent Rating Scale (CPRS-R): Factor Structure, Reliability, and Criterion Validity. J Abnorm Child Psychol. Kluwer Academic Publishers-Plenum Publishers; 1998;26: 257–268. doi: 10.1023/A:1022602400621 [DOI] [PubMed] [Google Scholar]
- 24.Conners CK. Conners’ Parenting Rating Scales–Revised Technical manual. New York: Multi-Health Systems Inc.; 1997. [Google Scholar]
- 25.Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and Restandardization of the Conners Teacher Rating Scale (CTRS-R): Factor Structure, Reliability, and Criterion Validity. J Abnorm Child Psychol. Kluwer Academic Publishers-Plenum Publishers; 1998;26: 279–291. doi: 10.1023/A:1022606501530 [DOI] [PubMed] [Google Scholar]
- 26.Conners CK. Conners’ Teacher Rating Scales–Revised Technical manual. New York: Multi-Health Systems Inc.; 1997. [Google Scholar]
- 27.Center for Disease Control and Prevention. About Child & Teen BMI [Internet]. 2012 [cited 25 Jun 2017]. Available: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html
- 28.Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side Effects of Metlyiphenidate in Children With Attention Deficit Hyperactivity Disorder: A Systemic, Placebo-Controlled Evaluation. Pediatrics. 1990;86 Available: http://pediatrics.aappublications.org/content/86/2/184.short [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. Springer-Verlag; 2007;39: 175–191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 30.Crowe FL, Murray Skeaff C, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr. 2008;99 doi: 10.1017/S000711450779387X [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. Bmj. 2010;340: c869–c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. DHASCO: Agency Response Letter GRAS Notice No. GRN 000041. In: GRAS Notice Inventory [Internet]. 2001 [cited 26 Jun 2017]. Available: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154126.htm
- 33.Ioannidis JPA. Why Most Published Research Findings Are False. PLoS Med. Public Library of Science; 2005;2: e124 doi: 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon J. REPRODUCIBILITY. Many psychology papers fail replication test. Science. American Association for the Advancement of Science; 2015;349: 910–1. doi: 10.1126/science.349.6251.910 [DOI] [PubMed] [Google Scholar]
- 35.Montgomery P, Underhill K, Gardner F, Operario D, Mayo-Wilson E. The Oxford Implementation Index: A new tool for incorporating implementation data into systematic reviews and meta-analyses. J Clin Epidemiol. Elsevier Inc; 2013;66: 874–882. doi: 10.1016/j.jclinepi.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Wurff ISM, Meyer BJ, de Groot RHM. A Review of Recruitment, Adherence and Drop-Out Rates in Omega-3 Polyunsaturated Fatty Acid Supplementation Trials in Children and Adolescents. Nutrients. 2017;9: 474 doi: 10.3390/nu9050474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Bmj. 2008;1655: a1655 doi: 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisner M. No effects in independent prevention trials: Can we reject the cynical view? J Exp Criminol. 2009;5: 163–183. doi: 10.1007/s11292-009-9071-y [Google Scholar]
- 39.Williamson P, Altman D, Blazeby J, Clarke M, Gargon E. Driving up the quality and relevance of research through the use of agreed core outcomes. J Health Serv Res Policy. 2012;17: 1–2. doi: 10.1258/jhsrp.2011.011131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All anonymized data and syntax files are available from the Open Science Framework project page: https://osf.io/9ynjf.