Abstract
Background and objectives:
A majority of children presenting with sepsis do not receive adequate fluid resuscitation and have a delay in antibiotic administration despite recommendations from the Surviving Sepsis Campaign. The objective of this study was to evaluate the association of measuring a complete set of five vital signs in the emergency department (ED) with recognition and treatment of septic children presenting to the ED.
Methods:
Records of 218 patients aged 1 month to 17 years treated between February 2011 and December 2011 in a single academic centre with clinical criteria of sepsis, severe sepsis or septic shock were retrospectively evaluated. The presence or absence of complete vital signs was analyzed in relation to timing of fluid resuscitation, and if antibiotics were given in the first hour of medical evaluation.
Results:
Seventy-six per cent of children who had all five vital signs measured in the ED received fluid resuscitation in the first hour after medical evaluation as opposed to 61% of those who had an incomplete set of vital signs (P<0.04). Twenty per cent of children who had all five vital signs measured received antibiotics in the first hour as opposed to 9% in children who had fewer vital signs measured (P<0.02).
Conclusion:
In our study population, the measurement of all vital signs in the ED, including blood pressure, was associated with faster administration of antibiotics and improved compliance with existing fluid bolus recommendations, which may have been the result of better recognition of sepsis in children through vital signs measurement.
Keywords: Blood pressure, Sepsis, Triage, Vital signs.
Abstract
L’effet de la mesure des signes vitaux pour dépister et traiter les enfants atteints de sepsis
Historique et objectifs:
La majorité des enfants ayant un sepsis ne reçoivent pas de solutés de réanimation et doivent attendre avant de se faire administrer des antibiotiques, malgré les recommandations de la Surviving Sepsis Campaign. La présente étude visait à évaluer l’association entre la mesure de l’ensemble des cinq signes vitaux à la salle d’urgence (SU) et le dépistage et le traitement des enfants atteints de sepsis qui s’y présentaient.
Méthodologie:
Les dossiers de 218 patients de 11 mois à 17 ans traités entre février et décembre 2011 dans un seul centre universitaire en raison de critères cliniques de sepsis, de grave sepsis ou de choc septique ont fait l’objet d’une évaluation rétrospective. Les chercheurs ont analysé le lien entre la présence ou l’absence de tous les signes vitaux et le moment d’administrer des solutés de réanimation et ont vérifié si des antibiotiques avaient été administrés dans l’heure suivant l’évaluation médicale.
Résultats:
Au total, 76 % des enfants dont les cinq signes vitaux avaient été mesurés à la SU avaient reçu des solutés de réanimation dans l’heure suivant leur évaluation médicale, par rapport à 61 % de ceux dont les signes vitaux n’avaient pas tous été mesurés (P<0,04). De plus, 20 % des enfants dont les cinq signes vitaux avaient été mesurés à la SU avaient reçu des antibiotiques dans l’heure suivant leur évaluation médicale, par rapport à 9 % de ceux dont les signes vitaux n’avaient pas tous été mesurés (P<0,02).
Conclusion:
Au sein de la population à l’étude, la mesure de tous les signes vitaux en SU, y compris la tension artérielle, s’associait à une administration plus rapide d’antibiotiques et à une meilleure compliance aux recommandations sur le bolus de liquide, ce qui peut être attribuable à un meilleur dépistage du sepsis chez les enfants grâce à la mesure des signes vitaux.
Sepsis remains one of the leading global causes of paediatric morbidity and mortality (1). Even in resource-intensive settings, severe sepsis represents up to 20% of paediatric intensive care unit (PICU) admissions and is the principal cause of noncardiac mortality in the PICU (2). Rapid recognition and treatment of these patients has a considerable influence on the prognosis of these patients (3,4). Based on a broad review of the available evidence, the Surviving Sepsis Campaign (SSC) recommends rapid shock reversal through multiple fluid boluses in the first hour of treatment (5). Shock reversal in these situations is determined primarily through continued reassessment of the patient’s clinical state and requires careful and systematic attention to vital signs. Once recognized, treatment for shock must not be delayed. In a 2003 report of out-of-hospital cases of septic shock, each hour of nonreversed shock was associated with a more than twofold increase in mortality (4). Equally important, according to the SSC guidelines, empiric, broad spectrum antibiotics should be rapidly administered. As described in adults, children with hypotensive septic shock who received appropriate antibiotic therapy in the first hour had better survival rates than those who did not (6).
The primary objective of this study was to evaluate the association of documentation of a complete set of vital signs versus incomplete set of vital signs on fluid resuscitation and antibiotic administration of septic children presenting to the emergency department (ED). The underlying hypothesis was that patients with an incomplete evaluation of vital signs would be less likely to be treated according to existing guidelines. The study also evaluated the frequency of blood pressure measurement in relation to patient age.
PATIENTS AND METHODS
This was an observational retrospective study. A retrospective health record review was performed on a cohort of patients evaluated and treated in an academic tertiary hospital where no formal institutional protocol regarding paediatric sepsis existed during the study period. The inclusion criteria were children aged 1 month to 17 years who presented between February and December 2011 with the clinical criteria of sepsis, severe sepsis or septic shock in the ED and were admitted to the paediatric ward or PICU. Sepsis was defined according to the clinical and laboratory criteria outlined in International Pediatric Sepsis Consensus Conference: Definitions for sepsis and organ dysfunction in pediatrics (7). These guidelines were chosen as they are the most widely accepted in the published literature and are the basis for most societal recommendations including the Society of Critical Care Medicine. In this document, sepsis is defined as a systemic inflammatory response syndrome in the presence of, or as a result of, suspected or proven infection. Severe sepsis is defined as sepsis plus one of the following: cardiovascular organ dysfunction, acute respiratory distress syndrome or dysfunction of two or more end organs. The definition of septic shock is ‘sepsis plus cardiovascular dysfunction manifested by clinical and biochemical signs of poor perfusion’. However, acknowledging that patients who met these criteria often do not have sepsis recorded as a primary or secondary diagnosis, the charts of all patients identified by the Health Record department with the diagnoses of pneumonia, meningitis, meningococcaemia, urinary tract infection, bacteraemia, viraemia, abscess, empyema, cellulitis, septic arthritis or necrotizing fasciitis as well as sepsis, severe sepsis or septic shock were analyzed and hand-reviewed. They were included in the study if, upon chart review, they met sepsis criteria (7). The diagnosis of viraemia was made by the treating physician based on clinical suspicion, positive viral culture or both. Exclusion criteria were children aged less than 1 month, immunosuppression, shock from nonseptic aetiologies and patients transferred from another hospital, as the latter often have unavailable documentation of initial vital signs and management.
A complete set of vital signs was defined as measurement of heart rate, respiratory rate, blood pressure, temperature and pulse oximetry taken in the ED at any time during the patient’s ED visit. When one or more vital signs were completely missing (i.e., never taken in the ER), the patient was classified as having an incomplete set of vital signs.
Adequate management was defined as the administration of fluid resuscitation with isotonic IV fluids (minimum of 20 mL/kg) and any type of IV antibiotics in the first hour following the first medical evaluation performed by an ED physician or trainee. Medical evaluation was defined as a patient being evaluated by a resident (who can prescribe fluids and antibiotics) or attending physician. As medical students at our centre do not evaluate febrile children with abnormal vital signs without direct supervision, this was not a concern for additional delay.
It was outside the scope of this study to evaluate the adequacy of antibiotic coverage or subsequent fluid resuscitation. Physiologic endpoints targeted by physicians were poorly documented, and in a retrospective study, we were unable to determine whether the quantity of subsequent fluid administered was adequate in relation to a patient’s needs. Ethics approval for this study was obtained from the local ethics committee, and all patient information was anonymized upon entry into the study database.
Statistical analysis
Descriptive analyses for quantitative variables were expressed as the average ± 1 SD for normally distributed variables while they were expressed as medians with an interquartile range for non-normally distributed variables. For the qualitative variables, descriptive analyses were expressed according to their frequency of distribution.
The primary analysis was the proportion of participants with complete vital signs who had an appropriate treatment (IV fluids and antibiotics in the first hour). As a secondary analysis, we also examined the correlation between frequency of blood pressure measurement and patient age.
Pearson’s or Spearman’s correlation coefficients were used. The associations between quantitative and qualitative variables were analyzed using the Wilcoxon rank-sum test or the Kruskal–Wallis test according to the distribution of the variables. Logistic regression and chi-square or Fischer’s exact test were used for multivariate analyses. Data were entered in an Excel database (Microsoft Inc., Richmond, WA) and analyzed using SPSS v21 software (IBM Software Group Inc.).
RESULTS
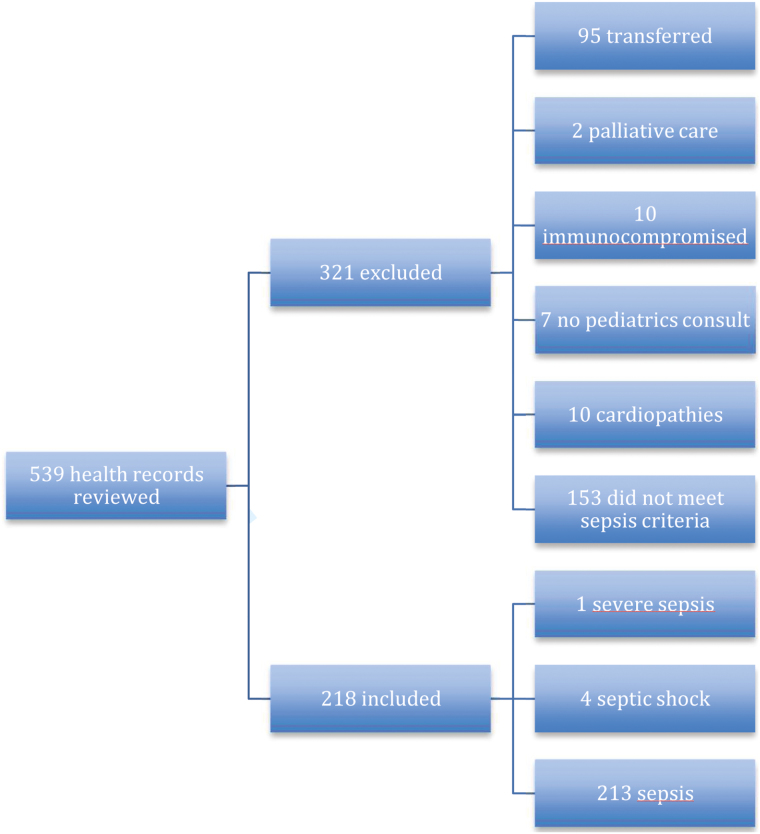
We reviewed 539 consecutive records and included 218 patients (Figure 1).
Figure 1.
Included and excluded patients
Ages were 1 month to 17 years (mean 3.6 years; SD 3.8 years) and 133 (61%) were boys. Two hundred and thirteen (97.7%) had sepsis, 1 (0.5%) had severe sepsis and 4 (1.8%) had septic shock. The infectious aetiologies of sepsis varied, with pneumonia 144 (66%) and viraemia 49 (22.5%) being the most frequent (Table 1).
Table 1.
Demographics
| Age | Mean | SD |
|---|---|---|
| 1 month–17 years | 3.6 | 3.8 |
| Sex | n | % |
| Female | 85 | 39 |
| Male | 133 | 61 |
| Diagnosis | n | % |
| Sepsis | 213 | 97.7 |
| Severe sepsis | 1 | 0.5 |
| Septic shock | 4 | 1.8 |
| Aetiologies | n | % |
| Meningococcemia | 1 | 0.5 |
| Pneumonia | 144 | 66 |
| Cellulitis | 5 | 2 |
| Urinary tract infection | 8 | 3.7 |
| Bacteraemia | 5 | 2 |
| Viraemia | 49 | 22.5 |
| Abscess | 5 | 2 |
| Other | 1 | 0.5 |
Of children who had all five vital signs measured in the ED, 76% received fluid resuscitation in the first hour after medical evaluation as opposed to 61% of those who had an incomplete set of vital signs (P<0.04). Of children who had all five vital signs measured in the ED, 20% received antibiotics in the first hour as opposed to 9% in children who had fewer vital signs measured (P<0.02).
Of children with an incomplete set of vital signs measured in the ED, 94% did not receive fluids, antibiotics or both in the hour after medical evaluation, as opposed to 84% in children who had all five vital signs measured in the ED (Table 2).
Table 2.
Vital signs measurement
| All vital signs recorded | Absence of one or more vital signs | P value | |||
|---|---|---|---|---|---|
| n=55 | % | n=163 | % | ||
| IV fluids in first hour | 42 | 76.4 | 100 | 61.3 | 0.0433 |
| No IV fluids in first hour | 13 | 23.6 | 63 | 38.7 | |
| Antibiotics in first hour | 11 | 20 | 14 | 8.6 | 0.0216 |
| No antibiotics in first hour | 44 | 80 | 149 | 91.4 | |
| IV fluids and antibiotics in first hour | 9 | 16.4 | 10 | 6.1 | 0.0275 |
| No IV fluids and no antibiotics in first hour | 46 | 83.6 | 153 | 93.9 | |
Measurement of blood pressure on the initial assessment was inversely proportional to patients’ age. Only 16% of children younger than 1 year had a blood pressure measured at the ED, versus 93% of children aged 13 and 17 (P<0.001) (Table 3).
Table 3.
Blood pressure and age
| Age | BP recorded | BP not recorded | ||
|---|---|---|---|---|
| n | % | n | % | |
| 1 month–1 year (n=95) | 15 | 15.8 | 80 | 84.2 |
| 2–5 years (n=85) | 19 | 22.4 | 66 | 77 |
| 6–12 years (n=24) | 10 | 41.7 | 14 | 58.3 |
| 13–17 years (n=14) | 13 | 92.9 | 1 | 7.1 |
Tendency test: P<0.0001. BP blood pressure
DISCUSSION
Our study shows that children who met the criteria for sepsis and had a complete set of vital signs, including blood pressure, measured in the ED were more likely to have received fluid resuscitation and antibiotics in accordance with the recommendations of the SSC than did those who did not. Considering that the rapid treatment of septic children has been shown to significantly influence prognosis, delays in treatment of these patients is a significant concern (3,4). These findings are consistent with previous reports that have shown that the recognition of paediatric sepsis is a principal barrier to adequate treatment (8). To this day, vital signs, specifically tachycardia and temperature, remain important clinical measures in sepsis recognition in the ED (9). To our knowledge, however, this is the first report that showed an association of a significant delay in treatment based on the completeness of vital sign measurement. While vital signs play a key role in recognition of sepsis, this recognition is only significant if it leads to treatment, namely fluid and antibiotic administration. Physicians in the ED have been shown to underestimate the probability of severe bacterial infections in paediatric patients, resulting in a delay in antibiotics (10). This was demonstrated in our current study, where all included patients met criteria for sepsis, yet only 20% of those with complete vital signs received antibiotics in the first hour. On one hand, the fact that this occurred in an academic, tertiary paediatric referral centre highlights the need for continued sepsis education and protocols that stress recognition and treatment. On the other hand, although SSC state that empiric antibiotics be administered within 1 hour of the identification of severe sepsis, clinical judgment is and will be required to assure that antibiotics are given rapidly to those with possible bacterial infections but not inappropriately administered to patients such as those unlikely to benefit. For example, as currently applied, sepsis algorithms recommend antibiotics for patients with alterations in vital signs, many of whom are not bacteraemic and would not benefit from antibiotic treatment. Care will have to be taken in further sepsis research to ensure that possible patient benefit is balanced against antibiotic overuse and its subsequent concerns.
This study also demonstrated that frequency of blood pressure measurement is inversely proportional to the patient’s age. Only 16% of infants younger than 1 year, with retrospectively confirmed sepsis, had their blood pressure measured in the ED. In our population, blood pressure was often not measured during initial evaluation, especially in younger children. Considering our results associating lack of complete vital signs and treatment delays, this would suggest that these infants were at higher risk for suboptimal sepsis treatment due to incomplete initial evaluation.
Our study has several limitations. The vast majority of our study population met the criteria for sepsis, but not severe sepsis or septic shock. This population of relatively well patients might have compounded the already existing difficulty in sepsis recognition and led to treatment delays. Moreover, vital signs were the main focus of this study and other indicators of severity of disease, such as signs of poor perfusion were not adequately documented to permit analysis. Furthermore, the incidence of poor outcomes PICU admission, mortality, or need of intubation was low, reflecting that our population was generally relatively well. Therefore, our study was underpowered to detect if the observed treatment delays affected these clinically relevant patient outcomes.
Our statistical analyses were based on the relationship between measuring a complete set of vital signs at any time during the patients’ evaluation and treatment in the ED and prompt initial sepsis treatment. It is possible that some of the vital signs (including blood pressure measurements) occurred after the administration of fluid or antibiotics. However, even if vital signs were completed after the initial treatment, the association between complete vital sign measurement and more rapid treatment was observed. As a retrospective observation, we are unable to suggest a causal relationship. It could be that early measurement of complete vital signs resulted in appropriately accelerated treatment, or it could be that treatment teams that measured vital signs more frequently generally had better compliance with other aspects of sepsis treatment guidelines. However, considering the previously cited evidence (3,4) linking treatment delays to poor prognosis, the measured delays may represent a significant outcome that places this vulnerable population at increased risk.
Despite these limitations, the findings suggest that the simple intervention of completely measuring vital signs in the ED may lead to better early treatment of one of the most common causes of paediatric morbidly and mortality.
CONCLUSION
Our study supports the importance of vital sign measurement in the initial evaluation of children at risk for sepsis. Efforts to improve sepsis recognition and treatment with an emphasis on vital sign monitoring might improve compliance with evidence-based treatment recommendations. Plans for future research include the re-evaluation of compliance after implementation of a multidisciplinary paediatric sepsis protocol. While the burden of paediatric sepsis is high, these data suggest that careful recognition of clinical status might help reduce its impact on our patients and our health care systems.
Acknowledgements
A.H. and M.-P.B. conceptualized and designed the study, designed the data collection, acquired the data, analyzed and interpreted the data, drafted and revised the manuscript. C.G. and M.W. helped with study design, analysis and interpretation of data, critically reviewed the manuscript and approved the final manuscript as submitted. Funding for this study was provided from funds available for resident research from the Pediatric and Emergency Departments of the Université Laval Faculté de Médecine. The authors have no financial relationship relevant to this article and no conflict of interest to disclose.
References
- 1. Ruth A, McCraken C, Fortenberry J, et al. Pediatric severe sepsis: Current trends and outcomes from the Pediatric Health Information Systems Database. Pediatr Crit Care Med 2014; e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2. Levy M, Dellinger R, Townsend S, et al. Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;38:367–74. [DOI] [PubMed] [Google Scholar]
- 3. Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Medicine. Crit Care Med 2009;37:666–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 2003;112:793–9. [DOI] [PubMed] [Google Scholar]
- 5. Dellinger R, Levy M, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care med 2013;41:580–635. [DOI] [PubMed] [Google Scholar]
- 6. Thompson M, Mayon-White R, Harnden A, Perera R, McLeoad D, Mant D. Using vital signs to assess children with acute infection: A survey of current practice. Br J Gen Pract 2008;58:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 8. Oliveira C, Noqueira de Sa F, Oliveira D, et al. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: Barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care 2008;12:810–15. [DOI] [PubMed] [Google Scholar]
- 9. Thompson G, Macias C. Recognition and management of sepsis in children: Practice patterns in the emergency department. J Emerg Med 2015. doi: 10.1016/j.jemermed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 10. Craig J, Williams G, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: Prospective cohort study of 15781 febrile illnesses. BMJ 2010;340:1594. [DOI] [PMC free article] [PubMed] [Google Scholar]