Abstract
Aim
Concerns of possible genotoxic effects of hyperbilirubinemia and phototherapy were raised from experimental and observational studies in neonates. The purpose of this study was to assess the effect of hyperbilirubinemia and phototherapy with three different methods on DNA damage by investigating sister chromatid exchange frequency.
Material and Methods
Patients whose gestational ages were >37 weeks and bilirubin levels above phototherapy limits were enrolled into three groups and each group was planned to receive 25 babies. Group 1 received enhanced light-emitting diode phototherapy, group 2 had light-emitting diode phototherapy, and group 3 received conventional phototherapy. Infants with hyperbilirubinemia but did not require phototherapy comprised the control group, which was subdivided into two groups regarding bilirubin levels (<or>10mg/dL). Blood samples were collected before and after phototherapy for sister chromatid exchange frequency and samples were examined by a biologist who was blinded to the study groups.
Results
The mean pre-treatment sister chromatid exchange frequency was 1.41±0.34/cell, post-treatment 2.65±0.68/cell, and 1.61±0.61/cell for the control group (p<0.05). A statistically significant increase in sister chromatid exchange frequency after phototherapy was observed in all three intervention groups (p values: 0.01, 0.01, and 0.008, respectively). When the treatment groups were compared with each other in terms of irradiance, no significant difference was found (p=0.08).
Conclusions
Phototherapy causes an increase in the frequency of sister chromatid exchange regardless of the irradiance. Phototherapy could have some genotoxic adverse effects on chromosomes; however, further investigations are warranted to enlighten as to whether these effects are permanent or clinically important.
Keywords: Hyperbilirubinemia, newborn, phototherapy
Introduction
Phototherapy has been used as a safe and efficient method in the treatment of neonatal hyperbilirubinemia for many years. Although phototherapy has some known adverse effects including skin rash, diarrhea, dehydration, and oxidative stress, no other method with fewer adverse effects that can be used conveniently has yet been developed for the treatment of hyperbilirubinemia (1, 2). In addition to the above-mentioned adverse effects, it has been shown that phototherapy might also have adverse effects on chromosomes in some animal and clinical studies (3–6).
The efficiency of phototherapy depends on the wavelength of the light administered, irradiance, body surface area where the light is applied, and the duration of the procedure (2). The American Academy of Pediatrics (AAP) defines intensive phototherapy as at least 30mW/(cm2 nm) in the 430–490 nm band and recommends this to provide rapid bilirubin reduction (7). More recently, high-intensity gallium nitride light-emitting diode (LED) light sources have been developed to be used in phototherapy treatment in neonatal jaundice (8). Blue LEDs emit high-intensity, narrow-spectrum light in the peak light spectrum required for bilirubin destruction. Therefore, it is thought that they potentially shorten the treatment period (8, 9). In addition, diode lamps are light and power saving sources that produce less heat and last longer (8–10). Many studies have been conducted in relation with the efficiency of diode lamps, which have been used in the treatment of neonatal jaundice for a long period (10). The adverse effects of conventional phototherapy on DNA and chromosomes were previously shown in clinical studies, but few studies have been conducted with LED phototherapy.
Sibling chromatid exchange (SCE) is among the cytogenetic markers used most commonly in determining the early biologic effects of agents that lead to DNA damage (2, 8). Sibing chromatid exchange is the exchange of sibling chromatids during DNA replication; it may occur as a normal characteristic of cellular division and it does not lead to a change in the structure of the chromosome, but its frequency increases when cellular DNA is damaged by genotoxic factors including ultraviolet light, radiation, and chemical drugs (11–13).
The aim of this study was to examine the adverse effects of phototherapy with different irradiances on chromosomes by investigating the frequency of SCE.
Material and Methods
This study was conducted between June 2009 and June 2010 in the Division of Neonatology in Zekai Tahir Burak Women’s Health Education and Research Hospital. The study was approved by the Ethics Committee and written consent was obtained from the parents of the newborns who were included in the study (14.09.2010–11). Newborns with a gestational age of >37 weeks and a postnatal age of >3 days who had bilirubin levels above the phototherapy limits according to the AAP and hospitalized in our hospital with the aim of phototherapy were included in the study. The control group consisted of healthy newborns born at term who presented to the Outpatient Clinic of Neonatology for screening. Newborns with direct bilirubinemia, systemic disease, phototherapy requirement in the first 72 hours of life, congenital anomalies, respiratory distress, glucose-6-phosphate dehydrogenase deficiency and clinical and confirmed sepsis were not included in the study.
The newborns included in the study were divided into three groups. Group 1 received enhanced LED phototherapy, group 2 received LED phototherapy, and group 3 received conventional phototherapy. Total bilirubin levels were monitored every 6 hours throughout the phototherapy. The necessary blood samples were obtained for SCE before and after phototherapy. When the bilirubin level was reduced to half of the blood exchange limit, phototherapy was discontinued. The bilirubin levels were evaluated at the 6th hour after discontinuation of phototherapy. The newborns included in the control group were examined in two subgroups, one with bilirubin levels below 10 mg/dL and the other with bilirubin levels between 10 and 15 mg/dL.
Baby LED force (Ertunç Özcan, Turkey, spectrum 450–470 nm, intensity 30–120 μW/cm2/nm) was used for enhanced phototherapy, Neoblue (NatusMedicalInc. San Carlos, CA, USA, spectrum 450–470 nm, intensity=35 μW/cm2/nm) was used for LED and AMS phototherapy (Spectrum 430–470 nm, intensity=12–16 μW/cm2/nm) including 6 fluorescence lamps was used for conventional phototherapy. Irradiances were measured before treatment and regularly, using IrradianceFluoro-lite 451® (Minolta/AirShields, USA) device. It was aimed to have an irradiance of >60, >35, and >10 μW/cm2/nm, respectively. Phototherapy devices were placed 30 cm away from the babies. Phototherapy was administered continuously with the eyes and genital region covered. Phototherapy was not paused except for cleaning the baby’s bottom and performing medical interventions.
The blood samples were placed into 1 mL heparin tubes just before initiating treatment for SCE and at the time of the risk of bilirubin re-increase. The samples were immediately sent to the genetics laboratory for incubation. Lymphocytes were incubated in appropriate liquids for 72 hours at 37°C and then spread on slides after adding colchicine and obtaining metaphase plates. After a one-night aging procedure, the slides were stained with 5% Giemsa. The slides were evaluated under a light microscope by an experienced genetics expert who was blinded to the patient groups. Sibling chromatid exhange frequency was reported as SCE/cell for each case.
Capillary tubes were used for the measurement of bilirubin values and studied using direct spectrophotometry.
Gestational age, birth weight, current weights, bilirubin values, postnatal age, and adverse effects including stool frequency, body temperature, and skin rash were recorded in all groups throughout the study.
Statistical Analysis
The SPSS 19 (SPSS Inc., Chicago, IL) package program was used to record the data and for statistical analysis. A p value of <0.05 was considered statistically significant. Parametric tests were used to analyze continuous variables showing normal distribution and nonparametric tests were used for those with abnormal distribution. Descriptive statistics that showed normal distribution are expressed as mean±standard deviation. The descriptive statistics that showed abnormal distribution are expressed as median. Kruskal–Wallis analysis and if required, the Mann–Whitney U test and Bonferroni correction were used in the assessment of significance. Pearson’s correlation analysis was used for correlation analysis and Wilcoxon analysis was used in the comparison of dependent variables.
Results
A total of 115 newborns were included in the study. Twenty-five newborns were included in each study group, but 27 newborns were excluded from the study because sufficient metaphase plates could not be obtained and SCE frequency could not be studied. Consequently, 24 newborns were examined in group 1, 18 newborns were examined in group 2, and 13 newborns were examined in group 3. In the control group, 22 newborns with a total bilirubin level of <10 mg/dL and 11 newborns with a total bilirubin level of 10–15 mg/dL were included in the analysis. The gestational age, body weight, sex, and mode of delivery, and age at the time of inclusion in the study were found to be similar in all groups (Table 1).
Table 1.
Basic characteristics of the groups
| Group 1 n=24 | Group 2 n=18 | Group 3 n=13 | Control 1 n=11 | Control 2 n=22 | p | |
|---|---|---|---|---|---|---|
| Gestational agea (weeks) | 38.2±1.1 | 38.5±1.1 | 38±1.2 | 38±1 | 38.7±0.7 | 0.20 |
| Body weighta (g) | 3 050±482 | 3 131±561 | 3 017±368 | 3 025±431 | 3 278±434 | 0.58 |
| Age at the time of inclusion in the studyb (days) | 4 (3–16) | 4 (3–14) | 5 (4–7) | 4 (1–5) | 4 (1–12) | 0.08 |
| Male, n (%) | 10 (41.6) | 5 (27.7) | 9 (69.2) | 6 (54.5) | 12 (54.5) | 0.06 |
| Cesarean section, n (%) | 13 (54.1) | 11 (61.1) | 7 (53.8) | 5 (45.4) | 11 (50) | 0.86 |
mean±standard deviation;
median(minimum–maximum)
The bilirubin levels of the groups are summarized in Table 2. Although the bilirubin levels at the time of hospitalization were found to be similar in the groups who received phototherapy, a statistically significant difference was observed in bilirubin levels in the comparison with the control group (p=0.18 and p<0.001) (Table 2). The bilirubin levels recorded in the 24th hour of hospitalization were found to be similar in all three phototherapy groups (Table 2). The total bilirubin levels that re-increased 6 hours after discontinuation of phototherapy were found to be similar (Table 2). The hourly bilirubin reduction rate was found to be statistically significantly lower in the group that received conventional phototherapy compared with the other groups (p=0.01, Table 2). The phototherapy period was found to be slightly longer in the group that received conventional phototherapy, but the difference was not statistically significant. The SCE frequencies studied before phototherapy were found to be similar in all groups (control and phototherapy) and in the different phototherapy groups. The SCE frequencies studied after phototherapy were found to be similar in the phototherapy groups (Table 2). In the dependent variable analysis, a significant increase in the SCE frequency was found after phototherapy (p<0.01). When the phototherapy groups were examined, the increase in each group was found to be significant one by one (p=0.011 for group 1, p=0.015 for group 2, p=0.008 for group 3). A linear relationship between bilirubin levels and SCE frequency was not found (Figure 1). No difference was found between the phototherapy groups in terms of adverse effects including skin rash, stool frequency, and body temperature recorded throughout the study (p>0.05).
Table 2.
Sibling chromatid exchange frequencies and bilirubin values of the groups
| Group 1 n=24 | Group 2 n=18 | Group 3 n=13 | pa | Control 1 n=11 | Control 2 n=22 | pb | |
|---|---|---|---|---|---|---|---|
| T0 Bil (mg/dL) | 19.3±2.1 | 20.3±2.4 | 19.2±3.1 | 0.52 | 12.7±0.9 | 7.5±5.4 | <0.001 |
| T24 bil (mg/dL) | 10.3±3.3 | 9.1±2 | 12.5±2.6 | 0.25 | - | - | - |
| TR6 Bil (mg/dL) | 9.6±1.9 | 8.3±2 | 9.7±2.8 | 0.21 | - | - | - |
| ΔBil (mg/dL/h) | 0.71±0.3 | 0.73±0.27 | 0.45±0.32 | 0.01 | - | - | - |
| Phototherapy time (s) | 19.5±5.5 | 19.2±4.4 | 23.1±5.8 | 0.07 | - | - | - |
| SCE1 | 1.49±0.35 | 1.54±0.51 | 1±0.45 | 0.08 | 1.48±0.62 | 1.62±0.6 | 0.18 |
| SCE2 | 2.5±0.7 | 2.65±0.72 | 2.56±0.84 | 0.94 |
All values are given as mean±standard deviation.
p value obtained from comparison of the groups who received phototherapy, Kruskal-Wallis test;
p value obtained from comparison of all groups, Kruskal-Wallis test;
SCE1: Sibling Chromatid Exchange frequency, before phototherapy; SCE2: Sibling Chromatid Exchange frequency, after phototherapy; T0Bil: bilirubin value at the time of hospitalization; T24Bil: total bilirubin value 24 hours after hospitalization; TR6 Bil: bilirubin value 6 hours after discontinuation of phototherapy; ΔBil: Hourly bilirubin reduction rate
Figure 1.
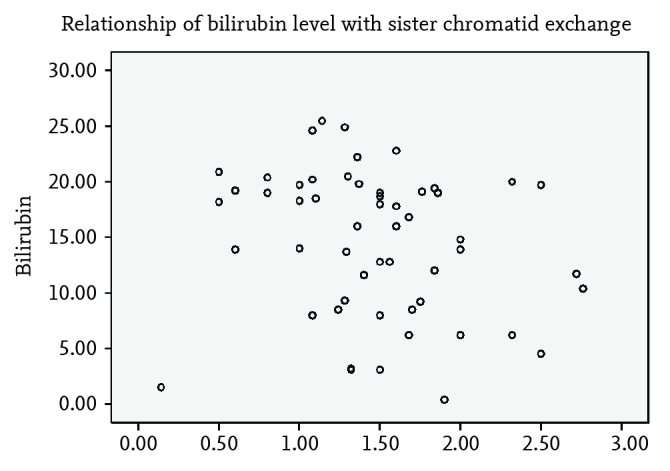
Relationship of bilirubin level with sister chromatid exchange frequency
Discussion
This study showed that the frequency of SCE increased in the lymphocytes of newborn babies who received phototherapy independent of the light irradiance. It has been thought for a long time that phototherapy and bilirubin, as a factor of increasing light sensitivity, might have harmful effects on DNA and some previous studies showed these effects. Christensen et al. (14) and Wu et al. (15) showed increased cytotoxicity and DNA damage in newborn lymphocytes that were exposed to blue light especially. Similar to our study, Karadag et al. (16) reported that both enhanced and conventional phototherapy increased the frequency of SCE.
Sibling chromatid exchange is used as a sensitive indicator of chromosomal exposure because increased frequency of SCE also occurs with exposure to genotoxic factors at low levels. Therefore, SCE frequency was selected as a method to evaluate genotoxicity in this study based on previous studies. The first clinical study that investigated the effect of phototherapy on the frequency of SCE was published by Goyanes-Villaescusa et al. (17). Three groups of newborns including a phototherapy group, a group with jaundice that did not receive phototherapy, and a group without jaundice were compared and it was shown that phototherapy increased the frequency of SCE significantly. In contrast, Schwartz et al. (18) reported that phototherapy did not cause an increase in the frequency of SCE. However, the number of patients in this study was considerably low compared with the other studies and a complex patient group was included in the study. Karadag et al. (16) showed that bilirubin levels in addition to phototherapy affected the frequency of SCE and demonstrated a linear relationship. However, we did not find a relationship between bilirubin levels and the frequency of SCE in our study.
Many studies in the literature investigated the effects of conventional phototherapy on chromosomes, but there is a paucity of studies investigating the effects of different radiances. Our study is unique in terms of comparing three different light irradiances with two different control groups that did not receive phototherapy. It was predicted that increased irradiance might be correlated with increased toxicity, but this hypothesis cannot be supported with the results of this study. The reason that this correlation could not be demonstrated might be the fact that the number of subjects included in the groups was insufficient. Exposure to phototherapy and the period of exposure seem to be related with genotoxicity independent of irradiance. In a recent study conducted by Kahveci et al. (19), it was shown that the frequency of SCE increased each day compared with the previous day during exposure to phototherapy. In our study, the phototherapy period was found to be longer in conventional phototherapy, though statistical significance was not reached.
One of the important limitations of this study was the fact that the frequency of SCE could not be determined in some patients because of technical reasons and these patients were excluded from the analysis. Another limitation is the fact that long-term effects of phototherapy in terms of genotoxicity were not investigated and we did not evaluate as to whether the increase in the frequency of SCE was permanent. Kahveci et al. (19) showed that the effect of phototherapy on SCE disappeared in the follow-up visits performed around the age of 3.5 years in their studies. Long-term follow-up was not performed in this study and the subjects were not monitored in terms of the clinical reflection of this change in the laboratory.
Although it has been proposed that the reduction in serum bilirubin levels reaches a saturation point when irradiance reaches 30–35 μW/cm2/nm and there is no more increase in the reduction rate when values above this point are reached, it is not clear if such a saturation point exists (20). In our study, increasing irradiance to >60 μW/cm2/nm did not cause an increase in the reduction rate of bilirubin and a significant reduction in the phototherapy time and an increase in the expected adverse effects was not found either.
Phototherapy is being used with a gradually increasing frequency in the treatment of neonatal jaundice. Some physicians may even initiate phototherapy at bilirubin levels below phototherapy limits because of the few adverse effects and convenient application. In some current publications, phototherapy has been shown to be related with increased risk of childhood and infancy cancers (21). It may be thought that the increase in the risk of cancer is related with the long-term results of the genotoxic effect of phototherapy or hyperbilirubinemia itself. In studies conducted with Comet assays, it was shown that phototherapy caused DNA damage in peripheral blood lymphocytes and triggered apoptosis (22, 23). It would be beneficial to consider such risks when deciding upon the initiation of therapy, especially in neonates with bilirubin levels below phototherapy limits.
In conclusion, phototherapy affects the frequency of SCE independent of irradiance. However, the clinical reflections and long-term effects of this change are questions that still need to be answered. Although this study is the first to investigate the effect of irradiance on SCE, the expected impact might not have been shown because of the limited number of subjects.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Zekai Tahir Burak Woman Health Training and Research Hospital.
Informed Consent: Written informed consent was obtained from patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.S.O., H.G.K.; Design - S.S.O., H.G.K.; Supervision - S.S.O., H.G.K.; Funding - S.S.O., H.G.K.; Materials - D.D., H.G.K.; Data Collection and/or Processing - N.O., A.Y.; Analysis and/or Interpretation - S.S.O., H.G.K.; Literature Review - N.O., H.G.K.; Writing - H.G.K, N.O.; Critical Review - S.S.O.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Vreman HJ, Wong JR, Stevenson DK. Phototherapy: current methods and future directions. Semin Perinatol. 2004;28:326–33. doi: 10.1053/j.semperi.2004.09.003. https://doi.org/10.1053/j.semperi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358:920–8. doi: 10.1056/NEJMct0708376. https://doi.org/10.1056/NEJMct0708376. [DOI] [PubMed] [Google Scholar]
- 3.Roll EB, Christensen T. Formation of photoproducts and cytotoxicity of bilirubin irradiated with turquoise and blue phototherapy light. Acta Paediatr. 2005;94:1448–54. doi: 10.1111/j.1651-2227.2005.tb01819.x. https://doi.org/10.1080/08035250510032655. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstein BS, Ducore JM. Enhancement by bilirubin of DNA damage induced in human cells exposed to phototherapy light. Pediatr Res. 1984;18:3–6. [PubMed] [Google Scholar]
- 5.Tatli MM, Minnet C, Kocyigit A, Karadag A. Phototherapy increases DNA damage in lymphocytes of hyperbilirubinemic neonates. Mutat Res. 2008;654:93–5. doi: 10.1016/j.mrgentox.2007.06.013. https://doi.org/10.1016/j.mrgentox.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Aycicek A, Kocyigit A, Erel O, Senturk H. Phototherapy causes DNA damage in peripheral mononuclear leukocytes in term infants. J Pediatr (Rio J) 2008;84:141–6. doi: 10.2223/JPED.1765. https://doi.org/10.2223/JPED.1765. [DOI] [PubMed] [Google Scholar]
- 7.Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant ≥35 weeks gestation: an update with clarification. Pediatrics. 2009;124:1193–8. doi: 10.1542/peds.2009-0329. https://doi.org/10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
- 8.Vreman HJ, Wong JR, Stevenson DK, et al. Light-emitting diodes: a novel light source for phototherapy. Pediatr Res. 1998;44:804–9. doi: 10.1203/00006450-199811000-00027. https://doi.org/10.1203/00006450-199811000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Ennever JF. Blue light, green light, white light, more light: treatment of neonatal jaundice. Clin Perinatol. 1990;17:467–81. [PubMed] [Google Scholar]
- 10.Seidman DS, Moise J, Ergaz Z, et al. A new blue light-emitting phototherapy device: a prospective randomized controlled study. J Pediatr. 2000;136:771–4. https://doi.org/10.1016/S0022-3476(00)75202-4. [PubMed] [Google Scholar]
- 11.Tucker JD, Preston JR. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges, and cancer risk assessment. Mutat Res. 1996;365:147–59. doi: 10.1016/s0165-1110(96)90018-4. https://doi.org/10.1016/S0165-1110(96)90018-4. [DOI] [PubMed] [Google Scholar]
- 12.Renault G, Gentil A, Chouroulinkov I. Kinetics of induction of sister-chromatid exchanges by X-rays through two cell cycles. Mutat Res. 1982;94:359–68. doi: 10.1016/0027-5107(82)90298-6. https://doi.org/10.1016/0027-5107(82)90298-6. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DM, Thompson LH. Molecular mechanisms of sister-chromatid exchange. Mutat Res. 2007;616:11–23. doi: 10.1016/j.mrfmmm.2006.11.017. https://doi.org/10.1016/j.mrfmmm.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Christensen T, Kinn G, Granli T, Amundsen I. Cells, bilirubin and light: formation of bilirubin photoproducts and cellular damage at defined wavelengths. Acta Paediatr. 1994;83:7–12. doi: 10.1111/j.1651-2227.1994.tb12943.x. https://doi.org/10.1111/j.1651-2227.1994.tb12943.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu FY, Iijima K, Takiguchi D, Nishida A, Higurashi M. Effect of phototherapy on sister-chromatid exchange in infants with Down syndrome. Mutat Res. 1992;283:65–7. doi: 10.1016/0165-7992(92)90123-y. https://doi.org/10.1016/0165-7992(92)90123-Y. [DOI] [PubMed] [Google Scholar]
- 16.Karadag A, Yesilyurt A, Unal S, et al. A chromosomal-effect study of intensive phototherapy versus conventional phototherapy in newborns with jaundice. Mutat Res. 2009;676:17–20. doi: 10.1016/j.mrgentox.2009.03.008. https://doi.org/10.1016/j.mrgentox.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Goyanes-Villaescusa VJ, Ugarte M, Vazquez A. Sister chromatid exchange in babies treated by phototherapy. Lancet. 1977;2:1084–5. doi: 10.1016/s0140-6736(77)91927-4. https://doi.org/10.1016/S0140-6736(77)91927-4. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AL, Cole FS, Fiedorek F, et al. Effect of phototherapy on sister chromatid exchange in premature infants. Lancet. 1978;2:157–158. doi: 10.1016/s0140-6736(78)91545-3. https://doi.org/10.1016/S0140-6736(78)91545-3 [DOI] [PubMed] [Google Scholar]
- 19.Kahveci H, Dogan H, Karaman A, Caner I, Tastekin A, Ikbal M. Phototherapy causes a transient DNA damage in jaundiced newborns. Drug Chem Toxicol. 2013;36:88–92. doi: 10.3109/01480545.2011.653491. https://doi.org/10.3109/01480545.2011.653491. [DOI] [PubMed] [Google Scholar]
- 20.Maisels MJ, Baltz RD, Bhutani VK, et al. American Academy of Pediatrics, Clinical Practice Guideline, Subcommittee on Hyperbilirubinemia. Management of the newborn 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. https://doi.org/10.1542/peds.114.1.297. [Google Scholar]
- 21.Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal phototherapy and infantile cancer. Pediatrics. 2016;137 doi: 10.1542/peds.2015-1353. pii: e20151353. https://doi.org/10.1542/peds.2015-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramy N, Ghany EA, Alsharany W, et al. Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatol. 2016;36:132–6. doi: 10.1038/jp.2015.166. https://doi.org/10.1038/jp.2015.166. [DOI] [PubMed] [Google Scholar]
- 23.Yahia S, Shabaan AE, Gouida M, et al. Influence of hyperbilirubinemia and phototherapy on markers of genotoxicity and apoptosis in full-term infants. Eur J Pediatr. 2015;174:459–64. doi: 10.1007/s00431-014-2418-z. https://doi.org/10.1007/s00431-014-2418-z. [DOI] [PubMed] [Google Scholar]


