Abstract
The vertebrate central nervous (CNS) is comprised of vast number of distinct cell types arranged in a highly organized manner. This high degree of complexity is achieved by cellular communication, including direct cell-cell contact, cell-matrix interactions, and cell-growth factor signaling. Among the several developmental signals controlling the development of the CNS, Wnt proteins have emerged as particularly critical and, hence, have captivated the attention of many researchers. With Wnts’ evolutionarily conserved function as primordial symmetry breaking signals, these proteins and their downstream effects are responsible for simultaneously establishing cellular diversity and tissue organization. With their expansive repertoire of secreted agonists and antagonists, cell surface receptors, signaling cascades and downstream biological effects, Wnts are ideally suited to control the complex processes underlying vertebrate neural development. In this review, we will describe the mechanisms by which Wnts exert their potent effects on cells and tissues and highlight the many roles of Wnt signaling during neural development, starting from the initial induction of the neural plate, the subsequent patterning along the embryonic axes, to the intricately organized structure of the CNS.
Keywords: Wnt, cell signaling, neural development, embryonic development
INTRODUCTION
The formation of the central nervous system (CNS) begins during gastrulation with the emergence of the ectoderm from the epiblast (human embryonic development days 14–18 [E14-E18]) (Sanes et al., 2012). During neural induction (E16-E18), naïve ectoderm becomes committed to the neural lineage as the neural plate forms along the dorsal midline of the embryo (Andoniadou and Martinez-Barbera, 2013; Kicheva and Briscoe, 2015). The neural plate continues to fold into the underlying mesoderm resulting in the formation of the neural groove surrounded on each side by the neural folds (E18-E20) (Andoniadou and Martinez-Barbera, 2013; Kicheva and Briscoe, 2015). As the neural groove elongates along the anterior-posterior axis the neural folds fuse to create the neural tube (E20-E24) (Andoniadou and Martinez-Barbera, 2013; Kicheva and Briscoe, 2015). Concomitantly with the formation of neural tube, the neural crest arises from non-neuronal ectodermal cells along the lateral edge of the neural plate (Huang and Saint-Jeannet, 2004). The neural crest will eventually give rise to a myriad of cell populations, including the neural cells of the sensory, sympathetic, and parasympathetic nervous system as well as various craniofacial musculoskeletal cell types (Huang and Saint-Jeannet, 2004; Sanes et al., 2012; Liu and Cheung, 2016). By the fourth week of development, the neural tube becomes further subdivided into three primary vesicles—the prosencephalon (forebrain), the mesencephalon (midbrain), and the rhombencephalon (hindbrain)—and the emerging spinal cord (Sanes et al., 2012). The prosencephalon will be further divided into the anterior telencephalon (which eventually matures into the cerebrum) and the posterior diencephalon (which will form the thalamic and hypothalamic regions) (Wilson and Houart, 2004). The rhombencephalon further separates into eight defined regions called rhombomeres (Guthrie and Lumsden, 1991; Glover, 2001). The anterior rhombomeres (R1-R3), which comprise the metencephalon, will differentiate into the pons and cerebellum (Carlson, 2014). The posterior rhombomeres (R4-R8), which constitute the myelencephalon, will generate the structures of the medulla oblongata (Carlson, 2014).
A variety of signaling molecule pathways act in a tightly regulated spatial and temporal manner to instruct the specification, development, and maturation of the CNS. In particular, the Wnt signaling pathway plays critical roles in every aspect of CNS development. In this review, we will focus of the role of Wnt signaling in the earliest stages of neurodevelopment and patterning from gastrulation to approximately week 5 of human in utero development (Figure 1). The mechanisms by which Wnt signaling regulates later CNS developmental processes including corticogenesis (Bielen and Houart, 2014; Inestrosa and Varela-Nallar, 2015; Fortress and Frick, 2016), neural crest emergence (De Calisto et al., 2005; Pegoraro and Monsoro-Burq, 2013; Mayor and Theveneau, 2014), eye morphogenesis (Cavodeassi, 2014; Fujimura, 2016), axon growth and guidance (Guan and Rao, 2003; Clark et al., 2012; Onishi et al., 2014; Petrova et al., 2014), synaptic formation and function (Sanes and Lichtman, 2001; Burden, 2002; Salinas, 2012; Dickins and Salinas, 2013; Poon et al., 2013; Stamatakou and Salinas, 2014), and adult neurogenesis (Bielen and Houart, 2014) have been reviewed extensively elsewhere.
Figure 1. Summary of early of neural development events regulated by Wnt signaling.
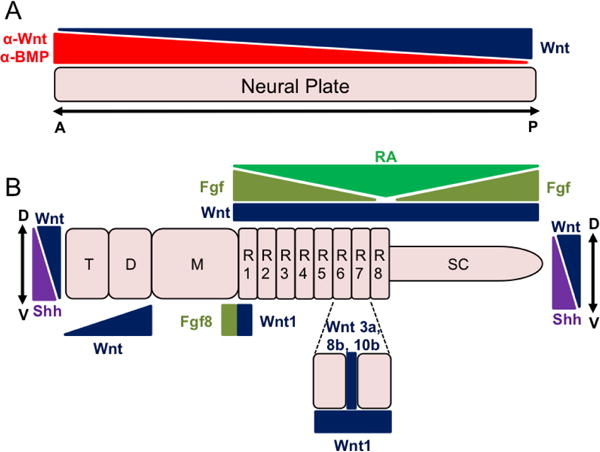
A. The neural plate initially emerges from the ectoderm through the action of BMP antagonists (α-BMPs). Opposing gradients of Wnt antagonists (α-Wnt) and Wnt ligands pattern the neural tube along the anterior-posterior (A/P) axis. B. As the neural tube continues to mature, it becomes further subdivided into primary vesicles (prosencephalon, mesencephalon [M], and the rhombencephalon) and the developing spinal cord (SC). The prosencephalon will be further divided into the telencephalon (T) and diencephalon (D). In addition, the rhombencephalon dives into eight rhombomeres (R1-R8). Wnt signaling along with other signaling molecules (e.g. FGFs, Shh, retinoic acid [RA]) regulates the further patterning and maturation of these regions along both the A/P as well as dorsal-ventral (D/V) axis.
Overview of Wnt Signaling
The importance of Wnt signaling in the biology of multicellular life was first appreciated in two independent lines of investigation: in a mouse model for breast cancer and, separately, in genetic analysis of Drosophila developmental mutants. Work by Nusse and Varmus demonstrated that proviral insertion of the mouse mammary tumor virus in a specific locus of the genome, termed int1, promoted tumorigenesis (Nusse and Varmus, 1982). A poly-adenylated RNA transcribed from within this locus was expressed in tumors only, and subsequent studies (Nusse et al., 1984; Fung et al., 1985) showed this RNA to encode a protein with two of the key hallmarks of all Wnt proteins: a hydrophobic amino terminus signifying targeting to the secretory pathway and an unusually high content of cysteine residues.
Predating these mouse studies, groundbreaking genetic screens in Drosophila led to the identification of a group of genes that controlled embryonic patterning (Nusslein-Volhard and Wieschaus, 1980). One of the groups of genes, called segment polarity genes, included wingless (wg), previously named after a hypomorphic allele that produced flies lacking wings (Sharma and Chopra, 1976). Molecular cloning of the Wg gene revealed it to be homologous to the mouse gene transcribed from the int1 locus. With the recognition that Wg and int1 represented prototypes for a much larger family of related genes, the mnemonic Wnt was coined (Nusse et al., 1991).
Wnt genes have been identified in all metazoan species, with the mammalian genome containing 19 distinct Wnt genes, some of which encode alternative transcripts. Because Wnt proteins and their signaling pathways govern a myriad of biological processes—from patterning of the fly embryo to cancer development in mammals—it may, at times, be difficult to discern a theme that underlies and unifies Wnt biology. Nonetheless, from an evolutionary perspective, it is clear that Wnt proteins are pivotal symmetry breaking signals that establish the primary body axis during embryonic development (reviewed in (Loh et al., 2016)). Consistent with this universal role for Wnts in organismal development, and as we will discuss in this review, Wnt signaling regulates anterior-posterior and dorsal-ventral patterning of the developing central nervous system. The study of Wnt signaling has extended into virtually all aspects of biology and the reader is encouraged to visit the Wnt homepage (wnt.stanford.edu) for additional information on Wnt across the spectrum of developmental biology.
Wnt proteins
Wnt genes encode secreted lipid-modified glycoproteins (Brown et al., 1987; Papkoff et al., 1987; Mason et al., 1992; Willert et al., 2003). A nearly invariant pattern of 22 cysteine residues, all of which are engaged in disulfide linkages, are essential for the proper folding of this growth factor into a two-domain hand-like structure, which was recently elucidated by X-ray crystallography (Janda et al., 2012). A covalently attached lipid extending from the tip of the “thumb” is critically important for Wnt function and renders it highly hydrophobic (Willert et al., 2003; Takada et al., 2006). The process of attaching this lipid, palmitoleic acid (a 16-carbon monounsaturated lipid), on a conserved Serine residue of the Wnt polypeptide chain, occurs in the endoplasmic reticulum and requires the activity of Porcupine (Porcn), an acyl-transferase (Hofmann, 2000; Tanaka et al., 2000). Since Porcn likely processes all Wnt proteins, blocking its function, either by mutation (Barrott et al., 2011; Biechele et al., 2011; Liu et al., 2012) or treatment with Porcn inhibitors (Chen et al., 2009; Liu et al., 2013; Proffitt et al., 2013), provides a powerful tool to interfere with all Wnt signaling activity and create an all-Wnt deficient state. Following processing by Porcn, Wnts transit through the secretory pathway and are eventually secreted from the cell, a process that requires an additional protein encoded by the wntless (Wls) gene (Banziger et al., 2006; Bartscherer et al., 2006; Herr and Basler, 2012).
The lipid renders the Wnt protein highly hydrophobic and, hence, insoluble in what is largely an aqueous environment. This feature serves to limit Wnt diffusion in the extracellular space, and for the majority of cases, Wnt proteins remain closely associated with the Wnt expressing cells. Of note, engineering a Wnt protein such that it remains tethered to the cell surface via a transmembrane domain does not significantly limit its signaling activity: flies expressing such an allele of wg are patterned normally and exhibit no major developmental defects other than a slight reduction in body size (Alexandre et al., 2014). These observations indicate that initiation of a Wnt signal through receptor engagement involves close cell-cell proximity, as in the case of Notch signaling, rather than the action of a diffusible growth factor that can act at a long distance from its original site of secretion. Nonetheless, Wnt proteins, in particular Wg, have been observed at significant distances from Wnt-secreting cells, which may be mediated by transport through cells via a transcytosis-like process (Dierick and Bejsovec, 1998; Yamazaki et al., 2016), by long membrane protrusions, such as cytonemes or filipodia (Hsiung et al., 2005; Stanganello et al., 2015), or by exosomes (Korkut et al., 2009; Gross et al., 2012).
Wnt receptors
The main class of Wnt receptors encoded by the Frizzled (Fzd) gene family is comprised of ten independent genes (Fzd1-10) in mammals. Wnt engages Fzd by grasping with its thumb and finger extensions, with the lipid cradled in a hydrophobic groove of the extracellular cysteine rich domain (CRD) of Fzd. Binding specificities between the 19 Wnts and 10 Fzds are poorly characterized, but it is generally accepted that interactions exhibit a certain degree of promiscuity. Specificity in binding and subsequent signaling is conferred through the interaction with co-receptors, in particular Lrp5/6. Wnt signaling is partially regulated by the availability of Fzd proteins on the cell surface: in particular, membrane spanning RING-type E3 ubiquitin ligases, such as Rnf43 and Znrf3, ubiquitinate Fzd thereby leading to reduced Fzd cell surface expression. The activity of these ubiquitin ligases is in turn regulated by association with a complex comprised of the transmembrane Lgr4/5 receptors and the R-spondin (Rspo1-4) ligands: formation of a Znrf3, Lgr, Rspo complex promotes Znrf3 turnover, thereby stabilizing Fzd levels and enhancing WNT signaling output (Hao et al., 2012; Chen et al., 2013; Xie et al., 2013; Zebisch et al., 2013). Therefore, even though Rspo by itself has no inherent Wnt signaling activity, it potently augments basal levels of Wnt signaling through increased Fzd receptor availability.
In addition to the Fzd/Lrp receptor complexes, several other Wnt receptors have been identified, including the receptor tyrosine kinase like orphan receptors 1 and 2 (Ror1 and 2) and the receptor-like tyrosine kinase Ryk (Green et al., 2014). Despite their homologies, tyrosine kinase activity has only been demonstrated for Ror2 (Mikels et al., 2009), with Ror1 and Ryk most likely functioning as pseudokinases. Ror1 and 2 carry an extracellular CRD such that Wnt likely engages these receptors in a manner similar to its binding to Fzd. In contrast, Ryk carries an extracellular Wnt inhibitory factor (WIF) domain that directly binds Wnt. Signaling mechanisms of these receptors are poorly understood, but is thought to involve formation of various types of receptor complexes, including oligomerization of Ror1 and Ror2 (Yu et al., 2016). In certain contexts Ryk signaling involves proteolytic cleavage to release a C-terminal fragment that translocates to the nucleus where it affects gene expression (Lyu et al., 2008), in a process reminiscent of signaling by the Notch intracellular domain. Similar proteolytic processing upon ligand binding may also occur with other Wnt receptors, including Fzd (Mathew et al., 2005; Mosca and Schwarz, 2010) and ROR1 (Tseng et al., 2010; Tseng et al., 2011). Ryk plays critical functions in Wnt-mediated axon guidance, as first demonstrated for the Derailed receptor, the Drosophila homolog of Ryk (Yoshikawa et al., 2003). Studies in vertebrate systems have cemented Ryk’s critical role as a regulator of neurite outgrowth, acting largely as repulsive cue that inhibits axon growth (Liu et al., 2005; Schmitt et al., 2006; Liu et al., 2008; Hollis et al., 2016) and dendritic arborization (Lanoue et al., 2017). In addition, Ryk has also been implicated in mediating neurite outgrowth (Lu et al., 2004).
Wnt signaling pathways
Wnt signaling is generally divided into two categories—commonly referred to as canonical and non-canonical Wnt signaling—that are portrayed as independent signaling pathways. Although it is clear that Wnt regulates multiple downstream effects, the distinction between canonical and non-canonical Wnt signaling is an artificial one reflective of experimental settings in which these pathways have been interrogated. A recent review by Nusse and colleagues (Loh et al., 2016) presents a more unified view and posits that integration of these pathways lies at the heart of Wnt’s role as a symmetry-breaking signal common to all metazoan life forms: by simultaneously regulating cell fate (= canonical Wnt signaling) and cell polarity (= non-canonical Wnt signaling), Wnts control not only the diversity of cell types but also their organization within the developing organism.
The Wnt cell fate signaling pathway (Figure 2) has garnered the most attention and interest. In this pathway, Wnt engages a Fzd-Lrp5/6 receptor complex leading to the inactivation of cytoplasmic protein complex, which, in the absence of Wnt signal input, acts to target β-catenin for ubiquitination and proteasomal degradation. Consequently, Wnt signaling stabilizes cytosolic β-catenin, which in turn enters the nucleus and displaces transcriptional corepressors of the Groucho family (TLE) to interact with the Tcf/Lef family of transcription factors (including Tcf7/Tcf1, Tcf7l1/Tcf3, Tcf7l2/Tcf4 and Lef1) and activate transcription of target genes. Some of the main components of the β-catenin destruction complex include the protein products of Adenomatous Polyposis Coli (APC), Axin, and Glycogen Synthase Kinase 3 (Gsk3). Another key component of this Wnt signaling pathway is Dishevelled (Dvl1-3), which associates with the intracellular C-terminus of Fzd and serves to recruit components of the destruction complex to the membrane, thereby releasing β-catenin from the destruction complex. Gsk3, together with the priming kinase Ck1, phosphorylates a series of conserved serine and threonine residues in the amino terminus of β-catenin to generate a binding site for the β-transducin repeat containing E3 ubiquitin protein ligase (Btrc). Mutation of these phosphorylation sites produces a constitutively active (CA) form of β-catenin; such mutations have been identified in various types of cancer, and CA-β-catenin has served as a powerful tool to study Wnt-independent activation of the pathway.
Figure 2. Schematic of Wnt signaling pathway.
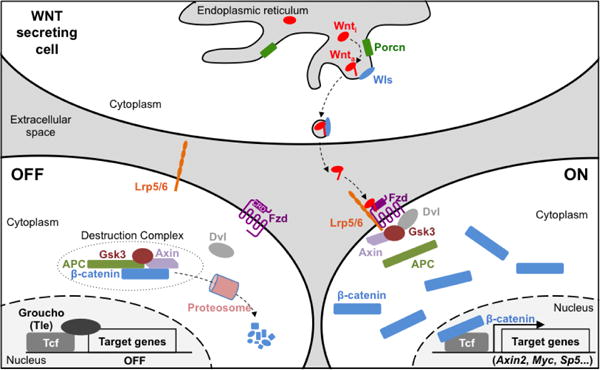
Upon translation and entry into the secretory pathway, Wnt proteins are acylated by Porcn and ushered out of the cell via Wls. In the off-state, a destruction complex composed of multiple proteins targets β-catenin for degradation by the proteasome. In the on-state, Wnt engages a Fzd/Lrp receptor complex leading to the disassembly of the destruction complex and re-localization of some of its components to the cell membrane. As a result, β-catenin is no longer degraded, accumulates in the cytoplasm and enters the nucleus where it associates with the Tcf transcription factors to activate expression of target genes.
Wnt/β-catenin target gene signatures are cell-type specific, however, a common class of target genes encode Wnt antagonists that act at multiple levels of the pathway, including directly on the Wnt protein (e.g. Sfrp and Notum), the receptors (Dkk, Znrf3, Rnf43), and the β-catenin destruction complex (Axin2, Nkd). In pluripotent stem cells (PSCs), activation of Wnt signaling induces the transcription of genes associated with endodermal and mesodermal lineages (ten Berge et al., 2008; Davidson et al., 2012), consistent with Wnt’s role in primitive streak formation and vertebrate axis formation (Liu et al., 1999; Huelsken et al., 2000). In contrast, ectopic Wnt pathway activation during the derivation of neural progenitor cells from human PSCs induces the expression of genes associated with posterior fates (Moya et al., 2014), again consistent with Wnt’s role in establishing the anterior-posterior identity.
Wnt signaling is under exquisitely tight control in the extracellular environment (Figure 3). Secreted Wnt antagonists can either bind Wnt to preclude receptor binding (including Secreted Frizzled Related Proteins [Sfrp1-5] and Wnt inhibitory factor-1 [WIF-1] (Hsieh et al., 1999)) or bind receptors to preclude Wnt-receptor interactions, as is the case for Dickkopf (Dkk1-4) and Wise (SOSTDC1), which bind Lrp5/6. Additionally, Wnt proteins can be modified by enzymatic mechanisms, such as by Notum, which catalyzes the deacylation of WNT proteins, thereby rendering Wnt unable to bind the CRDs of Fzd. Another protein, Tiki encoded by the TRABD2A and B genes, inactivates Wnt by cleaving the amino-terminal region (Zhang et al., 2012; Zhang et al., 2016). The activity and distribution of Wnt proteins is additionally influenced by the composition of the extracellular environment, and several proteoglycans, such as Syndecan and Glypican, as well as a host of diverse extracellular matrix proteins, physically interact with Wnt and regulate their signaling range (reviewed in (Perrimon and Bernfield, 2000; Zhu and Scott, 2004).
Figure 3. Extracellular regulation of Wnt signaling.
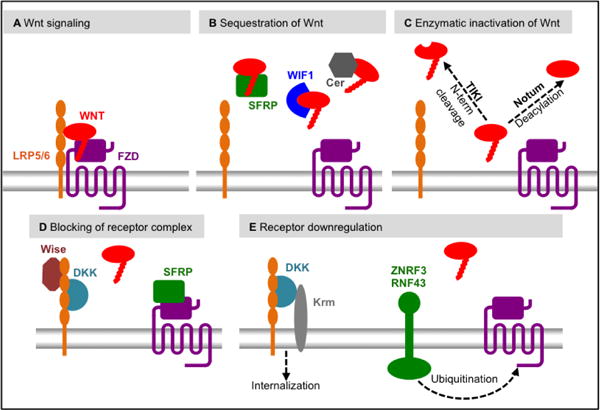
A. Wnt signaling is activated when a Wnt protein engages the receptor complex comprised of FZD and LRP. B. Wnt proteins can be sequestered by binding proteins, such as Cerebus (Cer), SFRP and WIF1, thereby preventing their interaction with the receptor complex. C. Wnt proteins can be inactivated irreversibly, either by deacylation by Notum or by amino-terminal cleavage by TIKI. D. Several secreted molecules interact directly with either FZD (e.g. SFRP) or LRP (DKK and Wise), thereby preventing Wnt-mediated receptor complex formation. E. Wnt receptors are subject to downregualtion, thus reducing Wnt signaling. FZD can be ubiquitinated by the E3 ligases ZNRF3 or RNF43 thereby leading to their degradation. LRP is internalized when in complex with the DKK co-receptor Kremen (Krm).
The Wnt/β-catenin pathway is frequently depicted as a two-state model with an OFF (no Wnt) and ON (Wnt binding its receptor), as shown in Figure 2. However, such a binary system is insufficient in explaining the complex and extensive downstream effects of Wnt signaling. Rather, multiple states of Wnt signaling are essential in generating cellular diversity and tissue patterning. Two mechanisms influencing such a multi-state system are likely at work. First, Wnts achieve distinct outcomes depending on their concentration. A concentration gradient of Wnt activity may be achieved by varying the amounts of the Wnt agonist, the availability of receptor(s), or the amounts of Wnt antagonists, such as Sfrp, Dkk and Notum. Wnt gradients have been visualized in several settings, such as in the Drosophila wing (Zecca et al., 1996), Xenopus anteroposterior neural patterning (Kiecker and Niehrs, 2001), mouse intestinal crypts (Farin et al., 2016), in caudalization of neural tissue in the chick embryo (Nordstrom et al., 2002), and the ventricular zone of the chick tectum (Schmitt et al., 2006). A second mechanism that imparts multi-state complexity on Wnt is through the integration with other developmental signaling systems, including TGFβ, Shh, FGF, RA and Notch. For example, the process of specifying the anteroposterior axis during gastrulation, as first described by Nieuwkoop and colleagues (Nieuwkoop, 1952), requires the cooperative actions of Wnt, BMP and FGF signaling (reviewed in (Hikasa and Sokol, 2013). Later sections will highlight examples of such cooperativity during neural development.
In contrast to the cell fate pathway, very few if any robust transcriptional target genes have been identified downstream of the Wnt cell polarity signaling pathway. Effects of this pathway are generally difficult to model in cell culture systems and, rather, are most clearly observed during organismal or tissue development and involve complex cell movements, as occur, for instance, during gastrulation, gut tube elongation, neural tube closure and neural crest migration. Certain signaling components, including Wnt, Fzd and Dvl are shared between cell fate and cell polarity pathways, however, some pathways are quite distinct, utilizing distinct receptors (e.g. Ror, Ryk and Ptk7) and signaling components, such as Vangl, Celsr and Prickle, that act in concert to organize cells within the plane of a tissue.
The literature is rich with descriptions of distinct Wnt pathways collectively referred to as “non-canonical” Wnt pathways. A variety of downstream signaling components mediate these non-canonical Wnt signals, including G proteins, small GTPases (e.g. Rac and Rho), kinases (e.g. Jnk, Tak1 and Nlk), second messengers (e.g. Calcium), transcription factors (e.g. NFκB), and more. Assays for these downstream effectors of non-canonical Wnt signaling are scarce and highly cell- and context-dependent. One robust downstream assay of a non-canonical pathway is the inhibition of the Wnt/β-catenin, as demonstrated for Wnt5a-Ror2 signaling (Ishitani et al., 2003; Mikels and Nusse, 2006; Mikels et al., 2009). Of note, although many studies refer to individual Wnts as either “canonical” or “non-canonical”, the downstream effects of any Wnt is dependent on the expression of receptors and signal transducers rather than by an intrinsic property of the Wnt. For example, the so-called non-canonical Wnt5a can act “canonically” (i.e. through β-catenin) in certain contexts (He et al., 1997; Mikels and Nusse, 2006). This review will largely focus on the role of the cell fate pathway, depicted in Figure 2, in neural development. However, it should be stressed that coordinated actions of Wnt in the cell fate and cell polarity pathways are critical in establishing the complexity of the neural system, both in terms of cellular diversity and tissue organization.
Activation or Repression? The Unresolved Role of Wnt Signaling in Neural Specification of Ectoderm
Wnt signaling is highly dynamic throughout all stages of human development with multiple Wnt ligands and antagonists expressed in a temporal and regionally specific manner during early embryonic neurodevelopment (Table 1). The first principal event of neurodevelopment that is influenced by Wnt signaling is the neural specification of embryonic ectodermal cells and the subsequent formation of the neural plate. Pioneering work conducted by Mangold and Spemann in the 1920s led to a proposed mechanism by which a subset of ectodermal cells acquire a neural identity through signaling supplied by a central “organizer” in the adjacent mesodermal tissue (now referred to as the Spemann-Mangold organizer; (Spemann and Mangold, 2001) published in 1923). Subsequently, it was discovered that FGF signaling and BMP antagonism supplied by the Spemann-Mangold organizer was responsible for neural induction of ectodermal tissue (Sasai et al., 1995; Fainsod et al., 1997; Harland, 2000; De Robertis, 2009). However, the role Wnt plays in the determination of neural versus non-neural ectodermal (i.e. epidermal) cell fates has been difficult to precisely ascertain, in part, due to species-specific differences (Stern, 2005).
Table 1.
Function of Wnt ligands in early neurodevelopment processes.
| Ligand | Neurodevelopment Process |
|---|---|
| Wnt1 | AP (Hollyday et al., 1995), FB (Molven et al., 1991; Hollyday et al., 1995; Lagutin et al., 2003), MHB (Wolda et al., 1993; Hollyday et al., 1995; Lagutin et al., 2003; Buckles et al., 2004), HB (Riley et al., 2004), SC (Parr et al., 1993) |
| Wnt2b | MHB (Kunz et al., 2004) |
| Wnt3 | AP, SC (Roelink and Nusse, 1991), FB (Braun et al., 2003) |
| Wnt3a | AP, FB (Hollyday et al., 1995), MHB (McMahon et al., 1992; Buckles et al., 2004), HB (Riley et al., 2004), SC (Parr et al., 1993) |
| Wnt4 | AP, FB, MHB (Hollyday et al., 1995), HB (McGrew et al., 1992) |
| Wnt5a | FB (Hollyday et al., 1995) |
| Wnt7b | FB (Hollyday et al., 1995) |
| Wnt8a | NE (Baker et al., 1999), HB (Hume and Dodd, 1993) |
| Wnt8b | FB (Hollyday et al., 1995; Houart et al., 2002), MHB (Kunz et al., 2004), HB (Riley et al., 2004) |
| Wnt10a | FB, HB (Kelly et al., 1993) |
| Wnt10b | MHB (Buckles et al., 2004), HB (Riley et al., 2004) |
Abbreviations: NE : Neural Specification of Ectoderm; AP : Anterior-Posterior Patterning of the Neural Tube; FB : Patterning of the Forebrain; MHB : Induction of the Midbrain-Hindbrain Boundary; HB : Specification and Segmentation of the Hindbrain; SC : Induction and Patterning of the Spinal Cord.
Early work in Xenopus embryos demonstrated that forced expression of Wnt8 or β-catenin inhibited the expression of BMP4, thereby allowing the ectoderm to respond to neural inducing signals, such as FGF (Baker et al., 1999) (Figure 4A). Additional studies in Xenopus revealed that active Wnt signaling promoted neural induction by stimulating the expression of secreted BMP antagonists (Baker et al., 1999) (Figure 4A). However, other studies implementing Xenopus as a model system have produced conflicting results suggesting that Wnt signaling interferes with neural induction (Heeg-Truesdell and LaBonne, 2006; Min et al., 2011). Likewise, work with chick embryos suggests that Wnt signaling promotes epidermal fates rather than neural ectodermal cell identities through the attenuation of FGF signaling and activation of BMP signaling (Wilson et al., 2001) (Figure 4B). It is implied that only in the absence of Wnt signaling does FGF upregulation occur, thereby leading to the generation of neural cell fates (Wilson et al., 2001). Work with pluripotent stem cell (PSC)-based models may provide an opportunity to resolve this controversy in a human system (see Emerging Trends: Human Pluripotent Stem Cell Models to Study Neurodevelopment).
Figure 4. Two proposed models for the role of Wnt signaling in determination of neural versus non-neural ectodermal fates.
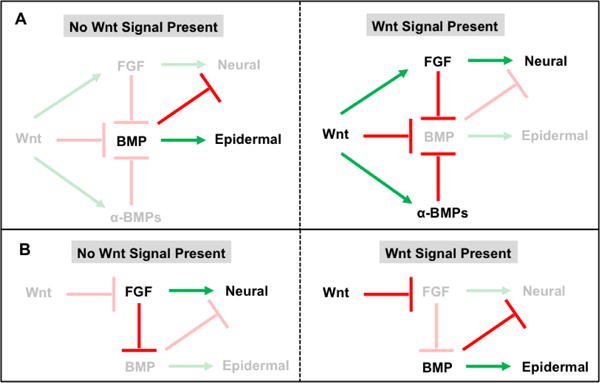
A. In the first model, in the absence of Wnt signaling BMP expression promotes epidermal fate while repressing neural specification through the inhibition of FGF signaling (upper left panel). Active Wnt signaling promotes ectodermal induction through activation of FGF signaling as well as direct and indirect (through the induction of BMP antagonists [α-BMPs]) repression of BMP signaling (upper right panel). B. In an alternative model, absence of Wnt signaling allows for active FGF signaling to induce neural cell fates (lower left panel). Active Wnt signaling attenuates FGF signaling thereby providing for BMP signals to promote epidermal cell identities (lower right panel).
Wnt Signaling Is Required for Posteriorization of the Neural Tube
Building on the initial work of Mangold and Spemann, Nieuwkoop proposed a two-step model of neural induction and patterning in which neural tissue of anterior identity is first induced by the Spemann-Mangold organizer and then subsequently patterned along the anterior-posterior (A/P) axis. More specifically, the role of Wnt signaling is multifaceted as a precise balance of both signaling inhibition and activation is required for the development of an endogenous Wnt gradient that specifies regional neural cell identities along the A/P axis (McGrew et al., 1995; McGrew et al., 1997).
Although naïve ectodermal cells acquire an initial anterior neural identity through BMP antagonism and the absence of active Wnt signaling, the maintenance of anterior identity during A/P patterning requires the active inhibition of Wnt activity. The initial evidence of this principle was displayed in zebrafish headless (hdl) mutants, which lack Tcf7l1/Tcf3-based transcriptional repression of Wnt signaling (Kim et al., 2000). As such, the hdl mutants lack several anterior structures thereby demonstrating that repression of Wnt signaling pathway activity is required for vertebrate head formation and patterning (Kim et al., 2000). Several Wnt antagonists, such as Dickkopf-1 (Dkk1; (Glinka et al., 1998; Hashimoto et al., 2000; Kazanskaya et al., 2000; Mukhopadhyay et al., 2001; Kimura-Yoshida et al., 2005) and Secreted Frizzled Related Proteins-1, -3, -5 (Sfrps; (Leyns et al., 1997; Kemp et al., 2005; Mii and Taira, 2009), secreted by the surrounding anterior visceral endoderm and anterior mesendoderm, play critical roles in anterior neural development. Several studies have shown that abolishing expression of these Wnt antagonists during A/P patterning results in the absence or malformation of anterior structures (Glinka et al., 1998; Kimura-Yoshida et al., 2005). Additional negative modulators of Wnt activity, including Cerberus (Glinka et al., 1997), Tiki (Zhang et al., 2012; Reis et al., 2014; Zhang et al., 2016) and Notum (Zhang et al., 2015), have also been implicated in anterior neural tissue formation.
Numerous studies have revealed that the direct action of Wnt proteins on anterior neural cells is required for their posteriorization into structures that will eventually become the prospective midbrain, hindbrain and spinal cord (Nordstrom et al., 2002). For example, early ex vivo studies that involved the overexpression of different Wnt ligands, β-catenin, or Tcfs in Xenopus animal cap cells demonstrated the posteriorizing effects of Wnt signaling at the expense of the expression of anterior neural markers (McGrew et al., 1995; Domingos et al., 2001). Complementing these gain-of-function studies, disruption or loss of Wnt1 (McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Augustine et al., 1993), Wnt3 (Liu et al., 1999), Wnt3a (Augustine et al., 1993; McGrew et al., 1997; Shimizu et al., 2005), and Wnt8 (Erter et al., 2001; Lekven et al., 2001) in Xenopus, zebrafish, or mouse embryos led to the expansion of the forebrain compartment and the improper development of midbrain, hindbrain and spinal cord structures. More recent studies suggest that the source of this graded posteriorizing Wnt signal is the paraxial dorsolateral mesoderm, which underlies the developing neural tube (Elkouby et al., 2010).
Multiple Roles of Wnt Signaling in Patterning of the Forebrain
After the initial patterning of the neural tube, the forebrain is further partitioned along the anterior-posterior (A/P) and dorsal-ventral (D/V) axis. More precisely, the anterior forebrain is patterned into the dorsal telencephalon (which will develop the cerebral cortex) and the ventral telencephalon (which will develop into the basal ganglia) while patterned posteriorly into the diencephalon (which will generate the thalamic tissues) (Wilson and Houart, 2004).
As it relates to A/P patterning of the developing forebrain, a general model has emerged in which local Wnt signaling is necessary for the diencephalic development while Wnt antagonism is necessary to promote emergence of telencephalic cell types (Wilson and Houart, 2004). Early evidence for this model was identified in zebrafish with masterblind (mbl) mutations in Axin1. The absence of Axin1-dependent repression of Wnt signaling in mbl mutants leads to the anterior expansion of diencephalic cell types and the elimination of telencephalic cell identities (Masai et al., 1997; Heisenberg et al., 2001). Along similar lines, ectopic expression of Wnt signaling during forebrain patterning led to the generation of embryos phenotypically similar to mbl mutants (van de Water et al., 2001). Corroborating work with chick (Braun et al., 2003) and mouse (Lagutin et al., 2003) models further demonstrated that proper regionalization of the forebrain requires the repression in Wnt signaling in the anterior compartments and Wnt activation in the posterior areas. Additional studies have implicated Wnt8b as the inducer of posterior diencephalic fates in the emerging forebrain whereas Tlc, a zebrafish Sfrp homolog secreted by the anterior neural ridge, antagonizes this Wnt signal in the anterior forebrain regions allowing for the development of telencephalic cell identities (Houart et al., 2002; Tian et al., 2002; Echevarria et al., 2003). Finally, several studies have provided insights into the transcriptional targets likely mediating these Wnt-inducing effects of the regionalization of the forebrain (Kobayashi et al., 2002; Braun et al., 2003; Lagutin et al., 2003; Gestri et al., 2005). Briefly, these studies have established that Irx3 expression is induced by Wnt signaling and acts directly to repress Six3 expression in the diencephalon, and conversely, Irx3 represses Six3 expression in the telencephalon (Figure 5A).
Figure 5. Wnt signaling patterns the forebrain along the anterior-posterior (A/P) and dorsal-ventral (D/V) axis.
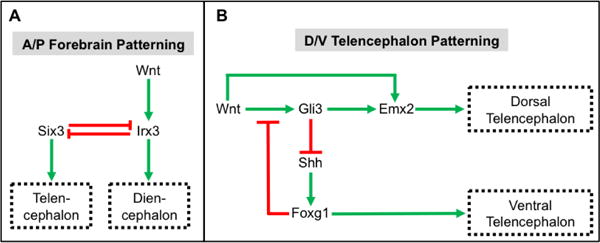
A. Wnt signals induce the expression of Irx3, which leads to the adoption of posterior diencephalonic cell identities. Six3 expression results in the generation of anterior telencephalic cell types. Cross-repressive activities of Six3 and Irx3 restrict expression of these transcription factors to the telencephalon and diencephalon, respectively. B. Wnt signaling establishes dorsal telencephalic (i.e. pallidal) cell fates through the direct and indirect (via Gli3) induction of Emx2 expression and the Gli3-mediated repression of Shh signaling. Ventral telencephalic (i.e. subpallidal) cell identities are established through the Shh stimulated expression of Foxg1. In addition, Foxg1 represses dorsal cell fates in the ventral telencephalon through direct inhibition of Wnt signaling.
Within the developing telencephalon, Wnt signaling plays a prominent role in specifying dorsal (i.e. pallidal) versus ventral (i.e. subpallidal) cell fates (Campbell, 2003). Initial experiments with chick embryos implicated Wnt1, Wnt4 and Wnt8b as likely inducers of the Wnt signaling response in the dorsal telencephalon (Hollyday et al., 1995). Additionally, ex vivo experiments with chick neural tissue explants demonstrated that soluble Wnt3a induced dorsal identities in ventral cells (Gunhaga et al., 2003). Conversely, dorsal telencephalic cells failed to emerge in explant cultures in which Wnt signaling was inhibited (Gunhaga et al., 2003). Similarly, targeted deletion of β-catenin in mouse embryos negatively impacted dorsal telencephalic development (Gulacsi and Anderson, 2008). Finally, numerous studies have revealed a complex gene regulatory network by which Wnt target genes such as Emx2 and Gli3 exert their dorsalizing effects on the immature telencephalon through the repression of Shh signaling (Theil et al., 2002; Alvarez-Medina et al., 2008) (Figure 5B). Conversely, in the ventral telencephalon Shh target genes, such as Foxg1, serve as transcriptional repressors of Wnt signaling (Danesin et al., 2009) (Figure 5B).
Wnt and Fgf Signaling Act Cooperatively to Induce the Midbrain-Hindbrain Boundary
The midbrain-hindbrain boundary (MHB) is a key organizing center that is critical for the proper formation of midbrain and anterior hindbrain (Rhinn and Brand, 2001; Harada et al., 2016). Examination of developing Xenopus (Schohl and Fagotto, 2002) and mouse (Maretto et al., 2003) embryos revealed a strong expression of nuclear β-catenin in the MHB, suggesting a role for Wnt signaling in MHB generation and maintenance. Indeed, Wnt1 mutants display prominent abnormalities in the midbrain and the metencephalic regions of the hindbrain (McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Thomas et al., 1991; McMahon et al., 1992). Subsequent studies revealed that Wnt1 expression stimulates cell proliferation and survival, possibly explaining the observed phenotype in Wnt1 mutants (Serbedzija et al., 1996; Megason and McMahon, 2002). In addition, Fz3/Fz6 double mutants exhibit significant defects in midbrain morphogenesis, suggesting that Wnt signals act through these receptors for proper MHB development (Stuebner et al., 2010).
Extensive studies have investigated the transcriptional mechanisms by which Wnt signaling regulates MHB induction (Figure 6) (Wurst and Bally-Cuif, 2001; Raible and Brand, 2004). Early studies with Wnt1 knockout mouse embryos revealed that loss of the midbrain and adjacent metencephalic regions was preceded by a complete loss of En1 and En2 expression (McMahon et al., 1992). Subsequent studies revealed that expression of En1 in the developing midbrain of Wnt1 mutant embryos is sufficient to rescue midbrain and anterior hindbrain developmental deficits (Danielian and McMahon, 1996). Likewise, complementary studies in Xenopus demonstrated that En2 is a direct target of the Wnt signaling pathway (McGrew et al., 1999). Collectively, these studies demonstrate that En1 and En2 act downstream of Wnt1 signaling activity to regulate MHB emergence and identity. Additional evidence suggests a more complex regulatory network in which Wnt1 signals through En1 to regulate FGF8 expression in the MHB (Wurst and Bally-Cuif, 2001; Ye et al., 2001; Raible and Brand, 2004). In turn, FGF8 acts through Otx2 and Gbx2 to establish and maintain MHB cell identity (Joyner et al., 2000).
Figure 6. Wnt1 and Fgf8 signaling establishes a gene regulatory network to control the induction and maintenance of the midbrain-hindbrain boundary (MHB).
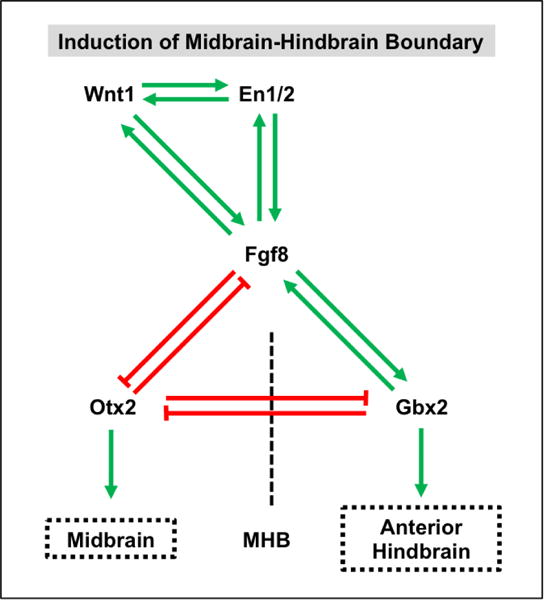
A positive regulatory loop established by the reciprocal expression of Wnt1, En1/2, and Fgf8 is responsible for establishing and maintaining the MHB. Concomitantly, mutual activation of Fgf8 and Gbx2 establishes anterior hindbrain (i.e. metencephalic) cell identities posterior to the MHB whereas mutual repression of Fgf8 and Otx2 induces midbrain cell fates anterior to the MHB. Reciprocal Otx2 and Gbx2 repression is also critical in establishing these cell types at the MHB.
Wnt Signaling Directs the Specification and Segmentation of the Hindbrain
As discussed previously, initial hindbrain development proceeds in two stages—the rhombencephalon first emerges from the developing neural tube and then next divides into eight defined rhombomeres (Guthrie and Lumsden, 1991; Glover, 2001). The Wnt signaling pathway is the primary inducer of the gene network that regulates this initial induction and subsequent segmentation (Figure 7).
Figure 7. Wnt signaling is refined by FGF and retinoic acid (RA) signaling to induce and pattern the hindbrain (HB) and spinal cord (SC).
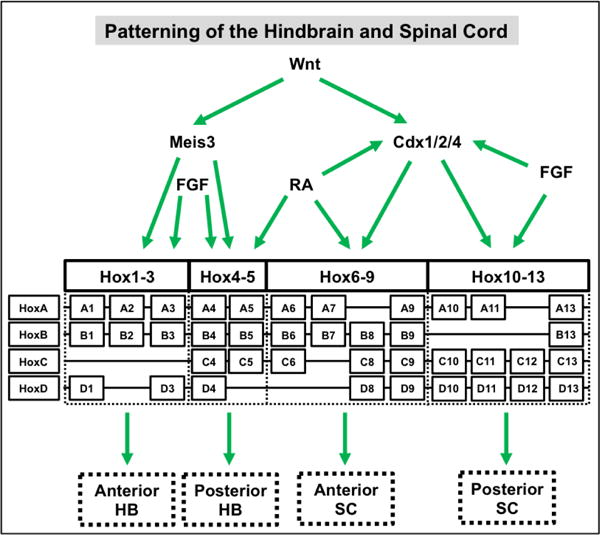
In the hindbrain, Wnt can directly (not shown) or indirectly (via Meis3) induce the expression of the Hox1–Hox5 paralog group genes. In addition, FGF signaling alone or in concert with RA signaling can further refine the expression pattern of Hox genes in the anterior and posterior hindbrain, respectively. In the spinal cord, Wnt-induced Cdx 1/2/4 expression leads to the induction of the Hox6-Hox13 paralog group genes. Moreover, RA signaling alone or in combination with FGF signaling can further refine the expression, directly or indirectly (via Cdx1), of Hox genes in the anterior and posterior spinal cord, respectively.
Wnt3a secreted from the neighboring paraxial dorsolateral mesoderm is the initial signaling event that governs the emergence of the rhombencephalon from the developing neural tube (Elkouby et al., 2010). This initial endogenous signal stimulates the downstream expression of Meis3, which is a direct target of Wnt signaling. Multiple studies have shown that misexpression of Meis3 results in loss of hindbrain, but not spinal cord, structures as well as expansion of anterior cell types (Dibner et al., 2001; Elkouby et al., 2010; Gutkovich et al., 2010). Corresponding studies have also shown that Meis3 expression is sufficient for hindbrain induction in the absence of exogenous Wnt signals (Elkouby et al., 2010). Although other hindbrain makers, such as Gbx2 (Li et al., 2009a) and Hox1–Hox5 paralog group genes (In der Rieden et al., 2010), have been shown to be direct targets of the Wnt pathway, their activation is dependent on the expression of Meis3 (Vlachakis et al., 2001; Dibner et al., 2004; Elkouby et al., 2010; Elkouby et al., 2012). Together, this suggests a mechanism by which Wnt signaling directly induces Meis3 expression to activate a downstream gene regulatory network to control hindbrain development. Finally, it should be noted this Wnt signal is further refined by FGF and retinoic acid (RA) signaling to further subdivide the prospective rhombencephalon (Partanen, 2007; Esain et al., 2010; Ishioka et al., 2011; Mazzoni et al., 2013).
Wnt signaling also plays a prominent role in rhombomere boundary formation and maintenance. Specifically, using zebrafish as a model system, Riley and colleagues found that several Wnt genes (e.g. Wnt1, Wnt3a, Wnt8b, Wnt10b) were elevated at rhombomere boundaries (Riley et al., 2004). Moreover, knockdown of Tcf-mediated Wnt gene transcription, resulted in complete elimination of boundary cell types. Additional work revealed that Wnt signaling, mainly via the action of Wnt1, maintains boundary cell identity by promoting neuronal differentiation in non-boundary regions of the rhombomere (Amoyel et al., 2005).
Wnt Acts in Concert with Multiple Signaling Molecules to Induce and Pattern the Spinal Cord
In a manner similar to that in the hindbrain, Wnt acts through a complex gene regulatory network to regulate the initial formation of the spinal cord (Melton et al., 2004; Elkouby and Frank, 2010) (Figure 7). Several studies have implicated Cdx1, 2, and 4 as the chief transcriptional mediators of the Wnt inducing response in the spinal cord (Lohnes, 2003). These Cdx genes have several Tcf binding sites in their regulatory regions and are direct targets of Wnt signaling (Prinos et al., 2001; Lickert and Kemler, 2002; Haremaki et al., 2003; Pilon et al., 2007; Wang and Shashikant, 2007). Moreover, gain- (Prinos et al., 2001; Shimizu et al., 2005; Keenan et al., 2006) and loss-of-function (Prinos et al., 2001; Shimizu et al., 2005) studies confirm the role of these Cdx genes in mediating downstream expression of spinal cord-specific Hox5-13 paralog group genes. It should be noted, though, that there appears to be some functional redundancy between these Cdx genes as knockdown of individual Cdx genes leads to a range of effects on Hox expression patterns whereas only the compound loss-of-function of Cdx 1, 2 and 4 leads to complete posterior truncation (van den Akker et al., 2002; Shimizu et al., 2005; Faas and Isaacs, 2009; Savory et al., 2009). Finally, both RA and FGF act in concert with Wnt signaling to further refine the Hox expression pattern in the developing spinal cord with RA inducing anterior cell fates and FGF promoting posterior identities (Bel-Vialar et al., 2002; Shimizu et al., 2006; Mazzoni et al., 2013; Philippidou and Dasen, 2013).
As the spinal cord continues to mature, Wnt signals from the roof plate oppose Shh signals from the notochord and ventral plate to pattern cells along the D/V axis (Jessell, 2000; Wilson and Maden, 2005; Ulloa and Marti, 2010). Early studies revealed that both Wnt1 and Wnt3a are highly expressed in the dorsal spinal cord (Parr et al., 1993). Studies in which Wnt1 and Wnt3a were knocked out confirmed the importance of these signaling molecules in dorsal spinal cord specification as Wnt1/Wnt3a double knockout mice showed a marked reduction in neural crest derivatives, dorsolateral neural precursors in the neural tube, and spinal neurons and a concomitant increase in ventral spinal neurons (Ikeya et al., 1997; Muroyama et al., 2002). Mechanistically, Wnt signaling induces the expression of Gli3 to directly repress Shh signaling (Alvarez-Medina et al., 2008; Yu et al., 2008; Ulloa and Marti, 2010) (Figure 8). In turn, this leads to a gradient of Shh ventral-inducing signaling activity that patterns the spinal cord into various D/V domains.
Figure 8. Wnt and SHH signaling act in a opposing manner during dorsal/ventral (D/V) patterning of the spinal cord.
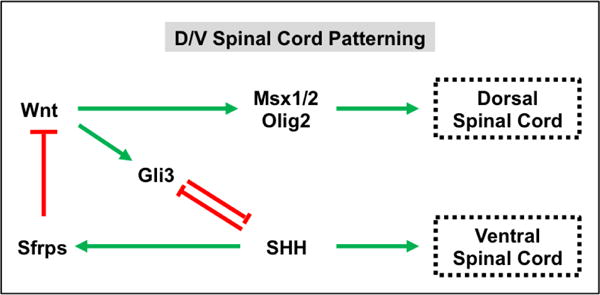
Wnt signaling promotes dorsal fate specification in the spinal cord through the activation of target genes such as Msx1/2 and Olig3. Wnt signaling also represses SHH signaling in the dorsal spinal cord by inducing Gli3 expression. Shh signaling promotes ventral spinal cord cell identities and represses Wnt signaling through induction of Wnt antagonists, such as Sfrps.
Wnt and neuro-developmental defects
With its nearly ubiquitous functions in development, it is not surprising that perturbations in Wnt signaling lead to a variety of neurodevelopmental defects in mammals, many of which have been discussed in the previous sections. In addition to the engineered Wnt knockouts described above, classic mouse mutations have also provided evidence for the importance of Wnt signaling in neural development. For example, the recessive mutation swaying (sw), which is characterized by ataxia, carries a frameshift mutation in the Wnt1 gene (Thomas et al., 1991), consistent with the phenotyope observed in the Wnt1 knockout model (McMahon and Bradley, 1990; Thomas and Capecchi, 1990). Furthermore, the recessive mutation vestigial tail (vt) is a hypomorphic allele of Wnt3a (Greco et al., 1996), which is required for hippocampal development (Lee et al., 2000), and—together with Wnt1 deficiency—for proper neural tube development (Ikeya et al., 1997). As the name implies, vt mice present with shortened tails due to loss of caudal vertebrae. In contrast, the engineered Wnt3a null mutation is embryonic lethal with a severe posterior truncation. As expected, allelic combinations of vt and the null mutation produce intermediate phenotypes.
Mutations in WNT genes in humans are extremely rare and the few that have been reported generally did not present with overt neurodevelopmental defects. For example, mutations in WNT1 are associated with a syndrome of severe bone fragility, called osteogenesis imperfecta (OI) (Fahiminiya et al., 2013; Keupp et al., 2013; Laine et al., 2013; Pyott et al., 2013), without overt neurological problems as one may expect given the mouse Wnt1 knockout phenotype. However, here it is important to note that the neurological defects in mice are quite variable, ranging from ataxia to neonatal death, indicating that loss of function phenotypes are variable in different genetic backgrounds. Furthermore, mice carrying the spontaneous Wnt1 mutation sw, aside from exhibiting the aforementioned neurodevelopmental defects, serve as useful model of OI (Joeng et al., 2014). Mutation of human WNT3 is embryonic lethal with tetra-amelia, which is characterized by complete loss of all four limbs (Niemann et al., 2004). This severe phenotype has precluded identification of associated neurological defects, as observed in the mouse Wnt3 knockout. Mutations in WNT5A produce Robinow syndrome (Person et al., 2010), which is characterized by multiple morphological defects of stature, skeleton, head, face and external genitalia. Neurodevelopmental defects, as observed in mouse Wnt5a-Wnt1 double mutants (Andersson et al., 2013), are not common in individuals with Robinow syndrome and intelligence is generally normal.
Mutations in Porcn and Wls, which encode critical Wnt processing enzymes, are predicted to block secretion of all Wnts and produce “all Wnt mutant” phenotypes. Consistent with this model, global mutations in these genes are embryonic lethal and fail to complete gastrulation, thus precluding analysis of later events, such as neural development. Use of the Cre-Lox recombination system to create conditional alleles of Porcn and Wls has confirmed the importance of Wnt signaling in various aspects of early neural patterning. For example, heterozygous Porcn deleted females are embryonic lethal with open neural tubes and decreased expression of the neuroectodermal markers Gbx2 and Sox2 (Liu et al., 2012). Deletion of Wls with a neural specific Cre driver (Wnt1-Cre) results in deletion in midbrain and hindbrain, a phenotype resembling Wnt1 mutation (Carpenter et al., 2010; Fu et al., 2011). Interestingly, PORCN mutations in humans produce a condition called focal dermal hypoplasia (FDH, also know as Goltz syndrome) (Grzeschik et al., 2007; Wang et al., 2007), which is characterized by skin lesions and defects of the gastrointestinal and cardiovascular system. The reason for the extreme pleiotropic phenotypes observed in FDH is due to the fact that PORCN is encoded on the X chromosome and hence is subject to random X-inactivation. Neurodevelopmental and cognitive defects are infrequently observed in FDH, which likely indicates that defects due to inactivation of PORCN in neural tissues are to severe to permit survival to birth.
Emerging Trends: Human Pluripotent Stem Cell Models to Study Neurodevelopment
Studies in model animal organisms, including frogs, chick and mouse, have provided important insights into the role of Wnt signaling in early neural development and patterning. However, the inherent complexities of these biological systems have made it difficult to dissect the underlying molecular mechanisms associated with complex neurodevelopmental processes. Human pluripotent stem cells (hPSCs) provide an emerging opportunity to study the mechanisms of human neurodevelopment in an easily controllable and accessible system. In addition, hPSC-based models enable for the resolution of species-specific differences that have been observed in some model systems. For example, studies with model organisms have provided conflicting evidence with regards to whether Wnt induces neural or non-neural ectodermal cell fates. To that end, Lee and colleagues showed that hPSC lines with amplified WNT3/WNT9B revealed that upregulation of Wnt signaling enhanced neural differentiation (Lee et al., 2015).
HPSC-based systems have also been used to model and elucidate the roles of Wnt signaling in early A/P patterning of the neural tube (Kriks et al., 2011; Kirkeby et al., 2012; Moya et al., 2014). For example, Moya et al. developed an in vitro hPSC-model model that mimics the same effects of the Wnt signaling gradient on the early A/P patterning of the neural tube observed during in vivo development (Moya et al., 2014). Moreover, this system allowed for genome-wide expression analysis of cell populations of various A/P regional identities. This analysis provided novel insight into the mechanisms by which Wnt signaling regulates A/P fate in that Wnt signaling appeared to be acting cell autonomously, with Wnt signaling activity restricted to those cells expressing Wnt genes. This suggests that during development WNT proteins may act in an autocrine, rather than paracrine, manner to specify and maintain A/P neural cell identities.
Experiments with hPSC-based models have also allowed for a better understanding of the mechanisms by which Wnt signaling regulates D/V pattering of the forebrain (Li et al., 2009b). Specifically, through the precise activation and inhibition of Wnt and Shh signaling, Li and colleagues were able to generate cells of various telencephalic D/V cell fates. Moreover, the authors identified a Gli3-dependent mechanism by which Wnt signaling represses Shh signaling to promote dorsal telencephalic fates.
More recently, studies with hPSCs have allowed for the unraveling of the temporal mechanisms by which Wnt patterns the hindbrain and spinal cord (Lippmann et al., 2015). Through the modulation of Wnt, RA, and FGF signaling, Lippmann et al. were able to generate early neural cells with HOX profiles corresponding to specific rhombomeric segments and cervical, thoracic, and lumbosacral identities. In addition, the authors discovered that Wnt, RA, and FGF act in a biphasic manner to induce discrete hindbrain and spinal cord regional identities.
In the future, the use of 3-D culture methods and organoid approaches may allow for the generation of hPSC-based models that more accurately mimic in vivo neural development (Lancaster et al., 2013; Meinhardt et al., 2014; Kelava and Lancaster, 2016). A recent study showed that modulation of endogenous Wnt signaling (with IWP2, a Porcn inhibitor) promoted the formation of spheroids that resembled ventral forebrain or the subpallium as monitored by strong induction of NKX2-1 and FOXG1 (Birey et al., 2017). Finally, the use of these systems with next-generation sequencing technologies, such as single-cell RNA sequencing, will allow for a better understanding of how individual cells make fate decisions in early neurodevelopmental processes (Yao et al., 2017).
CONCLUDING REMARKS
Despite the plethora of research to date, there is still much that needs to be unraveled about the role of Wnt signaling in the earliest stages of neural development. For instance, the mechanisms by which the Wnt cell polarity (= non-canonical) pathway acts in concert with the Wnt cell fate (= canonical) pathway in neural fate decisions has not been subject to extensive investigation. In addition, the complex manner in which Wnt interacts with other signaling pathways (e.g. BMP, Shh, FGF, RA, Notch) and components of the cellular microenvironment (e.g. mechanical forces, extracellular matrix proteins) to precisely regulate early neural developmental cell fate has yet to be ascertained. Finally, the downstream epigenetic and genetic gene regulatory networks by which Wnt imparts specific neural cell identities have not been fully identified. In the future, continued advancements with in vitro culture systems (e.g. hPSCs), genetic manipulation systems (e.g. CRISPR/Cas9), and molecular analysis techniques (e.g. next-generation sequencing), will allow for a more complete understanding of these multifaceted roles Wnt signaling plays in CNS development.
References
- Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Amoyel M, Cheng YC, Jiang YJ, Wilkinson DG. Wnt1 regulates neurogenesis and mediates lateral inhibition of boundary cell specification in the zebrafish hindbrain. Development. 2005;132:775–785. doi: 10.1242/dev.01616. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Salto C, Villaescusa JC, Cajanek L, Yang S, Bryjova L, Nagy II, Vainio SJ, Ramirez C, Bryja V, et al. Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells. Proc Natl Acad Sci U S A. 2013;110:E602–610. doi: 10.1073/pnas.1208524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniadou CL, Martinez-Barbera JP. Developmental mechanisms directing early anterior forebrain specification in vertebrates. Cell Mol Life Sci. 2013;70:3739–3752. doi: 10.1007/s00018-013-1269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine K, Liu ET, Sadler TW. Antisense attenuation of Wnt-1 and Wnt-3a expression in whole embryo culture reveals roles for these genes in craniofacial, spinal cord, and cardiac morphogenesis. Dev Genet. 1993;14:500–520. doi: 10.1002/dvg.1020140611. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Itasaki N, Krumlauf R. Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development. 2002;129:5103–5115. doi: 10.1242/dev.129.22.5103. [DOI] [PubMed] [Google Scholar]
- Biechele S, Cox BJ, Rossant J. Porcupine homolog is required for canonical Wnt signaling and gastrulation in mouse embryos. Dev Biol. 2011;355:275–285. doi: 10.1016/j.ydbio.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Bielen H, Houart C. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev Neurobiol. 2014;74:772–780. doi: 10.1002/dneu.22168. [DOI] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017 doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Brown AM, Papkoff J, Fung YK, Shackleford GM, Varmus HE. Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol. 1987;7:3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles GR, Thorpe CJ, Ramel MC, Lekven AC. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech Dev. 2004;121:437–447. doi: 10.1016/j.mod.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. doi: 10.1016/s0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Human embryology and developmental biology. Elsevier/Saunders; Philadelphia, PA: 2014. [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F. Integration of anterior neural plate patterning and morphogenesis by the Wnt signaling pathway. Dev Neurobiol. 2014;74:759–771. doi: 10.1002/dneu.22135. [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Chen X, Lin Z, Fang D, He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013;27:1345–1350. doi: 10.1101/gad.219915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CE, Nourse CC, Cooper HM. The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals. 2012;20:202–220. doi: 10.1159/000332153. [DOI] [PubMed] [Google Scholar]
- Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Danielian PS, McMahon AP. Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. doi: 10.1038/383332a0. [DOI] [PubMed] [Google Scholar]
- Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT. Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. 2009;126:925–941. doi: 10.1016/j.mod.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Elias S, Frank D. XMeis3 protein activity is required for proper hindbrain patterning in Xenopus laevis embryos. Development. 2001;128:3415–3426. doi: 10.1242/dev.128.18.3415. [DOI] [PubMed] [Google Scholar]
- Dibner C, Elias S, Ofir R, Souopgui J, Kolm PJ, Sive H, Pieler T, Frank D. The Meis3 protein and retinoid signaling interact to pattern the Xenopus hindbrain. Dev Biol. 2004;271:75–86. doi: 10.1016/j.ydbio.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Dickins EM, Salinas PC. Wnts in action: from synapse formation to synaptic maintenance. Front Cell Neurosci. 2013;7:162. doi: 10.3389/fncel.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA, Bejsovec A. Functional analysis of Wingless reveals a link between intercellular ligand transport and dorsal-cell-specific signaling. Development. 1998;125:4729–4738. doi: 10.1242/dev.125.23.4729. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Itasaki N, Jones CM, Mercurio S, Sargent MG, Smith JC, Krumlauf R. The Wnt/beta-catenin pathway posteriorizes neural tissue in Xenopus by an indirect mechanism requiring FGF signalling. Dev Biol. 2001;239:148–160. doi: 10.1006/dbio.2001.0431. [DOI] [PubMed] [Google Scholar]
- Echevarria D, Vieira C, Gimeno L, Martinez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Brain Res Rev. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, Klein PS, Root H, Liu KJ, Frank D. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development. 2010;137:1531–1541. doi: 10.1242/dev.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkouby YM, Frank D. Wnt/beta-Catenin Signaling in Vertebrate Posterior Neural Development. San Rafael (CA): 2010. [PubMed] [Google Scholar]
- Elkouby YM, Polevoy H, Gutkovich YE, Michaelov A, Frank D. A hindbrain-repressive Wnt3a/Meis3/Tsh1 circuit promotes neuronal differentiation and coordinates tissue maturation. Development. 2012;139:1487–1497. doi: 10.1242/dev.072934. [DOI] [PubMed] [Google Scholar]
- Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- Esain V, Postlethwait JH, Charnay P, Ghislain J. FGF-receptor signalling controls neural cell diversity in the zebrafish hindbrain by regulating olig2 and sox9. Development. 2010;137:33–42. doi: 10.1242/dev.038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas L, Isaacs HV. Overlapping functions of Cdx1, Cdx2, and Cdx4 in the development of the amphibian Xenopus tropicalis. Dev Dyn. 2009;238:835–852. doi: 10.1002/dvdy.21901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50:345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV, de Punder K, Angers S, Peters PJ, Maurice MM, et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. Hippocampal Wnt Signaling: Memory Regulation and Hormone Interactions. Neuroscientist. 2016;22:278–294. doi: 10.1177/1073858415574728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N. WNT/beta-Catenin Signaling in Vertebrate Eye Development. Front Cell Dev Biol. 2016;4:138. doi: 10.3389/fcell.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung YK, Shackleford GM, Brown AM, Sanders GS, Varmus HE. Nucleotide sequence and expression in vitro of cDNA derived from mRNA of int-1, a provirally activated mouse mammary oncogene. Mol Cell Biol. 1985;5:3337–3344. doi: 10.1128/mcb.5.12.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestri G, Carl M, Appolloni I, Wilson SW, Barsacchi G, Andreazzoli M. Six3 functions in anterior neural plate specification by promoting cell proliferation and inhibiting Bmp4 expression. Development. 2005;132:2401–2413. doi: 10.1242/dev.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Onichtchouk D, Blumenstock C, Niehrs C. Head induction by simultaneous repression of Bmp and Wnt signalling in Xenopus. Nature. 1997;389:517–519. doi: 10.1038/39092. [DOI] [PubMed] [Google Scholar]
- Glover JC. Correlated patterns of neuron differentiation and Hox gene expression in the hindbrain: a comparative analysis. Brain Res Bull. 2001;55:683–693. doi: 10.1016/s0361-9230(01)00562-7. [DOI] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff U, Hofling K, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- Guan KL, Rao Y. Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci. 2003;4:941–956. doi: 10.1038/nrn1254. [DOI] [PubMed] [Google Scholar]
- Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008;11:1383–1391. doi: 10.1038/nn.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Guthrie S, Lumsden A. Formation and regeneration of rhombomere boundaries in the developing chick hindbrain. Development. 1991;112:221–229. doi: 10.1242/dev.112.1.221. [DOI] [PubMed] [Google Scholar]
- Gutkovich YE, Ofir R, Elkouby YM, Dibner C, Gefen A, Elias S, Frank D. Xenopus Meis3 protein lies at a nexus downstream to Zic1 and Pax3 proteins, regulating multiple cell-fates during early nervous system development. Dev Biol. 2010;338:50–62. doi: 10.1016/j.ydbio.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- Harada H, Sato T, Nakamura H. Fgf8 signaling for development of the midbrain and hindbrain. Dev Growth Differ. 2016;58:437–445. doi: 10.1111/dgd.12293. [DOI] [PubMed] [Google Scholar]
- Haremaki T, Tanaka Y, Hongo I, Yuge M, Okamoto H. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development. 2003;130:4907–4917. doi: 10.1242/dev.00718. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr Opin Genet Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev Biol. 2000;217:138–152. doi: 10.1006/dbio.1999.9537. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev Biol. 2006;298:71–86. doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol. 2013;5:a007955. doi: 10.1101/cshperspect.a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, Richman A, Tury A, Wang SH, Pessian M, et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016;19:697–705. doi: 10.1038/nn.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hsiung F, Ramirez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Dodd J. Cwnt-8C: a novel Wnt gene with a potential role in primitive streak formation and hindbrain organization. Development. 1993;119:1147–1160. doi: 10.1242/dev.119.4.1147. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- In der Rieden PM, Vilaspasa FL, Durston AJ. Xwnt8 directly initiates expression of labial Hox genes. Dev Dyn. 2010;239:126–139. doi: 10.1002/dvdy.22020. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359:215–223. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- Ishioka A, Jindo T, Kawanabe T, Hatta K, Parvin MS, Nikaido M, Kuroyanagi Y, Takeda H, Yamasu K. Retinoic acid-dependent establishment of positional information in the hindbrain was conserved during vertebrate evolution. Dev Biol. 2011;350:154–168. doi: 10.1016/j.ydbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural basis of Wnt recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Joeng KS, Lee YC, Jiang MM, Bertin TK, Chen Y, Abraham AM, Ding H, Bi X, Ambrose CG, Lee BH. The swaying mouse as a model of osteogenesis imperfecta caused by WNT1 mutations. Hum Mol Genet. 2014;23:4035–4042. doi: 10.1093/hmg/ddu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–741. doi: 10.1016/s0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, Niehrs C. The role of Xenopus dickkopf1 in prechordal plate specification and neural patterning. Development. 2000;127:4981–4992. doi: 10.1242/dev.127.22.4981. [DOI] [PubMed] [Google Scholar]
- Keenan ID, Sharrard RM, Isaacs HV. FGF signal transduction and the regulation of Cdx gene expression. Dev Biol. 2006;299:478–488. doi: 10.1016/j.ydbio.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol. 2016;420:199–209. doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GM, Lai CJ, Moon RT. Expression of wnt10a in the central nervous system of developing zebrafish. Dev Biol. 1993;158:113–121. doi: 10.1006/dbio.1993.1172. [DOI] [PubMed] [Google Scholar]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Keupp K, Beleggia F, Kayserili H, Barnes AM, Steiner M, Semler O, Fischer B, Yigit G, Janda CY, Becker J, et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet. 2013;92:565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva A, Briscoe J. Developmental Pattern Formation in Phases. Trends Cell Biol. 2015;25:579–591. doi: 10.1016/j.tcb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Herrmann M, Wedlich D, Gradl D. Autoregulation of canonical Wnt signaling controls midbrain development. Dev Biol. 2004;273:390–401. doi: 10.1016/j.ydbio.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Joeng KS, Campeau PM, Kiviranta R, Tarkkonen K, Grover M, Lu JT, Pekkinen M, Wessman M, Heino TJ, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoue V, Langford M, White A, Sempert K, Fogg L, Cooper HM. The Wnt receptor Ryk is a negative regulator of mammalian dendrite morphogenesis. Sci Rep. 2017;7:5965. doi: 10.1038/s41598-017-06140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Bendriem RM, Kindberg AA, Worden LT, Williams MP, Drgon T, Mallon BS, Harvey BK, Richie CT, Hamilton RS, et al. Functional consequences of 17q21.31/WNT3-WNT9B amplification in hPSCs with respect to neural differentiation. Cell Rep. 2015;10:616–632. doi: 10.1016/j.celrep.2014.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009a;136:3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009b;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Kemler R. Functional analysis of cis-regulatory elements controlling initiation and maintenance of early Cdx1 gene expression in the mouse. Dev Dyn. 2002;225:216–220. doi: 10.1002/dvdy.10149. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, Ashton RS. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Reports. 2015;4:632–644. doi: 10.1016/j.stemcr.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JA, Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419:199–216. doi: 10.1016/j.ydbio.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Liu W, Shaver TM, Balasa A, Ljungberg MC, Wang X, Wen S, Nguyen H, Van den Veyver IB. Deletion of Porcn in mice leads to multiple developmental defects and models human focal dermal hypoplasia (Goltz syndrome) PLoS One. 2012;7:e32331. doi: 10.1371/journal.pone.0032331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, van Amerongen R, Nusse R. Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. Bioessays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lyu J, Yamamoto V, Lu W. Cleavage of the Wnt receptor Ryk regulates neuronal differentiation during cortical neurogenesis. Dev Cell. 2008;15:773–780. doi: 10.1016/j.devcel.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Heisenberg CP, Barth KA, Macdonald R, Adamek S, Wilson SW. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron. 1997;18:43–57. doi: 10.1016/s0896-6273(01)80045-3. [DOI] [PubMed] [Google Scholar]
- Mason JO, Kitajewski J, Varmus HE. Mutational analysis of mouse Wnt-1 identifies two temperature-sensitive alleles and attributes of Wnt-1 protein essential for transformation of a mammary cell line. Mol Biol Cell. 1992;3:521–533. doi: 10.1091/mbc.3.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor R, Theveneau E. The role of the non-canonical Wnt-planar cell polarity pathway in neural crest migration. Biochem J. 2014;457:19–26. doi: 10.1042/BJ20131182. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Mahony S, Peljto M, Patel T, Thornton SR, McCuine S, Reeder C, Boyer LA, Young RA, Gifford DK, et al. Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat Neurosci. 2013;16:1191–1198. doi: 10.1038/nn.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev. 1997;69:105–114. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Lai CJ, Moon RT. Specification of the anteroposterior neural axis through synergistic interaction of the Wnt signaling cascade with noggin and follistatin. Dev Biol. 1995;172:337–342. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Otte AP, Moon RT. Analysis of Xwnt-4 in embryos of Xenopus laevis: a Wnt family member expressed in the brain and floor plate. Development. 1992;115:463–473. doi: 10.1242/dev.115.2.463. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Takemaru K, Bates R, Moon RT. Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for Wnt signaling during neural patterning in Xenopus. Mech Dev. 1999;87:21–32. doi: 10.1016/s0925-4773(99)00136-7. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Brauninger M, Stec A, Schackert G, Lutolf M, Tanaka EM. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Reports. 2014;3:987–999. doi: 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton KR, Iulianella A, Trainor PA. Gene expression and regulation of hindbrain and spinal cord development. Front Biosci. 2004;9:117–138. doi: 10.2741/1202. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284:30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min TH, Kriebel M, Hou S, Pera EM. The dual regulator Sufu integrates Hedgehog and Wnt signals in the early Xenopus embryo. Dev Biol. 2011;358:262–276. doi: 10.1016/j.ydbio.2011.07.035. [DOI] [PubMed] [Google Scholar]
- Molven A, Njolstad PR, Fjose A. Genomic structure and restricted neural expression of the zebrafish wnt-1 (int-1) gene. EMBO J. 1991;10:799–807. doi: 10.1002/j.1460-2075.1991.tb08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Schwarz TL. The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development. Nat Neurosci. 2010;13:935–943. doi: 10.1038/nn.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya N, Cutts J, Gaasterland T, Willert K, Brafman DA. Endogenous WNT signaling regulates hPSC-derived neural progenitor cell heterogeneity and specifies their regional identity. Stem Cell Reports. 2014;3:1015–1028. doi: 10.1016/j.stemcr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD. Activation and Organization of the Central Nervous System in Amphibians .3. Synthesis of a New Working Hypothesis. J Exp Zool. 1952;120:83–108. [Google Scholar]
- Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, Moon R, Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991;64:231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307:131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Onishi K, Hollis E, Zou Y. Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol. 2014;27:232–240. doi: 10.1016/j.conb.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J, Brown AM, Varmus HE. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987;7:3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Partanen J. FGF signalling pathways in development of the midbrain and anterior hindbrain. J Neurochem. 2007;101:1185–1193. doi: 10.1111/j.1471-4159.2007.04463.x. [DOI] [PubMed] [Google Scholar]
- Pegoraro C, Monsoro-Burq AH. Signaling and transcriptional regulation in neural crest specification and migration: lessons from xenopus embryos. Wiley Interdiscip Rev Dev Biol. 2013;2:247–259. doi: 10.1002/wdev.76. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, Schleiffarth JR, Billington CJ, Jr, van Bokhoven H, Hoogeboom JM, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn. 2010;239:327–337. doi: 10.1002/dvdy.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova IM, Malessy MJ, Verhaagen J, Fradkin LG, Noordermeer JN. Wnt signaling through the Ror receptor in the nervous system. Mol Neurobiol. 2014;49:303–315. doi: 10.1007/s12035-013-8520-9. [DOI] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Savory JG, Lohnes D. Wnt signaling is a key mediator of Cdx1 expression in vivo. Development. 2007;134:2315–2323. doi: 10.1242/dev.001206. [DOI] [PubMed] [Google Scholar]
- Poon VY, Choi S, Park M. Growth factors in synaptic function. Front Synaptic Neurosci. 2013;5:6. doi: 10.3389/fnsyn.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Dev Biol. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- Pyott SM, Tran TT, Leistritz DF, Pepin MG, Mendelsohn NJ, Temme RT, Fernandez BA, Elsayed SM, Elsobky E, Verma I, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am J Hum Genet. 2013;92:590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible F, Brand M. Divide et Impera–the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–734. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Reis AH, Macdonald BT, Feistel K, Brito JM, Amado NG, Xu C, Abreu JG, He X. Expression and evolution of the Tiki1 and Tiki2 genes in vertebrates. Int J Dev Biol. 2014;58:355–362. doi: 10.1387/ijdb.140106ja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Brand M. The midbrain–hindbrain boundary organizer. Curr Opin Neurobiol. 2001;11:34–42. doi: 10.1016/s0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Riley BB, Chiang MY, Storch EM, Heck R, Buckles GR, Lekven AC. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231:278–291. doi: 10.1002/dvdy.20133. [DOI] [PubMed] [Google Scholar]
- Roelink H, Nusse R. Expression of two members of the Wnt family during mouse development–restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the nervous system. Elsevier Academic Press; Burlington, MA, USA: 2012. [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Savory JG, Pilon N, Grainger S, Sylvestre JR, Beland M, Houle M, Oh K, Lohnes D. Cdx1 and Cdx2 are functionally equivalent in vertebral patterning. Dev Biol. 2009;330:114–122. doi: 10.1016/j.ydbio.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Dickinson M, McMahon AP. Cell death in the CNS of the Wnt-1 mutant mouse. J Neurobiol. 1996;31:275–282. doi: 10.1002/(SICI)1097-4695(199611)31:3<275::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Hibi M. Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue. Development. 2006;133:4709–4719. doi: 10.1242/dev.02660. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- Stamatakou E, Salinas PC. Postsynaptic assembly: a role for Wnt signaling. Dev Neurobiol. 2014;74:818–827. doi: 10.1002/dneu.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanganello E, Hagemann AI, Mattes B, Sinner C, Meyen D, Weber S, Schug A, Raz E, Scholpp S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat Commun. 2015;6:5846. doi: 10.1038/ncomms6846. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Stuebner S, Faus-Kessler T, Fischer T, Wurst W, Prakash N. Fzd3 and Fzd6 deficiency results in a severe midbrain morphogenesis defect. Dev Dyn. 2010;239:246–260. doi: 10.1002/dvdy.22127. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Okabayashi K, Asashima M, Perrimon N, Kadowaki T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem. 2000;267:4300–4311. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Musci TS, Neumann PE, Capecchi MR. Swaying is a mutant allele of the proto-oncogene Wnt-1. Cell. 1991;67:969–976. doi: 10.1016/0092-8674(91)90369-a. [DOI] [PubMed] [Google Scholar]
- Tian E, Kimura C, Takeda N, Aizawa S, Matsuo I. Otx2 is required to respond to signals from anterior neural ridge for forebrain specification. Dev Biol. 2002;242:204–223. doi: 10.1006/dbio.2001.0531. [DOI] [PubMed] [Google Scholar]
- Tseng HC, Kao HW, Ho MR, Chen YR, Lin TW, Lyu PC, Lin WC. Cytoskeleton network and cellular migration modulated by nuclear-localized receptor tyrosine kinase ROR1. Anticancer Res. 2011;31:4239–4249. [PubMed] [Google Scholar]
- Tseng HC, Lyu PC, Lin WC. Nuclear localization of orphan receptor protein kinase (Ror1) is mediated through the juxtamembrane domain. BMC Cell Biol. 2010;11:48. doi: 10.1186/1471-2121-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Marti E. Wnt won the war: antagonistic role of Wnt over Shh controls dorso-ventral patterning of the vertebrate neural tube. Dev Dyn. 2010;239:69–76. doi: 10.1002/dvdy.22058. [DOI] [PubMed] [Google Scholar]
- van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H, Zivkovic D. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877–3888. doi: 10.1242/dev.128.20.3877. [DOI] [PubMed] [Google Scholar]
- van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, Meyer BI, Deschamps J. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development. 2002;129:2181–2193. doi: 10.1242/dev.129.9.2181. [DOI] [PubMed] [Google Scholar]
- Vlachakis N, Choe SK, Sagerstrom CG. Meis3 synergizes with Pbx4 and Hoxb1b in promoting hindbrain fates in the zebrafish. Development. 2001;128:1299–1312. doi: 10.1242/dev.128.8.1299. [DOI] [PubMed] [Google Scholar]
- Wang WC, Shashikant CS. Evidence for positive and negative regulation of the mouse Cdx2 gene. J Exp Zool B Mol Dev Evol. 2007;308:308–321. doi: 10.1002/jez.b.21154. [DOI] [PubMed] [Google Scholar]
- Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, Eble TN, Patel A, Thaller C, Fang P, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zamponi R, Charlat O, Ramones M, Swalley S, Jiang X, Rivera D, Tschantz W, Lu B, Quinn L, et al. Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep. 2013;14:1120–1126. doi: 10.1038/embor.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Palmer L, Alexandre C, Kakugawa S, Beckett K, Gaugue I, Palmer RH, Vincent JP. Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs. Nat Cell Biol. 2016;18:451–457. doi: 10.1038/ncb3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Mich JK, Ku S, Menon V, Krostag AR, Martinez RA, Furchtgott L, Mulholland H, Bort S, Fuqua MA, et al. A Single-Cell Roadmap of Lineage Bifurcation in Human ESC Models of Embryonic Brain Development. Cell Stem Cell. 2017;20:120–134. doi: 10.1016/j.stem.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Bouchard M, Stone D, Liu X, Vella F, Lee J, Nakamura H, Ang SL, Busslinger M, Rosenthal A. Distinct regulators control the expression of the mid-hindbrain organizer signal FGF8. Nat Neurosci. 2001;4:1175–1181. doi: 10.1038/nn761. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Yu J, Chen L, Cui B, Widhopf GF, 2nd, Shen Z, Wu R, Zhang L, Zhang S, Briggs SP, Kipps TJ. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2016;126:585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, McDonnell K, Taketo MM, Bai CB. Wnt signaling determines ventral spinal cord cell fates in a time-dependent manner. Development. 2008;135:3687–3696. doi: 10.1242/dev.021899. [DOI] [PubMed] [Google Scholar]
- Zebisch M, Xu Y, Krastev C, MacDonald BT, Chen M, Gilbert RJ, He X, Jones EY. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4:2787. doi: 10.1038/ncomms3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abreu JG, Yokota C, MacDonald BT, Singh S, Coburn KL, Cheong SM, Zhang MM, Ye QZ, Hang HC, et al. Tiki1 is required for head formation via Wnt cleavage-oxidation and inactivation. Cell. 2012;149:1565–1577. doi: 10.1016/j.cell.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, Jones EY, Abreu JG, He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell. 2015;32:719–730. doi: 10.1016/j.devcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, MacDonald BT, Gao H, Shamashkin M, Coyle AJ, Martinez RV, He X. Characterization of Tiki, a New Family of Wnt-specific Metalloproteases. J Biol Chem. 2016;291:2435–2443. doi: 10.1074/jbc.M115.677807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AJ, Scott MP. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. doi: 10.1101/gad.1233104. [DOI] [PubMed] [Google Scholar]


