Abstract
Osteosarcoma (OS) is the most common histological type of primary bone cancer. The present study was designed to identify the key genes and signaling pathways involved in the metastasis of OS. Microarray data of GSE39055 were downloaded from the Gene Expression Omnibus database, which included 19 OS biopsy specimens before metastasis (control group) and 18 OS biopsy specimens after metastasis (case group). After the differentially expressed genes (DEGs) were identified using the Linear Models for Microarray Analysis package, hierarchical clustering analysis and unsupervised clustering analysis were performed separately, using orange software and the self-organization map method. Based upon the Database for Annotation, Visualization and Integrated Discovery tool and Cytoscape software, enrichment analysis and protein-protein interaction (PPI) network analysis were conducted, respectively. After function deviation scores were calculated for the significantly enriched terms, hierarchical clustering analysis was performed using Cluster 3.0 software. Furthermore, logistic regression analysis was used to identify the terms that were significantly different. Those terms that were significantly different were validated using other independent datasets. There were 840 DEGs in the case group. There were various interactions in the PPI network [including intercellular adhesion molecule-1 (ICAM1), transforming growth factor β1 (TGFB1), TGFB1-platelet-derived growth factor subunit B (PDGFB) and PDGFB-platelet-derived growth factor receptor-β (PDGFRB)]. Regulation of cell migration, nucleotide excision repair, the Wnt signaling pathway and cell migration were identified as the terms that were significantly different. ICAM1, PDGFB, PDGFRB and TGFB1 were identified to be enriched in cell migration and regulation of cell migration. Nucleotide excision repair and the Wnt signaling pathway were the metastasis-associated pathways of OS. In addition, ICAM1, PDGFB, PDGFRB and TGFB1, which were involved in cell migration and regulation of cell migration may affect the metastasis of OS.
Keywords: osteosarcoma, differentially expressed genes, protein-protein interaction network, function deviation score
Introduction
As a cancerous tumor that originates from bone, osteosarcoma (OS) is the most frequent histological type of primary bone cancer (1). It is usually derived from tubular long bones, with 10% occurring in the humerus, 19% in the tibia and 42% in the femur (2). OS is the eighth most common type of cancer in pediatric patients, accounting for ~20% of all primary bone cancers and 2.4% of all malignancies in children (3). OS usually occurs in young adults and teenagers, and may be treated by surgical resection of the cancer (4). Although ~90% of OS patients undergo limb-salvage surgery, the subsequent complications (such as infection, local tumor recurrence, prosthetic non-union and loosening) may result in further surgery or amputation (5). Thus, revealing the underlying mechanisms of OS is considered to be important for improving therapeutic strategies.
Through activating the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, metastasis-associated lung adenocarcinoma transcript 1 contributes to proliferation and metastasis of OS and serves as a therapeutic target for OS patients (6,7). Transforming growth factor-α (TGF-α)/epidermal growth factor receptor (EGFR) interaction induces the activation of PI3K/Akt and nuclear factor-κB (NF-κB) signaling pathways, leading to intracellular cell adhesion molecule 1 (ICAM1) expression and promoting the metastasis of human OS cells (8). The low expression level of monocarboxylate transporter isoform 1 (MCT1) performs an antitumor role via the NF-κB signaling pathway, and the high expression level of MCT1 predicts poor overall survival in OS patients (9). As a negative regulator of Wnt signaling, naked cuticle homolog 2 functions in inhibiting tumor growth and invasion of human OS (10,11). Via activation of the cyclooxygenase-2 (COX-2) gene, p50-associated COX-2 extragenic RNA promotes the proliferation and invasion of OS cells (12). Although these studies have investigated the underlying mechanisms of OS metastasis, the key genes and signaling pathways involved in the metastasis of OS have not been fully reported.
In 2013, Kelly et al (13) performed formalin-fixed, paraffin-embedded assays to identify the miRNA biomarkers associated with OS prognosis, finding that the 14q32 locus was potential involved in the progression and outcome of OS. Using the data deposited by Kelly et al (13), the differentially expressed genes (DEGs) were identified between the OS biopsy specimens following metastasis and the OS biopsy specimens prior to metastasis. Subsequently, comprehensive bioinformatics analyses [including clustering analysis, enrichment analysis, protein-protein network (PPI) analysis, calculation of function deviation score and logistic regression analysis] were performed to identify the metastasis-associated terms. Finally, the metastasis-associated terms were further confirmed by other independent datasets.
Materials and methods
Microarray data
Gene expression profiling and clinical outcomes under GSE39055 were downloaded from the Gene Expression Omnibus database (GEO; http://www.ncbi.nlm.nih.gov/geo/) database, which was based on the GEO platform, GPL14951, Illumina HumanHT-12 WG-DASL v4.0 R2 expression BeadChip. GSE39055 included 19 OS biopsy specimens before metastasis and 18 OS biopsy specimens after metastasis. The samples were acquired from the pathology archives of Boston Children’s Hospital (Boston, USA) and Beth Israel Deaconess Medical Center (Boston, USA). Kelly et al (13) deposited GSE39055, and the study obtained the approval of the Institutional Review Boards at Boston Children’s Hospital and Beth Israel Deaconess Medical Center with a waiver of consent.
DEG screening and clustering analysis
Based on the platform annotation information, all probe IDs were transformed into gene symbols. When one gene symbol corresponded to multiple probes, the gene expression value was obtained by calculating the average value of the probes. Subsequently, Z-score normalization was performed for all gene expression values. Using the Linear Models for Microarray Analysis package (http://www.bioconductor.org/packages/release/bioc/html/limma.html) (14) in R, the DEGs between the OS biopsy specimens after metastasis (case group) and the OS biopsy specimens before metastasis (control group) were analyzed. P<0.05 and |logFC (fold change)| >0.6 were set as the thresholds.
To confirm whether the DEGs were able to effectively distinguish between the two groups of samples, hierarchical clustering analysis was conducted using Orange software (version 3.4, http://orange.biolab.si/citation/) (15) and the result was visualized using a distance map (16). Pearson correlation coefficient (17) and average linkage (18) were performed separately for similarity algorithms and similarity matrices. Additionally, unsupervised clustering analysis was conducted for the samples using the self-organization map (SOM) method (19).
Functional and pathway enrichment analysis
The Gene Ontology (GO; http://www.geneontology.org) database provides information regarding cellular components (CCs), molecular function (MF), and biological processes (BPs) for gene produces (20). The Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.ad.jp/kegg) is a database applied for conducting pathway analysis for genes or other molecules (21). Based on the Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/) tool (22), GO functional and KEGG pathway enrichment analyses were performed for the DEGs, with the threshold set at P<0.05.
PPI network analysis
Biological General Repository for Interaction Datasets (BioGrid; http://www.thebiogrid.org) is an interaction database that includes genetic and protein interactions for humans and all major model organism species (23). Human Protein Reference Database (HPRD; http://www.hprd.org/) is a comprehensive protein information resource that provides various features of human proteins (24). The PPI network data in BioGrid (23) and HPRD (24) databases were downloaded and merged. Then, the DEGs were mapped into the human PPI network to identify the PPIs among the DEGs. Subsequently, the PPI network for the DEGs was visualized using Cytoscape software (version 3.5.0, http://www.cytoscape.org) (25). In addition, the network analysis plug-in in Cytoscape software (25) was used to analyze network topological features to screen the hub nodes (26) in the PPI network.
Calculation of function deviation score
To obtain the results with stability and accuracy, function deviation scores were calculated for the significantly enriched terms. The formula was follows:
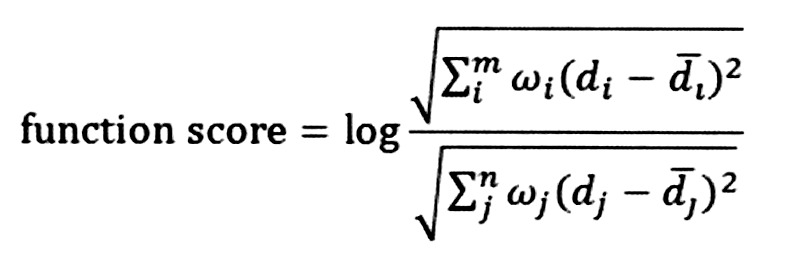 |
Where m and n separately represent the numbers of upregulated and downregulated genes enriched in term represents the mean value of the upregulated gene, i or downregulated gene, j in the control group. ω represents the node degree of a gene in the PPI network. The Euclidean distance is used to calculate the degree of deviation of the term P influenced by the upregulated and downregulated genes. Function score >0 indicates that the term P is upregulated in the case group compared with the control group. Function score <0 indicates that the term P is downregulated in the case group compared with the control group. Combined with the function deviation scores, hierarchical clustering analysis was performed using Cluster 3.0 software (27) to verify whether these significantly enriched terms clearly distinguished the case group from the control group.
Identification of significantly different terms
To identify the terms with significant differences in OS biopsy specimens before/after metastasis, logistic regression analysis was performed to calculate the significance of each term. The terms with P<0.05 were considered to be significantly different (also defined as metastasis-associated terms).
Validation of the metastasis-associated terms using other independent datasets
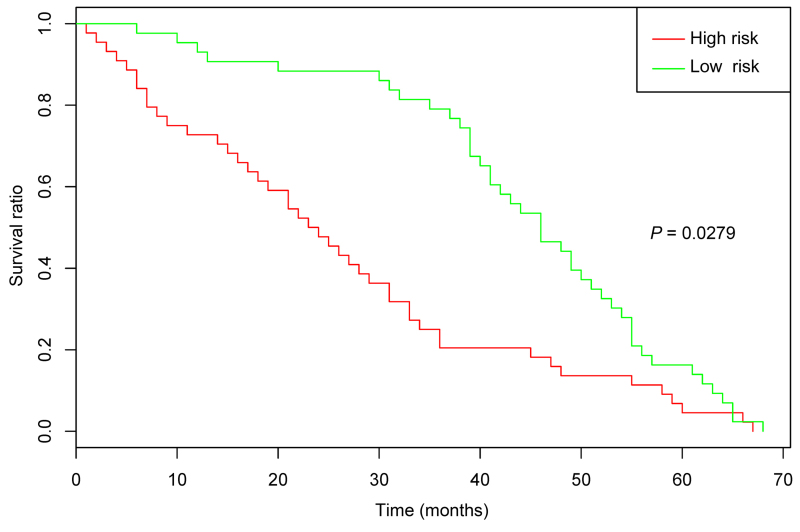
Two microarray datasets, GSE21257 (including 19 OS specimens without metastasis and 34 OS specimens with metastasis) and GSE32981 (including five OS specimens without metastasis and 18 OS specimens with metastasis) were downloaded from the European Molecular Biology Laboratory (http://www.ebi.ac.uk/embl/index.html) Nucleotide Sequence Database. Subsequently, the two datasets were merged and normalized. The OS specimens with/without metastasis were predicted, and the prediction accuracy was exhibited via receiver operating characteristic (ROC) curves. In addition, Kaplan-Meier (KM) survival analysis (28) was used to analyze the correlations between the survival times in GSE21257 and GSE16091 (downloaded from the GEO database) and the metastasis-associated terms.
The main risk factor for disease recurrence and metastasis is the sensitivity of patients to drug treatment, and metastasis/recurrence typically occurs when there is apparent drug resistance (29). Microarray data of GSE81892 (including 21 OS specimens with obvious resistance and 13 OS specimens with drug sensitivity) and GSE14827 (including 16 OS specimens with obvious resistance and 11 OS specimens with drug sensitivity) were downloaded from the GEO database. To confirm whether the metastasis-associated terms affect drug sensitivity and influence the risk of metastasis/recurrence, the terms were used to distinguish the OS specimens with obvious resistance and the OS specimens with drug sensitivity.
Results
DEG analysis
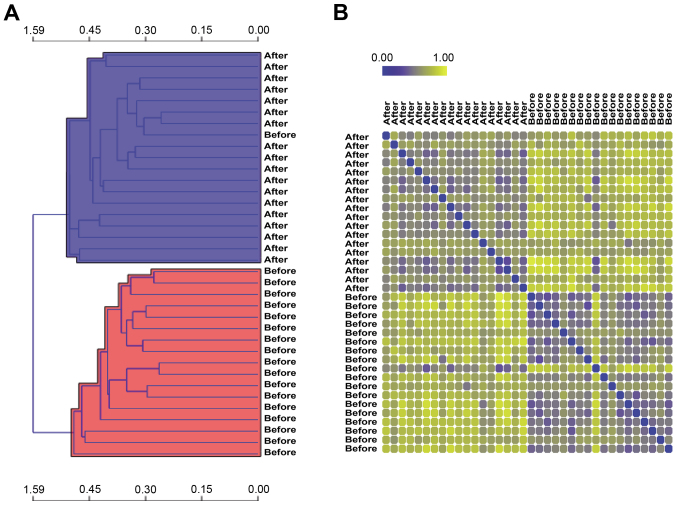
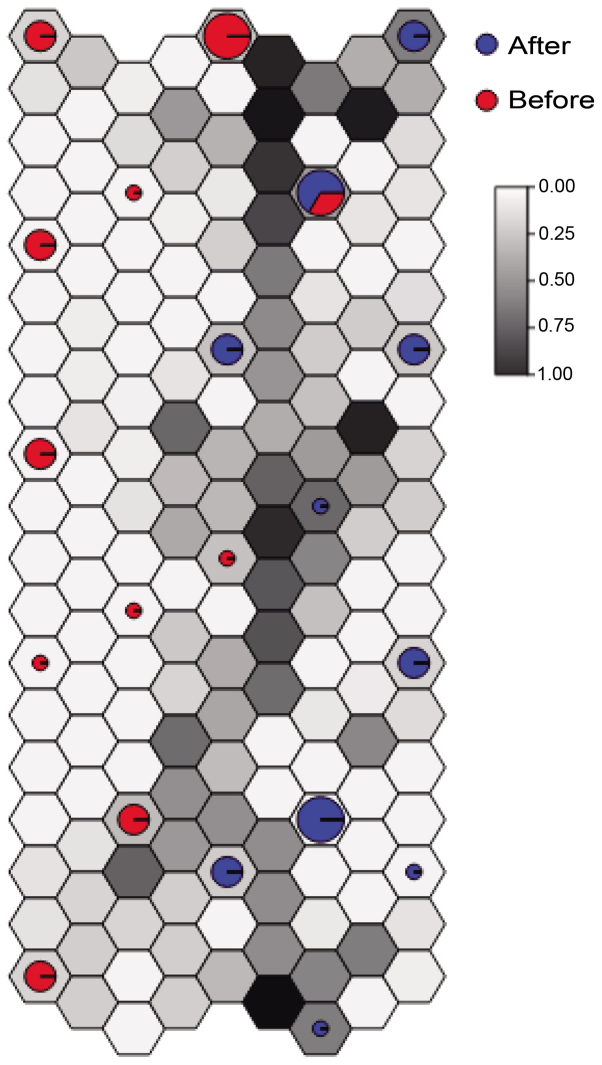
A total of 840 DEGs were identified in the case group compared with the control group, including 420 upregulated genes and 420 downregulated genes. After removing one abnormal sample, the remaining 36 samples were used for the subsequent analysis. Hierarchical clustering analysis demonstrated that 19 samples were included in the case group (accuracy, 95%) and 17 samples were included in the control group (accuracy, 100%) (Fig. 1A). To further evaluate the correlation and distance between the two groups of samples, the distances between the remaining 36 samples were visualized using a distance map (Fig. 1B). The distances between the intra-group samples tended to be closer, while the distances between the inter-group samples were relatively far apart. Meanwhile, unsupervised clustering analysis based on the SOM method demonstrated that the classification effect for the samples in the majority of neurons was good (Fig. 2).
Figure 1.
(A) Heat map of hierarchical clustering analysis (horizontal and vertical axes represent similarity distance and OS biopsy specimens before/after metastasis, respectively). (B) Distance map demonstrating the distances between the remaining 36 samples (blue and yellow separately represent close and far distances, respectively). OS, osteosarcoma.
Figure 2.
Result of unsupervised clustering analysis based on the self-organization map method. Red and blue represent osteosarcoma biopsy specimens before and after metastasis, respectively.
Functional and pathway enrichment analysis
The upregulated and downregulated genes separately underwent enrichment analysis. The GO terms that were enriched for the upregulated genes predominantly included regulation of cell migration (P=2.83E-12), blood vessel development (P=2.74E-10) and vasculature development (P=4.67E-10) (Table I). The upregulated genes were enriched in the KEGG pathways of viral myocarditis (P=7.85E-08), focal adhesion (P=3.70E-06) and endocytosis (P=1.44E-03) (Table II). In addition, the downregulated genes were predominantly enriched in the GO terms of RNA metabolic processes (P=1.11E-06), RNA processing (P=2.11E-06) and regulation of gene expression (P=5.20E-04) (Table III). Furthermore, the KEGG pathways enriched for the downregulated genes were cell adhesion molecules (P=2.32E-09), extracellular matrix-receptor interaction (P=1.49E-08), and pathways in cancer (P=3.04E-02) (Table IV).
Table I.
The Gene Ontology (GO) terms significantly enriched for the upregulated genes.
| Term | Count | P-value | Gene symbol |
|---|---|---|---|
| GO:0030334 - regulation of cell migration | 25 | 2.83E-12 | DLC1, PDGFB, ENPP2, KIT, ADA, TGFB1, ACE, S1PR1, INS, TEK, ROBO4, TIE1, LAMB1, ICAM1, PTPRM, IGF2, VASH1, THY1, KDR, LAMA4, LAMA3, CLIC4, PDGFRB, ARAP3, IGFBP3, F2R |
| GO:0001568 - blood vessel development | 27 | 2.74E-10 | EMCN, CAV1, ATP5B, COL3A1, WASF2, ELK3, CDH5, GJC1, PTK2, ACE, S1PR1, CD44, ROBO4, LOX, PLXND1, EPAS1, MYO1E, MMP19, COL15A1, UBP1, THY1, KDR, LAMA4, BGN, PLXDC1, NOTCH4, ENG |
| GO:0001944 - vasculature development | 27 | 4.67E-10 | EMCN, CAV1, ATP5B, COL3A1, WASF2, ELK3, CDH5, GJC1, PTK2, ACE, S1PR1, CD44, ROBO4, LOX, PLXND1, EPAS1, MYO1E, MMP19, COL15A1, UBP1, THY1, KDR, LAMA4, BGN, PLXDC1, NOTCH4, ENG |
| GO:0048514 - blood vessel morphogenesis | 22 | 4.70E-08 | CAV1, EMCN, EPAS1, ATP5B, MYO1E, MMP19, WASF2, COL15A1, ELK3, UBP1, GJC1, THY1, KDR, PTK2, ACE, S1PR1, BGN, PLXDC1, NOTCH4, ROBO4, PLXND1, ENG |
| GO:0016477 - cell migration | 24 | 2.82E-07 | ICAM1, PDGFB, ATP5B, PODXL, WASF2, ITGA1, KIT, TGFB1, KDR, VCAM1, NCK2, EDNRB, PTK2, ITGA6, CD44, CD34, ITGA5, FYN, PDGFRB, MSN, LAMC1, ENG, MYH10, THBS4 |
| GO:0007167 - enzyme linked receptor protein signaling pathway | 24 | 1.11E-05 | PDGFB, EFNA1, ADCYAP1R1, COL3A1, FKBP1A, KIT, EPHB3, EPHB4, TGFB1, PTK2, INS, TEK, TIE1, PTPRE, SOCS2, MYO1E, IGF2, KDR, LEFTY1, NCK2, PRLR, PDGFRB, TGFB1I1, ENG, GFRA2 |
| GO:0009966 - regulation of signal transduction | 42 | 4.27E-05 | DLC1, FGD2, CAV1, PDGFB, TBC1D9, EFNA1, AGFG2, PREX2, ASAP2, ASAP1, ITPKB, FKBP1A, KIT, CD74, MCF2L, TGFB1, ADA, S1PR1, NOD1, INS, RAPGEF5, RHOC, TRAF5, RAMP2, SOCS2, PSD3, ITGA1, IGF2, ARHGEF15, CD40, THY1, NCK2, TNFSF10, ARRB1, RGS5, TGFB1I1, GRK5, IGFBP2, ARAP3, ENG, IGFBP3, MAP3K11, F2R |
| GO:0009888 - tissue development | 31 | 8.58E-04 | DLC1, CAV1, COL3A1, FKBP1A, TIMP3, TGFB1, GJC1, EDNRB, S1PR1, CD44, SERPINE1, TIE1, F11R, BMP1, HSPG2, MECOM, SNAI2, COL5A2, FZD6, KDR, NOTCH3, LAMA3, SPRR1B, NOTCH4, PDGFRB, LAMC1, TGFB1I1, ENG, FABP5, F2R, MYH10 |
| GO:0008284 - positive regulation of cell proliferation | 22 | 1.26E-03 | GNAI2, PDGFB, BTC, CDK6, IGF2, KIT, CD40, PNP, ADA, TGFB1, KDR, VCAM1, NCK2, S1PR1, INS, NOTCH4, AVPR1A, PDGFRB, LAMC1, LAMB1, TRAF5, THPO, F2R |
| GO:0043067 - regulation of programmed cell death | 34 | 2.71E-03 | DLC1, FGD2, BID, CADM1, BTC, KIT, TIMP3, ADA, TGFB1, MCF2L, CD74, EDNRB, PEA15, PCGF2, NOD1, CASP4, CD44, INS, TRAF5, PHLDA1, HIP1, GIMAP5, ACTN4, SOCS2, ITGA1, IGF2, NLRP1, CASP10, TNFSF10, PRLR, ETS1, IGFBP3, FAIM2, F2R, MAP3K11 |
| GO:0007166 - cell surface receptor linked signal transduction | 64 | 3.44E-03 | PDGFB, EFNA1, PREX2, WASF2, LPAR4, ITPKB, TGFB1, LPHN2, EDNRB, S1PR1, ELTD1, TIE1, RAMP2, GPR176, SOCS2, CD40, OR10J1, THY1, LEFTY1, NCK2, CPE, CHRM2, LPAR6, GPR56, PDGFRB, TGFB1I1, GNAI2, GNAI1, ENPP2, ADCYAP1R1, COL3A1, GNG11, FKBP1A, GAST, KIT, EPHB3, EPHB4, APLNR, PTK2, DOCK1, INS, TEK, PTPRE, MYO1E, ITGA1, IGF2, OR51E2, CENPI, KDR, FZD6, NOTCH3, SEMA6A, LAMA3, PRLR, ITGA6, FYN, ITGA5, HEYL, NOTCH4, AVPR1A, GRK5, ENG, GFRA2, GPR116, F2R |
| GO:0042981 - regulation of apoptosis | 33 | 4.22E-03 | DLC1, FGD2, BID, CADM1, BTC, TIMP3, ADA, TGFB1, MCF2L, CD74, EDNRB, PEA15, PCGF2, NOD1, CASP4, CD44, INS, TRAF5, PHLDA1, HIP1, GIMAP5, ACTN4, SOCS2, ITGA1, IGF2, NLRP1, CASP10, TNFSF10, PRLR, ETS1, IGFBP3, FAIM2, F2R, MAP3K11 |
| hsa04514: Cell adhesion molecules (CAMs) | 22 | 2.32E-09 | F11R, ICAM1, PTPRM, CADM1, ICAM2, CLDN5, HLA-A, HLA-C, CD40, HLA-B, HLA-E, HLA-DMA, HLA-DQA1, HLA-G, CDH5, VCAM1, ITGA6, CD34, PECAM1, HLA-DPA1, HLA-DPB1, JAM2, HLA-DRA |
| hsa04512: ECM-receptor interaction | 17 | 1.49E-08 | COL4A2, COL4A1, COL3A1, ITGA1, HSPG2, COL5A2, VWF, LAMA4, LAMA3, CD44, ITGA6, ITGA5, COL6A3, SV2B, LAMC1, LAMB1, THBS4 |
| hsa05200: Pathways in cancer | 19 | 3.04E-02 | BID, COL4A2, COL4A1, PDGFB, EPAS1, CDK6, KIT, MECOM, TGFB1, FZD6, PTK2, LAMA4, LAMA3, ITGA6, ETS1, PDGFRB, LAMC1, LAMB1, TRAF5 |
Table II.
The KEGG pathways significant enriched for the upregulated genes.
| Term | Count | P-value | Gene symbol |
|---|---|---|---|
| hsa04514: Cell adhesion molecules (CAMs) | 22 | 2.32E-09 | F11R, ICAM1, PTPRM, CADM1, ICAM2, CLDN5, HLA-A, HLA-C, CD40, HLA-B, HLA-E, HLA-DMA, HLA-DQA1, HLA-G, CDH5, VCAM1, ITGA6, CD34, PECAM1, HLA-DPA1, HLA-DPB1, JAM2, HLA-DRA |
| hsa04512: ECM-receptor interaction | 17 | 1.49E-08 | COL4A2, COL4A1, COL3A1, ITGA1, HSPG2, COL5A2, VWF, LAMA4, LAMA3, CD44, ITGA6, ITGA5, COL6A3, SV2B, LAMC1, LAMB1, THBS4 |
| hsa05200: Pathways in cancer | 19 | 3.04E-02 | BID, COL4A2, COL4A1, PDGFB, EPAS1, CDK6, KIT, MECOM, TGFB1, FZD6, PTK2, LAMA4, LAMA3, ITGA6, ETS1, PDGFRB, LAMC1, LAMB1, TRAF5 |
Table III.
The Gene Ontology (GO) terms significant enriched for the downregulated genes.
| Term | Count | P-value | Gene symbol |
|---|---|---|---|
| GO:0016070 - RNA metabolic process | 49 | 1.11E-06 | EIF2C2, TCOF1, SYNCRIP, SKIV2L2, WTAP, SART1, SMNDC1, INTS8, IMP3, RRP1B, ASH2L, TRMT5, QKI, SUPT5H, IMP4, NFX1, KHDRBS3, AR, ZCCHC11, PRPF39, MED13, HNRNPU, C4ORF23, EIF4G1, HNRPDL, CELF1, SNRPF, MYNN, CPSF3L, ELL, SR140, WBP11, HSPA1A, POLR2C, PPAN, RPL7, PRKRA, C19ORF29, GTF3C5, TCEA1, SNRNP70, SNRNP35, NFATC3, PRPF40A, SMG5, TAF7, PPP1R8, POP1, DDX54 |
| GO:0006396 - RNA processing | 34 | 2.11E-06 | CPSF3L, EIF2C2, SR140, SYNCRIP, SKIV2L2, WBP11, WTAP, POLR2C, SART1, SMNDC1, INTS8, PPAN, IMP3, RRP1B, RPL7, TRMT5, PRKRA, C19ORF29, QKI, SNRNP70, SNRNP35, IMP4, PRPF40A, KHDRBS3, ZCCHC11, PRPF39, HNRNPU, C4ORF23, HNRPDL, PPP1R8, POP1, CELF1, DDX54, SNRPF |
| GO:0010468 - regulation of gene expression | 97 | 5.20E-04 | EIF2C2, ARNT2, STAT5B, MORF4L2, NAA15, SYNCRIP, CNOT1, CBX7, ZNF304, CRY2, DIRAS3, MED28, SUPT5H, EIF2B4, MYST3, GTPBP4, MED13, HNRNPU, BAZ1B, ZNF238, ZNF239, MNX1, MGA, ZZZ3, CELF1, PAF1, MYNN, SIVA1, CNBP, ELL, ADORA2A, HOXA11, TH1L, CYTL1, KEAP1, NR1H2, LYL1, EIF3H, PRKRA, LHX5, DNMT3A, TAF7, ZBTB44, UIMC1, SAFB2, HDAC5, NRF1, EIF4H, ATF7, SMARCC2, RFX1, DDX54, SOX21, E2F6, MITF, CTCF, PHF20, ZKSCAN5, CRX, AES, ASH2L, ACTR5, CCDC101, ZNF146, QKI, NFX1, ZNF281, AR, KHDRBS3, ZCCHC11, GMEB1, ZNF143, HNRPDL, EIF4G1, MED6, BPTF, CARM1, MTDH, ZBTB11, SCML1, GCN1L1, MORC3, HMGXB4, PPP3CB, TCEA1, THAP1, KDM3B, SNRNP70, NFATC3, TERF2, NACC2, CEBPE, SIRT4, ZNF669, PKNOX1, PPP1R8, RBM15 |
| GO:0016071 - mRNA metabolic process | 21 | 9.32E-04 | EIF2C2, KHDRBS3, SMG5, SYNCRIP, PRPF39, WBP11, SKIV2L2, HSPA1A, WTAP, POLR2C, HNRNPU, SART1, SMNDC1, PPP1R8, C19ORF29, QKI, CELF1, SNRNP35, SNRNP70, SNRPF, PRPF40A |
| GO:0010605 - negative regulation of macromolecule metabolic process | 33 | 1.34E-03 | HCRT, EIF2C2, MTDH, E2F6, TH1L, CTCF, ANAPC10, NR1H2, AES, PSMB1, PRKRA, SUPT5H, TERF2, EIF2B4, MYST3, NFX1, ZNF281, DNMT3A, ANAPC2, GTPBP4, NACC2, ZCCHC11, TAF7, SIRT4, UIMC1, HDAC5, ZNF238, BPTF, PSMA5, PSMC3, SMARCC2, CELF1, RBM15 |
| GO:0030163 - protein catabolic process | 29 | 1.68E-03 | RAD23B, KIAA0368, ATG12, UBE3B, UBE2V2, ANAPC10, UBE2D3, LONP1, USP27X, PSMB1, OTUD7B, RNF167, FBXO42, FBXO9, TPRKB, CUL1, NFX1, AXIN1, ANAPC2, SOCS6, SOCS5, KCMF1, CUL4A, PSMC3, PSMA5, UBR5, SIAH1, PCYOX1, FBXO11 |
| GO:0043632 - modification-dependent macromolecule catabolic process | 27 | 2.20E-03 | RAD23B, ATG12, UBE3B, KIAA0368, UBE2V2, ANAPC10, LONP1, USP27X, UBE2D3, PSMB1, OTUD7B, RNF167, FBXO42, FBXO9, CUL1, NFX1, ANAPC2, SOCS6, SOCS5, KCMF1, CUL4A, PSMC3, PSMA5, UBR5, SIAH1, PCYOX1, FBXO11 |
| GO:0010629 - negative regulation of gene expression | 24 | 3.64E-03 | ZNF281, DNMT3A, EIF2C2, NACC2, ZCCHC11, MTDH, E2F6, TH1L, TAF7, SIRT4, CTCF, UIMC1, NR1H2, HDAC5, AES, ZNF238, BPTF, PRKRA, SMARCC2, CELF1, SUPT5H, RBM15, MYST3, NFX1 |
| GO:0019219 - regulation of nucleic acid metabolic process | 91 | 4.03E-03 | EIF2C2, SOX21, E2F6, MORF4L2, MITF, STAT5B, ARNT2, NAA15, SYNCRIP, CNOT1, CTCF, PHF20, CBX7, ZKSCAN5, CRX, ZNF304, CRY2, AES, ASH2L, MED28, ACTR5, CCDC101, ZNF146, SUPT5H, MYST3, NFX1, ZNF281, AR, KHDRBS3, GTPBP4, GMEB1, ZNF143, MED13, HNRNPU, MED6, HNRPDL, ZNF238, BPTF, BAZ1B, ZNF239, MNX1, MGA, ZZZ3, PAF1, CARM1, MYNN, SIVA1, HCRT, CNBP, MTDH, ADORA2A, ELL, ZBTB11, HOXA11, SCML1, PTH1R, TH1L, CYTL1, UBE2V2, KEAP1, NR1H2, LYL1, HMGXB4, LHX5, THAP1, TCEA1, KDM3B, SNRNP70, NFATC3, TERF2, GUCA1B, DNMT3A, GUCA1A, NACC2, CEBPE, TAF7, SIRT4, ZNF669, ZBTB44, UIMC1, SAFB2, HDAC5, NRF1, P2RY11, PKNOX1, PPP1R8, ATF7, SMARCC2, RFX1, DDX54, RBM15 |
| GO:0044257 - cellular protein catabolic process | 27 | 4.27E-03 | RAD23B, ATG12, UBE3B, KIAA0368, UBE2V2, ANAPC10, LONP1, USP27X, UBE2D3, PSMB1, OTUD7B, RNF167, FBXO42, FBXO9, CUL1, NFX1, ANAPC2, SOCS6, SOCS5, KCMF1, CUL4A, PSMC3, PSMA5, UBR5, SIAH1, PCYOX1, FBXO11 |
| GO:0010558 - negative regulation of macromolecule biosynthetic process | 25 | 4.95E-03 | HCRT, EIF2C2, MTDH, E2F6, TH1L, CTCF, NR1H2, AES, SUPT5H, EIF2B4, TERF2, NFX1, MYST3, ZNF281, DNMT3A, GTPBP4, NACC2, TAF7, SIRT4, UIMC1, HDAC5, ZNF238, BPTF, SMARCC2, RBM15 |
| GO:0031325 - positive regulation of cellular metabolic process | 35 | 6.60E-03 | CNBP, ADORA2A, MORF4L2, ARNT2, MITF, STAT5B, NAA15, CYTL1, CTCF, ANAPC10, CRX, NR1H2, PSMB1, TCEA1, CD24, SUPT5H, NFATC3, TERF2, CUL1, MYST3, AXIN1, ANAPC2, AR, TAF7, MED13, UIMC1, MED6, HDAC5, PKNOX1, BPTF, PSMA5, PSMC3, SMARCC2, TNK2, RBM15 |
| GO:0031327 - negative regulation of cellular biosynthetic process | 25 | 6.64E-03 | HCRT, EIF2C2, MTDH, E2F6, TH1L, CTCF, NR1H2, AES, SUPT5H, EIF2B4, TERF2, NFX1, MYST3, ZNF281, DNMT3A, GTPBP4, NACC2, TAF7, SIRT4, UIMC1, HDAC5, ZNF238, BPTF, SMARCC2, RBM15 |
| GO:0031324 - negative regulation of cellular metabolic process | 30 | 6.85E-03 | HCRT, EIF2C2, MTDH, E2F6, TH1L, CTCF, ANAPC10, NR1H2, AES, PSMB1, SUPT5H, EIF2B4, TERF2, MYST3, NFX1, ZNF281, DNMT3A, ANAPC2, GTPBP4, NACC2, TAF7, SIRT4, UIMC1, HDAC5, ZNF238, BPTF, PSMC3, PSMA5, SMARCC2, RBM15 |
| GO:0044267 - cellular protein metabolic process | 77 | 7.19E-03 | EIF2C2, KIAA0368, NAA15, ART3, USP27X, LONP1, CRY2, FAU, SIK2, EIF2B4, CUL1, MYST3, NFX1, ANAPC2, SGK2, ROCK2, SECISBP2, SOCS6, SOCS5, DAPK3, ST13, EIF4G1, BAZ1B, PSMA5, UBR5, SIAH1, PAF1, PCYOX1, CARM1, TM4SF4, FBXO11, RAD23B, UBE3B, ATG12, STK11, BLK, CAMK2G, MAPKAPK5, ANAPC10, UBE2V2, AKAP9, MTMR3, UBE2D3, EIF3G, RPL7, PSMB1, MORC3, EIF3H, SH3GLB1, PRKRA, OTUD7B, PPP3CB, RNF167, FBXO42, RSL24D1, EIF3J, FBXO9, OBSCN, P4HB, ALPK3, RYK, EEF1A2, SIRT4, OXSR1, LOC653566, UIMC1, HDAC5, NMT1, CUL4A, KCMF1, RPL13A, PSMC3, PPID, EIF4H, CCT8, LGTN, TNK2 |
| GO:0031326 - regulation of cellular biosynthetic process | 93 | 7.26E-03 | EIF2C2, SOX21, E2F6, MORF4L2, STAT5B, ARNT2, MITF, NAA15, CNOT1, CTCF, PHF20, CBX7, ZKSCAN5, CRX, ZNF304, CRY2, AES, ASH2L, MED28, ACTR5, CCDC101, ZNF146, QKI, SUPT5H, EIF2B4, MYST3, NFX1, ZNF281, AR, KHDRBS3, GTPBP4, GMEB1, ZNF143, MED13, MED6, HNRPDL, EIF4G1, ZNF238, BPTF, BAZ1B, ZNF239, MNX1, MGA, ZZZ3, PAF1, CARM1, MYNN, SIVA1, HCRT, CNBP, MTDH, ADORA2A, ELL, ZBTB11, HOXA11, SCML1, PTH1R, TH1L, CYTL1, KEAP1, GCN1L1, NR1H2, EIF3H, LYL1, HMGXB4, LHX5, THAP1, TCEA1, KDM3B, NFATC3, TERF2, GUCA1B, DNMT3A, GUCA1A, NACC2, CEBPE, TAF7, SIRT4, ZNF669, ZBTB44, UIMC1, SAFB2, HDAC5, NRF1, P2RY11, PKNOX1, PPP1R8, EIF4H, ATF7, SMARCC2, RFX1, DDX54, RBM15 |
| GO:0010604 - positive regulation of macromolecule metabolic process | 34 | 7.79E-03 | CNBP, MORF4L2, ARNT2, MITF, STAT5B, NAA15, CYTL1, CTCF, ANAPC10, CRX, NR1H2, PSMB1, TCEA1, CD24, SUPT5H, NFATC3, TERF2, CUL1, MYST3, AXIN1, ANAPC2, AR, TAF7, MED13, UIMC1, MED6, HDAC5, PKNOX1, BPTF, PSMA5, PSMC3, SMARCC2, TNK2, RBM15 |
| GO:0045893 - positive regulation of transcription, DNA-dependent | 22 | 7.98E-03 | AR, CNBP, MORF4L2, ARNT2, STAT5B, MITF, TAF7, CYTL1, NAA15, CTCF, MED13, CRX, NR1H2, MED6, HDAC5, PKNOX1, BPTF, SMARCC2, TCEA1, SUPT5H, NFATC3, RBM15 |
| GO:0009890 - negative regulation of biosynthetic process | 25 | 8.53E-03 | HCRT, EIF2C2, MTDH, E2F6, TH1L, CTCF, NR1H2, AES, SUPT5H, EIF2B4, TERF2, NFX1, MYST3, ZNF281, DNMT3A, GTPBP4, NACC2, TAF7, SIRT4, UIMC1, HDAC5, ZNF238, BPTF, SMARCC2, RBM15 |
| GO:0051254 - positive regulation of RNA metabolic process | 22 | 8.70E-03 | AR, CNBP, MORF4L2, ARNT2, STAT5B, MITF, TAF7, CYTL1, NAA15, CTCF, MED13, CRX, NR1H2, MED6, HDAC5, PKNOX1, BPTF, SMARCC2, TCEA1, SUPT5H, NFATC3, RBM15 |
| GO:0045934 - negative regulation of nucleic acid metabolic process | 23 | 8.74E-03 | ZNF281, DNMT3A, HCRT, GTPBP4, NACC2, MTDH, E2F6, TH1L, TAF7, SIRT4, CTCF, UIMC1, NR1H2, HDAC5, AES, ZNF238, BPTF, SMARCC2, SUPT5H, RBM15, TERF2, MYST3, NFX1 |
| GO:0010556 - regulation of macromolecule biosynthetic process | 89 | 9.86E-03 | EIF2C2, SOX21, E2F6, MORF4L2, STAT5B, ARNT2, MITF, NAA15, CNOT1, CTCF, PHF20, CBX7, ZKSCAN5, CRX, ZNF304, CRY2, AES, ASH2L, MED28, ACTR5, CCDC101, ZNF146, QKI, SUPT5H, EIF2B4, MYST3, NFX1, ZNF281, AR, KHDRBS3, GTPBP4, GMEB1, ZNF143, MED13, MED6, HNRPDL, EIF4G1, ZNF238, BPTF, BAZ1B, ZNF239, MNX1, MGA, ZZZ3, PAF1, CARM1, MYNN, SIVA1, HCRT, CNBP, MTDH, ADORA2A, ELL, ZBTB11, HOXA11, SCML1, TH1L, CYTL1, KEAP1, GCN1L1, NR1H2, EIF3H, LYL1, HMGXB4, LHX5, THAP1, TCEA1, KDM3B, NFATC3, TERF2, DNMT3A, NACC2, CEBPE, TAF7, SIRT4, ZNF669, ZBTB44, UIMC1, SAFB2, HDAC5, NRF1, PKNOX1, PPP1R8, EIF4H, ATF7, SMARCC2, RFX1, DDX54, RBM15 |
Table IV.
The KEGG pathways significant enriched for the downregulated genes.
| Term | Count | P-value | Gene symbol |
|---|---|---|---|
| hsa05416: Viral myocarditis | 15 | 7.85E-08 | BID, ICAM1, CAV1, HLA-A, HLA-C, CD40, HLA-B, HLA-E, HLA-DMA, HLA-DQA1, HLA-G, FYN, HLA-DPA1, HLA-DPB1, MYH10, HLA-DRA |
| hsa04510: Focal adhesion | 22 | 3.70E-06 | CAV1, COL4A2, COL4A1, ACTN4, PDGFB, COL3A1, ITGA1, COL5A2, KDR, VWF, PTK2, LAMA4, LAMA3, DOCK1, ITGA6, ITGA5, FYN, COL6A3, PDGFRB, LAMC1, LAMB1, THBS4 |
| hsa04144: Endocytosis | 16 | 1.44E-03 | PSD3, ASAP2, HLA-A, ASAP1, HLA-C, KIT, HLA-B, HLA-E, HLA-G, KDR, ARRB1, GRK5, ARAP3, EHD2, EHD3, F2R, EHD4 |
PPI network analysis and function deviation score
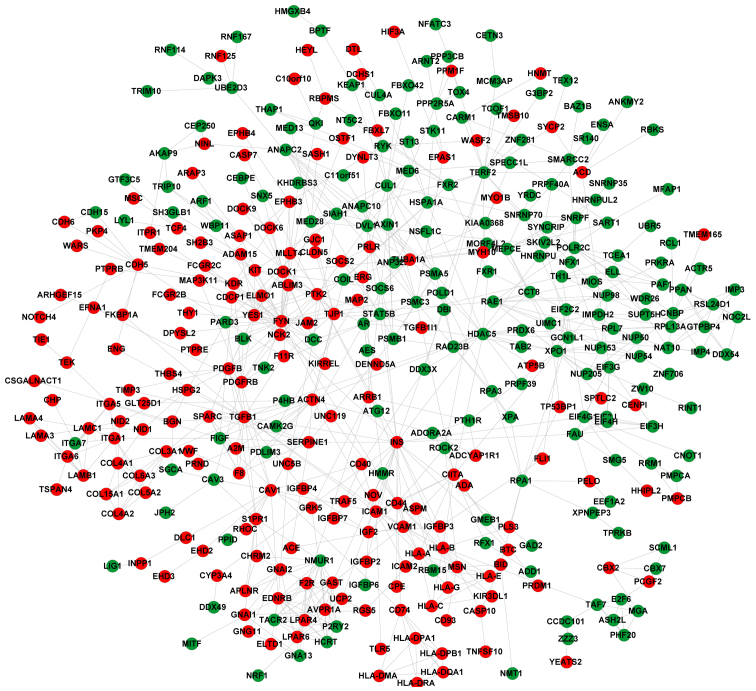
The PPI network for the DEGs contained 409 nodes (including 195 upregulated and 214 downregulated genes) (Fig. 3). Notably, there were various interactions [including ICAM1-TGF-β1, TGFB1-platelet-derived growth factor β (PDGFB), and PDGFB-platelet derived growth factor receptor β (PDGFRB)] in the PPI network. After calculating the function deviation scores of the significantly enriched terms, hierarchical clustering analysis was performed to verify whether these terms clearly distinguished the case group from the control group. The heat map demonstrated that almost all samples were clearly distinguished, indicating that the significantly enriched terms significantly changed at the functional level during OS metastasis (Fig. 4).
Figure 3.
Protein-protein interaction network constructed for the differentially expressed genes. Red and green represent upregulated and downregulated genes, respectively.
Figure 4.
Heat map of hierarchical clustering analysis with functional terms as features. Horizontal and vertical axes represent osteosarcoma biopsy specimens before (yellow)/after (blue) metastasis and the functional terms (red and blue represent upregulation and downregulation, respectively.
Identification of significantly different terms
To identify the significantly different terms in the two groups of samples, logistic regression analysis was performed. The top 25 terms with P<0.01 were listed in Table V, including regulation of cell migration (P=2.15E-05), nucleotide excision repair (P=1.31E-05), Wnt signaling pathway (P=2.32E-03), and cell migration (P=5.56E-03). Specifically, ICAM1, PDGFB, PDGFRB and TGFB1 were enriched in cell migration and regulation of cell migration.
Table V.
The top 25 terms with significant difference in the osteosarcoma biopsy specimens before/after metastasis.
| Term | P-value |
|---|---|
| Blood vessel development | 3.01E-05 |
| Vasculature development | 8.11E-06 |
| RNA processing | 3.97E-05 |
| Positive regulation of macromolecule metabolic process | 1.87E-07 |
| Negative regulation of gene expression | 6.75E-05 |
| Regulation of cell migration | 2.15E-05 |
| Blood vessel morphogenesis | 1.77E-05 |
| DNA replication | 3.78E-05 |
| Nucleotide excision repair | 1.31E-05 |
| ECM-receptor interaction | 5.27E-05 |
| Viral myocarditis | 3.09E-04 |
| Protein catabolic process | 7.33E-04 |
| Oocyte meiosis | 9.26E-04 |
| Negative regulation of metabolic process | 1.85E-03 |
| Mismatch repair | 2.12E-03 |
| Negative regulation of cellular biosynthetic process | 2.32E-03 |
| Wnt signaling pathway | 2.32E-03 |
| mRNA metabolic process | 3.70E-03 |
| Tissue development | 5.16E-03 |
| Cell migration | 5.56E-03 |
| RNA metabolic process | 7.41E-03 |
| Enzyme linked receptor protein signaling pathway | 8.33E-03 |
| Focal adhesion | 8.77E-03 |
| Pathways in cancer | 9.26E-03 |
| Regulation of apoptosis | 9.52E-03 |
Validation of the metastasis-associated terms using other independent datasets
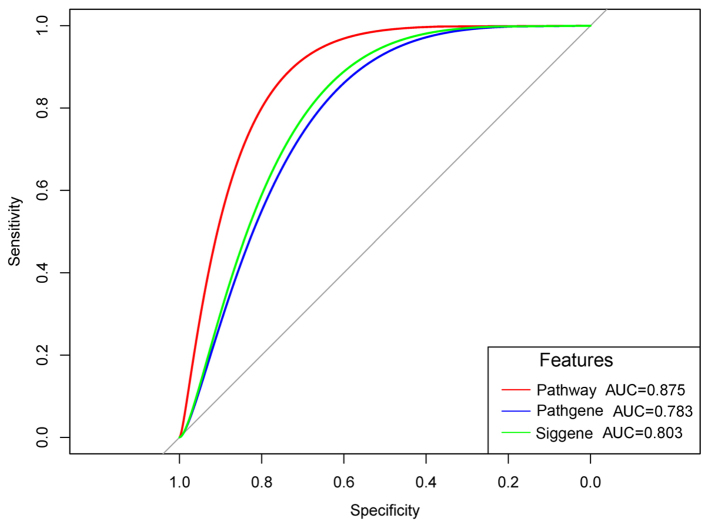
After the microarray datasets, GSE21257 and GSE32981 were merged and normalized, the OS specimens with/without metastasis were predicted. The ROC curves demonstrated that the prediction accuracies of the metastasis-associated terms, the genes enriched in the metastasis-associated terms, and all the DEGs were 0.875, 0.783 and 0.803, respectively (Fig. 5). Thus, the metastasis-associated terms optimized the number of characteristics without reducing the prediction accuracy. In addition, the KM survival curve demonstrated that the survival times of the samples with significant differences in the metastasis-associated terms were significantly changed (P=0.0279) (Fig. 6). In addition, a regression model was used to predict the samples in GSE81892 and GSE14827. The results demonstrated that the prediction accuracies of the model to OS specimens with obvious resistance and OS specimens with drug sensitivity were 82.4 and 65%, respectively. These indicated that the metastasis-associated terms affected drug sensitivity and had better predictive effects for OS specimens with obvious resistance.
Figure 5.
Receiver operating characteristic curves. Red curve indicates the prediction accuracies of the recurrent risk-associated terms (pathway); blue curve indicates the prediction accuracies of the genes enriched in the recurrent risk-associated terms (Pathgene); green curve indicates the prediction accuracies of the differentially expressed genes (Siggene). AUC, area under the curve.
Figure 6.
Kaplan-Meier survival curves. Red and green represent the predicted high- and low-risk groups, respectively.
Discussion
In the present study, a total of 840 DEGs (including 420 upregulated and 420 downregulated genes) were identified in the case group compared with the control group. Unsupervised clustering analysis indicated that the classification effect for the samples in the majority of neurons was good. Hierarchical clustering analysis indicated that the significantly enriched terms significantly changed at a functional level during OS metastasis. Regulation of cell migration, nucleotide excision repair, the Wnt signaling pathway, and cell migration were among the top 25 terms exhibiting significant differences. ROC curves indicated that the metastasis-associated terms optimized the number of characteristics without reducing the prediction accuracy. Additionally, the KM survival curve indicated that the survival times of the samples with significantly different metastasis-associated terms also demonstrated significant changes. In addition, the metastasis-associated terms affected drug sensitivity and had better predictive effect for OS specimens with obvious resistance.
The nucleotide excision repair pathway, which repairs bulky lesions and is correlated with platinum-based chemotherapy and tumor progression (30,31). ERCC excision repair 2, TFIIH core complex helicase subunit, a member of the nucleotide excision repair pathway, rs1799793 is a marker of OS and is associated with the survival of OS patients following platinum therapy (32). The Wnt signaling pathway has aberrant activation in OS, which is associated with the development and progression of this type of bone malignancy (33,34). The genes involved in Wnt and other Wnt signaling pathways have frequent deletions, indicating that the Wnt signaling pathway is genetically inactivated in patients with OS (35). Therefore, nucleotide excision repair and Wnt signaling pathways may be involved in the pathogenesis of OS.
The present study indicated that ICAM1, PDGFB and PDGFRB were enriched in cell migration and regulation of cell migration. Via promoting the expression of ICAM1, chemokine (C-X3-C motif) ligand 1 (termed fractalkine) contributes to migration and metastasis of OS cells, and thus represents a promising therapeutic target for inhibiting OS metastasis (36). Via the PI3K/Akt signaling pathway, ICAM1 expression is upregulated by amphiregulin and thus leads to enhanced cell metastasis of OS (37,38). Kubo et al (39) demonstrate that the expression levels of PDGF and PDGFR potentially predict the prognosis of OS. Takagi et al (40) observed that platelets promote PDGF function in the proliferation of OS cells via activation of the PDGFR-Akt signaling axis. Thus, ICAM1, PDGFB and PDGFRB may act in OS via cell migration and regulation of cell migration. In the PPI network, there were various interactions (including ICAM1-TGFB1, TGFB1-PDGFB, and PDGFB-PDGFRB). Thus, ICAM1, PDGFB and PDGFRB function in OS via interacting with other genes.
Furthermore, TGFB1 was enriched in cell migration and regulation of cell migration. The serum level of TGFB in OS patients with metastasis is higher than that in OS patients without metastasis, indicating that TGFB may be important in OS (41). TGFB2 activity is inhibited by lumican, which subsequently mediates cell adhesion of OS by regulating integrin-β1, phosphorylated SMAD family member 2 (SMAD2) and phosphorylated focal adhesion kinase (42). Wu et al (43) report that the 29 T>C single nucleotide polymorphism of the TGFB1 gene is correlated with the incidence and invasion of OS. The TGFB/SMAD3 and p53 signaling pathways are critical for the doxorubicin-induced cytotoxicity in OS cells (44). Antisense TGFB1 inhibits matrix metallopeptidase 2 and urokinase expression levels, which suppresses the invasion and metastasis of OS cells (45). Thus, TGFB1 contribute to the metastasis of OS via cell migration and regulation of cell migration.
Limited to the number of collected samples, we cannot validate the results of this study in clinical samples. However, we believe that these results based on large data analysis could provide valuable clues for the study of the pathogenesis of metastasis or may aid in the development of novel treatment strategies for OS.
In conclusion, a total of 840 DEGs were identified in the case group. In addition, nucleotide excision repair and the Wnt signaling pathway, as well as cell migration and regulation of cell migration involving ICAM1, PDGFB, PDGFRB and TGFB1 may represent the metastasis-associated pathways of OS.
References
- 1.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N, Eftekhari F, Raymond AK, Yasko AW. Pediatric and Adolescent Osteosarcoma. Springer; New York, NY: 2010. [Google Scholar]
- 3.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–vii325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 4.Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 5.Mavrogenis AF, Abati CN, Romagnoli C, Ruggieri P. Similar survival but better function for patients after limb salvage versus amputation for distal tibia osteosarcoma. Clin Orthop Relat Res. 2012;470:1735–1748. doi: 10.1007/s11999-011-2238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Liang G, Yuan B, Yang C, Gao R, Zhou X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015;36:1477–1486. doi: 10.1007/s13277-014-2631-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JH, Kang XH, Lu P, Miao ZH, Sun GS, Cao XJ, Cao F. Expression of long non-coding RNA MALAT1 in osteosarcoma and its effect on invasiveness and metastatic potential of osteosarcoma cells. Zhonghua Bing Li Xue Za Zhi. 2016;45:561–565. doi: 10.3760/cma.j.issn.0529-5807.2016.08.013. In Chinese. [DOI] [PubMed] [Google Scholar]
- 8.Hou CH, Lin FL, Tong KB, Hou SM, Liu JF. Transforming growth factor alpha promotes osteosarcoma metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem Pharmacol. 2014;89:453–463. doi: 10.1016/j.bcp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, Wu Y, Yin J, Xie X, Shen J, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett. 2014;342:150–158. doi: 10.1016/j.canlet.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Kurenbekova L, Gao Y, Roos A, Creighton CJ, Rao P, Hicks J, Man TK, Lau C, Brown AM, et al. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene. 2015;34:5069–5079. doi: 10.1038/onc.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun XZ, Liao Y, Zhou CM. NKD2 a novel marker to study the progression of osteosarcoma development. Eur Rev Med Pharmacol Sci. 2016;20:2799–2804. [PubMed] [Google Scholar]
- 12.Qian M, Yang X, Li Z, Jiang C, Song D, Yan W, Liu T, Wu Z, Kong J, Wei H, et al. p50-associated COX-2 extragenic RNA (PACER) overexpression promotes proliferation and metastasis of osteosarcoma cells by activating COX-2 gene. Tumour Biol. 2016;37:3879–3886. doi: 10.1007/s13277-015-3838-8. [DOI] [PubMed] [Google Scholar]
- 13.Kelly AD, Haibe-Kains B, Janeway KA, Hill KE, Howe E, Goldsmith J, Kurek K, Perez-Atayde AR, Francoeur N, Fan JB, et al. MicroRNA paraffin-based studies in osteosarcoma reveal reproducible independent prognostic profiles at 14q32. Genome Med. 2013;5:2. doi: 10.1186/gm406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demšar J, Curk T, Erjavec A, Gorup Č, Hočevar T, Milutinovič M, Možina M, Polajnar M, Toplak M, Starič A, et al. Orange: Data mining toolbox in Python. J Mach Learn Res. 2013;14:2349–2353. [Google Scholar]
- 16.Man D, Uda K, Ito Y, Nakano K. Accelerating computation of Euclidean distance map using the GPU with efficient memory access. Int J Parallel Emergent Distrib Syst. 2013;28:383–406. doi: 10.1080/17445760.2012.703195. [DOI] [Google Scholar]
- 17.Nahler G. Pearson correlation coefficient. Springer Top Signal Process. 2009;2:1–4. doi: 10.1007/978-3-642-00296-0_5. [DOI] [Google Scholar]
- 18.Zha W, Li M, Fu Y. Empirical analysis of highway traffic accident in average linkage clustering method. ECJTU. 2010 [Google Scholar]
- 19.Kohonen T. The self-organization map. Adv Inf Knowl Process. 2007;1:3–17. [Google Scholar]
- 20.Tweedie S, Ashburner M, Falls K, Leyland P, McQuilton P, Marygold S, Millburn G, Osumi-Sutherland D, Schroeder A, Seal R, et al. FlyBase Consortium FlyBase: Enhancing Drosophila gene ontology annotations. Nucleic Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatr-Aryamontri A, Breitkreutz BJ, Oughtred R, Boucher L, Heinicke S, Chen D, Stark C, Breitkreutz A, Kolas N, O’Donnell L, et al. The BioGRID interaction database: 2015 update. Nucleic Acids Res. 2015;43:D470–D478. doi: 10.1093/nar/gku1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, et al. Human Protein Reference Database - 2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T. A travel guide to Cytoscape plugins. Nat Methods. 2012;9:1069–1076. doi: 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langfelder P, Mischel PS, Horvath S. When is hub gene selection better than standard meta-analysis? PLoS One. 2013;8:e61505. doi: 10.1371/journal.pone.0061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roush ET, Das T, Nandana P. Cluster software upgrades. Journal. 2009 [Google Scholar]
- 28.May WL. Kaplan-Meier survival analysis. Encyclopedia of Cancer. 2011:1934–1937. doi: 10.1007/978-3-642-16483-5_3196. [DOI] [Google Scholar]
- 29.Yoshii T, Geng Y, Peyton S, Mercurio AM, Rotello VM. Biochemical and biomechanical drivers of cancer cell metastasis, drug response and nanomedicine. Drug Discov Today. 2016;21:1489–1494. doi: 10.1016/j.drudis.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoehlmacher J, Park DJ, Zhang W, Yang D, Groshen S, Zahedy S, Lenz HJ. A multivariate analysis of genomic polymorphisms: Prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br J Cancer. 2004;91:344–354. doi: 10.1038/sj.bjc.6601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat Rev. 1998;24:331–344. doi: 10.1016/S0305-7372(98)90056-1. [DOI] [PubMed] [Google Scholar]
- 32.Biason P, Hattinger CM, Innocenti F, Talamini R, Alberghini M, Scotlandi K, Zanusso C, Serra M, Toffoli G. Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics J. 2012;12:476–483. doi: 10.1038/tpj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Y, Cai T, Chen Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem. 2014;115:625–631. doi: 10.1002/jcb.24708. [DOI] [PubMed] [Google Scholar]
- 34.Lin CH, Ji T, Chen CF, Hoang BH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. 2014;804:33–45. doi: 10.1007/978-3-319-04843-7_2. [DOI] [PubMed] [Google Scholar]
- 35.Du X, Yang J, Yang D, Tian W, Zhu Z. The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer. 2014;14:450. doi: 10.1186/1471-2407-14-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu JF, Tsao YT, Hou CH. Fractalkine/CX3CL1 induced intercellular adhesion molecule-1-dependent tumor metastasis through the CX3CR1/PI3K/Akt/NF-κB pathway in human osteosarcoma. Oncotarget. 2016;8:54136–54148. doi: 10.18632/oncotarget.11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JF, Tsao YT, Hou CH. Amphiregulin enhances inter-cellular adhesion molecule-1 expression and promotes tumor metastasis in human osteosarcoma. Oncotarget. 2015;6:40880–40895. doi: 10.18632/oncotarget.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin YM, Chang ZL, Liao YY, Chou MC, Tang CH. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma. Cancer Lett. 2013;328:135–143. doi: 10.1016/j.canlet.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Kubo T, Piperdi S, Rosenblum J, Antonescu CR, Chen W, Kim HS, Huvos AG, Sowers R, Meyers PA, Healey JH, et al. Platelet-derived growth factor receptor as a prognostic marker and a therapeutic target for imatinib mesylate therapy in osteosarcoma. Cancer. 2008;112:2119–2129. doi: 10.1002/cncr.23437. [DOI] [PubMed] [Google Scholar]
- 40.Takagi S, Takemoto A, Takami M, Oh-Hara T, Fujita N. Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci. 2014;105:983–988. doi: 10.1111/cas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S, Yang S, Sun G, Huang W, Zhang Y. Transforming growth factor-beta polymorphisms and serum level in the development of osteosarcoma. DNA Cell Biol. 2014;33:802–806. doi: 10.1089/dna.2014.2527. [DOI] [PubMed] [Google Scholar]
- 42.Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN. Lumican regulates osteosarcoma cell adhesion by modulating TGFβ2 activity. Int J Biochem Cell Biol. 2011;43:928–935. doi: 10.1016/j.biocel.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Zhao J, He M. Correlation between TGF-β1 gene 29 T>C single nucleotide polymorphism and clinicopathological characteristics of osteosarcoma. Tumour Biol. 2015;36:5149–5156. doi: 10.1007/s13277-015-3168-x. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Xia P, Zhang H, Liu B, Shi Y. P53 is required for Doxorubicin-induced apoptosis via the TGF-beta signaling pathway in osteosarcoma-derived cells. Am J Cancer Res. 2015;6:114–125. [PMC free article] [PubMed] [Google Scholar]
- 45.Bao TZ, Zao HW, Zheng QX, Liu W, Liu Y. Effects of antisense transforming growth factor-β1 on the factors related to metastasis in osteosarcoma. Chin J Exp Surg. 2005;4:477–478. [Google Scholar]