Abstract
The present study aimed to perform microRNA (miRNA/miR) expression profiling of the thalamus (T), the anterior cingulate (AC), the dorsal horn of the spinal cord (DHSC) and the blood (B) in post-complete brachial plexus avulsion (CBPA) pain model, and analyze biological functions. Neuropathic pain was induced in Sprague-Dawley rats by CBPA. Animal behavioral tests were performed to differentiate the pain and control groups. DHSC, T, AC and B tissues were collected from the two groups for miRNA array analysis. The predicted mRNA targets were investigated by Gene Ontology analysis and pathway analysis. The results revealed that in the post-CBPA pain model, there were 10 differentially expressed miRNAs revealed among 4 different tissues. A total of 4 microRNAs in the AC and 3 microRNAs in the T were shown to be significantly upregulated. The functions of the differentially expressed miRNAs in the AC and T were synergetic in the aspect of positive regulation of neuron apoptotic process, inhibition of long-term potentiation and formation of synapse plasticity. miR-30c-1-3p and its predicted genes [calcium/calmodulin dependent protein kinase IIβ (Camk2b) and protein kinase Cγ (Prkcg)] existed in the AC and T groups with significant changes in expression. There were 2 miRNAs in the DHSC and B groups, respectively, with significant downregulation. The function of the change in miRNAs in the DHSC group was opposite to that in the AC and T groups. The differentially expressed microRNAs in the B group were revealed to be negative for the regulation of cell apoptosis. In conclusion, the central nerve groups (AC and T) and the peripheral nerve group (DHSC) exhibited contrasting effects on synapse plasticity and neuron apoptosis. miR-30c-1-3p and its predicted genes (Camk2b and Prkcg) existed in the AC and T groups with significant changes in expression.
Keywords: microRNA, brachial plexus, neuropathic pain, synapse plasticity, apoptosis
Introduction
Neuropathic pain caused by nerve injury remains an intractable disease due to a lack of satisfactory treatment (1–3). In 2009, Ciaramitaro et al (4) carried out a multicenter prospective study on the prevalence of neuropathic pain after traumatic brachial plexus injury. From the 107 patients enrolled, 56% had neuropathic pain. Neuropathic pain impaired the quality of life and caused depression. Brachial plexus avulsion (BPA) induces a characteristic persistent oppression and intermittent shooting pain, which is often difficult to cure (5,6). The pain could be experienced as burning or a feeling of compression. Pain after BPA is resistant to most traditional pain relief treatments (7) due to a lack of understanding of the cellular or molecular mechanisms in the occurrence and development of pain (8,9).
As long-term sensitivity changes in neuropathic pain are associated with changes in gene regulation, it is worthy of note to determine if the microRNAs (miRNAs/miRs) that regulate genes taking part in the nociceptive pathways affect the occurrence and development of pain (10). The key roles of miRNAs in nervous system development and pathophysiology is increasingly evident (11), however, certain facts remain to be elucidated. Understanding the gene regulatory events in miRNA-mediated neuropathic pain may provide a pathway for identifying biomarkers or finding novel therapeutic targets (12). Since pathophysiological changes in pain are associated with altered expression in pain-associated proteins, miRNA may be a promising tool for controlling inflammatory and neuropathic pain by regulating gene and protein expression in pain pathways (13). Few studies have been performed on pain following brachial plexus injury (14–16).
In the present study, miRNA expression profiling was performed of the thalamus (T), the anterior cingulate (AC), the dorsal horn of the spinal cord (DHSC) and the blood (B) in a neuropathic pain model 4 weeks after complete brachial plexus avulsion (BPA) surgery. The combination of experimental and bioinformatics methods was applied to identify the biological and cytological functions that were influenced by the changes in miRNA expression (17). These data provide further evidence in support of the hypothesis that post-complete BPA pain may be regulated by miRNA.
Materials and methods
Study approval
Animal handling and procedures used in this study were in agreement with the guidelines of the Animal Care and Use Committee of Fudan University (Shanghai, China). Neuropathic pain was induced in male Sprague-Dawley rats (n=40; weight, 200–250 g; age, 8 weeks; supplied by the Department of Laboratory Animal Science, Fudan University, Shanghai, China) by CBPA. The rats were kept in an environment with a temperature of 20°C and humidity of 50% and were maintained on a 12/12-h light/dark cycle and allowed free access to food and water. The animal use protocol was reviewed and approved by the Animal Ethics and Welfare Committee of Huashan Hospital affiliated to Fudan University.
Animal behavioral tests
Mechanical allodynia
The rats were placed on a metal mesh floor, covered by a transparent plastic box and raised 30 cm above the floor. The plantar surface of the paw was stimulated with a series of ascending force von Frey monofilaments. The threshold was taken as the lowest force that evoked a brisk withdrawal response to one of five repetitive stimuli (7). A withdrawal response was considered valid only when the paw was completely removed from the platform.
Cold allodynia
Cold allodynia was measured by an acetone spray test, as described by Choi et al (18). Briefly, 250 ml acetone was squirted onto the mid-plantar surface of the paw. The withdrawal responses were evaluated on a scale of 0–3 points: 0 points, the paw was not moved; 1 point, a response in which the paw had little or no weight born on it; 2 points, a response in which the paw was elevated and was not in contact with any surface; and 3 points, a vigorous response in which the rat licked, bit or shook the paw.
All behavioral tests were performed by the same technician who was blinded to the study groups and identification of animals in order to avoid subjective differences in interpretation, which could occur with different observers (19).
Surgery procedure
Following the animal behavioral tests, the rats were anesthetized with sodium pentobarbital injected intraperitoneally. The CBPA rat model (20) was used to induce neuropathic pain. The brachial plexus was approached through a horizontal supraclavicular incision. The sternocleidomastoid muscle was cut off and the omohyoid muscle was pulled aside, leaving the transverse cervical vessels intact. The brachial plexus was located in the scalene fissure, including upper, middle and lower trunks. The complete brachial plexus was grasped with forceps and extracted from the spinal cord by traction. The tissue layers were then brought together and the skin was closed with 4-0 silk sutures.
These rats were maintained on a 12/12 h light/dark cycle and allowed free access to food and water for one month. At the end of the 4 weeks, paw withdrawal threshold to mechanical stimuli was assessed using von Frey filaments and cold allodynia was measured by an acetone spray test, as previously described.
According to the comparison of the behavioral test results (both mechanical allodynia and cold allodynia tests) prior to and following surgery, the rats were divided into two groups: The pain and control group. In the pain group, the paw withdrawal thresholds decreased and cold allodynia points rose following surgery, while the two results did not significantly change following surgery in the control group.
At the completion of behavioral testing, each group (n=3) was euthanized using CO2. The flow rate for CO2 euthanasia displaced 20% of the chamber volume/min according to the 2013 edition of the American Veterinary Medical Association Guidelines for the Euthanasia of Animals. The following tissues were collected from these rats for microRNA analysis: DHSC, T, AC and B.
miRNA microarray
Total RNA was isolated using an miRNeasy mini kit and RNase-Free DNase set (both Qiagen, Inc., Valencia, CA, USA). RNA quality was checked with denaturing agarose gel (1.5%) electrophoresis and nucleic acid staining. Samples with 28S and 18S rRNA bands were resolved into two discrete bands that had no significant smearing below each band, and the 28S rRNA band intensity, which was approximately twice that of the 18S rRNA band, was used for subsequent procedures. Total RNA (1 µg) was labeled with Affymetrix® FlashTag™, Biotin HSR RNA Labeling kits (Affymetrix, Inc., Santa Clara, CA, USA). Next, samples were hybridized to a GeneChip® miRNA 4.0 array (Affymetrix, Inc.) at 60 rpm, at 48°C for 16 h. Fluorescent images of microarray slides were scanned using a GeneChip® Scanner 3000 7G (Affymetrix, Inc.). All CEL files from the miRNA 4.0 chips were normalized using Expression Console software (version 1.3.1; Affymetrix, Inc.). Affymetrix miRNA arrays are designed to contain all miRNAs in miRBase release 20 (http://tools.thermofisher.com/content/sfs/brochures/miRNA_4-0_and_4-1_datasheet.pdf).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
All procedures were performed according to the corresponding manufacturer's protocols. Expression of the miRNAs was examined with the SYBR-Green RT-PCR kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Results were normalized to U6 expression. RT was performed with 1 mg total RNA using the ThermoScript RT-PCR system (Invitrogen; Thermo Fisher Scientific. Inc.) for first-strand complementary DNA (cDNA) synthesis. For qPCR, cDNA was amplified using the MyiQ Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Quantitative cycle values were obtained from each amplification curve using iQ5 Optical System Software (version 2.1) provided by the manufacturer (Bio-Rad Laboratories, Inc.). The 2−ΔΔct method was used (21). Primer sequences will be provided upon request. The primers used were as follows: rno-miR-3573-5p forward, 5′-ACACTCCAGCTGGGTGAGGGGCAGTGATAGAAAGGA-3′ and reverese, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTC AGTTGAGTCCTTTC-3′; rno-miR-3074 forward, 5′-ACACTCCAGCTGGGGATATCAGCTCAGTAGGCACCG-3′ and reverse, CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGGTGCC-3′; rno-miR-25-5p forward, 5′-ACACTCCAGCTGGGAGGCGGAGACACGGGCAATTGC-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGCAATTGC-3′; rno-miR-702-3p forward, 5′-ACACTCCAGCTGGGTGCCCACCCTTTACCCCACTCCA-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGAGTG-3′; rno-miR-30c-1-3p forward, 5′-ACACTCCAGCTGGGCTGGGAGAGGGTTGTTTACTCC-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGAGTAAA-3′; rno-miR-93-3p forward, 5′-ACA CTCCAGCTGGGACTGCTGAGCTAGCACTTCCCGA-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCGGGAAG-3′; rno-miR-873-5p forward, 5′-ACACTCCAGCTGGGGCAGGAACTTGTGAGTCTCCT-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGGAGAC-3′; rno-miR-455-3p forward, 5′-ACACTCCAGCTGGGGCAGTCCACGGGCATATA-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGTGTATA-3′; rno-miR-32-3p forward, 5′-ACACTCCAGCTGGGTGGACGGAGAACTGAT-3′ and reverse, 5′-CTCA ACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACCCTTAT-3′; rno-miR-184 forward, 5′-ACACTCCAGCTGGGGCAATTTAGTGTGTGTGA-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAATATCAC-3′; universal miR reverse, 5′-TGTCGTGGAGTCGGCAATTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATA-3′ and reverse, 5′-ATATGGAACGCTTCACGAATTTGC-3′. The thermocycling conditions were as follows: stage 1 (1 cycle), pre-degeneration at 95°C for 5 sec; stage 2 (40 cycles), denaturation at 95°C for 5 sec and primer annealing at 61°C for 30 sec; stage 3 (1 cycle), sufficient extention at 72°C for 1 min and cooling down at 4-10°C.
Bioinformatic evaluation
For the differential regulation miRNAs, the predicted, but not (yet) verified mRNA targets were investigated. Since hundreds were found for each, Gene Ontology (GO) analysis (http://www.geneontology.org/page/go-database) was performed using the GO enrichment analysis software tool kit (22,23) and pathway analysis was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway/brite/module mapping tools (24) (http://www.kegg.jp/kegg/pathway.html#mapping) to define which gene molecular function terms were enriched that characterize the collection of target genes in comparison with the genome in general.
Statistical analysis
Due to the small sample size (n=3), the random variance model t-test was adopted to filter the differentially expressed microRNAs between the control and pain groups using GraphPad 5.0. Following the significance analysis and false discovery rate analysis, differentially expressed genes were selected according to their P-values. P<0.05 was considered to indicate a statistically significant difference.
Results
miRNA microarray
To functionally investigate a possible link between miRNA expression and the post-brachial plexus injury neuropathic pain, the differential expression of miRNAs between groups in 4 different tissues, DHSC, T, AC and B, was analyzed. The expression of 728 miRNAs was detected in each tissue and divided into three types according to the changes: Decrease, increase and no change. The statistical results of the microRNA array analysis of the 4 different tissues are shown in Table I (P<0.05; n=3). The statistical analysis software used was GraphPad 5.0.
Table I.
miRNAs with statistical differences in expression between the pain and control groups.
| miRNA | P-value | FDR | Fold-change | Regulation trend | Sequence length, nt | Sequence (5′-3′) |
|---|---|---|---|---|---|---|
| DHSC group | ||||||
| miR-3573-5p | 0.0068 | 1.000 | 0.70 | Down | 22 | UGAGGGGCAGUGAUAGAAAGGA |
| miR-3074 | 0.041413 | 1.000 | 0.90 | Down | 22 | GAUAUCAGCUCAGUAGGCACCG |
| miR-1193-3p | 0.025148 | 1.000 | 1.23 | Up | 22 | UAGGUCACCCGUUUUACUAUCC |
| miR-410-5p | 0.040567 | 1.000 | 1.23 | Up | 21 | AGGUUGUCUGUGAUGAGUUCG |
| miR-340-5p | 0.018692 | 1.000 | 1.48 | Up | 22 | UUAUAAAGCAAUGAGACUGAUU |
| AC group | ||||||
| miR-208a-3p | 0.018404 | 0.997 | 0.37 | Down | 18 | AUAAGACGAGCAAAAAGC |
| miR-6216 | 0.028229 | 0.997 | 0.71 | Down | 23 | GAUACACAGAGGCAGGAGGAGAA |
| miR-3580-3p | 0.034532 | 0.997 | 0.72 | Down | 22 | UGACUAGGGUAGUAUGAGUAGA |
| miR-205 | 0.045209 | 0.997 | 1.41 | Up | 23 | UCCUUCAUUCCACCGGAGUCUGU |
| miR-25-5p | 0.030307 | 0.997 | 1.46 | Up | 22 | AGGCGGAGACACGGGCAAUUGC |
| miR-702-3p | 0.035705 | 0.997 | 1.49 | Up | 23 | UGCCCACCCUUUACCCCACUCCA |
| miR-501-3p | 0.024475 | 0.997 | 1.65 | Up | 23 | AAUGCACCCGGGCAAGGAUUUGG |
| let-7f-5p | 0.035378 | 0.997 | 1.70 | Up | 22 | UGAGGUAGUAGAUUGUAUAGUU |
| miR-381-5p | 0.032536 | 0.997 | 1.80 | Up | 22 | AGCGAGGUUGCCCUUUGUAUAU |
| miR-30c-1-3p | 0.011214 | 0.997 | 1.87 | Up | 22 | CUGGGAGAGGGUUGUUUACUCC |
| miR-671 | 0.028852 | 0.997 | 1.87 | Up | 21 | UCCGGUUCUCAGGGCUCCACC |
| miR-184 | 0.006879 | 0.997 | 2.42 | Up | 22 | UGGACGGAGAACUGAUAAGGGU |
| T group | ||||||
| miR-34b-5p | 0.04851 | 0.971 | 0.62 | Down | 23 | AGGCAGUGUAAUUAGCUGAUUGU |
| miR-181d-3p | 0.0342 | 0.971 | 0.65 | Down | 20 | CCACCGGGGGAUGAAUGUCA |
| miR-484 | 0.027441 | 0.971 | 0.66 | Down | 22 | UCAGGCUCAGUCCCCUCCCGAU |
| miR-370-5p | 0.016922 | 0.971 | 0.70 | Down | 24 | CAGGUCACGUCUCUGCAGUUACAC |
| miR-9b-5p | 0.008269 | 0.971 | 0.78 | Down | 19 | UUCGGUUAUCUAGCUUUAU |
| miR-1912-3p | 0.017277 | 0.971 | 0.79 | Down | 19 | CACAGAACAUGCAGUGAGA |
| miR-759 | 0.018461 | 0.971 | 0.81 | Down | 22 | GCAGAGUGCAAACAAUUUUGAC |
| miR-463-5p | 0.036635 | 0.971 | 0.81 | Down | 21 | UACCUAAUUUGUUGUCCAUCA |
| miR-193-3p | 0.045474 | 0.971 | 0.81 | Down | 22 | AACUGGCCUACAAAGUCCCAGU |
| miR-802-5p | 0.040079 | 0.971 | 0.83 | Down | 21 | UCAGUAACAAAGAUUCAUCCU |
| miR-218a-5p | 0.019683 | 0.971 | 0.84 | Down | 21 | UUGUGCUUGAUCUAACCAUGU |
| miR-31b | 0.031174 | 0.971 | 0.84 | Down | 19 | CUAUGCCAGCAUCUUGCCU |
| miR-3593-5p | 0.033797 | 0.971 | 0.87 | Down | 22 | UGGCCUCCGCAGGGUUGAAGCU |
| miR-3570 | 0.039038 | 0.971 | 1.15 | Up | 22 | GGUACAAUCAACGGUCGAUGGU |
| miR-3588 | 0.018922 | 0.971 | 1.19 | Up | 22 | UCACAAGUUAGGGUCUCAGGGA |
| miR-664-3p | 0.039461 | 0.971 | 1.30 | Up | 22 | UAUUCAUUUACUCCCCAGCCUA |
| miR-488-3p | 0.025485 | 0.971 | 1.41 | Up | 21 | UUGAAAGGCUGUUUCUUGGUC |
| miR-30c-1-3p | 0.009129 | 0.971 | 2.03 | Up | 22 | CUGGGAGAGGGUUGUUUACUCC |
| miR-106b-3p | 0.018801 | 0.971 | 2.08 | Up | 22 | CCGCACUGUGGGUACUUGCUGC |
| miR-93-3p | 0.012217 | 0.971 | 2.69 | Up | 23 | ACUGCUGAGCUAGCACUUCCCGA |
| miR-28-5p | 0.041407 | 0.971 | 2.85 | Up | 22 | AAGGAGCUCACAGUCUAUUGAG |
| miR-873-5p | 0.02098 | 0.971 | 2.93 | Up | 21 | GCAGGAACUUGUGAGUCUCCU |
| B group | ||||||
| miR-455-3p | 0.028562 | 0.997 | 0.32 | Down | 22 | GCAGUCCACGGGCAUAUACACU |
| miR-32-3p | 0.030898 | 0.997 | 0.47 | Down | 22 | GCAAUUUAGUGUGUGUGAUAUU |
| miR-466b-2-3p | 0.032782 | 0.997 | 0.57 | Down | 21 | AUAUACAUACACACAUACACA |
| miR-702-3p | 0.035054 | 0.997 | 0.62 | Down | 23 | UGCCCACCCUUUACCCCACUCCA |
| miR-742-5p | 0.035279 | 0.997 | 0.72 | Down | 21 | UACUCACAUGGUUGCUAAUCA |
| miR-195-3p | 0.021132 | 0.997 | 0.73 | Down | 23 | CCAAUAUUGGCUGUGCUGCUCCA |
| miR-509-3p | 0.045567 | 0.997 | 0.77 | Down | 22 | UGAUUGACAUGUCUGCAGUGGA |
miRNA/miR, microRNA; FDR, false discovery rate; DHSC, dorsal horn of the spinal cord; AC, anterior cingulate; T, thalamus; B, blood.
In the DHSC group, 332 miRNAs exhibited decreased expression, 378 miRNAs exhibited increased expression and 18 miRNAs exhibited no change. The statistical analysis showed that 2 miRNAs were downregulated and 3 miRNAs were upregulated significantly.
In the T group, 305 miRNAs exhibited decreased expression, 409 miRNAs exhibited increased expression and 14 miRNAs exhibited no change. The statistical analysis showed that 13 miRNAs were downregulated and 9 miRNAs were upregulated significantly.
In the AC group, 348 miRNAs exhibited decreased expression, 359 miRNAs exhibited increased expression and 21 miRNAs exhibited no change. The statistical analysis showed that 3 miRNAs were downregulated and 9 miRNAs were upregulated significantly.
In the B group, 362 miRNAs exhibited decreased expression, 352 miRNAs exhibited increased expression and 14 miRNAs exhibited no change. The statistical analysis showed that 7 miRNAs were downregulated and no miRNAs were upregulated significantly.
PCR verification
To validate the microarray results, RT-qPCR was performed for these differentially regulated (diff-reg) miRNAs in the 4 groups. It was found that the relative expression of 10 miRNAs among them were significantly altered, which coincided with the results of the microarray. The 10 miRNAs were miR-3573-5p (DHSC), miR-3074 (DHSC), miR-30c-1-3p (AC and T), miR-702-3p (AC), miR-184 (AC), miR-25-5p (AC), miR-873-5p (T), miR-93-3p (T), miR-455-3p (B) and miR-32-3p (B). miR-30c-1-3p with a significant change appeared in both the AC and T groups. The other miRNAs could not be verified by PCR. The statistical analysis showed that miR-3573-5p (DHSC), miR-3074 (DHSC), miR-455-3p (B) and miR-32-3p (B) were downregulated, and that miR-30c-1-3p (AC and T), miR-702-3p (AC), miR-184 (AC), miR-25-5p (AC), miR-873-5p (T) and miR-93-3p (T) were upregulated significantly (Table II).
Table II.
miRNAs with differential expression by PCR verification between pain and control groups.
| miRNA | P-value | FDR | Fold-change | Regulation trend | Sequence length, nt | Sequence (5′-3′) |
|---|---|---|---|---|---|---|
| DHSC group | ||||||
| rno-miR-3573-5p | 0.0067996 | 1.000 | 0.70 | Down | 22 | UGAGGGGCAGUGAUAGAAAGGA |
| rno-miR-3074 | 0.0414127 | 1.000 | 0.90 | Down | 22 | GAUAUCAGCUCAGUAGGCACCG |
| AC group | ||||||
| rno-miR-25-5p | 0.0303073 | 0.997 | 1.46 | Up | 22 | AGGCGGAGACACGGGCAAUUGC |
| rno-miR-702-3p | 0.0357045 | 0.997 | 1.49 | Up | 23 | UGCCCACCCUUUACCCCACUCCA |
| rno-miR-30c-1-3p | 0.0112141 | 0.997 | 1.87 | Up | 22 | CUGGGAGAGGGUUGUUUACUCC |
| rno-miR-184 | 0.0068785 | 0.997 | 2.42 | Up | 22 | UGGACGGAGAACUGAUAAGGGU |
| T group | ||||||
| rno-miR-30c-1-3p | 0.0091288 | 0.971 | 2.03 | Up | 22 | CUGGGAGAGGGUUGUUUACUCC |
| rno-miR-93-3p | 0.0122168 | 0.971 | 2.69 | Up | 23 | ACUGCUGAGCUAGCACUUCCCGA |
| rno-miR-873-5p | 0.02098 | 0.971 | 2.93 | Up | 21 | GCAGGAACUUGUGAGUCUCCU |
| B group | ||||||
| rno-miR-455-3p | 0.0285622 | 0.997 | 0.32 | Down | 22 | GCAGUCCACGGGCAUAUACACU |
| rno-miR-32-3p | 0.0308978 | 0.997 | 0.47 | Down | 22 | GCAAUUUAGUGUGUGUGAUAUU |
miRNA/miR, microRNA; FDR, false discovery rate; DHSC, dorsal horn of the spinal cord; AC, anterior cingulate; T, thalamus; B, blood.
Bioinformatics analysis of the diff-reg miRNAs
For these diff-reg miRNAs with PCR verification, the predicted, but not (yet) verified, mRNA targets were investigated.
In the DHSC group, there were 27 intersection genes (Table III), which were involved in neuropathic pain according to GO and pathway analyses. GO analysis showed that the downregulation function of miR-3573-5p and miR-3074 associated with neuropathic pain included 'axon guidance', 'synaptic transmission', 'synapse maturation', 'excitatory post-synaptic membrane potential', 'neuron apoptotic process', 'macrophage activation involved in immune response', 'cell chemotaxis', 'neuron migration' and 'neuron differentiation'. The target genes of miR-3573-5p and miR-3074 took part in several pathways, including the 'calcium signaling pathway', 'cholinergic synapse', 'GABAergic synapse', 'glutamatergic synapse', the 'HIF-1 signaling pathway', the 'MAPK signaling pathway', the 'mTOR signaling pathway', the 'notch signaling pathway', the 'PI3K-Akt signaling pathway', 'synaptic vesicle cycle' and the 'Wnt signaling pathway'. Downregulation of miR-3573-5p and miR-3074 would result in upregulation of the target genes, which inhibited neuron apoptotic process, promoted long-term potentiation and synapse plasticity, and strengthened cell proliferation and differentiation.
Table III.
Intersection genes involved in neuropathic pain in GO and pathway analyses.
| Group | Genes |
|---|---|
| DHSC | Grin1, Grm4, Grm6, Itga5, Notch1, P2rx1, Prkcg, Shank1, Peg12, Shank3, Srf, Stx1b, Stxbp1, Syk, Vegfa, Vhl, Wnt8b, Wif1, Adra1b, Cacna1a, Cd38, Chat, Ddit4, Efna2, Fgfr3, Gad1, Gng11 |
| AC | Camk2b, Csf1, Hdac1, Kdr, Mapk3, Prkcg, Tgfa, Vegfa |
| T | Camk2b, Itgb1, Prkcg |
| B | Scn1a, Kdr, Nos3 |
Genes listed belong to the targets of differentially expressed microRNAs according to GO and pathway analyses. GO, Gene Ontology; DHSC, dorsal horn of the spinal cord; AC, anterior cingulate; T, thalamus; B, blood.
In the T group, there were 3 intersection genes, which were involved in neuropathic pain according to GO and pathway analyses (Table III). GO analysis showed that the upregulation function of miR-30c-1-3p, miR-873-5p and miR-93-3p associated with neuropathic pain included 'neurotransmitter uptake', 'neuron apoptotic process', 'sensory perception of pain', 'long-term memory' and 'neuron projection development'. The target genes of miR-30c-1-3p, miR-873-5p and miR-93-3p took part in several pathways, including the 'calcium signaling pathway', 'cholinergic synapse', 'dopaminergic synapse', the 'ErbB signaling pathway', 'GABAergic synapse', 'glutamatergic synapse', the 'hedgehog signaling pathway', the 'HIF-1 signaling pathway', 'long-term potentiation', the 'MAPK signaling pathway', the 'mTOR signaling pathway', the 'neurotrophin signaling pathway', the 'notch signaling pathway', the 'PI3K-Akt signaling pathway', 'retrograde endocannabinoid signaling', 'serotonergic synapse' and the 'Wnt signaling pathway'. Upregulation of these miRNAs would result in downregulation of the target genes, which promoted neuron apoptotic apoptosis, reduced long-term potentiation and synapse plasticity, and inhibited cell proliferation and differentiation.
In the AC group, there were 8 intersection genes, which were involved in neuropathic pain according to GO and pathway analyses (Table III). GO analysis indicated that the upregulation function of miR-30c-1-3p, miR-702-3p, miR-184 and miR-25-5p associated with neuropathic pain included 'neuron apoptotic process', 'regulation of neuronal synaptic plasticity', 'axon guidance', 'synaptic transmission (glutamatergic)', 'axon extension', 'axonogenesis', 'long-term synaptic potentiation', 'sensory perception of pain', 'response to cold', 'response to stress', 'neuron differentiation', 'neuron migration', 'neuron cell-cell adhesion' and 'long-term memory'. The target genes of miR-30c-1-3p, miR-702-3p, miR-184 and miR-25-5p took part in several pathways, including the 'calcium signaling pathway', 'cholinergic synapse', the 'ErbB signaling pathway', 'GABAergic synapse', 'glutamatergic synapse', the 'hedgehog signaling pathway', the 'HIF-1 signaling pathway', 'long-term depression', the 'mTOR signaling pathway', the 'MAPK signaling pathway', 'dopaminergic synapse', 'long-term potentiation', 'serotonergic synapse', the 'neurotrophin signaling pathway', the 'notch signaling pathway', the 'PI3K-Akt signaling pathway', 'retrograde endocannabinoid signaling' and the 'Wnt signaling pathway'. Upregulation of these miRNAs would result in downregulation of the target genes, which promoted neuron apoptotic apoptosis, reduced long-term potentiation and synapse plasticity, and inhibited cell proliferation and differentiation.
In the B group, there were 3 intersection genes, which were involved in neuropathic pain according to GO and pathway analyses (Table III). GO analysis indicated that the downregulation function of miR-455-3p and miR-32-3p associated with neuropathic pain included 'neuron projection morphogenesis', 'cell migration', 'cell proliferation' and 'apoptotic process'. The target genes of miR-455-3p and miR-32-3p took part in four pathways: The 'calcium signaling pathway', the 'HIF-1 signaling pathway', the 'PI3K-Akt signaling pathway' and the 'VEGF signaling pathway'. Downregulation of miR-455-3p and miR-32-3p would induce upregulation of the target genes, which inhibited cell apoptosis, and promoted vascularization, cell migration and proliferation.
In the post-brachial plexus injury neuropathic pain model, the central nerve groups (AC and T) and the peripheral nerve group (DHSC) exhibited contrasting effects on synapse plasticity and neuron apoptosis. The former showed downregulation of synapse plasticity, cell proliferation and differentiation, while the later showed upregulation.
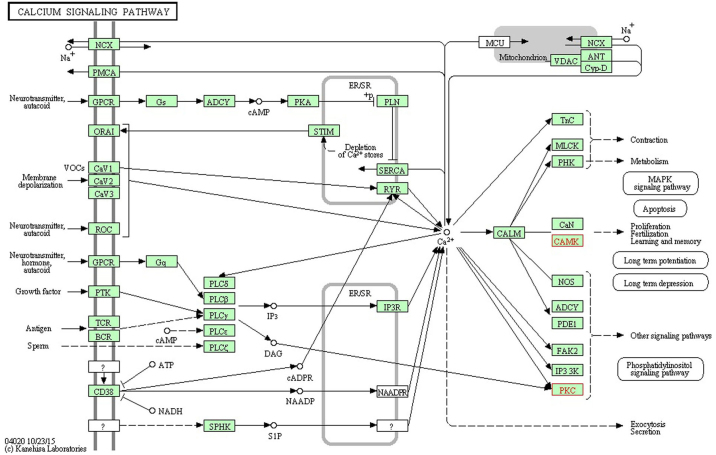
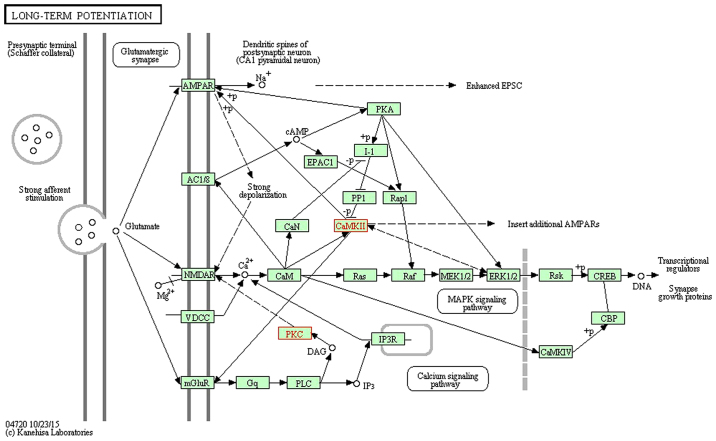
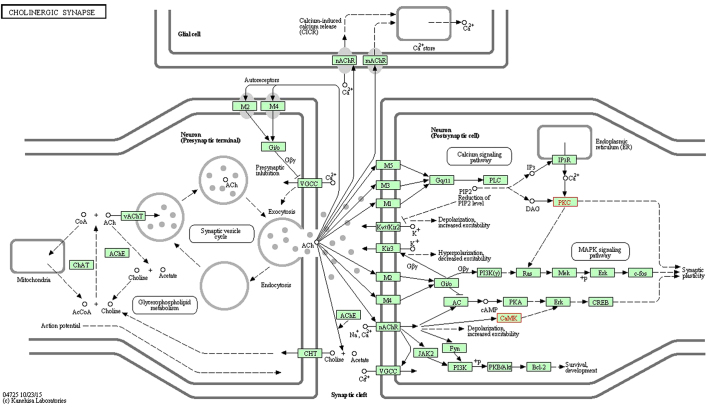
miR-30c-1-3p with a significant change appeared in the AC and T groups, which significantly increased in the pain model. The functions of miR-30c-1-3p associated with neuropathic pain included the 'calcium signaling pathway', 'cholinergic synapse', 'dopaminergic synapse', the 'HIF-1 signaling pathway', the 'ErbB signaling pathway', 'long-term potentiation', the 'neurotrophin signaling pathway', the 'Wnt signaling pathway', 'GABAergic synapse', 'glutamatergic synapse', the 'MAPK signaling pathway', the 'mTOR signaling pathway', 'retrograde endocannabinoid signaling', 'serotonergic synapse' and 'long-term depression'. The predicted genes of miR-30c-1-3p were calcium/calmodulin dependent protein kinase IIβ (Camk2b) and protein kinase Cγ (Prkcg), which were validated by PCR to exhibit significant downregulation in the AC and T groups. Camk2b and Prkcg took part in the 'calcium signaling pathway' (www.kegg.jp/dbget-bin/www_bget?map04020; Fig. 1), 'long-term potentiation' (www.kegg.jp/dbget-bin/www_bget?map04720; Fig. 2) and 'cholinergic synapse' (www.kegg.jp/dbget-bin/www_bget?map04725; Fig. 3). All figures were obtained using the KEGG pathway mapping tool in the KEGG database (24–26). The downregulation of Camk2b and Prkcg would inhibit cell proliferation and synapse growth protein formation, which made synapse plasticity decrease.
Figure 1.
Calcium signaling pathway, with red font indicating target genes. The downregulation of Camk2b and Prkcg would inhibit cell proliferation. This figure was obtained using the KEGG PATHWAY mapping tool in the KEGG database (www.kegg.jp/dbget-bin/www_bget?map04020) and published with permission from Kanehisa Laboratories (23–25). KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 2.
Long-term potentiation, with red font indicating target genes. The downregulation of Camk2b and Prkcg would inhibit synapse growth protein formation. This figure was obtained using the KEGG PATHWAY mapping tool in the KEGG database (www.kegg.jp/dbget-bin/www_bget?map04720) and published with permission from Kanehisa Laboratories (23–25). KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 3.
Cholinergic synapse, with red font indicating target genes. The downregulation of Camk2b and Prkcg would decrease synapse plasticity. This figure was obtained using the KEGG PATHWAY mapping tool in the KEGG database (www.kegg.jp/dbget-bin/www_bget?map04725) and published with permission from Kanehisa Laboratories (23-25). KEGG, Kyoto Encyclopedia of Genes and Genomes.
Discussion
It has previously been revealed that descending facilitatory modulation is a key mechanism underlying the induction and maintenance of neuropathic pain (27). It is well documented that spinal nociception is powerfully modulated by an endogenous descending inhibitory system (27), although descending control is bi-directional via inhibitory and facilitatory systems. When the brachial plexus was avulsed, the peripheral nerves were separated from the DHSC, which resulted in blockage of the descending inhibitory system. Sustained activation of descending facilitation increased the excitability of spinal nociceptive neurons and promoted long-term potentiation and synapse plasticity, which in turn evoked neuroplasticity of certain supraspinal structures, including the anterior cingulate cortex (ACC) and rostral ventral medulla (8–30), which underly certain states of neuropathic pain.
The roles of the ACC and T in pain conditions have been consistently demonstrated for the past several decades (31,32). Numerous animal studies have been performed to identify the T and ACC as potential mediators of the pain experience, which are not only involved in the transmission of pain sensation, but also serve a role in processing pain-related emotions (27). The ACC and T widely connect with the descending modulation system.
However, the miRNAs within the ACC and T that mediated the perception of nociceptive signals following brachial plexus injury remain poorly understood. In the present study, 4 miRNAs in the ACC and 3 miRNAs in the T exhibited significant upregulation, and were associated with neuropathic pain. The functions of the miRNAs in the ACC and T were synergetic in the aspect of positive regulation of neuron apoptotic process, and inhibition of long-term potentiation and synapse plasticity. Previous studies reported that peripheral injury induced long-term potentiation of excitatory synaptic responses in the ACC neurons (33,34).
miR-30c-1-3p and its predicted genes (Camk2b and Prkcg) existed in the AC and T groups, with significant changes, in the present study. Waggener et al (35) reported that calmodulin-dependent protein kinase II regulated oligodendrocyte maturation and central nervous system myelination. Fang et al (36) reported that Camk2b protected neurons from homocysteine-induced apoptosis with the involvement of the hypoxia-inducible factor 1α signal pathway. Miletic et al (37) demonstrated that Prkcg mediated the phosphorylation of glutamate receptor 1 in the postsynaptic density of spinal dorsal horn neurons, accompanied with neuropathic pain. Yeh et al (38) observed the expression of dorsal spinal protein kinase C γ-subunit and other pain-related molecules in the spinal area in neuropathic pain model animals. Together, these studies indicated that miR-30c-1-3p may serve an extremely important role in neuropathic pain.
Besides central sensitization, peripheral sensitization is also associated with neuropathic pain following brachial plexus avulsion, which may result from ectopic discharge (39), scar stimulation (40) and excitory conduction short circuit due to the lack of myelin sheaths, among others (41). The concrete mechanisms of these causes include five aspects: Neuronal apoptosis, axonal regeneration, immune cell infiltration, glial cell accumulation and chemokine transfer (42). The present study indicated that 2 microRNAs in the DHSC group and 2 microRNAs in the B group exhibited significant down-regulation in the pain model. GO analysis showed that these microRNAs had functions in 'axon guidance', 'synaptic transmission', 'synapse maturation', 'excitatory postsynaptic membrane potential', 'the neuronal apoptotic process', 'macrophage activation involved in the immune response', 'cell chemotaxis', 'neuron migration' and 'neuron differentiation', 'neuron projection morphogenesis', 'cell migration', 'cell proliferation' and 'apoptosis', which facilitated the peripheral sensitization and induced neuropathic pain. The functions of the microRNAs in the DHSC and B groups were synergetic in the aspect of inhibition of cell apoptosis and promotion of cell proliferation, which contrasted with the functions of the differentially expressed miRNAs in the AC and T groups. When sustained activation of descending facilitation system occurred, a number of neurotrophic factors and neurotransmitters were exhausted and synapse growth proteins decreased, which resulted in neuron apoptosis increasing and synapse plasticity decreasing in the AC and T.
There were 10 miRNAs with significant expressional changes in the pain group, namely miR-3573-5p (DHSC), miR-3074 (DHSC), miR-30c-1-3p (AC and T), miR-702-3p (AC), miR-184 (AC), miR-25-5p (AC), miR-873-5p (T), miR-93-3p (T), miR-455-3p (B) and miR-32-3p (B). Previous studies on the aforementioned miRNAs associated with neuropathic pain have included a study by Lu et al (43), which found that miR-702-3p was differentially expressed in rat cortex in the anesthetic treatment group, which indicated the miRNA may be associated with neuropathic pain. McAdams et al (44) reported that morphine decreased the expression of miR-455-3p in the hippocampus of stressed neonatal mice and that the miRNA was involved in neurodevelopment, neurotransmission and inflammation. Gong et al (45) investigated the differentially expressed miRNAs in the lumbar spinal dorsal horn of mice with streptozotocin-induced diabetic neuropathic pain (DNP) and found that aberrant expression of miR-184 may contribute to the pathogenesis of DNP, and was a potential target for therapeutic interventions following DNP. McKiernan et al (46) identified miR-184 as a novel contributor to neuronal survival following mild and severe seizures. Furthermore, Liu et al (47) found that high levels of miR-184 promoted proliferation, but inhibited differentiation of adult neural stem/progenitor cells, and that the miRNA regulated the expression of numblike, a known regulator of brain development.
In future research, the predicted genes (Camk2b and Prkcg) should be verified as the targets of miR-30c-1-3p by cytology. miR-30c-1-3p could be transfected into neuron cells through plasmids and then the amount of target proteins could be measured by PCR and western blot analysis. If Camk2b and Prkcg were validated as the targets of miR-30c-1-3p, an attempt would be made to inject an miR-30c-1-3p-expressing virus into the ACC of the rat and then behavioral tests would be performed to determine whether the rat was in pain or not.
In conclusion, in the present study, post-CBPA pain was regulated intricately by miRNAs from different tissues in different pathways. There were 10 miRNAs with significant changes in expression in 4 different tissues in the post-BPA pain model. The central nerve groups (AC and T) and the peripheral nerve groups (DHSC) exhibited contrasting effects on synapse plasticity and neuron apoptosis. miR-30c-1-3p and its predicted genes (Camk2b and Prkcg) existed in the AC and T groups with significant espressional changes.
Acknowledgments
The authors would like to thank Dr Jiali Li (Fudan University) for providing assistance with the data analysis. The present study was sponsored by the Ministry of Science and Technology of China (973 program; grant no. 2014CB542204), the Natural Science Foundation of Shanghai (grant no. STCSM 16ZR1404200) and the National Natural Science Foundation of China (grant no. NSFC 81572127).
References
- 1.Mendell JR, Sahenk Z. Clinical practice. Painful sensory neuropathy. N Engl J Med. 2003;348:1243–1255. doi: 10.1056/NEJMcp022282. [DOI] [PubMed] [Google Scholar]
- 2.Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Hansson PT, Dickenson AH. Pharmacological treatment of peripheral neuropathic pain conditions based on shared commonalities despite multiple etiologies. Pain. 2005;113:251–254. doi: 10.1016/j.pain.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ciaramitaro P, Padua L, Devigili G, Rota E, Tamburin S, Eleopra R, Cruccu G, Truini A, Neuropathic pain special interest group of the Italian Neurological Society Prevalence of neuropathic pain in patients with traumatic brachial plexus injury: A multicenter prospective hospital-based study. Pain Med. 2017 Mar 3; doi: 10.1093/pm/pnw360. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125:113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 6.Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199–207. doi: 10.1016/S0304-3959(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 7.Baruah S, Devi BI, Bhat DI, Shukla D. Drezotomy in the management of post brachial plexus injury neuropathic pain: Preliminary results. Indian J Neurotrauma. 2014;11:27–29. doi: 10.1016/j.ijnt.2014.04.003. [DOI] [Google Scholar]
- 8.Aley KO, Levine JD. Different peripheral mechanisms mediate enhanced nociception in metabolic/toxic and traumatic painful peripheral neuropathies in the rat. Neuroscience. 2002;111:389–397. doi: 10.1016/S0306-4522(02)00009-X. [DOI] [PubMed] [Google Scholar]
- 9.Erichsen HK, Blackburn-Munro G. Pharmacological characterisation of the spared nerve injury model of neuropathic pain. Pain. 2002;98:151–161. doi: 10.1016/S0304-3959(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 10.Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet. 2008;74:296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan PH, Pao YY, Cheng JK, Hung KC, Liu CC. MicroRNA-based therapy in pain medicine: Current progress and future prospects. Acta Anaesthesiol Taiwan. 2013;51:171–176. doi: 10.1016/j.aat.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Parry CB. Pain in avulsion lesions of the brachial plexus. Pain. 1980;9:41–53. doi: 10.1016/0304-3959(80)90027-5. [DOI] [PubMed] [Google Scholar]
- 15.Narakas AO. The effects on pain of reconstructive neurosurgery in 160 patients with traction and/or crush injury to the brachial plexus. In: Siegfried J, Zimmerman M, editors. Phantom and Stump Pain. 1st edition. Springer-Verlag; Berlin: 1981. pp. 126–147. [DOI] [Google Scholar]
- 16.Bruxelle J, Travers V, Thiebaut JB. Occurrence and treatment of pain after brachial plexus injury. Clin Orthop Relat Res. 1988;237:87–95. [PubMed] [Google Scholar]
- 17.von Schack D, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, et al. Dynamic changes in the MicroRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS One. 2011;6:e17670. doi: 10.1371/journal.pone.0017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Kroin JS, Kc R, Gibson G, Chen D, Corbett GT, Pahan K, Fayyaz S, Kim JS, van Wijnen AJ, et al. Altered spinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J Bone Miner Res. 2013;28:2512–2522. doi: 10.1002/jbmr.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Yuzhou L, Yingjie Z, Jie L, Xin Z. A new rat model of neuropathic pain: Complete brachial plexus avulsion. Neurosci Lett. 2015;589:52–56. doi: 10.1016/j.neulet.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. The Gene Ontology Consortium: Gene ontology: Tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Gene Ontology Consortium Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 2017;45:D331–D338. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Zhang Y, Zhao ZQ. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- 28.Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci. 1999;19:9346–9354. doi: 10.1523/JNEUROSCI.19-21-09346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/S0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 30.Robinson D, Calejesan AA, Zhuo M. Long-lasting changes in rostral ventral medulla neuronal activity after inflammation. J Pain. 2002;3:292–300. doi: 10.1054/jpai.2002.125183. [DOI] [PubMed] [Google Scholar]
- 31.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008;31:199–207. doi: 10.1016/j.tins.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waggener CT, Dupree JL, Elgersma Y, Fuss B. CaMKIIβ regulates oligodendrocyte maturation and CNS myelination. J Neurosci. 2013;33:10453–10458. doi: 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang M, Feng C, Zhao YX, Liu XY. Camk2b protects neurons from homocysteine-induced apoptosis with the involvement of HIF-1α signal pathway. Int J Clin Exp Med. 2014;7:1659–1668. [PMC free article] [PubMed] [Google Scholar]
- 37.Miletic G, Hermes JL, Bosscher GL, Meier BM, Miletic V. Protein kinase C gamma-mediated phosphorylation of GluA1 in the postsynaptic density of spinal dorsal horn neurons accompanies neuropathic pain, and dephosphorylation by calcineurin is associated with prolonged analgesia. Pain. 2015;156:2514–2520. doi: 10.1097/j.pain.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh CY, Chung SC, Tseng FL, Tsai YC, Liu YC. Biphasic effects of chronic intrathecal gabapentin administration on the expression of protein kinase C gamma in the spinal cord of neuropathic pain rats. Acta Anaesthesiol Taiwan. 2011;49:144–148. doi: 10.1016/j.aat.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Oh Y, Chung JM, Chung K. The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res Mol Brain Res. 2001;95:153–161. doi: 10.1016/S0169-328X(01)00226-1. [DOI] [PubMed] [Google Scholar]
- 40.Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain. 2009;5:64. doi: 10.1186/1744-8069-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel FT, Seifert F, Lanz S, Maihöfner C. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;2:81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Jian MY, Ouyang YB, Han RQ. Changes in rat brain microRNA expression profiles following sevoflurane and propofol anesthesia. Chin Med J (Engl) 2015;128:1510–1515. doi: 10.4103/0366-6999.157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAdams RM, McPherson RJ, Beyer RP, Bammler TK, Farin FM, Juul SE. Dose-dependent effects of morphine exposure on mRNA and microRNA (miR) expression in hippocampus of stressed neonatal mice. PLoS One. 2015;10:e0123047. doi: 10.1371/journal.pone.0123047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong Q, Lu Z, Huang Q, Ruan L, Chen J, Liang Y, Wang H, Yue Y, Feng S. Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem Biophys Res Commun. 2015;456:615–620. doi: 10.1016/j.bbrc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 46.McKiernan RC, Jimenez-Mateos EM, Sano T, Bray I, Stallings RL, Simon RP, Henshall DC. Expression profiling the microRNA response to epileptic preconditioning identifies miR-184 as a modulator of seizure-induced neuronal death. Exp Neurol. 2012;237:346–354. doi: 10.1016/j.expneurol.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]