Abstract
Aberrant activation of extracellular signal-regulated kinase 1/2 (ERK1/2) by phosphorylation modification can trigger tumor cell development in glioma. S-nitrosylation, which refers to the covalent addition of a nitric oxide (NO) group to a cysteine (Cys) thiol, is an important post-translational modification that occurs on numerous cancer-associated proteins. Protein S-nitrosylation can increase or decrease protein activity and stability, and subsequent signal transduction and cellular processes. However, the association between ERK1/2 S-nitrosylation and ERK1/2 phosphorylation, and the effects of ERK1 S-nitrosylation on glioma cell survival are currently unknown. U251 glioma cells were treated with NO donors sodium nitroprusside (SNP) or S-nitrosoglutathione (GSNO). CCK8 assay was used to assess the cell viability. NO levels in the medium were detected by Griess assay. Western blot analysis and biotin switch assay were employed to detect the ERK1/2 phosphorylation and S-nitrosylation. ERK1 wild-type and mutant plasmids were constructed, and used to transfect the U251 cells. Caspase-3 western blot analysis and flow cytometry were employed to assess cell apoptosis. The present study demonstrated that treatment with the NO donors SNP or GSNO led to an increase in ERK1/2 S-nitrosylation, and a reduction in ERK1/2 phosphorylation, which was accompanied by growth inhibition of U251 glioma cells. Mutational analysis demonstrated that Cys183 was vital for S-nitrosylation of ERK1, and that preventing ERK1 S-nitrosylation by replacing Cys183 with alanine partially reversed GSNO-induced cell apoptosis, and reductions in cell viability and ERK1/2 phosphorylation. In addition, increased ERK1/2 phosphorylation was associated with decreased ERK1/2 S-nitrosylation in human glioma tissues. These findings identified the relationship between ERK1/2 S-nitrosylation and phosphorylation in vitro and in vivo, and revealed a novel mechanism of ERK1/2 underlying tumor cell development and apoptotic resistance of glioma.
Keywords: nitric oxide, extracellular signal-regulated kinase 1/2, S-nitrosylation, phosphorylation, apoptosis, glioma
Introduction
Glioma is the most common type of intracranial primary malignant tumor, which is associated with a poor median survival time of <15 months (1). Existing therapies include surgical removal, chemotherapy and radiotherapy; however, they are often unsuccessful (1–3). The difficulties in curing glioma are due to uncontrollable proliferation and infiltrative growth (1), which are considered to be largely attributed to aberrant signaling (4).
Mitogen-activated protein kinase (MAPK) cascades have been widely studied and are reported to be markedly altered in glial tumors (4,5). Extracellular signal-regulated kinase 1/2 (ERK1/2) is a crucial member of the MAPK family, which contains a conserved and dual-specificity motif (T-E-Y) that can be phosphorylated on threonine (Thr)202 and tyrosine (Tyr)204 residues. ERK1/2 is involved in the regulation of cell cycle progression, proliferation, differentiation, senescence and apoptosis (6). In human glioma tissues, the expression levels of phosphorylated (p)-ERK1/2 are significantly increased compared with in normal brain tissues, and expression is correlated with glioma grade (7,8), thus suggesting that aberrant upregulation or activation of ERK1/2 may lead to malignant progression of glioma. However, pharmacological inhibitors of ERK1/2 are cytostatic at best, and only in a subset of patients (4), thus indicating that other unidentified factors or compensatory signals may affect the survival and growth of tumor cells.
Nitric oxide (NO) is a short-lived free radical, which serves critical roles in the regulation of cardiovascular, immune and central nervous systems (9). S-nitrosylation refers to the covalent addition of a NO group to a cysteine (Cys) thiol, and is considered one of the important ways through which NO functions (10). Protein S-nitrosylation can alter spatial structure of proteins, and increase or decrease protein activity and stability and subsequent signal transduction and cellular processes (11). Feng et al reported that ERK1 harbors six Cys residues and that Cys183 is the key site for ERK1 nitrosylation (12). The present study aimed to investigate the association between ERK1/2 nitrosylation and ERK1/2 phosphorylation, and the effects of ERK1 S-nitrosylation at Cys183 on glioma cell survival.
The results of the present study demonstrated that treatment with the NO donors sodium nitroprusside (SNP) or S-nitrosoglutathione (GSNO) induced an increase in ERK1/2 S-nitrosylation, and a reduction in ERK1/2 phosphorylation, which were accompanied by growth inhibition of U251 glioma cells. Mutational analysis [Cys183 to alanine (Ala)183] uncovered that S-nitrosylation of ERK1 attenuated ERK1/2 phosphorylation, inhibited cell survival and promoted apoptosis. In addition, the results detected an increase in phosphorylation of ERK1/2 and a decrease in ERK1/2 S-nitrosylation in human glioma tissues. These findings identified a novel mechanism of ERK1/2 underlying tumor cell development and apoptotic resistance in glioma.
Materials and methods
Reagents and antibodies
Methyl methylthiomethyl sulfoxide (MMTS), neocuproine, sodium ascorbate and GSNO were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). SNP was obtained from Beyotime Institute of Biotechnology (Haimen, China). PolyJet™ and Biotin-HPDP were purchased from Thermo fisher Scientific, Inc. (Waltham, MA, USA). Antibodies against Flag (F1084; 1:1,000; Sigma-Aldrich; Merck KGaA), ERK1/2 (ab17942; 1:1,000; Abcam, Cambridge, UK), p-ERK1/2 (sc-81492; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and caspase-3 (GTX110543; 1:1,000; GeneTex, Inc., Irvine, CA, USA) were commercially available.
Cell culture
The U251 glioma cell line was purchased from Shanghai Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) in a cell incubator containing 5% CO2 under saturated humidity at 37°C. Cells were treated at 37°C with NO donors SNP (0–2 µM) or GSNO (0–500 µM) for 36 h or with SNP (2 µM) or GSNO (500 µM) for different time (0–36 or 48 h) prior to further analysis.
Cell viability detection
Cell viability was measured using Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). A single cell suspension (5×103/ml, 100 µl) was seeded into a 96-well plate. Subsequently, 10 µl CCK-8 reagent was added to each well and the plates were incubated for 2 h at 37°C. Finally, the absorbance was measured at 450 nm using a scanning microplate reader. Cell viability at individual time-points was normalized to the untreated group.
Flow cytometric apoptotic assay
Cells were harvested and washed twice with ice-cold PBS, after which they were resuspended in 1X binding buffer [0.01 M HEPES/NaOH (pH 7.4), 0.14 M NaCl, 2.5 mM CaCl2] at a concentration of 106 cells/ml. Subsequently, 100 µl solution was transferred to a 5 ml cell culture tube and was treated with fluorescein isothiocyanate-conjugated Annexin V apoptosis detection kit I (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocol. The cells were analyzed using flow cytometry (DiVa 8.0.1; BD Biosciences) and a total of 10,000 cells/sample were analyzed to determine the percentage of apoptotic cells.
Western blot analysis
Total protein was extracted from the cells or tissues, as described previously (13). Protein concentrations were determined by a BCA protein assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Equal amounts of protein (30 µg) were mixed with SDS sample buffer, separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were then incubated with 3% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in PBS at room temperature for 2 h, and were treated with primary antibodies overnight at 4°C. β-actin (sc-47778; 1:1,000; Santa Cruz Biotechnology, Inc.) was used as a protein-loading control. The next day, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (31460)/mouse (31430) immunoglobulin G (1:4,000; Invitrogen; Thermo fisher Scientific, Inc.) at room temperature for 2 h and were then detected using a standard chemiluminescence detection system (Pierce; Thermo fisher Scientific, Inc.). Band densities were analyzed using ImageJ software (Image J 1.43u; National Institute of Health, Bethesda, MD, USA).
NO detection
NO levels were determined by Griess assay using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cell supernatants (100 µl) were thoroughly mixed with reagent I (200 µl). The supernatants were obtained simply by a 200 µl pipette from the cell medium. Subsequently, reagent II (100 µl) was added and mixed for 10 min at room temperature, followed by centrifugation at 1,700 × g for 15 min at room temperature. Finally, 160 µl supernatants were mixed with 80 µl chromogenic reagent for 15 min at room temperature. The optical density of the samples was measured using a spectrophotometer with absorbance set at 550 nm. Sodium nitrite was used as a standard.
Biotin switch assay
Samples were lysed in non-denaturing lysis buffer (25 mM HEPES, 50 mM NaCl, 0.1 mM EDTA, 1% NP-40 and 1X protease inhibitor cocktail, pH 7.4). Protein concentration was determined using the bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). Protein lysates (2 mg) were diluted to a final volume of 1.8 ml using HEN buffer (100 mM HEPES, 1 mM EDTA and 0.1 mM neocuproine). Subsequently, 0.2 ml 25% SDS and 20 µl 10% MMTS were added to block free thiols. After removing excess MMTS by acetone precipitation, the S-nitrosothiol (SNO) groups in the samples were reduced to thiols by 30 µl sodium ascorbate (200 mM) and biotinylated by 30 µl Biotin-HPDP (2.5 mg/ml). Finally, the biotinylated proteins were pulled down by streptavidin-agarose beads, and the beads were eluted by SDS loading buffer and subjected to western blot analysis.
Plasmids
Full-length wild-type ERK1 (ERK1WT) cDNA clone was purchased from Sino Biological, Inc. (Beijing, China) and was subcloned into the 3xFlag vector. The primer sequences used for construction of Flag-ERK1WT were as follows: Forward, 5′-CCGGAATTCATGGCGGCGGCGGCGGCTCA-3′ and reverse, 5′-CGCGGATCCGGGGGCCTCCAGCACTCCGG-3′. C183A mutant ERK1 (ERK1C183A) cDNA was obtained by polymerase chain reaction [PCR; Tiangen Biotech (Beijing) Co., Ltd., Beijing, China] with the Flag-ERK1WT plasmid used as the template, and was then subcloned into the 3xFlag vector. The primer sequences used were as follows: forward, 5′-CCTTAAGATTGCTGATTTCGGCCTGGC-3′ and reverse, 5′-GCCGAAATCAGCAATCTTAAGGTCGCAG-3′. PCR thermocycling was performed as follows: 94°C for 3 min; followed by 35 cycles at 90°C for 30 sec, 60°C for 45 sec and 72°C for 90 sec; 72°C for 10 min; hold at 4°C. The authenticity of the plasmids was confirmed by DNA sequencing. Briefly, when U251 cells reached a confluency of 40–50%, the cell medium was replaced with 2 ml fresh DMEM medium at 30 min before transfection. Plasmid (1 µg) and PolyJet™ reagent (3 µl) were mixed in high glucose DMEM medium at room temperature. The mixture was evenly added into the medium and the cells were incubated at 37°C for 6–8 h before replacing the medium with 2 ml fresh medium. After 48 h, the cells were used for the experiments. Transient transfection was performed using PolyJet™ according to the manufacturer's protocol. Expression of proteins was verified by western blot analysis using ERK1/2 and Flag antibodies.
Glioma and noncancerous human brain tissue collection
Human glioma specimens, collected during surgical resection, and noncancerous brain tissues, collected during internal decompression after cerebral trauma, were obtained from the Affiliated Hospital of Xuzhou Medical University (Xuzhou, China). The clinicopathological characteristics of all of the subjects are presented in Table I. Surgically removed tissues were sampled for histological diagnosis, and the remaining tissues were immediately frozen and stored in liquid nitrogen for further analysis. All glioma specimens had a confirmed pathological diagnosis and were classified according to World Health Organization criteria (14). Informed consent was obtained from all subjects, or legal guardians, and the present study was approved by the Medical Ethical Committee of Xuzhou Medical University.
Table I.
Clinicopathological characteristics of the studied subjects.
| Case no. | Code no. | Gender | Age (years) | Location | WHO grade |
|---|---|---|---|---|---|
| 1 | 919616 | M | 57 | Right cerebellum | Noncancerous |
| 2 | 912226 | F | 54 | Right temporal lobe | Noncancerous |
| 3 | 972078 | F | 49 | Not available | Noncancerous |
| 4 | 968605 | F | 69 | Not available | Noncancerous |
| 5 | 981488 | M | 41 | Cerebellum | Noncancerous |
| 6 | 1095392 | M | 32 | Not available | Noncancerous |
| 7 | 1004728 | M | 63 | Right frontal lobe | Noncancerous |
| 8 | 941814 | M | 20 | Cerebellum | Noncancerous |
| 9 | 928412 | M | 48 | Not available | Noncancerous |
| 10 | 970570 | M | 52 | Right frontal lobe | Grade II |
| 11 | 1157139 | M | 42 | Right frontal lobe | Grade II |
| 12 | 1145933 | F | 48 | Right frontal lobe | Grade II |
| 13 | 1140811 | F | 49 | Right frontal lobe | Grade II |
| 14 | 1164493 | M | 64 | Left insular lobe | Grade II |
| 15 | 1190502 | M | 31 | Left frontal lobe | Grade II |
| 16 | 1158620 | M | 43 | Right temporal lobe | Grade II |
| 17 | 1196273 | M | 40 | Left frontal lobe | Grade II |
| 18 | 1152968 | F | 63 | Left temporal lobe | Grade II |
| 19 | 1110685 | F | 27 | Right frontal lobe | Grade II |
| 20 | 1084447 | F | 52 | Not available | Grade II |
| 21 | 999737 | M | 52 | Left frontal-temporal lobe | Grade III |
| 22 | 920498 | M | 50 | Right temporal-parietal lobe | Grade III |
| 23 | 926714 | M | 56 | Bilateral temporal lobe | Grade III |
| 24 | 1164248 | F | 66 | Left frontal lobe | Grade III |
| 25 | 1191197 | M | 68 | Left parietal-occipital lobe | Grade III |
| 26 | 922050 | F | 19 | Right frontal lobe | Grade III |
| 27 | 1117547 | M | 47 | Right temporal lobe | Grade III |
| 28 | 1081283 | F | 58 | Left temporal lobe | Grade III |
| 29 | 947804 | F | 66 | Left frontal-temporal-parietal lobe | Grade III |
| 30 | 1145935 | M | 23 | Cervical cord | Grade III |
| 31 | 1029589 | M | 31 | Right frontal-temporal lobe | Grade III |
| 32 | 1147279 | F | 64 | Cerebellum | Grade IV |
| 33 | 1147166 | M | 58 | Left frontal-temporal lobe | Grade IV |
| 34 | 1141904 | M | 47 | Right temporal lobe | Grade IV |
| 35 | 1132842 | F | 62 | Left temporal lobe | Grade IV |
| 36 | 1119597 | F | 50 | Right frontal lobe | Grade IV |
| 37 | 1096129 | M | 61 | Left temporal lobe | Grade IV |
| 38 | 1077922 | M | 43 | Left temporal lobe | Grade IV |
| 39 | 1140776 | M | 26 | Right parietal-occipital lobe | Grade IV |
| 40 | 1088070 | F | 58 | Right temporal lobe | Grade IV |
| 41 | 1164493 | M | 66 | Left insular lobe | Grade IV |
| 42 | 1184604 | F | 34 | Right temporal lobe | Grade IV |
F, female; M, male; WHO, World Health Organization.
Statistical analysis
All quantitative data were obtained from at least three independent experiments and are presented as the means ± standard error of the mean [SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA)]. Data between two groups were assessed by Student's t-test, whereas one-way analysis of variance followed by Dunnett post hoc comparison was used to analyze data among three groups or more. P<0.05 was considered to indicate a statistically significant difference.
Results
NO donor treatment inhibits growth of glioma cells
According to the literature, treatment with the NO donor SNP for 30–40 h significantly inhibits the growth of human glioma cells (15); therefore, the present study conducted a CCK-8 assay to evaluate the concentration-dependent effects of SNP and GSNO on cell survival after 36 h in U251 cells. Cell viability was significantly reduced following treatment with 0.25 and 0.5 mM SNP or 100–200 µM GSNO, and was reduced to a greater extent following treatment with 1 and 2 mM SNP or 250 and 500 µM GSNO (Fig. 1A and B). To determine the appropriate duration of high-dose SNP or GSNO treatment, U251 glioma cells were treated with 2 mM SNP or 500 µM GSNO for 0, 6, 12, 24, 36 and 48 h. Significant inhibition of cell survival was observed when cells were exposed to 2 mM SNP for 36 h or 500 µM GSNO for 36 and 48 h (Fig. 1C and D). These results indicated that high doses of NO donors exert significant inhibitory effects on the growth of glioma cells.
Figure 1.
NO donor treatment inhibits the growth of U251 glioma cells. (A and B) Results of a CCK-8 assay indicated a concentration-dependent decrease in the viability of U251 glioma cells following exposure to 0–2 mM SNP or 0–500 µM GSNO for 36 h. (C and D) CCK-8 assay exhibited a reduction in cell viability at different time-points following SNP (2 mM) or GSNO (500 µM) treatment. Relative absorbance was normalized to the untreated group (0). *P<0.05 and ***P<0.001 compared with the untreated group (0). CCK-8, Cell Counting kit-8; GSNO, S-nitrosoglutathione; NO, nitric oxide; SNP, sodium nitroprusside.
NO donor treatment increases NO release into the cell supernatant
To ascertain whether the NO donors SNP and GSNO could release NO, the Griess method was used to measure NO levels in the supernatant of cultured U251 cells. Significant increases in NO release were detected following treatment with 1 or 2 mM SNP (Fig. 2A) and 250 or 500 µM GSNO (Fig. 2B). These data suggested that SNP and GSNO may breakdown to release NO into the culture supernatant of glioma cells.
Figure 2.
NO donors SNP and GSNO can release NO into the medium of cultured glioma cells. The Griess method was used to quantify NO levels in the culture supernatant of U251 cells following (A) 1 and 2 mM SNP or (B) 250 and 500 µM GSNO treatment. **P<0.01 and ***P<0.001 compared with the untreated group (0). GSNO, S-nitrosoglutathione; NO, nitric oxide; SNP, sodium nitroprusside.
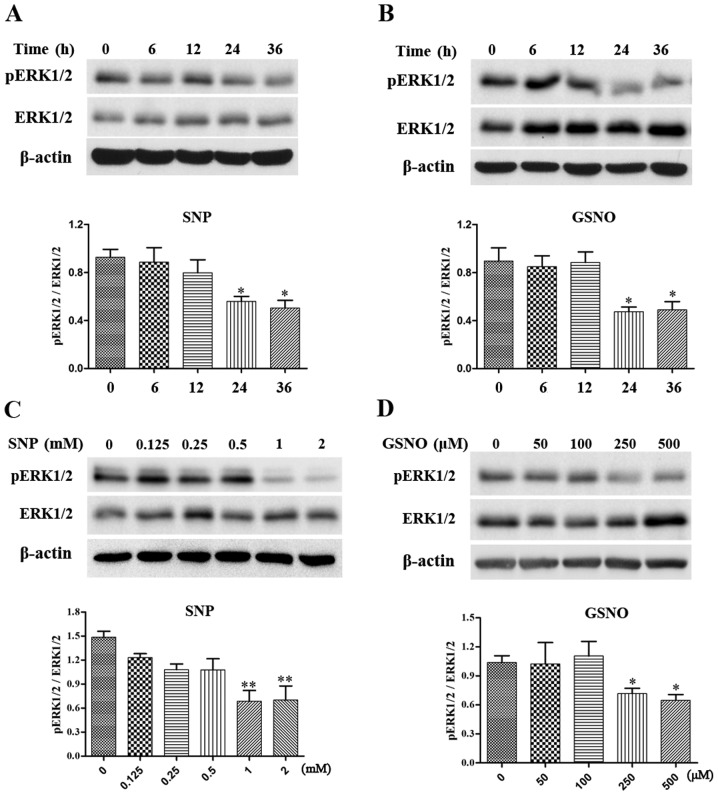
NO donor treatment attenuates phosphorylation of ERK1/2 in glioma cells
The MAPK pathway has been reported to serve a critical role in cell survival (6), and increased phosphorylation of ERK1/2 has been detected in various grades of glioma (7,8). The present study investigated the effects of NO donor treatment on the expression levels of p-ERK1/2 in glioma cells. Initially, a time-dependent assay was performed in U251 glioma cells. The expression levels of p-ERK1/2 were significantly reduced following treatment with 2 mM SNP or 500 µM GSNO treatment for 24 and 36 h (Fig. 3A and B). Subsequently, U251 cells were exposed to various concentrations of SNP or GSNO for 36 h. A concentration-dependent decrease in p-ERK1/2 expression was evident in response to SNP and GSNO treatment. Significant decreases in the expression levels of p-ERK1/2 were observed following treatment with 1 and 2 mM SNP (Fig. 3C). Similarly, p-ERK1/2 expression was significantly reduced following 250 and 500 µM GSNO treatment (Fig. 3D). These data indicated that NO donor treatment, particularly in high concentrations, may attenuate the phosphorylation of ERK1/2 in glioma cells.
Figure 3.
NO donor treatment attenuates ERK1/2 phosphorylation in U251 glioma cells. (A and B) Alterations in p-ERK1/2 expression at the indicated time-points following SNP (2 mM) or GSNO (500 µM) treatment were detected by western blotting and were semi-quantified. (C and D) Alterations in p-ERK1/2 expression following treatment with the indicated concentrations of SNP or GSNO were examined by western blotting and were semi-quantified. p-ERK1/2 expression was normalized to total ERK1/2. *P<0.05 and **P<0.01 compared with the control group (0). ERK1/2, extracellular signal-regulated kinase 1/2 GSNO, S-nitrosoglutathione; NO, nitric oxide; p-ERK1/2, phosphorylated-ERK1/2; SNP, sodium nitroprusside.
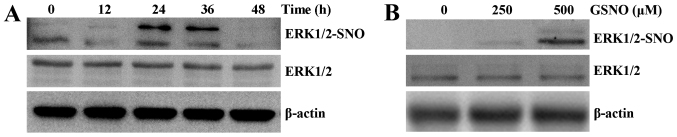
NO donor treatment promotes S-nitrosylation of ERK1/2 in glioma cells
To determine whether ERK could be nitrosylated by NO, S-nitrosylation of ERK1/2 (SNO-ERK1/2) was analyzed by a biotin switch assay, followed by western blot analysis. Time- and dose-dependent increases in the levels of SNO-ERK1/2 were detected in response to GSNO treatment of U251 cells. The levels of ERK1/2-SNO were markedly increased at 24 and 36 h, and then returned to control levels at 48 h (Fig. 4A). ERK1/2-SNO was initially detected following treatment with 250 µM GSNO and was amplified with 500 µM GSNO treatment (Fig. 4B). These results suggested that ERK1/2 may be nitrosylated in a time- and dose-dependent manner.
Figure 4.
NO donor treatment promotes S-nitrosylation of ERK1/2 in U251 glioma cells. (A) Cells were treated with 500 µM GSNO for the indicated time and ERK1/2-SNO was detected by biotin switch assay followed by western blotting. (B) Cells were treated with 250 and 500 µM GSNO for 24 h and the level of ERK1/2-SNO was detected as aforementioned. Total ERK1/2 was used as an endogenous control. ERK1/2, extracellular signal-regulated kinase1/2; GSNO, S-nitrosoglutathione; NO, nitric oxide; SNO, S-nitrosothiol.
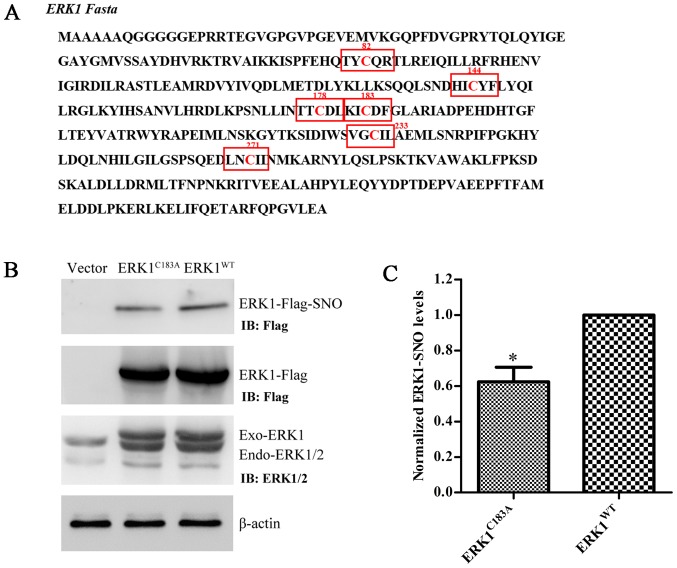
S-nitrosylation of ERK1 is prevented by mutation at Cys183
ERK1 has six Cys residues in its FASTA sequence (Fig. 5A). A preliminary computational prediction indicated that Cys183 is the most probable site for nitrosylation, according to the previously reported nitrosylation motif (K/R/H/D/E+C+D/E) (16). Therefore, Cys183 was replaced with Ala, and the ERK1 mutant plasmid (ERK1C183A) was constructed. Transfection efficiency of ERK1WT and ERK1C183A was verified by western blot analysis using anti-Flag and anti-ERK1/2 antibodies (Fig. 5B). The expression levels of ERK1-SNO were significantly attenuated following transfection of U251 cells with the ERK1C183A plasmid (Fig. 5B and C). These results suggested that mutation at Cys183 may partially prevent S-nitrosylation of ERK1/2 in glioma cells.
Figure 5.
Point mutation at Cys183 partially prevents S-nitrosylation of ERK1 in glioma cells. (A) Location of Cys and the adjacent amino acids in the ERK1 protein sequence. The red 'C' in the middle of each box is cysteine residue. (B) Transfection efficiency and S-nitrosylation of ERK1WT and ERK1C183A were determined by biotin switch assay, followed by western blotting. (C) Semi-quantitative analysis of ERK1-SNO levels. β-actin was used as a loading control. *P<0.05 compared with the ERK1WT group. Cys, cystein; ERK, extracellular signal-regulated kinase; SNO, S-nitrosothiol; IB, immunoblotting; WT, wild-type.
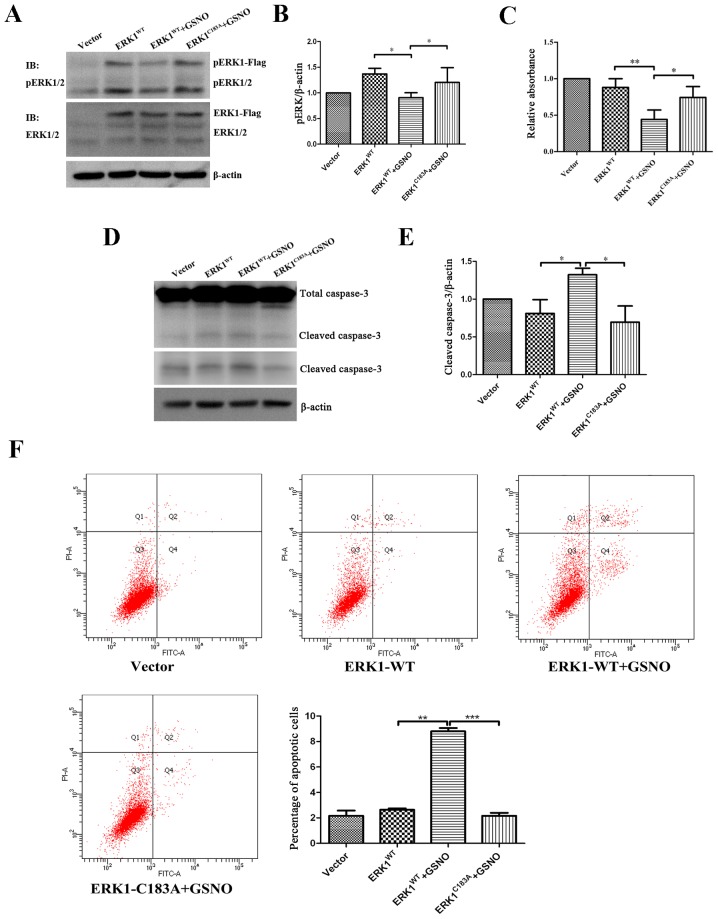
Preventing S-nitrosylation of ERK1 promotes ERK phosphorylation and cell survival
To determine the relationship between ERK1/2 nitrosylation and phosphorylation, U251 glioma cells were transfected with either ERK1WT or ERK1C183A plasmids, and were then treated with GSNO (500 µM). The results indicated that the expression levels of p-ERK were significantly decreased in the ERK1WT-transfected cells when GSNO was added (Fig. 6A and B), which was consistent with the previous findings presented in Fig. 3D. However, GSNO failed to reduce p-ERK1/2 levels when U251 cells were transfected with the ERK1C183A mutant (Fig. 6A and B). These findings indicated that preventing ERK1 S-nitrosylation may increase the phosphorylation of ERK1/2 in glioma cells.
Figure 6.
Preventing S-nitrosylation of ERK1 promotes ERK phosphorylation and cell survival, and suppresses apoptosis. Following transfection of U251 glioma cells with ERK1-Flag or ERK1 mutant form (ERKC183A), cells were treated with 500 µM GSNO for 24 h. (A and B) p-ERK1/2 was detected by western blotting and was semi-quantified. p-ERK1/2 levels were compared with β-actin levels, and results were normalized to vector group. (C) Cell Counting kit-8 assay was performed to examine the viability of U251 glioma cells. Cell survival percentage was normalized to the vector group. (D and E) Caspase-3 protein expression was detected by western blotting and semi-quantified. Cleaved caspase-3 levels were compared with β-actin levels, and results were normalized to the vector group. The lower panel of cleaved caspase-3 blot in part D was obtained after a longer exposure time compared with the upper panel. (F) Flow cytometric detection of apoptosis of U251 glioma cells. The percentage of apoptotic cells was quantified and compared. *P<0.05, **P<0.01 and ***P<0.001. IB, immunoblotting; ERK, extracellular signal-regulated kinase; fITC, fluorescein isothiocyanate; GSNO, S-nitrosoglutathione; p-ERK1/2, phosphorylated ERK1/2; PI, propidium iodide; WT, wild-type.
The present study also investigated the effects of ERK1 S-nitrosylation prevention on the survival of glioma cells. In line with the data presented in Fig. 1, cell viability was significantly reduced following treatment with GSNO; however, the reduction in cell viability was reversed by a point mutation at Cys183 of ERK1 (Fig. 6C). Furthermore, western blot analysis demonstrated that GSNO treatment induced an increase in cleaved caspase-3 expression; however, this was reversed following transfection with the ERK1C183A mutant (Fig. 6D and E). In addition, flow cytometric apoptotic assay indicated that the percentage of U251 apoptotic cells transfected with ERK1C183A mutant was significantly reduced following GSNO treatment compared with in the cells transfected with the ERK1WT plasmid (Fig. 6F). Taken together, these results suggested that preventing S-nitrosylation of ERK1, via transfection with a ERK1C183A mutant, partially reversed GSNO-induced decreases in ERK phosphorylation and cell survival in U251 glioma cells.
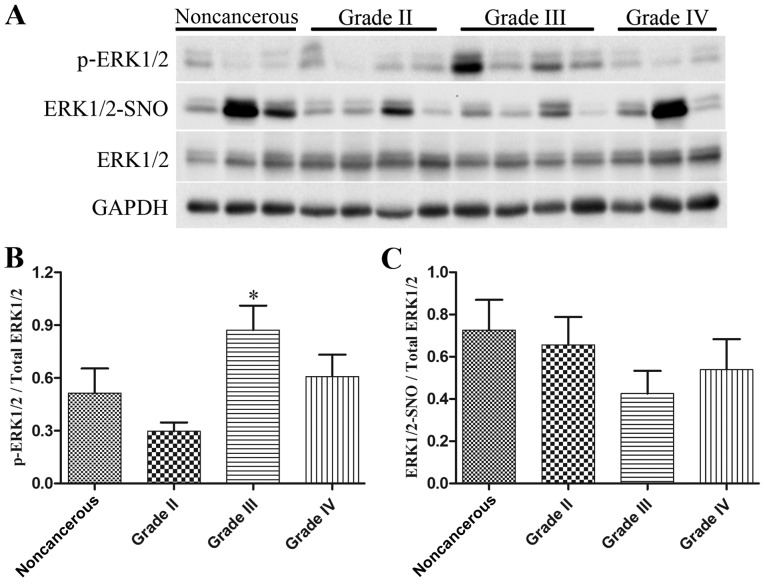
Alterations in ERK1/2 phosphorylation and S-nitrosylation levels in human glioma tissues
Western blot analysis was employed to detect p-ERK1/2 and total ERK1/2 levels, and a biotin switch assay followed by western blot analysis was used to measure ERK1/2-SNO levels, in 9 noncancerous brain tissues and 33 glioma specimens (n=11/grade). As presented in Fig. 7, the expression levels of p-ERK1/2 were increased in high-grade glioma tissues, particularly in glioma grade III (Fig. 7A and B), whereas there was a marked reduction in ERK1/2-SNO levels in glioma tissues, which was also evident in glioma grade III (Fig. 7A and C). These data provided in vivo evidence for the possible influence of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation during glioma proliferation.
Figure 7.
Alterations in the levels of ERK1/2 phosphorylation and S-nitrosylation in noncancerous and glioma tissues. In noncancerous brain samples (n=9) and various grades of glioma (n=11 for each grade), western blotting was used to detect the expression levels of p-ERK1/2 and total ERK1/2 levels, and biotin switch assay followed by western blotting was employed to detect ERK1/2-SNO. (A) Representative blot images are presented. Semi-quantification for the ratio of (B) p-ERK1/2/total ERK1/2 and (C) ERK1/2-SNO/total ERK1/2. *P<0.05 compared with the noncancerous group. ERK1/2, extracellular signal-regulated kinase 1/2; p-ERK1/2, phosphorylated-ERK1/2; SNO, S-nitrosothiol.
Discussion
NO donors, SNP and GSNO, breakdown to release NO and exert an inhibitory effect on cell survival in glioma cells. In the present study, NO donor treatment induced a significant decrease in p-ERK1/2 expression (Fig. 3) and a marked increase in ERK1/2-SNO levels (Fig. 4) in U251 cells, thus suggesting a link between ERK1/2-SNO and p-ERK/2. Further mutational analysis demonstrated that Cys183 was vital for S-nitrosylation of ERK1 (Fig. 5) and that preventing the formation of ERK-SNO by ERK1C183A mutation reversed NO-induced suppression of cell viability and p-ERK1/2 expression, and increased cell apoptosis of glioma cells (Fig. 6). In addition, increased p-ERK1/2 levels were observed in human glioma tissues, which were accompanied by a marked decrease in ERK1/2-SNO levels (Fig. 7). These findings indicated a novel mechanism underlying the antitumor role of NO-associated chemicals and provided insights into gene therapy targeting the ERK1/2 pathway in glioma.
NO is a free radical, which predominantly functions as a messenger or effector molecule. Previous studies have reported that the viability of U87 and C6 cells may be significantly inhibited following exposure to high concentrations of NO donors (15,17). The present study demonstrated that treatment with the NO donors SNP or GSNO resulted in a significant reduction in the viability of U251 cells. These data suggested that the inhibitory effects of NO on cell survival could be generalized in various glioma cell lines. However, previous evidence also suggests that NO displays stimulatory and inhibitory effects in the context of cell survival and apoptosis. Maejima et al reported that low doses of the NO donor S-mitroso-N-acetyl-D,L-penicillamine favor cell survival, whereas high doses may reduce cell viability of cardiomyocytes (18). Lechner et al demonstrated that low levels of NO produced by the tumor microenvironment favor tumor cell survival, whereas tumor cells with high NO levels undergo cell death (19). The dual effects of NO may be ascribed to the availability of enzymes, timing of apoptotic stimuli, redox state, donor doses, spatial location of key reactants and interactions with other molecules (20).
S-nitrosylation is involved in the regulation of numerous biological processes, including cell proliferation and survival, and particularly apoptosis (11,21). S-nitrosylation of B-cell lymphoma 2 enhances its stability, inhibits ubiquitous degradation in numerous tumor types and induces resistance to cis-platinum chemotherapy in breast cancer (22,23). Furthermore, S-nitrosylation of the death receptor Fas initiates its redistribution on lipid rafts and promotes Fas ligand-mediated apoptosis in cancer (24). ERK1 harbors six Cys residues, as indicated in Fig. 5A. Cys183 is the most probable site for S-nitrosylation, according to the S-nitrosylation motif (K/R/H/D/E+C+D/E) reported previously (16). The present study indicated that ERK1/2 may be nitrosylated by the NO donor GSNO, and that replacing Cys183 with alanine may prevent the S-nitrosylation of ERK1/2 in glioma cells. The small decrease in ERK1-SNO levels in response to Cys183 mutation indicates that other ERK1 Cys residues may also contribute to S-nitrosylation. Nevertheless, the ERK1C183A mutation significantly reversed GSNO-induced suppression of cell viability and enhancement of apoptosis of glioma cells. Together with a previous study in breast cancer cells (12), these findings suggested that ERK-SNO may promote tumor cell apoptosis.
Within the MAPK cascades, the ERK1/2 signaling pathway is a principle pathway that regulates cell proliferation and survival when activated by phosphorylation at Thr202 and Tyr204 residues of ERK1 (5,25). Activation of ERK1/2 signaling in glioma tissues, as determined by increased p-ERK1/2 levels, has been detected in the present study, as well as in previous studies (7,8). The S-nitrosylation site Cys183 is spatially close to Thr202 and Tyr204, thus suggesting the possibility of mutual regulation between S-nitrosylation and phosphorylation of ERK1/2. The present in vitro results indicated that treatment with GSNO induced a reduction in p-ERK1/2 expression, an increase in ERK1/2-SNO levels, and cell growth inhibition in glioma cells. In vivo, the results demonstrated that p-ERK1/2 levels were increased, whereas ERK1/2-SNO levels were decreased in glioma tissues, particularly in glioma grade III. Furthermore, a point mutation at Cys183 confirmed that preventing formation of ERK1-SNO significantly increased p-ERK1/2 expression and reversed GSNO-induced cell apoptosis in U251 glioma cells. These findings suggested a regulatory role of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation, which may provide novel information regarding ERK1/2 targeting in glioma therapy.
In addition to ERK1/2, other important signaling proteins are also modified by S-nitrosylation. Murillo-Carretero et al reported that S-nitrosylation of epidermal growth factor receptor (EGFR) inhibited EGFR phosphorylation and cell proliferation in neuroblastoma cells (26). In head and neck squamous cell carcinoma, S-nitrosylation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor (NF)-κB inhibited phosphorylation of STAT3 and activation of NF-κB, and decreased cell proliferation and increased apoptosis (27). These studies indicated a critical role of S-nitrosylation in the regulation of protein phosphorylation and cellular biological functions. Notably, several NO-hybridized drugs have been developed to inhibit cancer cell growth in vitro and in vivo (28,29), thus suggesting the potential translational relevance of NO-mediated S-nitrosylation in the future.
A few limitations should be mentioned with regards to the present study. All in vitro work presented in this study was performed in U251 glioma cells. In this respect, duplication of efforts in other glioma cell lines would be beneficial. In addition, it is necessary to perform brain xenograft experiments to confirm the inhibitory role of ERK1 S-nitrosylation on ERK1/2 phosphorylation and glioma growth.
In conclusion, NO donor treatment inhibited cell survival and induced apoptosis of U251 glioma cells. S-nitrosylation of ERK1/2 and ERK1/2 phosphorylation exhibited inverse alterations in GSNO-treated glioma cells and in human glioma tissues. Preventing ERK1 nitrosylation via a mutation at Cys183 partially reversed NO-induced decreases in ERK phosphorylation and cell survival. These findings revealed a novel mechanism of ERK1/2 underlying tumor cell development and apoptotic resistance of glioma.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant nos. 31400930, 81472345 and 81302175), the Natural Science Foundation of Jiangsu province (grant no. BK20140217), the China Postdoctoral Science Foundation (grant nos. 2015M570480 and 2016T90505) and the Key Research and Development Plan of Jiangsu Province (grant no. BE2016646).
References
- 1.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 5.Pandey V, Bhaskara VK, Babu PP. Implications of mitogen-activated protein kinase signaling in glioma. J Neurosci Res. 2016;94:114–127. doi: 10.1002/jnr.23687. [DOI] [PubMed] [Google Scholar]
- 6.Dong Chen, Waters SB, Holt KH, Pessin JE. SOS phosphorylation and disassociation of the Grb2-SOS complex by the ERK and JNK signaling pathways. J Biol Chem. 1996;271:6328–6332. doi: 10.1074/jbc.271.11.6328. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskara VK, Panigrahi M, Challa S, Babu PP. Comparative status of activated ERK1/2 and PARP cleavage in human gliomas. Neuropathology. 2005;25:48–53. doi: 10.1111/j.1440-1789.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 8.Xie H, Xue YX, Liu LB, Wang P, liu YH, Ying HQ. Expressions of matrix metalloproteinase-7 and matrix metalloproteinase-14 associated with the activation of extracellular signal-regulated kinase1/2 in human brain gliomas of different pathological grades. Med Oncol. 2011;28(Suppl 1):S433–S438. doi: 10.1007/s12032-010-9660-7. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Lipton SA. Protein S-nitrosylation as a therapeutic target for neurodegenerative diseases. Trends Pharmacol Sci. 2016;37:73–84. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012;320:123–129. doi: 10.1016/j.canlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Feng X, Sun T, Bei Y, Ding S, Zheng W, lu Y, Shen P. S-nitrosylation of ERK inhibits ERK phosphorylation and induces apoptosis. Sci Rep. 2013;3:1814. doi: 10.1038/srep01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen A, Gao S, Ben Z, Wang H, Jia J, Tao T, Niu S, Li X, Cheng C. Identification and potential role of PSD-95 in Schwann cells. Neurol Sci. 2008;29:321–330. doi: 10.1007/s10072-008-0989-z. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. IARC WHO Classification of Tumours; Lyon: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurimoto M, Endo S, Hirashima Y, Hamada H, Ogiichi T, Takaku A. Growth inhibition and radiosensitization of cultured glioma cells by nitric oxide generating agents. J Neurooncol. 1999;42:35–44. doi: 10.1023/A:1006160305294. [DOI] [PubMed] [Google Scholar]
- 16.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/S0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 17.Weyerbrock A, Baumer B, Papazoglou A. Growth inhibition and chemosensitization of exogenous nitric oxide released from NONOates in glioma cells in vitro. J Neurosurg. 2009;110:128–136. doi: 10.3171/2008.6.17607. [DOI] [PubMed] [Google Scholar]
- 18.Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol. 2005;38:163–174. doi: 10.1016/j.yjmcc.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin Cancer Biol. 2005;15:277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Lancaster JR, Jr, Xie K. Tumors face NO problems. Cancer Res. 2006;66:6459–6462. doi: 10.1158/0008-5472.CAN-05-2900. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Chen C, Loake GJ, Chu C. Nitric oxide: Promoter or suppressor of programmed cell death. Protein Cell. 2010;1:133–142. doi: 10.1007/s13238-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azad N, Vallyathan V, Wang L, Tantishaiyakul V, Stehlik C, leonard SS, Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J Biol Chem. 2006;281:34124–34134. doi: 10.1074/jbc.M602551200. [DOI] [PubMed] [Google Scholar]
- 23.Chanvorachote P, Nimmannit U, Stehlik C, Wang L, Jiang BH, Ongpipatanakul B, Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 24.Leon-Bollotte L, Subramaniam S, Cauvard O, Plenchette-Colas S, Paul C, Godard C, Martinez-Ruiz A, Legembre P, Jeannin JF, Bettaieb A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 2011;140:2009–2018. 2018.e2001–2004. doi: 10.1053/j.gastro.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 25.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 26.Murillo-Carretero M, Torroglosa A, Castro C, Villalobo A, Estrada C. S-nitrosylation of the epidermal growth factor receptor: A regulatory mechanism of receptor tyrosine kinase activity. Free Radic Biol Med. 2009;46:471–479. doi: 10.1016/j.freeradbiomed.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Kaliyaperumal K, Sharma A K, McDonald DG, Dhindsa JS, Yount C, Singh AK, Won JS, Singh I. S-nitrosoglutathione-mediated STAT3 regulation in efficacy of radiotherapy and cisplatin therapy in head and neck squamous cell carcinoma. Redox Biol. 2015;6:41–50. doi: 10.1016/j.redox.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay M, Goswami S, Rodes DB, Kodela R, Velazquez CA, Boring D, Crowell JA, Kashfi K. NO-releasing NSAIDs suppress NF-κB signaling in vitro and in vivo through S-nitrosylation. Cancer Lett. 2010;298:204–211. doi: 10.1016/j.canlet.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Szabo C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat Rev Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]