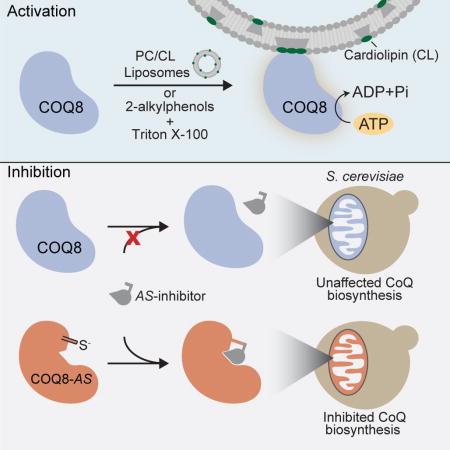
Summary
Human COQ8A (ADCK3) and Saccharomyces cerevisiae Coq8p (collectively COQ8) are UbiB family proteins essential for mitochondrial coenzyme Q (CoQ) biosynthesis. However, the biochemical activity of COQ8 and its direct role in CoQ production remain unclear, in part due to lack of known endogenous regulators of COQ8 function and of effective small molecules for probing its activity in vivo. Here we demonstrate that COQ8 possesses evolutionarily conserved ATPase activity that is activated by binding to membranes containing cardiolipin and by phenolic compounds that resemble CoQ pathway intermediates. We further create an analog-sensitive version of Coq8p and reveal that acute chemical inhibition of its endogenous activity in yeast is sufficient to cause respiratory deficiency concomitant with CoQ depletion. Collectively, this work defines lipid and small molecule modulators of an ancient family of atypical kinase-like proteins and establishes a chemical genetic system for further exploring the mechanistic role of COQ8 in CoQ biosynthesis.
eTOC blurb
Reidenbach et al. discover small molecule and lipid ligands for the atypical kinase-like protein COQ8 that enhance its ATPase activity. They develop an analog-sensitive version of COQ8 that can be covalently inhibited, enabling acute inhibition of COQ8 ATPase activity in vitro and coenzyme Q biosynthesis in vivo.
Introduction
The UbiB family of atypical protein kinase-like (PKL) genes is conserved across all three domains of life (Leonard et al., 1998). In eukaryotes, UbiB proteins are found exclusively in mitochondria and plastids where they have been linked to diverse processes involving cell migration, tumor cell viability, and lipid metabolism (Lundquist et al., 2012; Simpson et al., 2008; Wiedemeyer et al., 2010). An orthologous subset of UbiB proteins, including Escherichia coli UbiB, S. cerevisiae Coq8p, and human COQ8A (ADCK3) and COQ8B (ADCK4), support the biosynthesis of coenzyme Q (ubiquinone, CoQ) (Do et al., 2001; Poon et al., 2000), and mutations in COQ8A and COQ8B give rise to CoQ-related neurologic and kidney disorders, respectively (Ashraf et al., 2013; Horvath et al., 2012; Lagier-Tourenne et al., 2008; Mollet et al., 2008; Stefely et al., 2016). Despite these connections, the specific biochemical activities, regulation, and direct roles of UbiB proteins in cellular processes remain largely unclear, in part because effective tools for addressing these questions have been lacking.
To date, the only biochemical activities observed for UbiB family proteins are COQ8 cis autophosphorylation, which is seemingly spurious, and a low-level COQ8 ATPase activity (Stefely et al., 2016; Stefely et al., 2015). Additionally, structural analyses revealed that the COQ8 active site is likely sterically inaccessible to proteinaceous substrates, suggesting that this family possesses unorthodox kinase functionality in lieu of canonical protein kinase activity. However, many members of the protein kinase-like (PKL) superfamily are subject to activation by lipids and small molecules, including AMPK (AMP) (Ferrer et al., 1985), PKA (cAMP) (Walsh et al., 1968), PKC (diacylglycerol) (Takai et al., 1979a; Takai et al., 1979b), and AKT (phosphoinositides) (Burgering and Coffer, 1995; Franke et al., 1995), indicating that COQ8 activity may be altered or enhanced in a similar manner. Consistently, we recently found that Coq8p copurifies with intermediates in the CoQ pathway (Stefely et al., 2016), and have shown that the single-pass transmembrane domain of COQ8A can induce dimerization (Khadria et al., 2014), together suggesting that COQ8 activity may particularly be regulated by interactions at the membrane; however, this remains unexplored.
A second limitation for defining the functions of UbiB proteins has been the lack of specific inhibitors that can be used to disrupt protein activity. While the use of COQ8 active site point mutants in growth assays has implied the importance of COQ8 catalytic activity for CoQ production (Stefely et al., 2015; Xie et al., 2011), this has not been tested directly by chemical inhibition in vivo. Chemical-genetic strategies have proven effective for kinase inhibition when specific inhibitors for the wild type kinase are lacking. This approach involves mutating a “gatekeeper” residue in the ATP binding pocket to create a larger space capable of accommodating custom, cell-permeable inhibitors that are too bulky to bind to other kinases (Bishop et al., 2000). Additionally, to enable covalent, irreversible inhibition, a conserved valine near the active site can be mutated to a cysteine (Cohen et al., 2005; Rodriguez-Molina et al., 2016; Snead et al., 2007). When successful, this technique enables acute and selective inhibition of kinase activity in vivo, thereby overcoming the caveats associated with long-term adaptions that can arise from genetic manipulation. Although this approach has been used for various classes of kinases (Cohen et al., 2005; Coudreuse and Nurse, 2010; Ferreira-Cerca et al., 2012; Oh et al., 2007; Rodriguez-Molina et al., 2016; Snead et al., 2007), it has never been attempted for the much more divergent and atypical UbiB family, nor for a mitochondria-localized kinase in general.
To address these limitations in investigating the UbiB family, we combined biophysical, biochemical, computational, and chemical genetic analyses to explore the activity and regulation of COQ8. We found that ATPase activity is an evolutionarily conserved feature of COQ8 orthologs from E. coli to humans that is enhanced by phenolic compounds resembling CoQ biosynthetic precursors. Remarkably, we also find that mature COQ8 specifically associates with, and is activated by, cardiolipin-containing liposomes. Finally, we establish an analog-sensitive version of COQ8 in yeast and show that acute inhibition of endogenous COQ8 activity is sufficient to disrupt CoQ production and cause respiratory deficiency. Overall, our work reveals lipid and small molecule modulators of this ancient kinase-like protein, and establishes a chemical genetic tool for the further exploration of its direct role in CoQ biosynthesis.
Results
UbiB family ATPase activity is enhanced by phenols
To explore the ability of UbiB proteins to bind lipids and small molecules, we purified a maltose binding protein (MBP)-tagged version of E. coli UbiB that lacks its predicted C-terminal transmembrane (TM) domains (UbiBCΔ47) and analyzed copurifying lipids by mass spectrometry (Figure S1A). We found that MBP-UbiBCΔ47 preferentially copurifies with E. coli CoQ biosynthesis intermediates octaprenylphenol (OPP) and octaprenylhydroxybenzoate (OHB) and that this copurification depends on the integrity of active site residues (Figures 1A, S1B–E, and Table S1). These data are highly consistent with our previous lipid-binding data for Coq8p, indicating that the binding of CoQ precursors to UbiB family members is conserved from bacteria to eukaryotes (Stefely et al., 2016).
Figure 1. UbiB family members bind CoQ precursor-like lipids and small molecules.
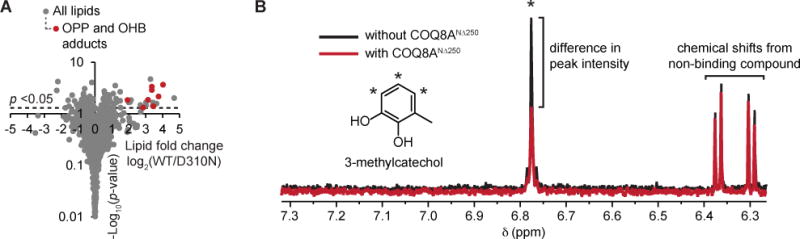
(A) Fold changes in the abundances of lipids copurifying with UbiB protein variants (log2[MBP-UbiBCΔ47/MBP-UbiBCΔ47 D310N], n = 3) as quantified by LC-MS/MS versus statistical significance. Different OPP and OHB adducts (i.e. OPP [M-H]− and OPP [M+Cl]−) are shown in red. (B) A selected region of the 1H NMR spectra for a mixture containing 3-methylcatechol (3-MC) with and without COQ8ANΔ250. Asterisk (*) indicates the three benzyl hydrogens on 3-MC peak that exhibit line-broadening. The peaks shown around 6.3 – 6.4 ppm belong to another compound (cis-3-chloroacrylic acid) in the mixture that is not interacting with COQ8ANΔ250. See also Figure S1 and Tables S1–S3.
As an orthogonal approach to assessing protein-small molecule interactions, we next performed a one-dimensional (1D) 1H NMR ligand-affinity screen of a diverse 417 compound library using COQ8ANΔ250 (COQ8A lacking its mitochondrial localization sequence and TM domain). To do so, we introduced COQ8ANΔ250 to mixtures of 3–5 compounds and analyzed the resulting line broadening and/or chemical shift changes of the 1H peaks for compounds interacting with COQ8ANΔ250. In addition to its expected binding to adenosine, COQ8ANΔ250 interacted with a total of 25 compounds including multiple that, like OPP and OHB, are phenol derivatives (Figures 1B and S1F and Table S2). Collectively, these data establish that COQ8 possesses an evolutionarily conserved capacity to bind CoQ precursor-like molecules, suggesting that these molecules are potential regulators or direct substrates of COQ8.
The CoQ precursors that copurify with Coq8p and UbiB are difficult to synthesize and are not commercially available; as such, to explore the functional ramifications of these COQ8-ligand interactions, we opted to screen a variety of CoQ headgroup-like analogs (phenols and hydroquinones) for their effect on COQ8 activity. To monitor ATP consuming activity, we used the ADP-Glo assay, which measures ADP produced as a proxy for phosphoryl transfer activity. We included the nonionic detergent Triton X-100 in our screens, as it has been shown to activate certain membrane bound or membrane associated proteins (Kanoh et al., 1991; Yoon and Robyt, 2005). We found that 2-alkylphenols (2-allylphenol and 2-propylphenol), which are structurally similar to OPP, strongly activated UbiB family members when in the presence of Triton X-100 (Figures 2A and S2A). This increase in activity was seen both for wild type (WT) and A339G (A-to-G) nucleotide-binding loop (P-loop) mutant, which has a higher baseline ATPase and cis autophosphorylation activity likely due to enhanced ATP binding (Stefely et al., 2016) (See Table S3 and Figure S2F for explanations of mutants used in this study). The negative control D507N (D-to-N) mutant, which lacks ATP binding ability, showed no activity (Figures 2A and S2A).
Figure 2. UbiB family members are activated by Triton X-100 and 2-alkylphenols.
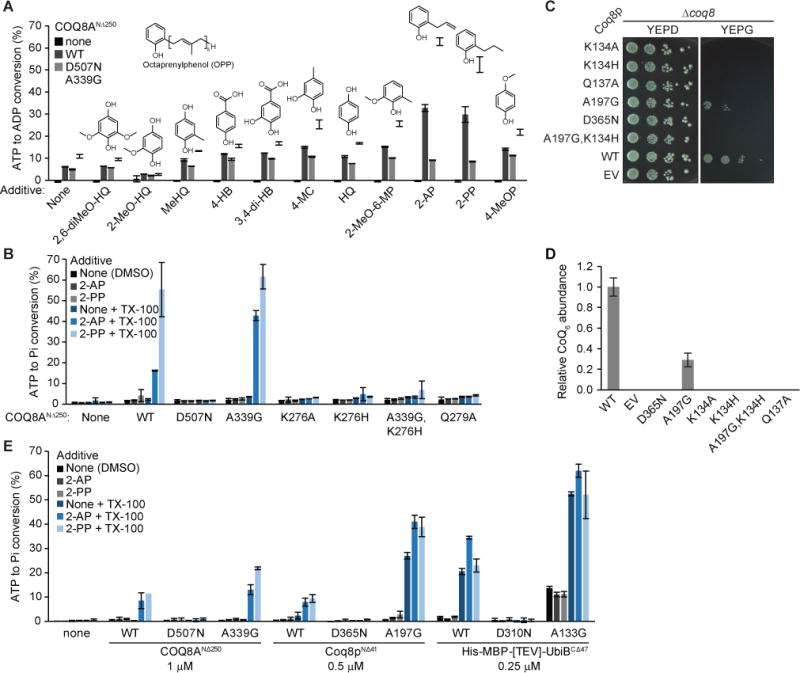
(A) Screen of CoQ intermediate-like compounds with Triton X-100 in an ADP-Glo assay. 2,6-diMeO-HQ, 2,6-dimethoxyhydroquinone; 2-MeO-HQ, 2-methoxyhydroquinone; MeHQ, methylhydroquinone; 4-HB, 4-hydroxybenzoic acid; 3,4-di-HB, 3,4-dihydroxybenzoic acid; 4-MC, 4-methylcatechol; HQ, hydroquinone; 2-MeO-6-MP, 2-methoxy-6-methylphenol; 2-AP, 2-allylphenol; 2-PP, 2-propylphenol; 4-MeOP, 4-methoxyphenol. (B) Malachite green ATPase assay with COQ8ANΔ250 KxGQ mutants, Triton X-100, and 2-alkylphenols. Pi, inorganic phosphate. (C) Drop assay of Δcoq8 transformed with KxGQ mutants. (D) Relative CoQ abundance from Δcoq8 transformed with Coq8p variants. (E) Malachite green ATPase assay of UbiB family members from human, yeast, and E. coli with 2-alkylphenols and Triton X-100. Concentration of protein used is listed under the protein name, and reactions were incubated at 30 °C for 10 minutes rather than 45 minutes. See also Figure S2 and Table S3.
Our previous structure of COQ8 revealed the presence of a water molecule in the active site bound by the glutamine (Q) of the invariant KxGQ motif that could serve as the nucleophile for an ATPase reaction (Stefely et al., 2015). As such, we hypothesized that the majority of our observed ADP production was due to ATP hydrolysis with water, which could be detected by the production of free phosphate using a malachite green assay. Indeed, we found a clear correlation between the amount of activity observed in the ADP-Glo assay and the malachite green assay, demonstrating that 2-alkylphenols with Triton X-100 increase the ATPase activity of UbiB family members (Figures 2B and S2B). Consistently, COQ8 KxGQ domain mutations that possess markedly enhanced cis autophosphorylation (e.g., the double A-to-G, K-to-H mutant) (Stefely et al., 2016; Stefely et al., 2015) show little ATPase activity, are unable grow in respiratory media, and fail to enable CoQ6 production in vivo (Figures 2C, 2D, S2C, and S2D). This further demonstrates that ATPase activity, and not protein kinase-related activity, tracks most closely with the endogenous function of COQ8.
Notably, compounds that more closely mimic the mature CoQ headgroup, such as 2,6-dimethoxyhydroquinone or CoQ1, did not greatly enhance COQ8 activity, in agreement with our CoQ precursor binding data (Figure S2E) (Stefely et al., 2016). Furthermore, the activation by 2-alkylphenols with Triton X-100 is conserved in UbiB, Coq8p, and COQ8A, mirroring the conservation of CoQ precursor binding (Figure 2E). Overall, these data suggest that COQ8 possesses an evolutionarily conserved ability to bind early CoQ intermediates and to be activated by 2-alkylphenols.
Mature COQ8 is activated by liposomes
Endogenous COQ8 contains a TM domain that anchors it to the inner mitochondrial membrane (IMM) facing the matrix where the other complex Q proteins reside and where it can potentially interact with CoQ intermediates (Cullen et al., 2016; Tauche et al., 2008; Vogtle et al., 2017). This suggests that membrane association might be another important modulator of its activity, as has been shown for other membrane-bound kinases (Jura et al., 2009). The mature N-termini of COQ8A and Coq8p (i.e., the N-termini present after cleavage of the mitochondrial localization sequences) begin 163 residues and 42 residues from the unprocessed N-terminus, respectively (Calvo et al., 2017; Cullen et al., 2016; Stefely et al., 2015; Vogtle et al., 2009). To test the effect of an intact TM domain on COQ8 membrane association and activity, we purified recombinant versions of the mature forms of COQ8A (COQ8ANΔ162) and Coq8p (Coq8pNΔ41) (Figure S3A).
Given that mature COQ8 resides on the IMM, we reasoned that mixing the protein with liposomes that contain IMM-enriched lipids, such as cardiolipin (CL) and phosphatidylethanolamine (PE) (de Kroon et al., 1997; Horvath and Daum, 2013; Zinser et al., 1991), along with the abundant membrane lipid phosphatidylcholine (PC), could have an effect on COQ8 membrane binding and activity. To test this, we first used a liposome flotation assay to determine how effectively COQ8 binds to liposomes (Figure 3A). Without the TM domain, COQ8ANΔ250 exhibited moderate affinity for PC/PE/CL liposomes; however, the addition of the TM domain enabled near complete COQ8ANΔ162 and Coq8pNΔ41 liposome binding (Figures 3B). Strikingly, this association markedly enhanced the ATPase activity of COQ8ANΔ162 and Coq8pNΔ41 (Figure 3C), which is several orders of magnitude higher than autophosphorylation (Figure S3B). Similar to the results observed with phenols and Triton X-100, KxGQ mutants were not activated by liposomes (Figure S3C). These data imply that UbiB proteins, like other PKL family members, may be activated endogenously by binding to particular membrane environments.
Figure 3. COQ8 binds to liposomes.
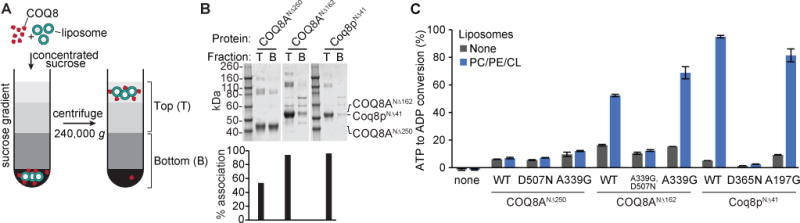
(A) Schematic of a liposome flotation assay. (B) Coomassie stained SDS-PAGE from a liposome flotation assay with COQ8 and PC/PE/CL liposomes. T; top fraction, B, bottom fraction. (C) ADP-Glo assay with COQ8 variants and PC/PE/CL liposomes. Error bars represent s.d. of two independent experiments performed in technical triplicate. See also Figure S3.
Cardiolipin specifically increases COQ8 membrane interaction and ATPase activity
To determine whether the COQ8 membrane binding and activity is driven by a particular membrane component, we tested the ability of multiple individual lipids to activate COQ8 when reconstituted in PC carrier liposomes. Remarkably, from amongst the eight individual species tested, only CL was able to activate COQ8 (Figure 4A). This increase in activity was matched by the superior ability of CL to facilitate binding of COQ8 to liposomes, indicating that CL activation of COQ8 is directly linked to its ability to mediate COQ8-membrane interactions (Figures 4B and S4A). Interestingly, COQ8 lacking its TM still preferentially bound to CL-containing liposomes (Figure S4B), but was not activated by them (Figure 3C), suggesting that the TM domain itself is a regulatory feature of COQ8.
Figure 4. CL enhances the ATPase activity of COQ8 and liposome binding.
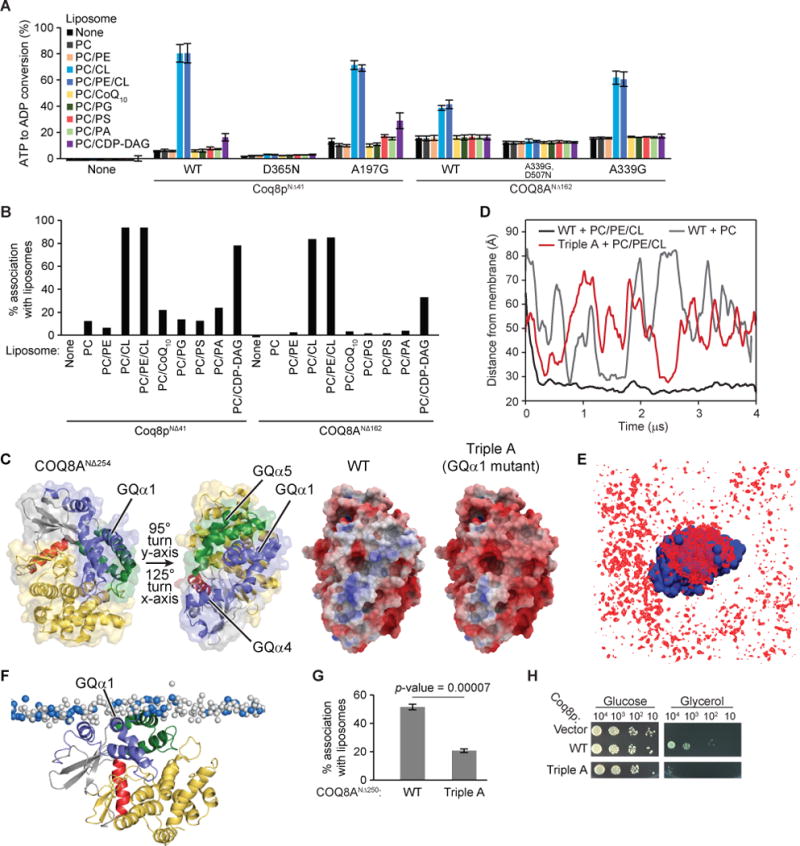
(A) ADP-Glo assay with a panel of liposomes and TM domain containing COQ8. Error bars represent s.d. of two independent experiments performed in technical duplicate. (B) Liposome flotation assay with a panel of PC based liposomes and TM-containing COQ8. (C) The two structures of COQ8ANΔ254 on the left are colored according to Stefely et al., 2015 and turned to show the orientation of the electrostatic maps. The KxGQ domain is colored in purple and green in the structures on the left. On the right, there are electrostatic maps of COQ8ANΔ254 showing the positively charged region spanning GQα1, GQα4, and GQα5 [negative (−5 kcal/e.u. charge): red, via white, to positive (+5 kcal/e.u. charge): blue]. (D) Time evolution of the distance between the center of mass of the protein and the center of mass of the phosphate heads of the leaflet it interacts with for CG-MD simulations of COQ8ANΔ254 with PC or PC/PE/CL liposomes or of the Triple A mutant with PC\PE\CL liposomes. (E) Average occupancy of CL phosphate heads (red) when COQ8ANΔ254 (blue) is centered throughout the trajectory. (F) Snapshot from a CG-MD simulation (t ~ 4 μs) showing the interaction of COQ8ANΔ254 with a PC/PE/CL membrane. CL phosphate heads shown in blue and PC/PE phosphate heads shown in white. (G) Percent protein associating with liposomes from a liposome flotation assay with COQ8ANΔ250 WT and the Triple A mutant (R262A,R265A,K269A). (H) Serial dilutions of Δcoq8 transformed with Coq8p WT or the Triple A mutant (R120A,K124A,K127A) on synthetic complete glucose (2% w/v) or glycerol (3% w/v) containing media. See also Figure S4.
To test whether liposome/CL binding specifically enhances the ATPase activity of COQ8, we performed [γ-32P]ATP kinase reactions with liposomes to follow the location of the gamma phosphate. We also assessed autophosphorylation using SDS-PAGE and potential phosphorylation of copurifying lipid substrates or CL using thin-layer chromatography. Consistent with the activation of COQ8 by phenols, no autophosphorylation or lipid phosphorylation was observed (data not shown), further supporting the hypothesis that COQ8 acts as an ATPase (Figures S4C). Additionally, liposome activation of COQ8 did not alter its inability to phosphorylate purified protein substrates, including complex Q proteins and myelin basic protein (Figures S4D and S4E). Collectively, these data demonstrate that CL enables COQ8 membrane binding and enhances COQ8 ATPase activity.
COQ8 interacts with the membrane through its signature KxGQ domain
To further explore the nature of COQ8 membrane binding and activation, we used molecular dynamics to simulate the binding of COQ8A to membranes. Examination of the electrostatic surface potential of the soluble domain of COQ8A revealed an extensive positive patch generated primarily by the GQα1 and GQα4 helices that likely interacts with negatively charged phospholipids found in the IMM, such as the CL used in our liposomes (Figures 4C and S4F). We then used coarse-grained molecular dynamics (CG-MD) simulations to investigate the binding of COQ8A to PC/PE/CL, mimicking the IMM-like membranes used in our liposome flotation assays, or to PC alone, mimicking a non-IMM membrane model. Invariably, the presence of CL at physiological concentration (Horvath and Daum, 2013) was required to induce rapid (within the first microsecond) membrane association (Figure 4D and Movies S1 and S2) mediated by the GQα1 and GQα4 helices (Figures 4F), providing independent validation of our liposome flotation assay results. Interestingly, anchoring of COQ8A at the IMM is associated with local reorganization of lipids, featuring a strong segregation of CL species to the COQ8A interface (Figure 4E).
Our CG-MD simulations further predicted three positively charged residues of the GQα1 helix (R262, R265, and K269) as the key anchor points driving initial encounter of the soluble domain with the membrane via electrostatic interactions with cardiolipin phosphate heads (Figures S4G and S4H). To validate the importance of these residues, we purified a triple mutant of COQ8ANΔ250 (R262A,R265A,K269A; Triple A) and measured its ability to bind liposomes. Indeed, this mutant demonstrated a large decrease in liposome association (Figures 4G and S4I). When the corresponding mutations were introduced into COQ8, its ability to rescue Δcoq8 respiratory deficiency was significantly diminished, suggesting that these residues may have important ramifications for membrane binding and/or orientation in vivo (Figures 4H, S4J and S4K). Altogether, these simulations reinforce the importance of CL for COQ8 membrane association, and suggest that the COQ8–CL interaction is driven by conserved residues in the signature KxGQ domain.
Inhibition of Coq8p-AS activity disrupts CoQ biosynthesis
Our analyses above suggest that COQ8 is a conserved ATPase that is activated by lipids found in its native environment on the mitochondrial inner membrane. We next sought to determine whether acute chemical inhibition of COQ8 activity in this native environment would be sufficient to disrupt CoQ biosynthesis and respiratory function in yeast. To do so, we attempted to generate an analog-sensitive (AS) version of Coq8p (Coq8p-AS) that could be covalently modified by halomethyl ketone inhibitors (Bishop et al., 2000; Cohen et al., 2005; Snead et al., 2007).
To create Coq8p-AS, we first identified the putative Coq8p gatekeeper and conserved valine residues by performing sequence and structural alignments of Coq8p with RSK2, a kinase amenable to covalent inhibition by halomethyl ketone inhibitors (Figures 5A and 5B) (Cohen et al., 2005). These analyses suggested that methionine 303 (M303) is the Coq8p gatekeeper residue and that V202 occupies the position of a nonconserved cysteine present in mammalian p90 RSK family kinases. To further test this in silico, we mutated the gatekeeper to a smaller residue (M303C), and mutated V202 to a cysteine to enable reactivity with halomethyl ketone inhibitors (Figure 5C). Our models indicated that the WT Coq8p nucleotide binding pocket was incapable of accepting CMK—a halomethyl ketone inhibitor designed specifically for kinases with a small gatekeeper and a cysteine on β-strand 2 in place of a conserved valine—due to steric clashes with the methionine side chain (Figures 5D and S5A). However, the M303C and V202C mutations alleviated these steric clashes and enabled covalent modification by CMK, respectively (Figures 5E and 5F).
Figure 5. Creation of analog-sensitive Coq8p.
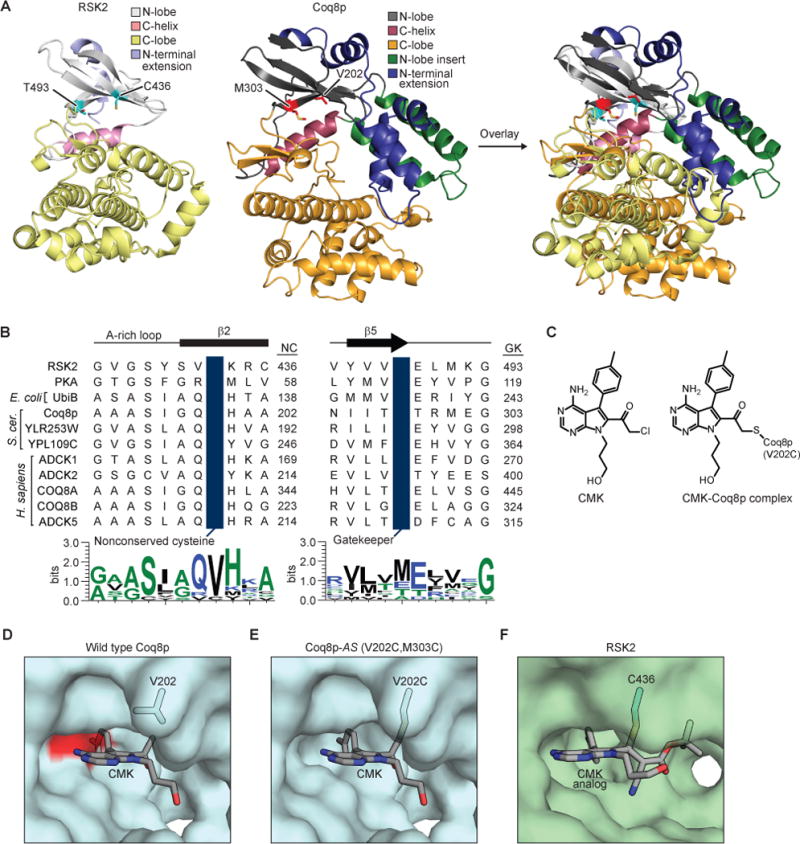
(A) Structural alignment of Coq8p homology model based on COQ8A (PDB: 5I35) with RSK2 (PDB: 4D9U). The nonconserved cysteine C436 and gatekeeper T493 residues are shown in cyan. The V202 and gatekeeper M303 residues of Coq8p are shown in red. (B) Primary sequence alignment of the nonconserved cysteine and gatekeeper residues from human RSK2, human PKA, and the UbiB family. Sequence logos are shown for the displayed amino acid sequences. NC, nonconserved cysteine; GK, gatekeeper. (C) Structure of CMK and its covalent interaction with Coq8p. (D) Modeling of CMK (lacking the chlorine atom) in the active site of the homology model of Coq8p. The surface of M303 is colored in red demonstrating a steric clash with CMK. (E) Mutation of valine 202 and methionine 303 to cysteine (V202C,M303C) creates a binding pocket for and allows covalent modification by CMK. (F) Structure of RSK2 (PDB: 4D9U) with a cyanoacrylate CMK analog (Serafimova et al., 2012). See also Figure S5.
To generate this putative Coq8p-AS, we expressed and purified Coq8pNΔ41 V202C,M303C from E. coli (Figure S5B). Using differential scanning fluorimetry (Niesen et al., 2007), we tested the ability of Coq8pNΔ41 V202C,M303C to bind CMK. Consistent with the modeling predictions, only the V202C,M303C mutant was able to bind to the inhibitor CMK, which increased the melting temperature of the mutant by a remarkable 15 degrees (Figures 6A and 6B). LC-MS/MS of an in vitro reaction containing Coq8p-AS and CMK revealed selective covalent modification of residue C202 by CMK (Figure S6A). The V202C,M303C mutant was able to rescue Δcoq8 respiratory function (Figure 6C), demonstrating that the endogenous function of the protein is intact. Accordingly, in an in vitro ATPase assay, Coq8pNΔ41 V202C,M303C had activity in the presence of PC/CL liposomes, but was completely inactivated by CMK (Figure 6D).
Figure 6. Acute inhibition of Coq8p-AS (V202C,M303C) with CMK decreases de novo CoQ biosynthesis.
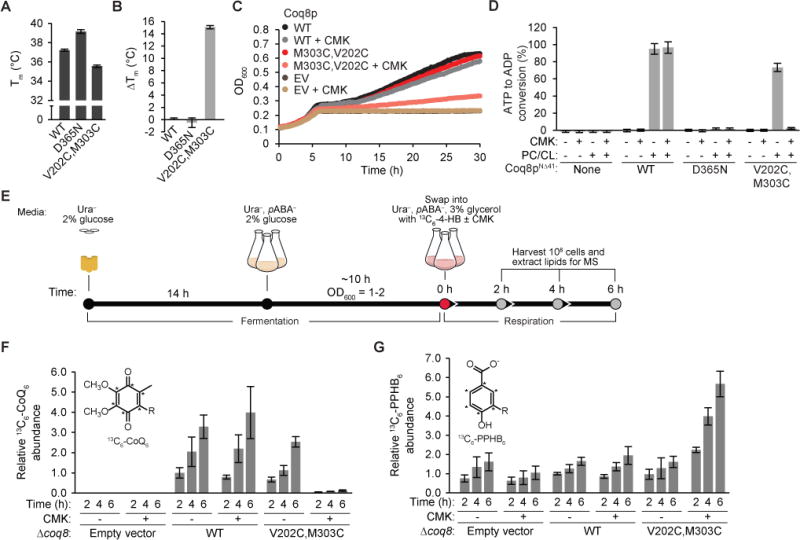
(A) Melting temperature of Coq8p variants measured with DSF. (B) ΔTm for COQ8 variants with the inhibitor CMK. Error bars represent s.d. of three independent experiments performed in technical triplicate. (C) Growth curve of Δcoq8 yeast transformed with Coq8p-AS and treated with CMK (20 μM) in Ura−, pABA− glucose (0.1% w/v) and glycerol (3% w/v) media. EV, empty vector. (D) ADP-Glo assay with Coq8p variants, PC/CL liposomes and the inhibitor CMK. Error bars represent s.d. of three independent experiments performed in technical triplicate. (E) Experimental scheme for heavy isotope labeling of CoQ6 and PPHB6 using 13C6-4HB. (F) and (G) Relative abundance of 13C6-CoQ6 (de novo synthesized CoQ6) and 13C6-PPHB6 from Δcoq8 yeast transformed with Coq8p-AS and treated with 13C6-4HB (50 μM) and CMK (20 μM) after 2, 4, and 6 hours in Ura−, pABA− glycerol (3% w/v) media measured using targeted LC-MS/MS. Asterisk (*) indicates 13C atoms; R, six isoprene units. Error bars represent s.d. from three biological replicates normalized to a CoQ10 internal standard. See also Figure S6.
To determine the effects of endogenous Coq8p inhibition, we treated Δcoq8 yeast expressing Coq8p V202C,M303C with CMK and measured respiratory function and the de novo production of CoQ. Treatment of Coq8p-AS yeast with CMK caused a marked decrease in respiratory function, as indicated by severely delayed growth in liquid culture, whereas this treatment had very little effect on WT or empty vector (EV)-transformed yeast (Figure 6C). To assess de novo CoQ biosynthesis, yeast were grown first in glucose-based media, and were then swapped into glycerol-based media containing heavy isotope labeled 4-hydroxybenzoic acid (13C6-4-HB), a precursor of the CoQ head group, and CMK (Figures 6E and S6B). Both media lacked para-amino benzoate (pABA), an alternative CoQ precursor. Treatment of the V202C,M303C strain with CMK caused a rapid and near complete inhibition of de novo CoQ biosynthesis concomitant with an increase in the CoQ precursor, PPHB (Figures 6F and 6G). These data reveal that CMK added to yeast liquid culture is capable of accessing and inhibiting Coq8p-AS in its proper endogenous location within the mitochondrial matrix, and that COQ8 catalytic activity is required for CoQ precursor processing downstream of PPHB, consistent with earlier analyses of Δcoq8 yeast and of strains harboring COQ8 mutations. Collectively, our work defines lipid and small molecule modulators—both endogenous and custom AS-compounds—of an ancient atypical kinase-like protein. Additionally, our establishment of a chemical genetic system for acutely inactivating COQ8 will enable further dissection of the precise mechanistic role of COQ8 in CoQ biosynthesis.
Discussion
Coenzyme Q was discovered 60 years ago as a key component of the mitochondrial electron transport chain, yet multiple aspects of its biosynthesis remain obscure. One such aspect is the unexplained need for multiple auxiliary proteins that have no clear catalytic role in the pathway, including COQ8—a highly conserved member of the large protein kinase-like (PKL) superfamily. Given its PKL membership, COQ8 has been proposed to fill a regulatory gap in CoQ biosynthesis by phosphorylating other proteins in the CoQ pathway (Xie et al., 2011). However, to date, no COQ8 trans protein kinase activity has been observed, demanding that other models for its activity be explored.
To further investigate the biochemical function of COQ8, we sought to study it in its native environment and to establish new tools to modulate its function. Multiple members of the PKL superfamily become activated by binding to endogenous molecules found at their sites of cellular activity, such as the activation of PKC or Akt by diacylglycerol and phosphatidylinositol 3,4,5-trisphosphate, respectively, at the plasma membrane (Burgering and Coffer, 1995; Franke et al., 1995; Nishizuka, 1992). Furthermore, protein-lipid interactions in general are becoming increasingly recognized as regulatory and structural features of proteins (Gupta et al., 2017; Moravcevic et al., 2012; Yeagle, 2014), indicating that it may be important to identify the full set of molecules that interact with COQ8 in order to elucidate its biochemical function.
Consistent with our recent results using Coq8p, our binding assays point to a conserved and selective interaction between COQ8 and precursors in the CoQ biosynthesis pathway. Curiously, these interactions seem to rely on the integrity of the COQ8 active site and result in an increase in COQ8 ATPase activity without affecting its inability to phosphorylate proteinaceous substrates. In mitochondria, these precursors would most likely be found buried between the leaflets of the inner mitochondrial membrane (IMM) due to their extreme hydrophobicity. These data might suggest that COQ8 is poised to sense CoQ precursors within the membrane and to then couple the hydrolysis of ATP to the partial extraction of these lipids into the aqueous matrix environment where they could be modified by other CoQ biosynthesis enzymes. Alternatively, it remains possible that the observed ATPase activity is a proxy for small molecule kinase activity in vivo; however, as COQ8-dependent small molecule phosphorylation was not observed in our assays, these data indicate that endogenous small molecule substrate(s) would likely be distinct from the free hydroxyl group(s) of CoQ intermediates, the most intuitive candidate substrates.
Similar to PKC and Akt, our data also indicate that COQ8 is activated via interaction with a specific lipid—cardiolipin (CL). CL is a unique anionic, four-tailed lipid found at particularly high concentrations in the IMM where it influences the activity and stability of various mitochondrial membrane proteins (Claypool, 2009; Claypool et al., 2008; Gebert et al., 2009; Planas-Iglesias et al., 2015), and promotes the formation of respiratory chain supercomplexes (Pfeiffer et al., 2003). In addition to mere binding, our CG-MD simulations indicate that CL also directs the orientation of the soluble COQ8 domain along the membrane surface, perhaps thereby enabling COQ8’s known interactions with other COQ proteins (Cullen et al., 2016; Floyd et al., 2016) and/or with membrane-embedded CoQ precursors. Furthermore, CL is thought to be distributed non-uniformly throughout the IMM (Mileykovskaya and Dowhan, 2009), suggesting that the interaction of COQ8 with CL might seed complex Q formation at strategic sub-mitochondrial locations. These observations suggest models whereby the proper positioning of COQ8 at CL-rich domains on the mitochondrial inner membrane activates its ATPase activity as a requisite first step in enabling complex Q assembly and/or CoQ biosynthesis, and that exposure to CoQ intermediates at those sites can further enhance COQ8 function (Figure 7). Within this context, CG-MD shows once more to be a powerful resource to understand the molecular determinants underlying protein-lipid interplay.
Figure 7. Model for how COQ8 ATPase ability could facilitate CoQ biosynthesis.
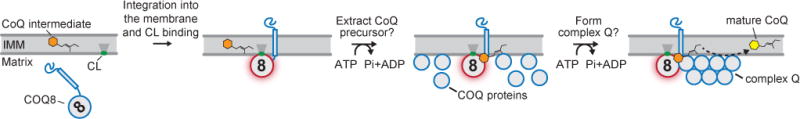
First, COQ8 is imported in to the matrix. Binding to CL then facilitates COQ8’s association with, and insertion into, the IMM with its soluble domain properly oriented along the membrane surface. Next, the CL-induced activation of COQ8 (red glow) allows it to advance CoQ biosynthesis by coupling the hydrolysis of ATP to the extraction of CoQ precursors out of the IMM and/or to the formation of complex Q.
More broadly, these results suggest an important and underappreciated connection between CoQ and CL—two quintessential mitochondrial lipids that enable oxidative phosphorylation (OxPhos). Indeed, CL has long been known to enable electron transfer between CI and CIII (Fry and Green, 1981), and in plants, CL was found to have a positive effect on the electron transfer rate from CoQ to the photosynthetic reaction center (Giustini et al., 2005). Similarly, CL enhances electron transport from Complex I to CoQ in Drosophila mitochondrial lysate (Vos et al., 2017), and the CL remodeling phospholipase Cld1p was shown to rescue coq7 hypomorphs in S. cerevisiae harboring mutations in Coq7p’s hydrophobic α-helix that is predicted to bind the IMM (Kar et al., 2016). These observations in conjunction with our data suggest a model whereby enhanced CL biosynthesis may augment OxPhos function in part by enabling the biosynthesis of CoQ via COQ8.
Testing these and other models of COQ8 function require new tools to modulate COQ8 activity in its endogenous setting where these activating molecules are present. To that end, we developed an analog-sensitive (AS) version of yeast Coq8p (Coq8p-AS). This chemical genetic strategy has proven to be highly effective for elucidating the functions of other PKL family members, but has never been attempted for the highly divergent UbiB family. Furthermore, this approach has not been attempted for kinases that reside within organelles where they would potentially be inaccessible to inhibitors. Our work demonstrates that endogenous chemical inhibition of COQ8 is sufficient to rapidly disrupt de novo CoQ biosynthesis, further suggesting that the catalytic activity of COQ8 is important for this pathway. Moreover, our demonstration that a UbiB family member is amenable to a chemical genetic system suggests that this tool can likewise be used to interrogate the functions of other family members, including Ypl109c and Ylr253w (Mcp2p) in yeast, the ABC1Ks in plants, and ADCK1, 2 & 5 in humans. These proteins have been implicated in diverse biological functions in cell migration (Simpson et al., 2008), tumor cell viability (Brough et al., 2011; Wiedemeyer et al., 2010), and lipid metabolism (Lundquist et al., 2012; Martinis et al., 2013; Tan et al., 2013), each through unclear means. A chemical genetic approach would offer the opportunity to identify functions for these proteins without the added complexity of compensatory changes that accompany full gene knockouts.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dave Pagliarini (dpagliairini@morgridge.wisc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Escherichia coli strain DH5α (NEB) was used for all cloning applications and grown at 37 °C in LB media [tryptone (10 g/L), yeast extract (5 g/L), NaCl (5 g/L)] with antibiotics. Escherichia coli strain BL21-CodonPlus (DE3)-RIPL (Agilent) was used for all protein expression and purification purposes and grown in autoinduction media (900 mL TB+G, 100 mL PO4− mix, 50 mL 20× 80155, 25 mL 15% aspartate buffer, 2 mL 1M MgSO4, 2 mL 15 mg/mL chloramphenicol stock (in ethanol), and 2 mL 60.6 mg/mL kanamycin stock). TB+G: tryptone (12 g/L), yeast extract (24 g/L), 10% (w/v) glycerol (40 mL/L) and autoclaved to sterilize. PO4− mix: KH2PO4 (23.1 g/L), K2HPO4 (125.4 g/L) and autoclaved to sterilize. 15% aspartate buffer: aspartate (150 g/L) and NaOH (44.6 g/L) pH 6.5–7.0 and autoclaved to sterilize. 20× 80155: glucose (3 g/L), α-lactose monohydrate (100 g/L), glycerol (160 g/L) and autoclaved to sterilize. 1M MgSO4, 15 mg/mL chloramphenicol, and 60.6 mg/mL kanamycin stocks were sterilized by filtration (0.22 μm pore size). See the following method below for more details: Recombinant Protein Expression and Purification. For yeast rescue assays in Figures 2C, 2D, 4H, S4J and S4K, Saccharomyces cerevisiae strain W303 was used. For the analog-sensitive experiments (Figure 6C, 6F and 6G), Saccharomyces cerevisiae strain BY4742 was used. Ura− media contained Ura− drop-out mix (1.92 g/L), yeast nitrogen base (6.7 g/L, with ammonium sulfate and without amino acids), and a carbon source of glucose and/or glycerol. Ura− media contained Ura−, pABA− drop-out mix (770 mg/L), yeast nitrogen base (6.7 g/L, with ammonium sulfate and without amino acids), and a carbon source of glucose and/or glycerol. Synthetic Ura− media were sterilized by filtration (0.22 μm pore size). See the following methods below for more details: Yeast Drop Assay and Growth Curves, Growth of Yeast and Extraction of Yeast CoQ6 for LC-MS Quantitation, and Immunoblotting of Yeast Coq8p.
METHOD DETAILS
NMR screening
One dimensional (1D) NMR ligand-affinity screens provide a quick and effective approach to identify small molecules from a large library of compounds that interact with a protein (Fischer and Jardetzky, 1965; Lepre et al., 2004; Mercier et al., 2006). We used a 1D 1H NMR ligand affinity screen to identify interacting small molecules from a library of 417 compounds, which consists primarily of metabolites, substrates, inhibitors, etc (Table S2). To minimize the materials required to evaluate every compound with COQ8ANΔ250, the compounds were combined into 89 mixtures of 3–5 compounds each. The mixtures were designed to minimize 1H peak signal overlap between the compounds using NMRmix (Stark et al., 2016). Two sets of NMR samples were created for each mixture: one with COQ8ANΔ250 and one without COQ8ANΔ250. The NMR samples were prepared in 15 mM bis-Tris-d19 buffer at pH 7.4 with 250 μM NaCl, 0.04% NaN3, and 15 μM DSS in in 99.99% D2O and placed in 3 mm SampleJet NMR tubes. Each NMR sample had a 75 μM final concentration for each compound in the mixture and a final COQ8ANΔ250 concentration of 6 μM in the set of samples containing protein. The NMR spectra were collected on a Bruker Avance III 600 MHz spectrometer equipped with a 5 mM cryoprobe and a SampleJet sample changer set at 25 °C, and Topspin v3 (Bruker) and NMRbot were used to automate the data collection process (Clos et al., 2013). The 1D 1H spectra were collected for all samples using a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with f1 presaturation and 128 averaged transients consisting of 16,384 time-domain points and a sweep width of 12 ppm.
The 1D 1H spectra were phased, baseline corrected, referenced to DSS, and analyzed using MestReNova 11 (Mestrelab Research). The spectra of the mixtures with COQ8ANΔ250 were visually compared to the spectra of the mixtures without COQ8ANΔ250. If the peak intensities of a compound in a mixture showed a substantial decrease (typically >50%) in the presence of COQ8ANΔ250, the compound would be considered a “hit” or interacting compound. The screen of the compound library with COQ8ANΔ250 identified 25 interacting compounds (Table S2).
Computational Modeling and Coarse-Grained Molecular Dynamics Simulations
All simulations were performed using the GROMACS (Abraham et al., 2015) simulation package version 5.0.4. The systems were described with the MARTINI (Marrink et al., 2007) CG force field, together with the ElNeDyn (Periole et al., 2009) approach to maintain the secondary and tertiary structure of the protein. CG-MD simulations based on the MARTINI force field have been previously used with success for different systems to investigate protein-lipid interactions (Periole et al., 2007; Schafer et al., 2011; Stansfeld et al., 2009; van den Bogaart et al., 2011).
The systems were built with center of mass of COQ8A (PDB: 4PED) 70 Å away from the surface of the membrane and checked for whether the protein approaches and interacts with the membrane. Inner mitochondrial membrane (IMM) models were built according to the experimental molecular ratio of CL observed for bovine heart mitochondria, namely 15 to 20% of the phosphorus content (Daum, 1985). Therefore, IMM mimics were featuring a lipid concentration of 16% CL/41% POPC/37% POPE/6% DSPC, while generic membrane models featuring 100% POPC lipids were built as control. Lipid bilayers of 130 × 130 Å2 were generated using the insane (INSert membrANE) (Wassenaar et al., 2015) method of MARTINI. Systems were solvated with polarizable CG water and counter ions were added to neutralize the system. Following solvation, systems were energy minimized with a timestep of 5 fs. Successive equilibrations with decreasing restraints were performed in order to obtain a fully equilibrated system (force constants of 1000, 500, 250 and 0 kJ/mol applied on protein particles, 10 ns of run with a timestep of 10 fs for each). In the production phase, which is 4 μs for all simulations, the protein, membrane and the aqueous phase (water and ions) were coupled independently to an external bath at 310 K and 1 bar. Three MD repeats with randomized initial velocities were performed for each membrane system and they yielded consistent results. Average occupancy of cardiolipins was calculated using VMD’s VolMap plugin (Humphrey et al., 1996).
Atomistic models of the different GQα mutated COQ8A proteins were built in PyMOL by mutating the following residues to alanine: R262, R265, K269 for ΔGQα1; K310, K314 for ΔGQα4; R262, R265, K269, K310, K314 for ΔGQα1α4; Q366, S367, N369, S370, N373, N374 for ΔGQα5. The effect of these mutations on the electrostatic potential surface of the protein was visualized through the ICM-REBEL module of the ICM program (Abagyan et al., 1994). CG-MD simulations were performed for these four mutants following the procedure explained above, where the residues listed for each mutant were altered by the removal of charges of the polarizable CG beads, to knockout Coulomb interactions without changing the residue types.
Modeling of Coq8p-AS with CMK
A homology model of as-Coq8p was generated by SWISS-MODEL using the nucleotide-bound form of Coq8A (PDB:5I35) as a template (RMSD 0.35Å over 2264 of 2590 aligned atoms) (Biasini et al., 2014). To understand the likely position of the bulky analog CMK bound to Coq8p-AS, CMK was superposed on the adenine ring and C1’ atoms of the nucleotide in 5i35 (RMSD 0.064Å over 11 paired atoms.) Clashes were relieved in UCSF Chimera by a small rigid body displacement of CMK, adjusting torsion angles of CMK (Pettersen et al., 2004). The cealign Pymol command was used to overlay the Coq8p homology model and RSK2 (PDB:4D9U) (RMSD of 4.75 Å over 120 residues). Sequence logos were created with WebLogo3 (Crooks et al., 2004).
DNA Constructs and Cloning
Cloning of COQ8ANΔ250, Coq8pNΔ41, PKA, and p426 GPD COQ8 plasmids was previously described (Stefely et al., 2016; Stefely et al., 2015). For MBP-UbiBCΔ47 and COQ8ANΔ162 standard PIPE cloning methods (Klock et al., 2008) were used. PIPE reactions were DpnI digested and transformed into DH5α competent E. coli cells. Plasmids were isolated from transformants and DNA sequencing was used to identify those containing the correct constructs. pVP68K, a plasmid for expression of recombinant proteins in bacteria [8His-cytoplasmically-targeted maltose-binding protein (MBP) with a linker including a tobacco etch virus (TEV) protease recognition site fused to the protein construct (8His-MBP-[TEV]-Protein)], has been described previously (Blommel et al., 2009). Oligonucleotides were purchased from IDT (Coralville, IA, USA). For yeast rescue experiments, COQ8 was cloned into the p426 GPD vector (Mumberg et al., 1995). Point mutations were introduced by PCR-based mutagenesis and confirmed by DNA sequencing.
Recombinant Protein Expression and Purification
Human COQ8ANΔ162 and COQ8ANΔ250
Method adapted from (Stefely et al., 2016; Stefely et al., 2015). COQ8A constructs were overexpressed in E. coli by autoinduction (Fox and Blommel, 2009). Cells were isolated and frozen at −80 °C until further use. For protein purification, cells were thawed, resuspended in Lysis Buffer [20 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM 2-mercaptoethanol (BME) or 0.5 mM tris(2-carboxyethyl)phosphine (TCEP), 0.25 mM phenylmethylsulfonyl fluoride (PMSF)] (4 °C). The cells were lysed by sonication (4 °C, 75% amplitude, 10 s × 3). The lysate was clarified by centrifugation (15,000 g, 30 min, 4 °C). The cleared lysate was mixed with cobalt IMAC resin (Talon resin) and incubated (4 °C, 1 h). The resin was pelleted by centrifugation (700 g, 2 min, 4 °C) and washed four times with Wash Buffer [50 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME, 0.25 mM PMSF, 10 mM imidazole]. His-tagged protein was eluted with Elution Buffer [20 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME or 0.5 mM TCEP, 0.25 mM PMSF, 100 mM imidazole]. The eluted protein was concentrated with a MW-cutoff spin filter (50 kDa MWCO) and exchanged into Storage Buffer [20 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME or 0.5 mM TCEP]. The concentration of 8His-MBP-[TEV]-COQ8ANΔ250 was determined by its absorbance at 280 nm (ε = 96,720 M−1 cm−1) (MW = 89.9 kDa) and 8His-MBP-[TEV]-COQ8ANΔ162 (ε = 96,720 M−1 cm−1) (MW = 99.26 kDa). The fusion protein was incubated with Δ238TEV protease (1:50, TEV/fusion protein, mass/mass) (1 h, 20 °C). The TEV protease reaction mixture was mixed with cobalt IMAC resin (Talon resin), incubated (4 °C, 1 h). The resin was pelleted by centrifugation (700 g, 3 min, 4 °C). The unbound COQ8A was collected and concentrated with a MW-cutoff spin filter (30 kDa MWCO) and exchanged into storage buffer. The concentration of COQ8ANΔ250 was determined by its absorbance at 280 nm (ε = 28,880 M−1 cm−1) (MW = 45.6 kDa). The concentration of COQ8ANΔ162 was determined by its absorbance at 280 nm (ε = 28,880 M−1 cm−1) (MW = 54.99 kDa). The protein was aliquoted, frozen in N2(l), and stored at −80 °C. Fractions from the protein preparation were analyzed by SDS-PAGE.
Yeast Coq8pNΔ41
Coq8pNΔ41 plasmid constructs were transformed into RIPL competent E. coli cells for protein expression. 8His-MBP-[TEV]-Coq8pNΔ41 was overexpressed in E. coli by autoinduction. Cells were isolated by centrifugation, frozen in N2(l), and stored at −80 °C until further use. For protein purification, cells were thawed on ice, resuspended in Lysis Buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 5% glycerol, 1 mM BME, 0.25 mM PMSF, 1 mg/mL lysozyme, pH 7.5] and incubated (1 h, 4 °C). The cells were lysed by sonication (4 °C, 6 V, 60 s × 4). The lysate was clarified by centrifugation (15,000 g, 30 min, 4 °C). The cleared lysate was mixed with cobalt IMAC resin (Talon resin) and incubated (4 °C, 1 h). The resin was pelleted by centrifugation (700 g, 5 min, 4 °C) and washed four times with Wash Buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 5% glycerol, 1 mM BME, 0.25 mM PMSF, 10 mM imidazole, pH 7.5] (10 resin bed volumes). His-tagged protein was eluted with Elution Buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 5% glycerol, 1 mM BME, 100 mM imidazole, pH 7.5]. The eluted protein was concentrated with a MW-cutoff spin filter (50 kDa MWCO) and exchanged into storage buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 5% glycerol, 1 mM BME, pH 7.5]. The concentration of 8His-MBP-[TEV]-Coq8pNΔ41 was determined by its absorbance at 280 nm (ε = 109,210 M−1 cm−1) (MW = 96.2 kDa). The fusion protein was incubated with Δ238TEV protease (1:50, TEV/fusion protein, mass/mass) (1 h, 20 °C). The TEV protease reaction mixture was mixed with cobalt IMAC resin (Talon resin) and incubated (4 °C, 1 h). The unbound Coq8pNΔ41 was isolated and concentrated with a MW-cutoff spin filter (30 kDa MWCO) and exchanged into Storage Buffer. The concentration of Coq8pNΔ41 was determined by its absorbance at 280 nm (ε = 41,370 M−1 cm−1) (MW = 52 kDa). The protein was aliquoted, frozen in N2(l), and stored at −80 °C. Fractions from the protein preparation were analyzed by SDS-PAGE.
Mouse PKA
8-His-MBP-PKA and PKA (mouse PKA, Prkaca) were isolated as described above for Coq8pNΔ41. The concentration of 8His-MBP-[TEV]-PKA was determined by its absorbance at 280 nm (ε = 121,700 M−1 cm−1) (MW = 84.8 kDa). The concentration of PKA was determined by its absorbance at 280 nm (ε = 53,860 M−1 cm−1) (MW = 40.5 kDa).
E. coli MBP-UbiBCΔ47 and MBP
8His-MBP-[TEV]-UbiBCΔ47 and 8His-MBP-[TEV]- were overexpressed in E. coli by autoinduction. Cells were isolated and frozen at −80 °C until further use. For protein purification, cells were thawed, resuspended in Lysis Buffer [50 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol (w/v), 5 mM BME, 0.25 mM PMSF] (4 °C). The cells were lysed by sonication (4 °C, 75% amplitude, 20 s × 2). The lysate was clarified by centrifugation (15,000 g, 30 min, 4 °C). The cleared lysate was mixed with cobalt IMAC resin (Talon resin) and incubated (4 °C, 1 h). The resin was pelleted by centrifugation (700 g, 5 min, 4 °C) and washed four times with Wash Buffer [20 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME or 0.5 mM TCEP, 0.25 mM PMSF, 10 mM imidazole]. His-tagged protein was eluted with Elution Buffer [50 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME, 0.25 mM PMSF, 100 mM imidazole]. The eluted protein was concentrated with a MW-cutoff spin filter (50 kDa MWCO) and exchanged into storage buffer [50 mM HEPES (pH 7.2), 300 mM NaCl, 10% glycerol, 5 mM BME]. The concentration of 8His-MBP-[TEV]-MBP-UbiBCΔ47 was determined by its absorbance at 280 nm (ε = 142,670 M−1 cm−1) (MW = 102.1 kDa). MBP-[TEV]- (ε = 67,840 M−1 cm−1) (MW = 44.284 kDa). The protein was aliquoted, frozen in N2(l), and stored at −80 °C. Fractions from the protein preparation were analyzed by SDS-PAGE.
Nucleotide Binding
The general differential scanning fluorimetry (DSF) method (thermal shift assay) has been described previously (Niesen et al., 2007). Mixtures (20 μL total volume) of Coq8pNΔ41 (2 μM) were prepared with SYPRO Orange dye (Life Tech.) (2×), NaCl (150 mM), HEPES (100 mM, pH 7.5), and ligands (e.g. MgATP). Otherwise, the general DSF method, ligand screen, and dissociation constant experiments were conducted as described for COQ8ANΔ250 (Stefely et al., 2015). Coq8pNΔ41 proteins used for DSF analysis were prepared as described above for COQ8A constructs.
ADP-Glo Assay
ADP-Glo (Promega) was performed according to the manufacturer’s instruction with the following modifications. All solutions were diluted in HBS (150 mM NaCl, 20 mM HEPES pH 7.5). Coq8pNΔ41 (1 μM), COQ8ANΔ250 (2 or 4 μM), COQ8ANΔ162 (2 μM), or MBP-UbiBCΔ47 (0.5 μM) were mixed with liposomes (~3.33 mM), ATP (100 μM), MgCl2 (4 mM), CMK (20 μM) or DMSO (0.2% v/v), Triton X-100 (1 mM) or reduced Triton X-100 (1 mM) (Sigma Aldrich) and CoQ headgroup analogs (Sigma Aldrich) dissolved as 200 mM stock solutions in DMSO (2-PP, 2-propylphenol; 2-MeO-6-MP, 2-methoxy-6-methylphenol; 4-methylcatechol; 4-HB, 4-hydroxybenzoic acid; 2-MeO-HQ, 2-methoxyhydroquinone; 4-MeOP, 4-methoxyphenol; 2-AP, 2-allylphenol; HQ, hydroquinone; 3,4-di-HB, 3,4-dihydroxybenzoic acid; MeHQ, methylhydroquinone; 2,6-diMeO-HQ, 2,6-dimethoxyhydroquinone) (1 mM) (final concentrations for reaction components). Reactions were incubated (30 °C, 45 min). Then ADP-Glo Reagent (5 μL) was added and incubated (40 min, r.t. [~21 °C], covered). Kinase Detection Reagent was added (10 μL) and incubated (40 min, r.t., covered). Luminescence was read using default values on a Biotek Cytation 3 plate reader. An ADP/ATP standard curve was made according to the manufacturer’s instructions. Error bars represent s.d. of technical triplicate measurements unless otherwise specified.
Cytophos ATPase Assay
The Cytophos ATPase assay (Malachite green) was performed according to the manufacturer’s instruction with the following modifications. All solutions were diluted in HBS (150 mM NaCl, 20 mM HEPES pH 7.5). COQ8ANΔ250 (1 μM final), Coq8pNΔ41 (1 μM final), COQ8ANΔ162 (1 μM final) or MBP-UbiBCΔ47 (0.25 μM final) with 100 μM ATP, 4 mM MgCl2, 1 mM 2-alkylphenol, 0.5 mM CoQ1, 1 mM Triton X-100 or reduced Triton X-100. CoQ1 was reduced by adding 1.2 fold molar excess of NaBH4 (Sigma) (10 min, r.t.). Reactions were incubated (30 °C, 45 min) and 35 μL of Cytophos reagent was added and incubated (10 min, r.t.). Absorbance was measured at 650 nm. Reactions in Figure 2E were incubated for 10 min rather than 45 min and [Coq8pNΔ41] was 0.5 μM. The standard curve ranged from 0–50 μM phosphate. Error bars represent s.d. of technical triplicate measurements unless otherwise specified.
In Vitro [γ-32P] Autophosphorylation and ATPase Comparison Assay
Method for Figure S3B
Coq8pNΔ41 (4 μM) was mixed with [γ-32P]ATP (0.25 μCi/μL, 100 μM [ATP]total) and MgCl2 (4 mM) in an aqueous buffer (20 mM HEPES, 150 mM NaCl, pH 7.5) and incubated (30 °C, 45 min, 20 μL total reaction volume) (final concentrations for reaction components). 10 μL of reaction was quenched with 10 μL 0.75 M potassium phosphate pH 3.3, 1 μL of quenched reaction was spotted on a PEI-cellulose TLC plate and developed using the method above. The other 10 μL of the reaction was quenched with 4 μL 4× LDS buffer and 10 μL was loaded onto an SDS-PAGE gel. [γ-32P]ATP was separated from Coq8pNΔ41 by SDS-PAGE (10% Bis-Tris gel, MES buffer, 150 V, 80 min). The gel was stained with Coomassie Brilliant Blue, dried under vacuum, and imaged by digital photography. The same storage phosphor screen was exposed to the TLC plate and the SDS-PAGE gel (1 hr) and then imaged with a Typhoon (GE) to generate the phosphorimages used for quantification of Pi and autophosphorylation.
In Vitro [γ-32P]ATP ATPase Assay
Method for Figure S4C
Unless otherwise indicated, COQ8ANΔ162 (2 μM) or Coq8pNΔ41 (1 μM) was mixed with liposomes (~3.33 mM), [γ-32P]ATP (0.01 μCi/μL, 100 μM [ATP]total), and MgCl2 (4 mM) in an aqueous buffer (20 mM HEPES, 150 mM NaCl, pH 7.5) and incubated (20 μL total volume, 30 °C, 45 min, 700 rpm) (final concentrations for reaction components). Reactions were quenched with 0.75 M potassium phosphate pH 3.3 (20 μL, 4 °C). 1 μL of quenched reaction was spotted on PEI-cellulose TLC (Millipore) and developed with 0.5 M LiCl in 1 M formic acid(aq). After drying, a storage phosphor screen was exposed to the PEI-cellulose TLC plate (~5 hours) and then imaged with a Typhoon (GE) to generate the phosphorimages.
In Vitro [γ-32P]ATP Kinase Assay
Method for Figures S4D–S4F
Unless otherwise indicated, COQ8ANΔ162, Coq8pNΔ41 (4 μM), or PKA (0.2 μM) was mixed with [γ-32P]ATP (0.25 μCi/μL, 100 μM [ATP]total) and MgCl2 (4 mM) in an aqueous buffer (20 mM HEPES, 150 mM NaCl, pH 7.5) and incubated (30 °C, 4 5 min, 700 rpm) (final concentrations for reaction components). Myelin basic protein (1 μM) and a cell free expressed COQ protein complex consisting of COQ3, COQ4, COQ5, COQ6, COQ7-strep, and COQ9 (~1.5–0.1 μM). Half (10 μL) of each reaction was quenched with 4× LDS buffer. [γ-32P]ATP was separated from COQ8 by SDS-PAGE (10% Bis-Tris gel, MES buffer, 150 V, 1.5 h). The gel was stained with Coomassie Brilliant Blue, dried under vacuum, and imaged by digital photography. The other half (10 μL) of the reaction was quenched with 1:1 CHCl3:MeOH (50 μL, 4 °C) and 1 M HCl (12.5 μL, 4 °C). Reactions were mixed by vortexing (3 × 10 s), centrifuged (3000 g, 1 min, 4 °C) and the aqueous layer was discarded. 10 μL of the organic layer was spotted on silica TLC and lipids were separated with CHCl3/MeOH/30% NH4OH(aq)/H2O (50:40:8:2, v/v/v/v). A storage phosphor screen was exposed to the gel or TLC plate (~5 days) and then imaged with a Typhoon (GE) to generate the phosphorimages.
Yeast Drop Assay and Growth Curves
Yeast cultures for drop assays. S. cerevisiae (BY4742) Δcoq8 yeast were transformed as previously described (Gietz and Woods, 2002) with p426 GPD plasmids encoding Coq8p variants and grown on uracil drop-out (Ura−) synthetic media plates containing glucose (2%, w/v). Individual colonies of yeast were used to inoculate Ura− media (2%, w/v) starter cultures, which were incubated (30 °C, ~18 h, 230 rpm). Seria l dilutions of yeast (104, 103, 102, or 10 yeast cells) were dropped onto Ura− agar media plates containing either glucose (2%, w/v) or glycerol (3%, w/v) and incubated (30 °C, 2 d). To assay yeast growth in liquid media, yeast from a starter culture were swapped into Ura− media with glucose (0.1%, w/v) and glycerol (3%, w/v) at an initial density of 5×106 cells/mL. The cultures were incubated in a sterile 96 well plate with an optical, breathable cover seal (shaking at 1140 rpm). Optical density readings were obtained every 10 min. For Coq8p-AS experiments, individual colonies of S. cerevisiae (BY4742) were used to inoculate Ura− media (20 g/L glucose) starter cultures, which were incubated (30 °C, ~12 h, 230 rpm). Yeast were diluted to 2.5×104 cells/mL in Ura−, pABA− media (20 g/L glucose) and incubated until early log phase (30 °C, 16 h, 2 30 rpm). Yeast were swapped into Ura−, pABA− media with glucose (0.1%, w/v) and glycerol (3%, w/v) at an initial density of 5×106 cells/mL with or without 20 μM CMK. The cultures were incubated in a sterile 96 well plate with an optical, breathable cover seal (shaking at 1140 rpm). Optical density readings were obtained every 10 min.
Growth of Yeast and Extraction of Yeast CoQ6 for LC-MS Quantitation
Method for Figure 2D adapted from (Stefely et al., 2015). 2.5×106 yeast cells (as determined by OD600 of a starter culture) were used to inoculate a 25 mL culture of Ura− media (10 g/L glucose), which was incubated (30 °C, 230 rpm) for 23 h. At 2 3 h, the yeast cultures were ~4 h past the diauxic shift and the media was depleted of glucose. The OD600 of the culture was measured and used to determine the volume of culture needed to isolate 1×108 yeast cells. 1×108 yeast cells were pelleted by centrifugation in 15 mL conical tubes (4,000 g, 3 min, 4 °C) and transferred to 1.5 mL tubes and spun again (16,000 g, 0.5 min, 4 °C), the supernatant was discarded, and the yeast pellet was flash frozen in liquid N2 and stored at −80 °C. A frozen pellet of yeast (108 yeast cells) was thawed on ice and mixed with phosphate buffered saline (200 μL) and glass beads (0.5 mm diameter, 100 μL). The yeast were lysed by vortexing with the glass beads (30 s). Coenzyme Q10 (CoQ10) was added as an internal standard (10 μM, 10 μL), and the lysate was vortexed (30 s). Hexanes/2-propanol (10:1, v/v) (500 μL) was added and vortexed (2 × 30 s). The samples were centrifuged (3,000 g, 1 min, 4 °C) to complete phase separation. 400 μL of the organic phase was transferred to a clean tube and dried under Ar(g). The organic residue was reconstituted in ACN/IPA/H2O (65:30:5, v/v/v) (100 μL) by vortexing (30 s) and transferred to a glass vial for LC-MS analysis. Samples were stored at −80 °C.
For analog-sensitive COQ8 experiments (Figure 6), 3 mL starter cultures in Ura− (2% glucose, w/v) were inoculated with a single colony of yeast and incubated (30 °C, 14 h). Next, 50 mL cultures in Ura− pABA− (2% glucose, w/v) were inoculated with 2.5×107 cells and incubated (30 °C, 230 rpm) until reaching an OD600 between 1–2. Then, 1.1×108 cells were centrifuged (3220 g, 3 min, r.t.). The cells were resuspended in Ura−, pABA− media (3% glycerol, w/v) containing 13C6-4HB (50 μM) and either CMK (20 μM) or DMSO (0.2%, v/v) and incubated (30 °C, 230 rpm). At 2, 4 and 6 h after the media s wap, the OD600 of the culture was measured and used to determine the volume of culture needed to isolate 1×108 yeast cells. 1×108 yeast cells were pelleted by centrifugation in 15 mL conical tubes (3,000 g, 3 min, 4 °C) and transferred to 1.5 mL tubes and spun again (16,000 g, 0.5 min, 4 °C), the supernatant was discarded, and the yeast pellet was flash frozen in liquid N2 and stored at −80 °C. A frozen pellet of yeast (1×108 yeast cells) was thawed on ice and mixed with water (200 μL), coenzyme Q10 (CoQ10) was added as an internal standard (20 μM, 10 μL), and glass beads (0.5 mm diameter, 100 μL). The yeast were lysed by vortexing with the glass beads (2×30 s), and the lysate was vortexed (30 s). Cold CHCl3/MeOH (2:1, v/v) (900 μL) was added and vortexed (2 × 30 s). Samples were acidified with HCl (1 M, 200 μL) and vortexed (2 × 30 s). The samples were centrifuged (5,000 g, 2 min, 4 °C) to complete phase separation. 400 μL of the organic phase was transferred to a clean tube and dried under Ar(g). The organic residue was reconstituted in ACN/IPA/H2O (65:30:5, v/v/v) (100 μL) by vortexing (2 × 30 s) and transferred to a glass vial for LC-MS analysis. Samples were stored at −80 °C.
Targeted LC-MS for Yeast CoQ6
Method for Figure 2D. LC-MS analysis was performed on an Acquity CSH C18 column held at 50 °C (100 mm × 2.1 mm × 1.7 μL particle size; Waters) using (400 μL/min flow rate; Thermo Scientific). Mobile phase A consisted of 10 mM ammonium acetate in ACN/H2O (70:30, v/v) containing 250 μL/L acetic acid. Mobile phase B consisted of 10 mM ammonium acetate in IPA/ACN (90:10, v/v) with the same additives. Initially, mobile phase B was held at 40% for 6 min and then increased to 60% over 3 min followed by an increase to 85% over 15 s. Mobile phase B was then increased to 99% over 75 s where it was held for 30 s. The column was reequilibrated for 4 min before the next injection. 10 μL of sample was injected for each sample. The LC system was coupled to a Q Exactive mass spectrometer (Build 2.3 SP2) by a HESI II heated ESI source kept at 300 °C. The inlet capillary was kept at 300 °C, sheath gas was set to 25 units, and auxiliary gas to 10 units. For identification of CoQ6 and CoQ10 species, the MS was operated in positive mode (5 kV) and masses (591.44 and 880.71, respectively) were targeted for fragmentation. AGC target was set to 1×106 and resolving power was set to 140,000. Quantitation was performed in Xcalibur (Thermo) by monitoring the product ion 197.08 Th, corresponding to the Q headgroup, for each targeted mass. Error bars represent s.d. of three biological replicates.
For Coq8p-AS lipid samples (Figure 6D), LC-MS analysis was performed on an Acquity CSH C18 column held at 50 °C (100 mm × 2.1 mm × 1.7 μL particle size; Waters) using an Vanquish Binary Pump (400 μL/min flow rate; Thermo Scientific). Mobile phase A consisted of 10 mM ammonium acetate in ACN/H2O (70:30, v/v) containing 250 μL/L acetic acid. Mobile phase B consisted of 10 mM ammonium acetate in IPA/ACN (90:10, v/v) with the same additives. Mobile phase B was held at 50% for 1.5 min and then increased to 99% over 7.5 min where it was held for 2 min. The column was then reequilibrated for 2.5 min before the next injection. 10 μL of sample were injected by a Vanquish Split Sampler HT autosampler (Thermo Scientific). The LC system was coupled to a Q Exactive mass spectrometer by a HESI II heated ESI source kept at 300 °C (Thermo Scientific). The inlet capillary was kept at 300 °C, sheath gas was set to 25 units, auxiliary gas to 10 units, and the spray voltage was set to 4,000 V. The MS was operated in positive mode and masses (597.46, 591.44 and 880.71, respectively) were targeted for fragmentation. Automatic gain control (AGC) target was set to 5×105 and resolving power was set to 120,000. Quantification was performed in Xcalibur (Thermo Scientific) by monitoring the product ion 203.10 Th (heavy CoQ6) and 197.08 Th (light CoQ6 and CoQ10), corresponding to the Q headgroup, for each targeted mass. The MS was operated in negative mode and masses (551.42, 550. 44, 545.40 and 544.42, respectively) were targeted to quantify heavy and light CoQ intermediates, PPHB6. The resulting LC-MS data were processed using TraceFinder 3.3 (Thermo Scientific). Metabolite signals were integrated and normalized to the CoQ10 internal standard. Error bars represent s.d. of three biological replicates.
AP-MS Lipidomics of 8His-MBP-[TEV]-MBP-UbiBCΔ47
8His-MBP-[TEV]-MBP-UbiBCΔ47 protein purifications were performed in biological triplicate according to the method used above. Eq/S buffer (50 mM HEPES [pH 7.2], 300 mM NaCl, 10% glycerol [w/v]) was added to 25 nmol of 8His-MBP-[TEV]-MBP-UbiBCΔ47 to bring total sample volume to 682 μL. 20 μL of 25 μM CoQ6 internal standard (in CHCl3:MeOH, 1:1, v/v) (Avanti Polar Lipids) was added to each sample and vortex 10 s (500 pmols) (protein precipitated upon mixing). Cold CH3Cl:MeOH (1:1, v/v, 4.3 mL) was added to each sample and mixed to form one phase by vortexing (1 × 30 s, 4 °C). Samples were a cidified by adding HCl (1 M, 30 μL) and vortexing (2 × 30 s). Phase separation was induced by addition of saturated NaCl(aq) (100 μL) and mixed by vortexing (10 s). Phase separation was completed by centrifugation (3000 g, 1 min, 4 °C). Aqueous layer was discarded and 2.9 mL of the organic layer was transferred to new glass tube and dry under Ar(g) (r.t., ~2 hr). The organic residue was reconstituted in ACN/IPA/H2O (65:30:5, v/v/v) (200 μL) by vortexing (30 s, 4 °C), bath sonication (60 s, 4 °C), and vortexing (30 s, 4 °C). Transfer 180 μL to an autosampler vial labeled, stored under Ar(g) at −80 °C.
LC-MS Discovery and Targeted Methods for AP-MS
Both targeted and discovery-based lipidomics of UbiB were done using the instrumentation, column, and mobile phases described in Targeted LC-MS for yeast CoQ6.
Discovery Lipidomics
Mobile phase B started at 2% and increased to 85% over 20 min, then increased to 99% over 1 min, where it was held for 7 min. The column was then reequilibrated for 2 min before the next injection. The LC system was coupled to a Q Exactive mass spectrometer by a HESI II heated ESI source kept at 300 °C (Thermo Scientific). The inlet capillary was kept at 300 °C, sheath gas was set to 25 units, auxiliary gas to 10 units, and the spray voltage was set to 3,000 V. The MS was operated in polarity switching mode, acquiring both positive mode and negative mode MS1 and MS2 spectra. The AGC target was set to 1×106 and resolving power to 17,500. Ions from 200-1600 m/z were isolated (Top 2) and fragmented by stepped HCD collision energy (20, 30, 40). The resulting LC-MS/MS data were processed using Compound Discoverer 2.0 (Thermo Fisher) and an in-house-developed software suite. Briefly, all m/z peaks were aggregated into distinct chromatographic profiles (i.e., compound groups) using a 10 p.p.m. mass tolerance. These chromatographic profiles were then aligned across all LC-MS/MS experiments using a 0.2 min retention time tolerance. All compound groups were compared against a matrix run and only compound groups whose intensity was 5-fold greater were retained. MS/MS spectra were searched against an in-silico generated lipid spectral library containing 35,000 unique molecular compositions representing 31 distinct lipid classes. Spectral matches with a dot product score greater than 650 and a reverse dot product score greater than 750 were retained for further analysis. Identifications were further filtered using precursor mass, MS/MS spectral purity, retention time, and chromatographic profile. Lipid MS/MS spectra which contained no significant interference (<75%) from co-eluting isobaric lipids were identified at the individual fatty acid substituent level of structural resolution. Otherwise, lipid identifications were made with the sum of the fatty acid substituents. All lipid intensities were normalized to the total intensity of all lipids in the chromatogram following the void volume. Intensities of each individual lipid were divided by the sum of the intensities for each sample and averaged across all three biological replicates. These normalized intensities were then divided to determine fold enrichment in one condition versus another (i.e. MBP-UbiBCΔ47/MBP-UbiBCΔ47 D310N). Student’s t-test was used to determine statistical significance. Nomenclature for lipid acyl chains is from (Liebisch et al., 2013).
Targeted Lipidomics for CoQ and its Intermediates
LC-MS analysis was performed on an Acquity CSH C18 column held at 50 °C (100 mm × 2.1 mm × 1.7 μL particle size; Waters) using an Ultimate 3000 RSLC Binary Pump (400 μL/min flow rate; Thermo Scientific). Mobile phase A consisted of 10 mM ammonium acetate in ACN/H2O (70:30, v/v) containing 250 μL/L acetic acid. Mobile phase B consisted of 10 mM ammonium acetate in IPA/ACN (90:10, v/v) with the same additives. Mobile phase B was held at 50% for 1.5 min and then increased to 95% over 6.5 min where it was held for 2 min. The column was then reequilibrated for 3.5 min before the next injection. 10 μL of sample were injected by an Ultimate 3000 RSLC autosampler (Thermo Scientific). The LC system was coupled to a Q Exactive mass spectrometer by a HESI II heated ESI source kept at 300 °C (Thermo Scientific). The inlet capillary was kept at 300 °C, sheath gas was set to 25 units, auxiliary gas to 10 units, and the spray voltage was set to 4,000 V and 5,000 V for positive and negative mode respectively. CoQ and its intermediates were targeted for quantification. The MS was operated in positive or negative mode depending on the intermediate being targeted. Metabolite signals were integrated and normalized to a CoQ6 internal standard. Error bars represent s.d. of biological triplicate measurements. See Table S1 for targeted CoQ species.
Liposomes
Liposomes were made by drying down lipids in a 5 mL plastic tube under Ar(g) at room temperature until a film was left. Liposomes were made of the following lipid (Avanti Polar Lipids) compositions: PC, PC/NBD-PE 99.9/0.1; PC/PE, PC/PE/NBD-PE 89.9/10/0.1; PC/CL, PC/CL/NBD-PE 89.9/10/0.1; PC/PE/CL, PC/PE/CL/NBD-PE 79.9/10/10/0.1; PC/CoQ10, PC/CoQ10/NBD-PE 97.9/2/0.1; PC/PG, PC/PG/NBD-PE 89.9/10/0.1; PC/PS, PC/PS/NBD-PE 89.9/10/0.1; PC/PA, PC/PA/NBD-PE 89.9/10/0.1; PC/CDP-DAG, PC/CDP-DAG/NBD-PE 89.9/10/0.1; all mol %. The lipids were dried in a vacuum chamber overnight at 25-30 inHg vacuum. The dry lipid film was reconstituted in HBS buffer (20 mM HEPES pH 7.5, 150 mM NaCl) at 30–35 °C for one hour with occasional pipetting to resuspend the lipid film. The total concentration of lipids in solution was 10 mM. 2 μL of the liposomes were taken before and after extrusion and diluted with 22 μL of HBS to determine how much the liposomes were diluted during extrusion by measuring the fluorescence of NBD-PE (excitation: 460 nm, emission: 535 nm). Liposomes were extruded through 100 nm membranes (Avanti Polar Lipids), 15 passes, 30–35 °C. Liposomes were made fresh before each experiment.
Liposome Flotation Assay
Liposome flotation assay is adapted from (Connerth et al., 2012) with the following modifications. Liposomes (100 μL) were mixed with protein (50 μL 6× COQ8) at 4 °C then incubated (r.t., ~10 min). Final concentrations of reagents are as follows: 2–4 μM protein and 3.33 mM liposomes in HBS (150 mM NaCl, 20 mM HEPES pH 7.5). 2.72 M sucrose (110 μL) was added to the protein liposome mixture (1.15 M [sucrose] final). The sucrose-liposome-protein mixture (250 μL) was added to the ultracentrifuge tube. The sucrose gradient was made by layering 300 μL HBS 0.86 M sucrose, 250 μL HBS 0.29 M sucrose, and 150 μL HBS on top of the sucrose-liposome-protein mix. This gradient was centrifuged (240,000 g, 1 h, 4 °C). 450 μL was removed for the top layer and 450 μL for the bottom layer. Liposome location was determined by mixing 8 μL of top or bottom fraction with 16 μL HBS and reading NBD-PE fluorescence for (excitation: 460 nm, emission: 535 nm). To concentrate the protein from the top and bottom fractions, a CHCl3:MeOH precipitation was performed according to (Wessel and Flugge, 1984). Methanol (1800 μL, 4 volumes) was added to a 450 μL fraction. After thorough mixing chloroform (450 μL, one volume) was added and vortexed. Water (1350 μL, three volumes) was added and the mixture was vortexed again then centrifuged immediately at full speed (~4,200 g, 5 min). A white disc of protein should form between the organic layer (bottom) and the aqueous layer (upper). Discard most of the upper aqueous layer, and be careful not to disturb the protein pellet. Methanol was added (1000 μL) to the tube, inverted 3 times, and centrifuged at full speed (5 min, 16,000 g). All of the liquid was removed and the pellet was air or vacuum dried. The precipitated protein pellet was resuspend in 30 μL 1× LDS with 10 mM DTT, incubated (95 °C, ~10 min), and analyzed with 4–12% Novex NuPAGE Bis-Tris SDS-PAGE (Invitrogen) gels (1 hr, 150 V). Band quantification was done with imaging and densitometry on a LI-COR Odyssey CLx (700 nm) using Image Studio v5.2 software. Error bars for Figure 4G represent s.d. of three independent floats. Student’s t-test was used to determine statistical significance.
Coq8p V202C,M303C with CMK LC-MS/MS
CMK labeling reaction
20 μL of Coq8p (1 μM final concentration) and 20 μL of CMK (20 μM final concentration) were mixed and incubated (30 °C, 15 min). The samples were then flash frozen in N2(l) and stored at −80 °C.
Sample preparation
Samples were diluted 10× in 100 mM Tris pH 8, 2 M urea, and 40 mM chloroacetamide and digested with trypsin (25:1 protein to enzyme) overnight. Sample cleanup was performed by desalting over a polystyrene-divinylbenzene solid phase extraction (PS-DVB SPE) cartridge (Phenomenex, Torrance, CA).
LC-MS/MS
Peptides were dissolved in 0.2% formic acid and 10% was injected onto the column for each analysis. Separations were performed over in-house fabricated 75 μm inner diameter × 360 μm outer diameter columns with an integrated nano electrospray emitter packed 30 cm long with 1.7 μm C18 bridged ethylene hybrid particles (Waters, Milford, MA). Samples were loaded in 100% A (0.2% formic acid), followed by gradient elution in increasing % B (0.2% formic acid/70% acetonitrile), and re-equilibration times in 100% A. All separations were performed with a Thermo Dionex Ultimate 3000 RSLC-nano liquid chromatography instrument and an in house fabricated column heater to perform separations at 50 °C (Hebert et al., 2014). Eluted peptides were analyzed on an Orbitrap Lumos Fusion platform. All MS analyses were performed in the Orbitrap with 60,000 resolving power. Following each MS1 survey scan, MS/MS analyses were performed on the most intense precursors for 1 second. Precursors were filtered with the quadrupole using a 2.0 Da isolation window, fragmented with HCD collision energy = 30 NCE, and fragment ions were analyzed in the Orbitrap. Dynamic exclusion was set to 45 seconds, MonoIsotope Precursor Selection (MIPS) was toggled on for all runs and charge states unknown, 1, or > 6 were excluded.
Data analysis
Raw files were converted to text files and scored against theoretical spectra from a target-decoy reference proteome database, using the OMSSA search engine. The database was generated by downloading the P27697 sequence from Uniprot concatenating a clone of the P27697 sequence but with sites 202 and 303 converted to cysteines. Tryptic peptides were searched with one missed cleavage. Cysteine carbamidomethylation, and CMK modification (+322.1429 Da) and methionine oxidation were set as variable modifications. Peptides were searched with 25 ppm tolerance on the precursor mass and 0.01 Da tolerance on fragment ion masses. The COMPASS software suite was used to filter search results to a 1% unique peptide FDR (based on E-value and ppm mass error) (Wenger et al., 2011).
Immunoblotting of Yeast Coq8p
Single yeast colonies (strain W303) were picked and grown in Ura− glucose (2% w/v) media (230 rpm, overnight, 30 °C). 50 mL cultures of Ura− glucose (0.1% w/v) and glycerol (3% w/v) were seeded with 2.5 × 106 cells and incubated (30 °C, 25 h, 230 rpm). 1 × 10 8 cells were collected, flash frozen, and stored (−80 °C). The c ells were resuspended in 150 μL NaOH/BME (prepared from 1 mL 2 M NaOH + 80 μL β-mercaptoethanol), and incubated on ice for 10 minutes, mixing every 2 minutes. 150 μL 50% TCA was added to each sample and incubated on ice for an additional 10 minutes, mixing every 2 minutes. Samples were centrifuged (14,000 g, 2 min), and the pellet was washed with 1 mL acetone, mixed briefly, and centrifuged (14,000 g, 2 min). The supernatant was removed and the pellet allowed to dry. Samples were resuspended in 60 μL 0.1 M NaOH. 25 μL 6× LDS sample buffer with 10 mM DTT was added and samples were incubated (95 °C, ~10 min). Proteins were analyzed with 4–12% Novex NuPAGE Bis-Tris SDS-PAGE (Invitrogen) gels (1 hr, 150 V). The gel was transferred to PVDF membrane at 20 V 1 hr with transfer buffer (192 mM glycine, 25 mM Tris, 20% methanol [v/v]). The membrane was blocked with 5% nonfat dry milk (NFDM) in TBST (20 mM Tris pH 7.4, 150 mM NaCl, 0.05% Tween 20 [v/v]) (1 h with agitation). Affinity purified Coq8p antibody (1:100) and the β-actin antibody (1:5000) were diluted in 1% NFDM in TBST and incubated with the PVDF (overnight, 4 °C with agitation). The PVDF was washed three times in TBST and the secondary antibodies were diluted 1:15,000 in 1% NFDM in TBST (1 h, r.t.). The membrane was washed three times in TBST and imaged. Band quantification was done with imaging and densitometry on a LI-COR Odyessey CLx using Image Studio v5.2 software. Error bars for Figure S4K represent s.d. of three independent experiments.
Coq8p Antibody Affinity Purification
A 4–12% Bis Tris SDS-PAGE gel was loaded with 20 μg Coq8pNΔ41 (5 μg per lane, 4 lanes total). The Coq8pNΔ41 was transferred to PVDF according to the procedure above. The PVDF membrane was stained with 1× Ponceau solution (10–15 min, r.t) and washed several times with water. The Coq8p band was cut out from the membrane. The membrane pieces were blocked with 2% NFDM in TBST (2 h, r.t.) and washed with TBST several times. Coq8p rabbit antisera (Catherine Clarke’s lab) was added to the membrane (overnight, 4 °C with agitation then 1 h, r.t.). Membrane pieces were washed 6–7 times TBST. To elute bound antibodies, 600 μL of 0.2 M glycine, pH 3.0 was added to the membrane pieces and mixed (1 min). Extract was transferred to a tube containing 36 μL (can be scaled up depending on the antisera added) of 1 M Tris-HCl pH 8.8 to obtain a neutral pH. Affinity enriched antibody was flash frozen in N2(l) and stored at −80 °C.
QUANTIFICATION AND STATISTICAL ANALYSIS
See each individual method for the associated statistical analysis. In general, p-values were calculated using an unpaired, two-tailed, Student’s t-test. In all cases, n represents independent replicates of an experiment.
Supplementary Material
4 microseconds of production run of a CG-MD simulation of COQ8A (green) (PDB:4PED) with PC bilayer (phosphate heads in gray). The positively charged residues R262, R265, K269 are colored blue.
4 microseconds of production run of a CG-MD simulation of COQ8A (green) (PDB:4PED) with PC/PE/CL bilayer (PC/PE phosphate heads in gray and CL phosphate heads in red). The positively charged residues R262, R265, K269 are colored blue.
Table S1, related to Figure 1. MBP-UbiBCΔ47 AP-MS lipidomics data.
Table S2, related to Figure 1. List of compounds and hits from NMR line-broadening screen.
Significance.
UbiB proteins comprise a large and ancient family of protein kinase-like (PKL) molecules that spans all three domains of life. These proteins have been implicated in diverse cellular processes and are mutated in human diseases; however, little is known about their direct biochemical roles and activities. The main orthologous UbiB proteins investigated in this work—S. cerevisiae Coq8p and human COQ8A (jointly referred to as COQ8)—exemplify this problem: they are known to enable the biosynthesis of the key mitochondrial lipid, coenzyme Q (CoQ), but the mechanism is unclear. Investigations into the functions of other PKL family members have benefitted greatly from the identification of activating lipids and small molecules, and from the use of custom inhibitors, suggesting that such tools may likewise be beneficial in elucidating how UbiB proteins operate; however, such resources have not been established for this family. Our work helps to address these deficiencies by defining lipid and small molecule modulators of COQ8. We demonstrate that COQ8 exhibits a conserved ATPase activity that is activated by CoQ-like precursors and by binding to membranes that contain cardiolipin—a prevalent mitochondrial lipid enriched at the IMM where COQ8 resides. We also establish an “analog-sensitive” version of Coq8p that can be inhibited by small molecules in vivo and use this tool to demonstrate that acute inhibition of Coq8p is sufficient to cause CoQ deficiency and respiratory dysfunction. Collectively, our work advances our understanding of the core COQ8 biochemical function across evolution (ATPase activity), reveals how the positioning of COQ8 on the IMM is key to its activation, and provides effective new tools for the further investigation of the role of COQ8 in CoQ biosynthesis.
Highlights.
UbiB family has a conserved ATPase activity that is activated by CoQ-like phenols
Mature COQ8 binds specifically to cardiolipin-containing liposomes
Cardiolipin significantly increases COQ8’s ATPase activity
Covalent inhibition of analog-sensitive COQ8 acutely disrupts CoQ biosynthesis
Acknowledgments
We thank Professor Kevan Shokat and Flora Rutaganira for useful discussions and reagents. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01GM112057 (to D.J.P.), T32GM008505 (to A.G.R.), T32HL007899 (to E.W), and R35GM118110 (to J.J.C). This work was further supported by SNSF grants 31003A_170154 and 200021_157217 (to M.D.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
A.G.R. and D.J.P. conceived of the project and its design. A.G.R., Z.A.K., D.A., A.J., M.T.M., P.D.H., J.L.S., J.A.S., T.R., A.S.H., E.M.W., I.E.J., C.A.B., and M.D.P. performed experiments and data analysis. D.A. and M.D.P designed and performed molecular modeling and simulation that supported the experimental design. J.L.M., J.J.C., M.D.P., and D.J.P. provided key experimental resources and/or aided in experimental design. A.G.R. and D.J.P. wrote the manuscript.
References
- Abagyan R, Totrov M, Kuznetsov D. Icm - a New Method for Protein Modeling and Design - Applications to Docking and Structure Prediction from the Distorted Native Conformation. J Comput Chem. 1994;15:488–506. [Google Scholar]
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–. 2015;2:19–25. [Google Scholar]
- Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, Fang H, Song X, Cattran DC, Avila-Casado C, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Blommel PG, Martin PA, Seder KD, Wrobel RL, Fox BG. Flexi vector cloning. Methods Mol Biol. 2009;498:55–73. doi: 10.1007/978-1-59745-196-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough R, Frankum JR, Sims D, Mackay A, Mendes-Pereira AM, Bajrami I, Costa-Cabral S, Rafiq R, Ahmad AS, Cerone MA, et al. Functional viability profiles of breast cancer. Cancer Discov. 2011;1:260–273. doi: 10.1158/2159-8290.CD-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- Calvo SE, Julien O, Clauser KR, Shen H, Kamer KJ, Wells JA, Mootha VK. Comparative Analysis of Mitochondrial N-Termini from Mouse, Human, and Yeast. Mol Cell Proteomics. 2017;16:512–523. doi: 10.1074/mcp.M116.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim Biophys Acta. 2009;1788:2059–2068. doi: 10.1016/j.bbamem.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Boontheung P, McCaffery JM, Loo JA, Koehler CM. The cardiolipin transacylase, tafazzin, associates with two distinct respiratory components providing insight into Barth syndrome. Mol Biol Cell. 2008;19:5143–5155. doi: 10.1091/mbc.E08-09-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos LJ, 2nd, Jofre MF, Ellinger JJ, Westler WM, Markley JL. NMRbot: Python scripts enable high-throughput data collection on current Bruker BioSpin NMR spectrometers. Metabolomics. 2013;9:558–563. doi: 10.1007/s11306-012-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerth M, Tatsuta T, Haag M, Klecker T, Westermann B, Langer T. Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein. Science. 2012;338:815–818. doi: 10.1126/science.1225625. [DOI] [PubMed] [Google Scholar]
- Coudreuse D, Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468:1074–1079. doi: 10.1038/nature09543. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JK, Abdul Murad N, Yeo A, McKenzie M, Ward M, Chong KL, Schieber NL, Parton RG, Lim YC, Wolvetang E, et al. AarF Domain Containing Kinase 3 (ADCK3) Mutant Cells Display Signs of Oxidative Stress, Defects in Mitochondrial Homeostasis and Lysosomal Accumulation. PLoS One. 2016;11:e0148213. doi: 10.1371/journal.pone.0148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim Biophys Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Do TQ, Hsu AY, Jonassen T, Lee PT, Clarke CF. A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J Biol Chem. 2001;276:18161–18168. doi: 10.1074/jbc.M100952200. [DOI] [PubMed] [Google Scholar]
- Ferreira-Cerca S, Sagar V, Schafer T, Diop M, Wesseling AM, Lu H, Chai E, Hurt E, LaRonde-LeBlanc N. ATPase-dependent role of the atypical kinase Rio2 on the evolving pre-40S ribosomal subunit. Nat Struct Mol Biol. 2012;19:1316–1323. doi: 10.1038/nsmb.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A, Caelles C, Massot N, Hegardt FG. Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl coenzyme A reductase kinase by adenosine 5′-monophosphate. Biochem Biophys Res Commun. 1985;132:497–504. doi: 10.1016/0006-291x(85)91161-1. [DOI] [PubMed] [Google Scholar]
- Fischer JJ, Jardetzky O. Nuclear Magnetic Relaxation Study of Intermolecular Complexes. The Mechanism of Penicillin Binding to Serum Albumin. J Am Chem Soc. 1965;87:3237–3244. doi: 10.1021/ja01092a040. [DOI] [PubMed] [Google Scholar]
- Floyd BJ, Wilkerson EM, Veling MT, Minogue CE, Xia C, Beebe ET, Wrobel RL, Cho H, Kremer LS, Alston CL, et al. Mitochondrial Protein Interaction Mapping Identifies Regulators of Respiratory Chain Function. Mol Cell. 2016;63:621–632. doi: 10.1016/j.molcel.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, et al. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustini M, Castelli F, Husu I, Giomini M, Mallardi A, Palazzo G. Influence of cardiolipin on the functionality of the Q(a) site of the photosynthetic bacterial reaction center. J Phys Chem B. 2005;109:21187–21196. doi: 10.1021/jp054104d. [DOI] [PubMed] [Google Scholar]
- Gupta K, Donlan JA, Hopper JT, Uzdavinys P, Landreh M, Struwe WB, Drew D, Baldwin AJ, Stansfeld PJ, Robinson CV. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature. 2017;541:421–424. doi: 10.1038/nature20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AS, Richards AL, Bailey DJ, Ulbrich A, Coughlin EE, Westphall MS, Coon JJ. The one hour yeast proteome. Mol Cell Proteomics. 2014;13:339–347. doi: 10.1074/mcp.M113.034769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R, Czermin B, Gulati S, Demuth S, Houge G, Pyle A, Dineiger C, Blakely EL, Hassani A, Foley C, et al. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J Neurol Neurosurg Psychiatry. 2012;83:174–178. doi: 10.1136/jnnp-2011-301258. [DOI] [PubMed] [Google Scholar]
- Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X, Kuriyan J. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell. 2009;137:1293–1307. doi: 10.1016/j.cell.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H, Kanaho Y, Nozawa Y. Activation and solubilization by Triton X-100 of membrane-bound phospholipase D of rat brain. Lipids. 1991;26:426–430. doi: 10.1007/BF02536068. [DOI] [PubMed] [Google Scholar]
- Kar A, Beam H, Borror MB, Luckow M, Gao X, Rea SL. CLD1 Reverses the Ubiquinone Insufficiency of Mutant cat5/coq7 in a Saccharomyces cerevisiae Model System. PLoS One. 2016;11:e0162165. doi: 10.1371/journal.pone.0162165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadria AS, Mueller BK, Stefely JA, Tan CH, Pagliarini DJ, Senes A. A Gly-zipper motif mediates homodimerization of the transmembrane domain of the mitochondrial kinase ADCK3. J Am Chem Soc. 2014;136:14068–14077. doi: 10.1021/ja505017f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock HE, Koesema EJ, Knuth MW, Lesley SA. Combining the polymerase incomplete primer extension method for cloning and mutagenesis with microscreening to accelerate structural genomics efforts. Proteins. 2008;71:982–994. doi: 10.1002/prot.21786. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Tazir M, Lopez LC, Quinzii CM, Assoum M, Drouot N, Busso C, Makri S, Ali-Pacha L, Benhassine T, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Aravind L, Koonin EV. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- Lepre CA, Moore JM, Peng JW. Theory and applications of NMR-based screening in pharmaceutical research. Chem Rev. 2004;104:3641–3676. doi: 10.1021/cr030409h. [DOI] [PubMed] [Google Scholar]
- Liebisch G, Vizcaino JA, Kofeler H, Trotzmuller M, Griffiths WJ, Schmitz G, Spener F, Wakelam MJ. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res. 2013;54:1523–1530. doi: 10.1194/jlr.M033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Davis JI, van Wijk KJ. ABC1K atypical kinases in plants: filling the organellar kinase void. Trends Plant Sci. 2012;17:546–555. doi: 10.1016/j.tplants.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- Martinis J, Glauser G, Valimareanu S, Kessler F. A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis. Plant Physiol. 2013;162:652–662. doi: 10.1104/pp.113.218644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier KA, Baran M, Ramanathan V, Revesz P, Xiao R, Montelione GT, Powers R. FAST-NMR: functional annotation screening technology using NMR spectroscopy. J Am Chem Soc. 2006;128:15292–15299. doi: 10.1021/ja0651759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, Lombes A, Boddaert N, Desguerre I, de Lonlay P, de Baulny HO, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravcevic K, Oxley CL, Lemmon MA. Conditional peripheral membrane proteins: facing up to limited specificity. Structure. 2012;20:15–27. doi: 10.1016/j.str.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oh H, Ozkirimli E, Shah K, Harrison ML, Geahlen RL. Generation of an analog-sensitive Syk tyrosine kinase for the study of signaling dynamics from the B cell antigen receptor. J Biol Chem. 2007;282:33760–33768. doi: 10.1074/jbc.M704846200. [DOI] [PubMed] [Google Scholar]
- Periole X, Cavalli M, Marrink SJ, Ceruso MA. Combining an Elastic Network With a Coarse-Grained Molecular Force Field: Structure, Dynamics, and Intermolecular Recognition. J Chem Theory Comput. 2009;5:2531–2543. doi: 10.1021/ct9002114. [DOI] [PubMed] [Google Scholar]
- Periole X, Huber T, Marrink SJ, Sakmar TP. G protein-coupled receptors self-assemble in dynamics simulations of model bilayers. J Am Chem Soc. 2007;129:10126–10132. doi: 10.1021/ja0706246. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–52880. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- Planas-Iglesias J, Dwarakanath H, Mohammadyani D, Yanamala N, Kagan VE, Klein-Seetharaman J. Cardiolipin Interactions with Proteins. Biophys J. 2015;109:1282–1294. doi: 10.1016/j.bpj.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon WW, Davis DE, Ha HT, Jonassen T, Rather PN, Clarke CF. Identification of Escherichia coli ubiB, a gene required for the first monooxygenase step in ubiquinone biosynthesis. J Bacteriol. 2000;182:5139–5146. doi: 10.1128/jb.182.18.5139-5146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Molina JB, Tseng SC, Simonett SP, Taunton J, Ansari AZ. Engineered Covalent Inactivation of TFIIH-Kinase Reveals an Elongation Checkpoint and Results in Widespread mRNA Stabilization. Mol Cell. 2016;63:433–444. doi: 10.1016/j.molcel.2016.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer LV, de Jong DH, Holt A, Rzepiela AJ, de Vries AH, Poolman B, Killian JA, Marrink SJ. Lipid packing drives the segregation of transmembrane helices into disordered lipid domains in model membranes. Proc Natl Acad Sci U S A. 2011;108:1343–1348. doi: 10.1073/pnas.1009362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafimova IM, Pufall MA, Krishnan S, Duda K, Cohen MS, Maglathlin RL, McFarland JM, Miller RM, Frodin M, Taunton J. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol. 2012;8:471–476. doi: 10.1038/nchembio.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- Snead JL, Sullivan M, Lowery DM, Cohen MS, Zhang C, Randle DH, Taunton J, Yaffe MB, Morgan DO, Shokat KM. A coupled chemical-genetic and bioinformatic approach to Polo-like kinase pathway exploration. Chem Biol. 2007;14:1261–1272. doi: 10.1016/j.chembiol.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld PJ, Hopkinson R, Ashcroft FM, Sansom MS. PIP(2)-binding site in Kir channels: definition by multiscale biomolecular simulations. Biochemistry. 2009;48:10926–10933. doi: 10.1021/bi9013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Eghbalnia HR, Lee W, Westler WM, Markley JL. NMRmix: A Tool for the Optimization of Compound Mixtures in 1D (1)H NMR Ligand Affinity Screens. J Proteome Res. 2016;15:1360–1368. doi: 10.1021/acs.jproteome.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefely JA, Licitra F, Laredj L, Reidenbach AG, Kemmerer ZA, Grangeray A, Jaeg-Ehret T, Minogue CE, Ulbrich A, Hutchins PD, et al. Cerebellar Ataxia and Coenzyme Q Deficiency through Loss of Unorthodox Kinase Activity. Mol Cell. 2016;63:608–620. doi: 10.1016/j.molcel.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefely JA, Reidenbach AG, Ulbrich A, Oruganty K, Floyd BJ, Jochem A, Saunders JM, Johnson IE, Minogue CE, Wrobel RL, et al. Mitochondrial ADCK3 employs an atypical protein kinase-like fold to enable coenzyme Q biosynthesis. Mol Cell. 2015;57:83–94. doi: 10.1016/j.molcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979a;254:3692–3695. [PubMed] [Google Scholar]
- Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979b;91:1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tan T, Ozbalci C, Brugger B, Rapaport D, Dimmer KS. Mcp1 and Mcp2, two novel proteins involved in mitochondrial lipid homeostasis. J Cell Sci. 2013;126:3563–3574. doi: 10.1242/jcs.121244. [DOI] [PubMed] [Google Scholar]
- Tauche A, Krause-Buchholz U, Rodel G. Ubiquinone biosynthesis in Saccharomyces cerevisiae: the molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 2008;8:1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtle FN, Burkhart JM, Gonczarowska-Jorge H, Kucukkose C, Taskin AA, Kopczynski D, Ahrends R, Mossmann D, Sickmann A, Zahedi RP, et al. Landscape of submitochondrial protein distribution. Nat Commun. 2017;8:290. doi: 10.1038/s41467-017-00359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtle FN, Wortelkamp S, Zahedi RP, Becker D, Leidhold C, Gevaert K, Kellermann J, Voos W, Sickmann A, Pfanner N, et al. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell. 2009;139:428–439. doi: 10.1016/j.cell.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Vos M, Geens A, Bohm C, Deaulmerie L, Swerts J, Rossi M, Craessaerts K, Leites EP, Seibler P, Rakovic A, et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J Cell Biol. 2017;216:695–708. doi: 10.1083/jcb.201511044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–3765. [PubMed] [Google Scholar]
- Wassenaar TA, Ingolfsson HI, Bockmann RA, Tieleman DP, Marrink SJ. Computational Lipidomics with insane: A Versatile Tool for Generating Custom Membranes for Molecular Simulations. J Chem Theory Comput. 2015;11:2144–2155. doi: 10.1021/acs.jctc.5b00209. [DOI] [PubMed] [Google Scholar]
- Wenger CD, Phanstiel DH, Lee MV, Bailey DJ, Coon JJ. COMPASS: a suite of pre- and post-search proteomics software tools for OMSSA. Proteomics. 2011;11:1064–1074. doi: 10.1002/pmic.201000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, Zhuang L, Rosenbluh J, Chen S, Xiao Y, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A. 2010;107:11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LX, Hsieh EJ, Watanabe S, Allan CM, Chen JY, Tran UC, Clarke CF. Expression of the human atypical kinase ADCK3 rescues coenzyme Q biosynthesis and phosphorylation of Coq polypeptides in yeast coq8 mutants. Biochim Biophys Acta. 2011;1811:348–360. doi: 10.1016/j.bbalip.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle PL. Non-covalent binding of membrane lipids to membrane proteins. Biochim Biophys Acta. 2014;1838:1548–1559. doi: 10.1016/j.bbamem.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Robyt JF. Activation and stabilization of 10 starch-degrading enzymes by Triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzyme and Microbial Technology. 2005;37:556–562. [Google Scholar]
- Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
4 microseconds of production run of a CG-MD simulation of COQ8A (green) (PDB:4PED) with PC bilayer (phosphate heads in gray). The positively charged residues R262, R265, K269 are colored blue.
4 microseconds of production run of a CG-MD simulation of COQ8A (green) (PDB:4PED) with PC/PE/CL bilayer (PC/PE phosphate heads in gray and CL phosphate heads in red). The positively charged residues R262, R265, K269 are colored blue.
Table S1, related to Figure 1. MBP-UbiBCΔ47 AP-MS lipidomics data.
Table S2, related to Figure 1. List of compounds and hits from NMR line-broadening screen.


