Abstract
Objectives
Despite the high burden of hepatitis C virus (HCV)-related morbidity and mortality among HIV-positive people who use illicit drugs (PWUD), uptake of interferon-based treatments for HCV infection has been negligible among this group. Direct-acting antiviral (DAA) therapies offer an opportunity to expand treatment access among this population. The aim of this study was to explore willingness to use DAA-based regimens among HIV/HCV co-infected PWUD in Vancouver, Canada.
Methods
Data was drawn from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), a prospective cohort of HIV-positive PWUD. Using logistic regression analyses we investigated factors associated with willingness to use DAA-based regimens among HIV/HCV co-infected participants.
Results
Of 418 HIV/HCV co-infected PWUD surveyed between June 2014 and May 2015, 295 (71%) were willing to use DAA-based regimens. In multivariable analysis, participants enrolled in methadone maintenance therapy (Adjusted Odds Ratio [AOR] = 1.61, 95% Confidence Interval [CI]: 1.04–2.51), those with a recent assessment by an HCV specialist (AOR = 2.02, 95% CI: 1.28–3.19), and those who perceived that HCV infection was affecting their health (AOR = 2.49, 95% CI: 1.41–4.37) were more likely to be willing to use DAA-based regimens.
Conclusions
Overall, this study found high prevalence of willingness to use DAA-based regimens among HIV/HCV co-infected PWUD in Vancouver. Importantly, enrollment in methadone maintenance therapy was positively associated with willingness, suggesting that integrated models of HIV, HCV and addiction care should be explored as a way to address HCV-related morbidity and mortality among HIV/HCV co-infected PWUD.
Keywords: hepatitis C, HIV, people who use drugs, direct-acting antiviral agents, methadone maintenance therapy
INTRODUCTION
As a consequence of shared routes of transmission, HIV and hepatitis C virus (HCV) co-infection is common, with approximately 3-12% of the 36.9 million people living with HIV (PLWH) globally being co-infected with HCV (1). Among PLWH, people who use drugs (PWUD) are disproportionately affected by HCV, with the prevalence of co-infection (i.e., HCV Ab positivity) ranging between 50% and 90%, leading to interrelated epidemics of HIV, HCV and substance use disorders (2, 3). In Canada, co-infection estimates for HIV-positive PWUD are over 80% (1).
Several studies show that dual infection with HIV and HCV may negatively impact the course of the other disease. In particular, PLWH are less likely to spontaneously clear HCV infection, and also experience accelerated rates of progression to end-stage liver disease, hepatocellular carcinoma, and death (2, 4). Indeed, HCV-related disease has become one of the leading causes of morbidity and mortality among PLWH, particularly in regions where antiretroviral therapy (ART) is widely available (5). Importantly, HCV eradication (i.e., sustained virological response [SVR]) is associated with significant reductions in HCV-related morbidity and all-cause mortality (4, 6). As such, treatment of HCV has become a priority for this population (7, 8).
Despite the high burden of HCV disease among HIV-infected PWUD, and their role as key drivers of HCV transmission in many settings (9), access and uptake of HCV treatment among this population have been consistently low (10, 11). Factors contributing to this low uptake include barriers at the patient-(e.g., fear of side effects, cost), provider-(e.g., discrimination, misconception of PWUD’s potential to adhere), and macro-structural level (e.g., criminalization of drug use, low access to treatment) (12). Furthermore, until recently most HCV treatment guidelines systematically excluded PWUD from HCV treatment (7).
Encouragingly, in recent years, increasing success of HCV treatment among PWUD has contributed to a reversal of this trend. In particular, empirical evidence has indicated that, when appropriately supported, PWUD achieve similar rates of SVR compared to the general population (13, 14). As a result, many international bodies, including the World Health Organization (15), the American Association for the Study of Liver Disease (AASLD)/Infectious Diseases Society of America (IDSA) (16), and the European Study for the Association of the Liver (EASL) (17), now recommend HCV treatment for PWUD.
The increasing availability of direct-acting antiviral (DAA) agents has marked a shift in the paradigm of HCV treatment from lengthy and poorly-tolerated interferon-based regimens to highly efficacious (regardless of HIV status), safer and shorter all-oral regimens (18). This shift has brought renewed optimism regarding the prospects of controlling the HCV epidemic (8). Given the key role that HIV/HCV co-infected PWUD play in perpetuating the HCV epidemic, expanding access to HCV treatment to this population should be a public health priority (7, 9), as optimal deployment of DAA-based HCV therapy to PWUD will offer the dual benefit of improving individual HCV clinical outcomes (e.g., reduced HCV-related morbidity and mortality), as well as reducing the risk of onward HCV transmission (19). The feasibility of scaling up access to HCV treatment among HIV/HCV co-infected PWUD depends, at least in part, on their willingness to use DAA-based treatments. However, to our knowledge, this issue has never been systematically assessed. Thus, the objective of this study was to examine the prevalence and correlates of willingness to use DAA-based regimens among a community-based cohort of HIV/HCV co-infected PWUD in Vancouver, Canada.
MATERIALS AND METHODS
Study design and population
Data for this study was drawn from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), an ongoing prospective cohort of HIV-positive adults who use illicit drugs in Vancouver, Canada that began recruitment in 2005, and that has been described in detail previously (20, 21). In brief, individuals are eligible for inclusion if they are HIV-positive, ≥18 years, live within greater Vancouver, and have used illicit drugs other than cannabis in the previous month. Participants are recruited through snowball sampling and extensive street outreach with a focus in the Downtown Eastside neighborhood, an area with an open drug market and high levels of illicit drug use, poverty and HIV/HCV infection.
After providing written informed consent, at baseline and on a semi-annual basis thereafter, participants undergo an interviewer-administered questionnaire, provide blood samples for serological testing (e.g., HCV antibodies), and are examined by a study nurse, who provides referrals to health services when needed. The questionnaire elicits information on socio-demographic characteristics, drug use patterns, health care access and utilization, including HIV, HCV and addiction care, as well as other relevant exposures. Starting in June 2014, questions regarding knowledge about HCV and willingness to use new DAA were added to the questionnaire. In addition, as has been described elsewhere (20), information gathered at each semi-annual visit is augmented by confidential data linkages with the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program, which provides HIV care, including free ART to all PLWH in the province of British Columbia. These linkages allow for a complete longitudinal clinical and laboratory profile for each participant, including all CD4 counts, plasma HIV viral load (VL) tests, and ART dispensation. Participants receive CAD$ 30 stipend at each study visit. The ACCESS study has received ethical approval by the University of British Columbia/Providence Health Care Research Ethics Board. The analytic sample for the current study was restricted to HCV-seropositive participants, who completed at least one study visit between June 2014 and May 2015. In the event of multiple observations for one individual, the most recent observation was used.
Measures
The primary outcome of interest was willingness to use DAA-based regimens, defined as responding “yes” to the question: “New HCV treatments have been developed that take less time, are more likely to cure you and have fewer side effects. If these became available to you, would you be interested in taking them?” Participants who responded affirmatively to this question were subsequently asked to indicate their treatment willingness under the following efficacy scenarios: <40%, 40-59, 60-79%, and >80%.
We selected a range of explanatory variables that based on previous studies were hypothesized to influence uptake of HCV treatment among PLWH and PWUD (3, 22–24). Socio-demographic characteristics considered included: age at baseline (per year older); gender (male versus female); and self-reported Indigenous ancestry (including self-reporting First Nations, Métis or Inuit ancestry, yes versus no). We also considered substance use behaviours including high-intensity injection drug use (≥ daily versus < daily); high-intensity non-injection drug use (≥ daily versus < daily); and heavy alcohol use (≥4 drinks/day versus <4 drinks/day), as well as living in unstable housing conditions, defined as living in a single-room occupancy hotel, shelter, hostel, treatment/recovery house, living on the street or having no fixed address. Access to and utilization of health services, as well as clinical status, was explored through the following variables: assessment by a HCV specialist; engagement in methadone maintenance therapy (MMT); other addiction treatments (i.e., detox, recovery house, counselling, 12-step programs, residential treatment); use of ART (≥1 day ART dispensed, using pharmacy refill data); and HIV viral load suppression, defined as having a HIV viral load < 50 copies/mL plasma (yes versus no) in the previous six months. In the case of multiple VL measurements within a six-month follow-up period, the median of all the observations was utilized. This number was then used to dichotomize participants at < or ≥ 50 c/mL as virally suppressed or not for that period. In the event of no tests in the six-month period, individuals were defined as suppressed if they were ART-exposed and dispensation records indicated they had been dispensed medications for the entire period (i.e., ≥ six pick-ups of medication.) Finally, we examined previous exposure to HCV treatment, and participants’ perceptions of the impact of HCV on their health. Except for the socio-demographic variables and previous exposure to HCV treatment, all other variables refer to the six-month period prior to the follow-up visit of interest.
Statistical analysis
First, we described characteristics of participants stratified by their willingness to receive DAA-based regimens. To examine bivariable associations between each independent variable and the outcome of interest, we then used the bivariable logistic regression. Next, to determine the independent correlates of willingness to receive DAA-based regimens, we fit a multivariable logistic model, using an a priori-defined backward stepwise procedure that has been used extensively in several earlier studies (25). Starting with a full model containing all variables associated with the outcome at p<0.10 in bivariable analyses, the Akaike information criterion (AIC) of the model is noted, and the variable with the largest p-value is dropped to fit a reduced model. This iterative process is continued until no variables are left. The model with the lowest AIC value is selected as the final model. Unadjusted and adjusted ORs (AOR) with 95% CIs are reported. All statistical analyses were performed using SAS version 9.4 (SAS Institute, USA).
RESULTS
Between June 2014 and May 2015, 572 HIV-positive PWUD completed at least one study visit, of whom 418 (73%) were HCV-seropositive and provided valid answers to the primary outcome, and thus were included in the present study. Characteristics of study participants stratified by willingness to use DAA-based regimens are presented in Table 1. The median age was 44 years (Interquartile range [IQR] 37-48), and 265 (63%) were male. Access and utilization of relevant health care was heterogeneous, as reflected by the fact that although the majority were on ART (395, 95%) and two-thirds had seen a HCV specialist in the last six months (279, 67%), only about half were enrolled in MMT (220, 53%), and less than 10% had ever been treated for HCV (39, 9%). Overall, 295 participants (71%) reported that they would be willing to use DAA-based regimens. As expected, as hypothetical efficacy scenarios increased, more participants would consider HCV treatment: ranging from 12% in a <40% efficacy scenario to 45% for >80% efficacy scenario (Figure 1).
Table 1.
Characteristics of 418 HIV/HCV positive PWUD stratified by willingness to use DAA-based regimens, Vancouver, Canada
| Characteristic | Total, n (%) (N = 418) |
Willingness to use DAA-based regimens, n (%) | p - value | |
|---|---|---|---|---|
| Yes (n = 295) |
No (n = 123) |
|||
| Individual-level factors | ||||
| Age (median, IQR) | 44 (37–48) | 43 (37–48) | 45 (37–49) | 0.304 |
| Male gender | 265 (63.4) | 187 (63.4) | 78 (63.4) | 0.996 |
| Indigenous ancestry | 193 (46.2) | 138 (46.8) | 55 (44.7) | 0.678 |
| ≥ Daily injection drug use* | 115 (27.5) | 85 (28.8) | 30 (24.4) | 0.357 |
| ≥ Daily non-injection drug use* | 76 (18.2) | 50 (16.9) | 26 (21.1) | 0.312 |
| Heavy alcohol use | 26 (6.2) | 18 (6.1) | 8 (6.5) | 0.877 |
| HIV care-related factors | ||||
| ≥ 1 day ART dispensation* | 395 (94.5) | 276 (93.6) | 119 (96.7) | 0.201 |
| HIV VL < 50 copies/mL* | 321(76.8) | 222 (75.3) | 99 (80.5) | 0.249 |
| Addiction care-related factors | ||||
| Enrolment in MMT* | 220 (52.6) | 167 (56.6) | 53 (43.1) | 0.013 |
| Other addiction treatments (e.g., psychosocial and residential treatment, detox)* | 23 (5.5) | 14 (4.7) | 9 (7.3) | 0.297 |
| HCV care-related factors | ||||
| Ever treated for HCV infection | 39 (9.3) | 28 (9.5) | 11 (8.9) | 0.861 |
| Assessed by a HCV specialist* | 279 (66.7) | 215 (72.9) | 64 (52.0) | <0.001 |
| Perceived HCV as threat to ones’ health* | 129 (30.9) | 109 (36.9) | 20 (16.3) | <0.001 |
| Other structural-level factors | ||||
| Unstable housing* | 295 (70.6) | 211 (71.5) | 84 (68.3) | 0.668 |
DAA, direct-acting antiviral; MMT, methadone maintenance therapy; ART, antiretroviral therapy.
Refers to the 6-month period prior to the interview
Figure 1. Willingness to use DAA-based regimens under different efficacy treatment scenarios among HIV/HCV co-infected PWUD, Vancouver, Canada (n=283)*.
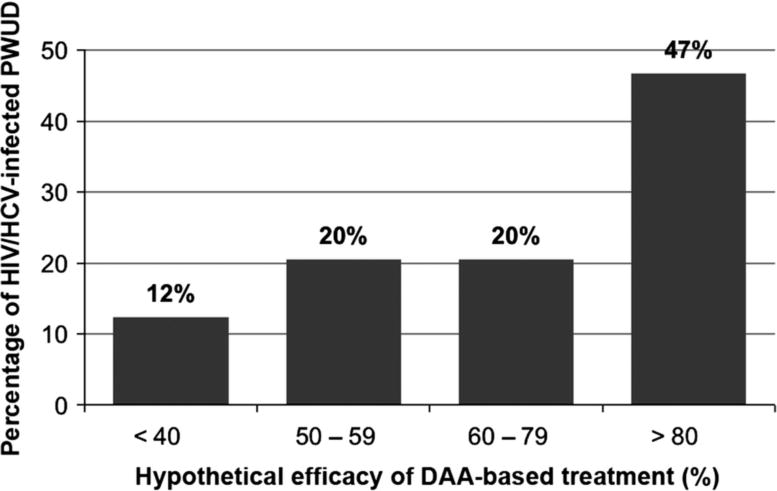
PWUD, people who use drugs; DAA, direct-acting antiviral.
* Missing information for 12 participants. Percentages do not sum 100% due to rounding error.
Table 2 shows the results of unadjusted and adjusted logistic regression analyses of factors associated with willingness to use DAA-based regimens. In bivariable analyses, engagement in MMT, recent assessment by a HCV specialist, and self-perception that HCV was affecting their health were positively associated with willingness to use DAA-based regimens. In the final multivariable model, engagement in MMT (AOR = 1.61, 95% Confidence Interval [CI]: 1.04–2.51), recent assessment by a HCV specialist (AOR = 2.02, 95% CI: 1.28–3.19), and self-perception that HCV was affecting threatening participant’s health (AOR = 2.49, 95% CI: 1.41–4.37) remained independently and positively associated with willingness to use DAA-based regimens.
Table 2.
Unadjusted and adjusted logistic regression analyses of factors associated with willingness to use DAA-based regimens among HIV/HCV positive PWUD in Vancouver, Canada
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age (per year older) | 0.99 (0.96 – 1.01) | |
| Male gender (yes vs. no) | 1.00 (0.65 – 1.55) | |
| Indigenous ancestry (yes vs. no) | 1.09 (0.72 – 1.67) | |
| ≥ Daily injection drug use (yes vs. no)* | 1.25 (0.77 – 2.03) | |
| ≥ Daily non-injection drug use (yes vs. no)* | 0.76 (0.45 – 1.29) | |
| Heavy alcohol use (yes vs. no)* | 0.93 (0.39 – 2.21) | |
| ART dispensation (≥ 1 day vs. 0 days)* | 0.49 (0.16 – 1.47) | |
| HIV VL (< 50 vs. ≥ 50 copies/mL)* | 0.74 (0.44 – 1.24) | |
| Enrolment in MMT (yes vs. no)* | 1.71 (1.12 – 2.62)† | 1.61 (1.04 – 2.51) |
| Other addiction treatments (yes vs. no)* | 0.63 (0.27 – 1.50) | |
| Ever treated for HCV infection (yes vs. no) | 1.07 (0.51 – 2.22) | |
| Assessed by a HCV specialist (yes vs. no)* | 2.48 (1.60 – 3.84)† | 2.02 (1.28 – 3.19) |
| Perceived HCV as threat to ones’ health (yes vs. no)* | 3.02 (1.77 – 5.15)† | 2.49 (1.41 – 4.37) |
| Unstable housing (yes vs. no)* | 1.11 (0.70 – 1.76) |
DAA, direct-acting antiviral; MMT, methadone maintenance therapy; ART, antiretroviral therapy; VL, viral load.
Refers to the 6-month period prior to the interview
p<0.10 and considered in the multivariable model selection process
DISCUSSION
In this study, we found a high prevalence of willingness to use DAA-based regimens among HIV/HCV co-infected PWUD in Vancouver, Canada. In particular, engagement in addiction (i.e., MMT) and HCV care (i.e., recent assessment by a HCV specialist), as well as holding the perception that HCV was affecting participant’s health, were associated with increased odds of reporting willingness to use DAAs.
To our knowledge, this is the first study to assess willingness to use DAA-based regimens among HIV/HCV co-infected PWUD, a key population within the HCV epidemic. Previous studies among HCV mono-infected PWUD have shown similarly high proportions (up to 86%) of willingness to use HCV treatment, even with older, less efficacious and poorly tolerated interferon-based regimens (3, 22–24). This high willingness to undergo HCV treatment contrasts with historical low HCV treatment access and uptake among PWUD (10, 11), suggesting a substantial unmet need in HCV treatment coverage for this population. For example, a recent survey among Canadian HCV specialists found that less than 20% would consider offering HCV treatment to individuals actively injecting drugs (26). This is highly concerning since expanding access to optimized HCV treatment to PWUD is a potentially highly cost-effective public health intervention, given the increased risk of HCV-related progression (particularly among PWUD co-infected with HIV) and associated health care costs, and given the key role that PWUD play in HCV transmission (19, 27). Altogether, these findings highlight the urgent need for interventions to ensure equitable access to HCV care for PWUD. These should include educating health care providers, reducing costs of HCV treatment, simplifying HCV care delivery, integration with HIV and addiction services, and the removal of punitive criminal laws and policies against PWUD (28–30).
In line with past research, engagement in MMT was associated with increased willingness to use DAA-based regimens (22, 24). A more stable lifestyle afforded by reduced opioid use and dependence symptoms, as well as frequent contact with health services (e.g., daily supervised ingestion of methadone) may result in a sub-population of PWUD who is more readily and amenable to consider engaging in HCV treatment. Indeed, a growing number of studies suggest that opioid agonist treatment programs may serve as an important platform to engage PWUD with opioid use disorders in the HCV continuum of care (31). In contrast, we did not find any impact on willingness to use DAAs of other addiction treatment approaches with less evidence-base, including psychosocial-only interventions or withdrawal management alone strategies. Altogether, these results support global calls to expand and sustain access to opioid agonist treatment as part of a broader public health effort to address the interrelated epidemics of HIV, HCV and substance use disorders (15, 32). Likewise, these findings underscore the urgent need to identify and develop effective pharmacotherapies for stimulant use disorders to improve addiction treatment outcomes, which, in turn may be of benefit to support engagement in HCV care for this population (33).
Absence of symptoms or limited knowledge about the natural history of HCV infection and its treatment have been described as important individual-level barriers to treatment uptake (12, 23, 28, 34). Therefore, it is not surprising that in line with previous work, participants in our study who perceived that HCV was affecting their health were more willing to consider HCV treatment (23). Similarly, higher willingness to use DAA-based regimens among HIV/HCV PWUD with a recent assessment by a HCV specialist might be a reflection of higher knowledge about their HCV disease status (e.g., HCV RNA, fibrosis stage), and risks/benefits of treatment due to information and assessments provided by the HCV specialist. These findings emphasize the key role that a trusting and respectful relationship between patients and health care providers may play in informing and supporting patients along the decision making process about HCV treatment (23, 28, 34). Accordingly, educational activities tailored to both patients and providers may help improve uptake of HCV treatment among PWUD. In particular, educational activities tailored to health care providers may reduce stigmatizing interactions with PWUD (e.g., misconceptions of PWUD’s potential to adhere to treatment), a major obstacle to the initiation of discussions about the potential benefits of HCV treatment for this population. Ultimately, successful scale-up of HCV treatment will rely, at least in part, on HCV care providers and patients readiness to engage in evidence-informed dialogues about HCV treatment.
This study has a number of limitations. First, as our sample was not randomly selected, results from this study may not be generalizable to other populations of HIV/HCV co-infected PWUD. In particular, more hidden sub-populations of PWUD may be underrepresented in this sample. Second, due to the cross-sectional nature of this study we were unable to determine temporal and causal relationships between the explanatory variables and outcome. For instance, this could be the case for the relationship between assessment by a HCV specialist and willingness to use DAA-based regimens, which could have suffered from reverse causality. Third, many measures for these analyses relied on self-reported data, which can be subject to social-desirability and recall biases. We attempted to mitigate these potential sources of biases by conducting all interviews in confidential environments by experienced interviewers with strong community rapport. Past research indicates that PWUD’s reports in these conditions are reliable (35, 36). Finally, due to the lack of systematic access to HCV RNA and tests for liver fibrosis staging (e.g., transient elastography), we were not able to evaluate the number of participants with chronic HCV or who would be eligible for publicly-funded HCV treatment under current Canadian guidelines (e.g., >F2) (37). However, giving increasing rates of progression to chronic HCV (up to 85%) and ESLD among HIV/HCV co-infected individuals it is anticipated that a large proportion would meet these eligibility criteria.
In summary, this study found high rates of willingness to use DAA-based regimens among HIV/HCV PWUD in this setting. Bridging the gap between PWUD’s HCV treatment willingness and actual treatment uptake should be a priority for this population moving forward. Importantly, enrollment in MMT was positively associated with willingness to use newer HCV treatments. This finding, alongside previous successful experiences of integration of HIV, HCV and addiction treatment, suggest that multidisciplinary one-stop-shop models of care for PWUD may play a critical role in increasing access to and improving HCV treatment outcomes among this population (29–31). As such, the role of addiction treatment on HCV treatment and prevention outcomes should be further explored (14). The impact of new DAA-based regimens on the HCV epidemic will remain limited as long as so-called core transmitters, including HIV/HCV co-infected PWUD, are not prioritized for access to optimized treatment for HCV infection.
Acknowledgments
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We would specifically like to thank: Kristie Starr, Deborah Graham, Tricia Collingham, Carmen Rock, Jennifer Matthews, Steve Kain, Benita Yip and Guillaume Colley for their research and administrative assistance.
This study was supported by the National Institute on Drug Abuse (NIDA) at the US National Institutes of Health [NIH; grant number R01-DA021525]. MES is supported by a Michael Smith Foundation for Health Research postdoctoral fellowship award and a Canada Addiction Medicine Research Fellowship (NIDA, grant number R25-DA037756). M-JM is supported, in part, by the NIH [grant number R01-DA021525]. JM is supported by the British Columbia Ministry of Health and by NIDA at the NIH [grant number R01-DA036307]. LT is supported by a Canadian Institutes of Health Research Fellowhip. The University of British Columbia has received unstructured funding from NG Biomed, Ltd., to support M-JM’s research. JM has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Footnotes
Statement of interests: All other authors report no potential conflicts.
References
- 1.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV coinfection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 2.Taylor LE, Swan T, Matthews GV. Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral-based therapy. Clin Infect Dis. 2013;57(Suppl 2):S118–24. doi: 10.1093/cid/cit326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207(Suppl 1):S19–25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mira JA, Rivero-Juarez A, Lopez-Cortes LF, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56:1646–53. doi: 10.1093/cid/cit103. [DOI] [PubMed] [Google Scholar]
- 5.Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS. 2015;10:297–302. doi: 10.1097/COH.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 6.Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730–40. doi: 10.1093/cid/civ396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26:1028–38. doi: 10.1016/j.drugpo.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockstroh JK, Hardy WD. Current treatment options for hepatitis C patients co-infected with HIV. Expert Rev Gastroenterol Hepatol. 2016:1–7. doi: 10.1586/17474124.2016.1145545. [DOI] [PubMed] [Google Scholar]
- 9.Jacka B, Applegate T, Krajden M, et al. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology. 2014;60:1571–80. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iversen J, Grebely J, Topp L, Wand H, Dore G, Maher L. Uptake of hepatitis C treatment among people who inject drugs attending Needle and Syringe Programs in Australia, 1999–2011. J Viral Hepat. 2014;21:198–207. doi: 10.1111/jvh.12129. [DOI] [PubMed] [Google Scholar]
- 12.Bruggmann P. Accessing Hepatitis C patients who are difficult to reach: it is time to overcome barriers. J Viral Hepat. 2012;19:829–35. doi: 10.1111/jvh.12008. [DOI] [PubMed] [Google Scholar]
- 13.Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(Suppl 2):S80–9. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]
- 14.Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56:806–16. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO; 2014. p. 124. [PubMed] [Google Scholar]
- 16.AASLD/IDSA. Recommendations for testing, managing, and treating hepatitis C. 2015;2016 [Google Scholar]
- 17.European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez JA, Lawitz EJ, Poordad F. Interferon-free, direct-acting antiviral therapy for chronic hepatitis C. J Viral Hepat. 2015;22:861–70. doi: 10.1111/jvh.12422. [DOI] [PubMed] [Google Scholar]
- 19.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–4. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 21.Strathdee SA, Palepu A, Cornelisse PG, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–9. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 22.Alavi M, Micallef M, Fortier E, et al. Effect of treatment willingness on specialist assessment and treatment uptake for hepatitis C virus infection among people who use drugs: the ETHOS study. J Viral Hepat. 2015;22:914–25. doi: 10.1111/jvh.12415. [DOI] [PubMed] [Google Scholar]
- 23.Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40(Suppl 5):S304–12. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005;40(Suppl 5):S313–20. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- 25.Socias ME, Shannon K, Montaner JS, et al. Gaps in the hepatitis C continuum of care among sex workers in Vancouver, British Columbia: Implications for voluntary hepatitis C virus testing, treatment and care. Can J Gastroenterol Hepatol. 2015;29:411–6. doi: 10.1155/2015/381870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myles A, Mugford GJ, Zhao J, Krahn M, Wang PP. Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: results from a national specialist survey in Canada. Can J Gastroenterol. 2011;25:135–9. doi: 10.1155/2011/810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin NK, Vickerman P, Miners A, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 28.Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int J Drug Policy. 2015;26:922–35. doi: 10.1016/j.drugpo.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford N, Wiktor S, Kaplan K, et al. Ten priorities for expanding access to HCV treatment for people who inject drugs in low- and middle-income countries. Int J Drug Policy. 2015;26:1088–93. doi: 10.1016/j.drugpo.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61. doi: 10.1093/cid/cit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlman DC, Jordan AE, Uuskula A, et al. An international perspective on using opioid substitution treatment to improve hepatitis C prevention and care for people who inject drugs: Structural barriers and public health potential. Int J Drug Policy. 2015;26:1056–63. doi: 10.1016/j.drugpo.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO, UNODC and UNAIDS. Technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users. Vol. 2012. Geneva: World Health Organization; 2012. Revision. [Google Scholar]
- 33.Dieperink E, Knott A, Thuras P, Pocha C. The effect of stimulant use on antiviral treatment in an integrated hepatitis clinic. Gen Hosp Psychiatry. 2013;35:387–92. doi: 10.1016/j.genhosppsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Zeremski M, Dimova RB, Zavala R, et al. Hepatitis C virus-related knowledge and willingness to receive treatment among patients on methadone maintenance. J Addict Med. 2014;8:249–57. doi: 10.1097/ADM.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Irala J, Bigelow C, McCusker J, Hindin R, Zheng L. Reliability of self-reported human immunodeficiency virus risk behaviors in a residential drug treatment population. Am J Epidemiol. 1996;143:725–32. doi: 10.1093/oxfordjournals.aje.a008806. [DOI] [PubMed] [Google Scholar]
- 36.Langendam MW, van Haastrecht HJ, van Ameijden EJ. The validity of drug users’ self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol. 1999;28:514–20. doi: 10.1093/ije/28.3.514. [DOI] [PubMed] [Google Scholar]
- 37.Myers RP, Shah H, Burak KW, Cooper C, Feld JJ. An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol Hepatol. 2015;29:19–34. doi: 10.1155/2015/692408. [DOI] [PMC free article] [PubMed] [Google Scholar]


