Summary
The bifunctional farnesyl/geranylgeranyl diphosphate synthase (FPPS/GGPPS) is a key branchpoint enzyme in isoprenoid biosynthesis in Plasmodium falciparum (malaria) parasites. PfFPPS/GGPPS is a validated, high-priority antimalarial drug target. Unfortunately current bisphosphonate drugs that inhibit FPPS and GGPPS enzymes by acting as a diphosphate substrate analog show poor bioavailability and selectivity for PfFPPS/GGPPS. We identified a new non-bisphosphonate compound, MMV019313, which is highly selective for PfFPPS/GGPPS and showed no activity against human FPPS or GGPPS. Inhibition of PfFPPS/GGPPS by MMV019313, but not bisphosphonates, was disrupted in an S228T variant, demonstrating that MMV019313 and bisphosphonates have distinct modes-of-inhibition. Molecular docking indicated that MMV019313 did not bind previously characterized substrate sites in PfFPPS/GGPPS. Our finding uncovers a new, selective small molecule binding site in this important antimalarial drug target with superior druggability compared to the known inhibitor site and sets the stage for development of Plasmodium-specific FPPS/GGPPS inhibitors.
Keywords: malaria, drug discovery, and high-throughput screening, non-bisphosphonate inhibitor
eTOC
Gisselberg et al. identified a non-bisphosphonate inhibitor of the bifunctional FPPS/GGPPS in malaria parasites. Using this inhibitor, they uncover a new, highly selective small molecule binding site in this validated antimalarial drug target, overcoming previous limitations to developing malaria-specific FPPS/GGPPS inhibitors.
Introduction
There is an urgent need for antimalarials with novel mechanisms-of-action to circumvent resistance to frontline drugs. The biosynthesis of cellular isoprenoids is an essential process in Plasmodium parasites that cause malaria. A number of antimalarial compounds target enzymes in isoprenoid biosynthetic pathways leading to parasite growth inhibition. First, Plasmodium parasites depend on the 7-enzyme prokaryotic 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway in its plastid organelle, the apicoplast, to produce isopentenyl pyrophosphate (IPP) and its isomer dimethyallyl pyrophosphate (DMAPP) (Jomaa et al., 1999). IPP and DMAPP are the C5 building blocks for all isoprenoids. The antibiotic fosmidomycin inhibits the MEP enzyme, Dxr/IspC, in both bacteria and Plasmodium parasites (Jomaa et al., 1999).
Second, at least three isoprenoid synthases (PF3D7_1128400.1, PF3D7_0202700, PF3D7_0826400) catalyze the condensation of IPP and DMAPP into longer prenyl chains (Artz et al., 2011; Jordão et al., 2013; Tonhosolo et al., 2005). In particular, farnesyl pyrophosphate synthase (FPPS) and geranylgeranyl pyrophosphate synthase (GGPPS) are key branch point enzymes that synthesize C15 and C20 prenyl chains, respectively, for multiple downstream enzymes. In Plasmodium parasites, these reactions are catalyzed by a single bifunctional enzyme, the farnesyl/geranylgeranyl diphosphate synthase (Artz et al., 2011; Jordão et al., 2013). Nitrogen-containing bisphosphonates, blockbuster drugs which inhibit human FPPS, also inhibit the bifunctional Plasmodium FPPS/GGPPS (Ghosh et al., 2004; Jordão et al., 2011; Martin et al., 2001; No et al., 2012; Singh et al., 2010).
Finally, prenyl chains are cyclized and/or conjugated to small molecule and protein scaffolds by a variety of prenyltransferases to biosynthesize final isoprenoid products required for parasite growth and replication. Tetrahydroquinolines (THQ) have been shown to potently inhibit the Plasmodium protein farnesyltransferase (Eastman et al., 2005; 2007; Nallan et al., 2005). Other inhibitors may interfere with isoprenoid biosynthesis indirectly by disrupting transporters that supply starting substrates or export products or blocking pathways that provide cofactors for isoprenoid biosynthetic enzymes.
Importantly fosmidomycin, bisphosphonates, and tetrahydroquinolines have all shown efficacy in mouse models of malaria infection, validating the key importance of isoprenoid biosynthesis as an antimalarial drug target (Jomaa et al., 1999; Nallan et al., 2005; No et al., 2012; Singh et al., 2010). Fosmidomycin is currently being tested in human clinical trials, while a THQ lead candidate was investigated in preclinical studies (Fernandes et al., 2015; Nallan et al., 2005). However, novel chemical scaffolds that disrupt isoprenoid biosynthetic pathways in Plasmodium remain desirable to overcome unfavorable drug properties of these known inhibitors. For example, bisphosphonates avidly bind bone mineral, and both fosmidomycin and THQs have short half-lives in vivo (Cremers et al., 2005; Sinigaglia et al., 2007; Tsuchiya et al., 1982; Van Voorhis et al., 2007).
In 2011 the Medicine for Malaria Venture (MMV) distributed the Open-Access Malaria Box to accelerate antimalarial drug discovery (Spangenberg et al., 2013). The Malaria Box consists of 400 structurally diverse compounds, curated from >20,000 hits generated from large-scale screens, that inhibit the growth of blood-stage Plasmodium falciparum parasites (Gamo et al., 2010; Guiguemde et al., 2010; Rottmann et al., 2010). A major goal of sharing these compounds was to facilitate elucidation of their antimalarial mechanism-of-action and open new classes of validated chemical scaffolds and drug targets. Compounds that disrupt isoprenoid metabolism can be detected by “rescue” of their growth inhibition upon supplementation of isoprenoids in the growth media (Yeh and Derisi, 2011). Previously, we and two other groups screened the Malaria Box for compounds whose growth inhibition were rescued by addition of IPP and identified MMV008138 (Bowman et al., 2014; Wu et al., 2015). We and our collaborators demonstrated that MMV008138 inhibits IspD, an enzyme in the MEP pathway that produces IPP (Wu et al., 2015).
Using a quantitative high-throughput screen (qHTS), we report a second compound in the Malaria Box, MMV019313, that shows an IPP rescue phenotype but was not identified in screens performed by other groups (Bowman et al., 2014; DeRisi, 2014; Van Voorhis et al., 2016). We demonstrate that the target of MMV019313 is the P. falciparum FPPS/GGPPS, a cytosolic isoprenoid synthase that utilizes IPP and the key branch point enzyme in isoprenoid biosynthesis in parasites. MMV019313 represents the first new class of specific non-bisphosphonate inhibitors of PfFPPS/GGPPS.
Results
A quantitative high-throughput screen (qHTS) for growth and IPP rescue identifies MMV019313 as an inhibitor of isoprenoid biosynthesis
Previous IPP rescue screens of the Malaria Box tested compounds at a single, high concentration >5 μM (Bowman et al., 2014; DeRisi, 2014; Van Voorhis et al., 2016). While testing compounds at a single concentration is useful for identifying phenotypes that occur at a threshold concentration (e.g. growth inhibition), growth rescue is expected to occur within a specific range of concentrations. For example, doxycycline inhibits P. falciparum growth with an EC50= 0.3 μM that increases to 3.2 μM upon addition of IPP (Yeh and Derisi, 2011). At concentrations <0.3 μM, doxycycline does not cause growth inhibition. However, at concentrations >3.2 μM, it is no longer specific for its target and causes growth inhibition through additional targets that cannot be IPP rescued. Therefore the concentration range in which IPP rescue can be observed is greater than the EC50 of the compound for its specific, IPP-rescuable target but less than that for any nonspecific targets.
To increase the sensitivity for detecting IPP chemical rescue, we screened the Malaria Box for growth inhibition of blood-stage P. falciparum in the presence and absence of IPP over 8–12 drug concentrations from 0.01–27 μM (Table S1). Of 397 compounds tested (3 compounds were not available), 383 showed growth inhibition at ≤27 μM. Initial hits showing IPP rescue of growth inhibition at one or more drug concentrations were commercially sourced and retested. Along with the previously reported compound, MMV008138, we confirmed a second compound in the Malaria Box showing an IPP rescue phenotype, MMV019313 (Figure 1A). MMV019313 inhibited P. falciparum growth measured in a single replication cycle with EC50=268 nM (250–289 nM) in the absence of IPP; in the presence of IPP (200 μM), the EC50 was over 13-fold more at 3.6 μM (3.2–4.0 μM) (Figure 1B). Notably, at concentrations >3.6 μM, MMV019313 inhibits a secondary target that can no longer be IPP rescued, which explains why it was not identified in previous screens. Incidentally, using this quantitative HTS also permits the screen to be performed with geranylgeraniol, which is significantly less costly than IPP. So far all identified inhibitors that rescue with IPP also show at least a partial rescue of parasite growth inhibition with geranylgeraniol (Yeh and Derisi, 2011; Zhang et al., 2011).
Figure 1. IPP rescues growth inhibition by MMV019313.

A. The structure of MMV019313. B. Dose-dependent growth inhibition in the absence (solid line) and presence (dotted line) of IPP. Parasitemia is normalized to that of an untreated control. Results shown for experiments performed in biological triplicate with technical duplicate. Shown plotted as mean ±SD.
An unusual feature of blood-stage Plasmodium is that IPP can rescue complete loss of the apicoplast, the plastid organelle which houses the MEP pathway, since production of IPP is the only essential function of the apicoplast. Compounds like doxycycline and actinonin that disrupt apicoplast biogenesis cause growth inhibition rescued by IPP and result in parasites lacking an apicoplast (Amberg-Johnson et al., 2017; Yeh and Derisi, 2011). In contrast, inhibitors of isoprenoid biosynthesis, like fosmidomycin and MMV008138, cause growth inhibition rescued by IPP with an intact apicoplast (Wu et al., 2015; Yeh and Derisi, 2011). We determined whether MMV019313 disrupted the biogenesis of the apicoplast. Both the replication of the apicoplast genome and import of an apicoplast-targeted GFP were intact in MMV019313-treated and IPP-rescued parasites (Figure S1) (Yeh and Derisi, 2011). Altogether MMV019313 causes growth inhibition rescued by IPP with no defect in apicoplast biogenesis, suggesting that, like fosmidomycin and MMV008138, MMV019313 blocks isoprenoid biosynthesis (Wu et al., 2015).
MMV019313-resistant parasites contain a mutation in the farnesyl/geranylgeranyl diphosphate synthase
Metabolomic profiling of MMV019313 by other groups did not look for isoprenoid intermediates so disruption of these pathways could not be detected (Allman et al., 2016; Creek et al., 2016). To gain further insight into the mechanism-of-action of MMV019313, we identified mutations that confer resistance to MMV019313. A single attempt to select drug-resistant parasites from a bulk culture of 1010 parasites was not successful, though this same protocol was effective in selecting drug resistance against MMV008138 when performed in parallel (Wu et al., 2015). Therefore, we treated 108 P. falciparum W2 parasites with sub-lethal doses of ethyl methanesulfonate (EMS), an alkylating agent, to increase the likelihood of selecting MMV019313-resistant parasites. Resistant parasites, which showed EC50 values 3-9-fold greater than that of the initial susceptible population, emerged in all mutagenized cultures. Growth inhibition of two MMV019313-resistant populations (designated 019313R1 and 019313R2) are shown in Figure 2A. Significantly, in the presence of IPP, the EC50 of MMV019313 against 019313R1 and 019313R2 was similar to that observed in susceptible strains, and growth inhibition could no longer be rescued by addition of IPP (Figure S2). These results suggest that, as expected, 019313R1 and 019313R2 populations were completely resistant to inhibition of its specific IPP-rescuable target but had not developed resistance to secondary targets.
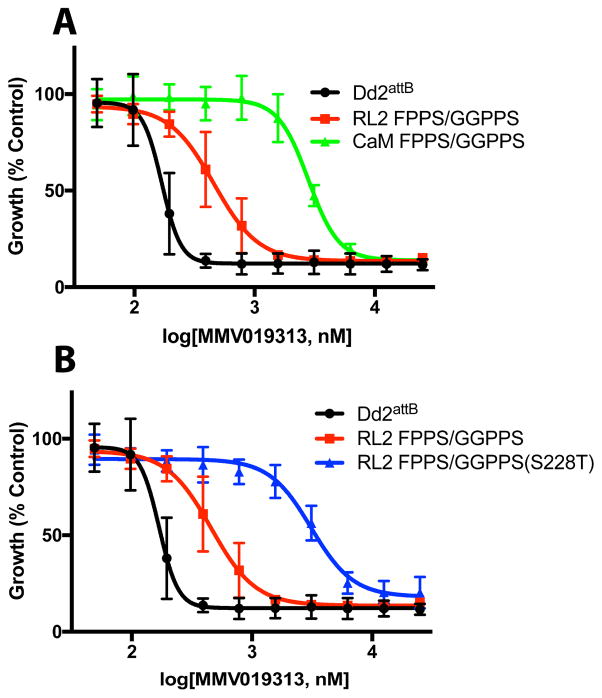
Figure 2. MMV019313-resistant parasites contain a mutation in the bifunctional farnesyl and geranylgeranyl diphosphate synthase.
A. Dose-dependent growth inhibition of parental W2 parasites (black, solid line), W2 parasites with addition of IPP (black, dotted line), and two resistant populations (019313R1, red line, and 019313R2, green line). The fold change in EC50 compared to W2 parasites is indicated. Results shown for experiments performed in biological triplicate with technical duplicate and plotted as mean ±SD. B. Mutation determined by whole genome sequencing of resistant populations 019313R1 and 019313R2, highlighted in red C. Schematic of PfFPPS/GGPPS protein. The S228T residue is highlighted in bold black while conserved KT and SARM residues are highlighted in bold grey and underlined.
019313R1, 019313R2, and their respective mutagenized parent strains used to initiate drug selection, WT1 and WT2, were subjected to whole-genome sequencing. Comparison of the resistant and parent genome sequences identified a single nucleotide variant (SNV) common to both resistance populations, which was present in 100% of reads in 019313R1 and 63% of reads in 019313R2 but not present in either susceptible parent genomes (Table 1). No other SNV were detected at >40% prevalence in either resistant populations relative to the corresponding parent populations (Table S2). The identified SNV was a T-to-A mutation in the gene PF3D7_1128400 characterized as a bifunctional FPPS/GGPPS and resulted in a Ser228-to-Thr change in the protein (Figure 2B) (Artz et al., 2011; Jordão et al., 2013). IPP is a known substrate of the FPPS/GGPPS, and Ser228 is within a conserved region required for catalysis in all homologous FPPS and GGPPS enzymes (Figure 2C). PfFPPS/GGPPS was a strong candidate for further validation as the molecular target of MMV019313.
Table 1.
Summary of mutations identified by whole genome sequencing
| Gene IDa | Descriptionb | Base Callc | AA change | Population | Read Numberd | %e | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | WT | EMS | WT | R | WT | R | ||||
| PF3D7_1128400 | Geranylgeranyl pyrophosphate synthase (GGPPS) | 682 | T | A | S228T | 019313R1 | 219 | 163 | 100 | 100 |
| 019313R2 | 186 | 181 | 100 | 62.4 | ||||||
PlasmoDB gene identification number
Based on PlasmoDB functional assignments
WT calls match 3D7 reference genome
Read depth corresponding to WT call (IGV) and Mut call (SnpEff) respectively
Percent of reads corresponding to WT call (IGV) and Mut call (SnpEff) respectively
Overexpression and an S228T variant of PfFPPS/GGPPS confer resistance to MMV019313
We determined whether overexpression of wildtype PfFPPS/GGPPS was sufficient to confer resistance to MMV019313. A transgene encoding FPPS/GGPPS-GFP under the control of either the ribosomal L2 protein (RL2; PF3D7_1132700) or calmodulin (CaM; PF3D7_1434200) promoter was integrated into an engineered attB locus in Dd2attb parasites (Nkrumah et al., 2006). Expression driven by the CaM promoter is 10 to 50-fold greater across the life cycle than that of the RL2 promoter based on RNA-seq data, resulting in appreciably greater FPPS/GGPPS-GFP protein (Figure S3A) (Otto et al., 2010). Therefore, we compared the effect of no, moderate (RL2), and high (CaM) levels of FPPS/GGPPS-GFP overexpression on susceptibility to MMV019313. As shown in Figure 3A and Table S3, overexpression of FPPS/GGPPS-GFP results in a dose-dependent increase in the EC50 of MMV019313 with intermediate-level resistance to MMV019313 observed in the RL2-FPPS/GGPPS-GFP strain and high-level resistance observed in the CaM-FPPS/GGPPS-GFP strain. Notably, growth inhibition by MMV019313 in the CaM-FPPS/GGPPS-GFP strain could no longer be rescued by IPP, indicating that complete resistance to inhibition of the specific IPP-rescuable target had been achieved (Figure S3B).
Figure 3. Overexpression of WT and an S228T variant of PfFPPS/GGPPS confer resistance to MMV019313.
A. Dose-dependent growth inhibition of parental Dd2attB parasites (black) and parasites overexpressing FPPS/GGPPS-GFP under the “moderate” RL2 (red) or “high” CaM (green) promoter. B. Dose-dependent growth inhibition of parental Dd2attB parasites (black) and parasites overexpressing wildtype (red) or mutant (S228T, blue) FPPS/GGPPS-GFP under the RL2 promoter. Results shown for experiments performed in biological duplicate with technical duplicate and plotted as mean ±SD.
We also determined whether the S228T variant of GGPPS confers MMV019313 resistance. Initially we attempted to introduce the S228T mutation into the fpps/ggpps gene in the P. falciparum genome using CRISPR-Cas9 mutagenesis but were unable to recover mutant parasites. As an alternative, we overexpressed the FPPS/GGPPS(S228T)-GFP variant using the “moderate” RL2 promoter and compared its effect on MMV019313 susceptibility with that of the wildtype RL2-FPPS/GGPPS-GFP. Overexpression of the FPPS/GGPPS(S228T)-GFP variant, even at moderate levels, caused an 18-fold increase in the EC50 which did not increase further with IPP rescue, indicating that complete resistance to inhibition of the specific, IPP-rescuable target had been achieved (Figure 3B and S3C; Table S3). Because moderate overexpression of the S228T variant caused greater MMV019313 resistance than moderate overexpression of wildtype PfFPPS/GGPPS, we conclude that the S228T variant is sufficient to confer resistance independent of overexpression. Altogether these results clearly demonstrated that the mechanism-of-action of MMV019313 is dependent on PfFPPS/GGPPS.
MMV019313 inhibits the enzymatic activity of PfFPPS/GGPPS but not human FPPS or GGPPS
To confirm that PfFPPS/GGPPS is the molecular target of MMV019313, we directly measured MMV019313 inhibition in enzymatic assays. As previously reported, purified PfFPPS/GGPPS catalyzed the production of both farnesyl(C15)-PP and geranylgeranyl(C20)-PP (Jordão et al., 2013). To measure the inhibitory effect of MMV019313, we determined its IC50 for FPP and GGPP production. MMV019313 was identified by its IPP rescue phenotype in cell growth assays wherein addition of exogenous substrate increases enzyme activity, indicating that physiological conditions are likely non-saturating. Consistent with this, the IC50 value was 330 nM for FPP production under non-saturating substrate conditions, comparable to its EC50 value in cellular growth inhibition assays (Figure 1B and S4; Table S3). Under saturating substrate conditions, IC50 values were 2.0 μM for FPP production and 9.8 μM for GGPP production (Figure 4A; Table S3).
Figure 4. The mode-of-inhibition of MMV019313 is specific for PfFPPS/GGPPS over human FPPS and distinct from bisphosphonates.
Dose-dependent inhibition of wildtype PfFPPS/GGPPS (solid), PfFPPS/GGPPS S228T variant (dotted), and human FPPS (dashed) production of farnesyl diphosphate by A. MMV019313 or B. BPH-703. Data plotted as mean±SD.
We also measured MMV019313 inhibition of PfFPPS/GGPPS S228T, human FPPS, or human GGPPS activity under saturating conditions which gives higher signal-to-noise. The S228T variant, which conferred resistance to MMV019313 in growth inhibition assays, was also 10-fold less susceptible to MMV019313 inhibition of its enzymatic activity (Figure 3B and 4A; Table S3). Significantly, MMV019313 at concentrations up to 200 μM did not inhibit human FPPS or GGPPS activity, indicating that it was at least 100-fold selective for PfFPPS/GGPPS over human homologs (Figure 4A; Table S3). In contrast, the most selective bisphosphonate identified by a previous study, BPH-703, showed only a 2.6-fold selectivity for PfFPPS/GGPPS versus human FPPS and 3.6-fold selectivity versus human GGPPS in enzymatic assays (Figure 4B; Table S3)(No et al., 2012). We performed preliminary structure-activity relationship studies varying the length of the linker and the ring size of the amide substituent. Though potency was reduced in these analogs, the high selectivity for PfFPPS/GGPPS over human FPPS as well as resistance of the S228T variant was maintained, suggesting that specificity for PfFPPS/GGPPS may be conferred by the tricyclic moiety (Table S4).
MMV019313 binds a novel site on PfFPPS/GGPPS, distinct from that of bisphosphonates
The lack of inhibition of human FPPS and GGPPS by MMV019313 was intriguing since PfFPPS/GGPPS is structurally related to human FPPS and GGPPS and all are inhibited by bisphosphonates (No et al., 2012). Three small molecule binding sites have been identified in this class of enzymes (Figure 5): Both the allylic substrate (DMAPP, GPP, or FPP) and IPP sites have been observed in the closely-related P. vivax FPPS/GGPPS (Artz et al., 2011). An allosteric site was identified in HsFPPS but has not been structurally characterized in Plasmodium FPPS/GGPPS (Jahnke et al., 2010; Park et al., 2017). Co-crystal structures of zoledronate and BPH-703 with PvFPPS/GGPPS show that bisphosphonates bind in the allylic substrate site, similar to what is observed in bisphosphonate-mammalian FPPS complexes (Kavanagh et al., 2006; No et al., 2012; Rondeau et al., 2006; Yokoyama et al., 2015; Zhang et al., 2013). To determine whether MMV019313 and bisphosphonates share a common binding site, we assessed whether the MMV019313-resistant S228T variant caused cross resistance with the bisphosphonate BPH-703. Unlike MMV019313, BPH-703 inhibited wildtype and the S228T variant PfFPPS/GGPPS activity with similar potency (Figure 4B). Furthermore, moderate overexpression of the S228T variant, which conferred resistance to MMV019313, did not affect parasite growth inhibition by BPH-703 (Figure 3B and S5). These results demonstrate that MMV019313 has a novel binding mode, distinct from bisphosphonates.
Figure 5. Location of Ser248 relative to small molecule binding sites in the P. vivax FPPS/GGPPS structure.
The structure of PvFPPS/GGPPS (PDB: 3EZ3) shows the relative positions of the allylic site (with zoledronate bound in teal), the IPP site (with residues which interact with IPP shown in light cyan) and the location of a potential allosteric site. Serine248 in PvFPPS/GGPPS (which corresponds to S228 in PfFPPS/GGPPS which confers resistance to MMV019313 when mutated to threonine) is shown in red.
S228 in PfFPPS/GGPPS corresponds to S248 in PvFPPS/GGPPS, which is located near the IPP site (but does not make any direct contacts with this substrate) and at the base of the putative allosteric site (Figure 5; Artz et al., 2011). The conformational dynamics of FPPS and GGPPS enzymes preclude straightforward interpretation of the mode-of-inhibition from conventional kinetic assays (Dunford et al., 2008; Taylor, 2004). Moreover PvFPPS/GGPPS could not be crystallized with bound MMV019313 using conditions in which bisphosphonate-PvFPPS/GGPPS crystals were obtained (personal communication, Dr. Raymond Hui). Therefore we used molecular docking to evaluate the likelihood of MMV019313 binding at substrate sites in PfFPPS/GGPPS. Rigid body docking was used to generate poses with MMV019313 bound to allylic substrate and IPP sites and compare the binding free energy of each MMV019313 pose with that of the known ligands (Friesner et al., 2004; 2006; Halgren et al., 2004). MMV019313 was predicted to have binding affinities >108 weaker than bisphosphonate at the allylic site and >105 weaker than IPP at its site (Table S5). The modeling suggests that MMV019313 is unlikely to bind either the allylic substrate or IPP site. Docking of MMV019313 into the putative allosteric site in the PvFPPS/GGPPS apo structure gave inconclusive results.
Discussion
Farnesyl and geranylgeranyl diphosphate synthase (FPPS and GGPPS) are key branch point enzymes in isoprenoid biosynthesis. Human cells contain separate FPPS and GGPPS enzymes. An important class of clinical drugs, nitrogen-containing bisphosphonates, inhibits human FPPS in osteoclasts and block their function and proliferation (Kavanagh et al., 2006). Because osteoclasts are responsible for bone resorption, bisphosphonates are highly effective for treatment of osteoporosis and other bone remodeling diseases. Bisphosphonates are chemically stable analogs of inorganic pyrophosphate containing a P-C-P bond in place of the phosphodiester, which accounts for both its inhibition of FPPS (acting as an analog of the allylic diphosphate substrate) and its high selectivity for osteoclasts (depositing in bone mineral which is composed of calcium and phosphate). Unfortunately the charge state of bisphosphonates is a major liability in other therapeutic applications, as they are poorly bioavailable, rapidly cleared by the kidney, and do not achieve therapeutic levels in serum for treatment of non-bone diseases (Cremers et al., 2005; Sinigaglia et al., 2007). Due to these limitations, there have been efforts to develop modified bisphosphonates or non-bisphosphonate FPPS inhibitors with improved pharmacokinetic properties for soft-tissue cancer and infectious disease applications (Chen et al., 2013; Jahnke et al., 2010; Liu et al., 2014; Marzinzik et al., 2015; Zhang et al., 2009). As an antimicrobial, there is the additional concern that broad inhibition of human FPPS could result in toxicity (Kotz, 2010).
PfFPPS/GGPPS, the molecular target of MMV019313 as demonstrated in this study, closely resembles mammalian FPPS enzymes in sequence, structure, and inhibition by bisphosphonates (Artz et al., 2011; Jordão et al., 2013; No et al., 2012). Like human FPPS, it is a central node in cellular isoprenoid biosynthesis vulnerable to drug inhibition (Artz et al., 2011; Kavanagh et al., 2006; Luckman et al., 1998). In Plasmodium, FPP and GGPP are required for the biosynthesis of prenylated proteins, the prenyl modification of ubiquinone, and other isoprenoid products, such that inhibition of PfFPPS/GGPPS disrupts multiple cellular pathways (Chakrabarti et al., 2002; de Macedo et al., 2002; Gabriel et al., 2015; Gisselberg et al., 2017; Suazo et al., 2016; Tonhosolo et al., 2005; 2009). Previously lipophilic bisphosphonates modified with an alkyl chain to increase their cell permeability were shown to inhibit PvFPPS/GGPPS and clear both blood- and liver-stage Plasmodium parasites in mice infection models (No et al., 2012; Singh et al., 2010). Inhibition of isoprenoid biosynthesis likely does not block gametocyte development (Lell et al., 2003; Van Voorhis et al., 2016). Importantly, these results validated Plasmodium FPPS/GGPPS as an antiparasitic drug target for both acute malaria treatment and malaria chemoprophylaxis.
MMV019313’s distinct mode-of-inhibition addresses key impediments in the development of PfFPPS/GGPPS inhibitors as antimalarial drugs. First, it is the first non-bisphosphonate inhibitor of Plasmodium FPPS/GGPPS with drug-like physicochemical properties satisfying the Rule of 5 (Van Voorhis et al., 2016). Unlike bisphosphonates, it does not mimic a charged diphosphate substrate to achieve FPPS/GGPPS inhibition. As a compound in the Malaria Box library, the results of >300 assays characterizing this library as part of an innovative “open source” drug discovery effort by many groups will be a rich source of information (Allman et al., 2016; Paul et al., 2016; Ullah et al., 2017; Van Voorhis et al., 2016). Second, MMV019313 showed high selectivity for PfFPPS/GGPPS with no inhibition of human FPPS and GGPPS at up to 200 μM, minimizing the potential for mechanism-based (e.g. “on-target”) toxicity. Consistent with its lack of enzymatic inhibition, MMV019313 showed no cytotoxity against a panel of 60 human cancer cell lines at 10 μM (Van Voorhis et al., 2016). In contrast, the most selective bisphosphonate BPH-703 identified so far showed 2.6–3.6-fold selectivity in our enzymatic assays, corresponding to a reported therapeutic index of 193 in growth inhibition assays (No et al., 2012). Overall, we demonstrate a novel mode-of-inhibition of PfFPPS/GGPPS that circumvents the inherent liabilities of previous bisphosphonate inhibitors.
MMV019313 likely binds a non-substrate site in PfFPPS/GGPPS that is either absent from or substantially different in the human homologs, accounting for its high selectivity. One possibility is the allosteric site observed in human FPPS structures. Unfortunately, this site has not been characterized in Plasmodium FPPS/GGPPS homologs and molecular docking in the apo PvFPPS/GGPPS structure was inconclusive. Another clue is the resistance caused by the S228T variant, which could be explained by a direct contact between Ser228 and MMV019313 in a new binding pocket. But since the change from Ser to Thr is quite conservative, the addition of a methyl group, Ser228 may also be involved in conformational dynamics important for catalysis. Structural analysis of this variant enzyme may reveal altered conformational states underlying the resistance to MMV019313.
Identification of lead compounds, based on MMV019313 or alternative scaffolds, that target this new small molecule binding pocket will accelerate antimalarial drug discovery. Optimization of potency and pharmacokinetic properties of lead compounds will be a prerequisite for testing in mouse models of Plasmodium infection. For example, we found that while MMV019313 is stable to human liver microsomal enzymes (t½> 159 min), its t½ in mouse liver microsomes was 4 min. This metabolic instability may account for a <1μM peak serum concentration following oral administration in mice (Van Voorhis et al., 2016). Importantly, our study has generated several tools for identifying specific PfFPPS/GGPPS inhibitors as lead compounds. The luciferase-based enzymatic assays used in our study can easily be adapted to high-throughput screening of libraries to identify analogs with 1) improved potency that 2) maintain selectivity for PfFPPS/GGPPS and 3) are dependent on residue S228 (in contrast we found a commercially-available colorimetric assay was not sufficiently sensitive) (Crowther et al., 2011). P. falciparum strains overexpressing wildtype or the S228T variant can also be retooled as secondary cellular assays for on-target specificity. Thus our finding provides critical foundation for development of specific PfFPPS/GGPPS inhibitors
Significance
There is an urgent need for antimalarials with novel mechanisms-of-action to circumvent resistance to frontline drugs. The bifunctional farnesyl/geranylgeranyl diphosphate synthase (FPPS/GGPPS) is a high-priority drug target in Plasmodium falciparum (malaria) parasites that has been validated in mouse models of malaria infection. Unfortunately, existing bisphosphonate inhibitors of PfFPPS/GGPPS have poor bioavailability and selectivity, related to their mode-of-inhibition mimicking the disphosphate substrate. Herein we identify the first non-bisphosphonate inhibitor of PfFPPS/GGPPS. We demonstrate that this inhibitor has a distinct mode-of-inhibition of PfFPPS/GGPPS that circumvents the inherent liabilities of bisphosphonates with improved drug properties and high specificity for the Plasmodium enzyme over human homologs. The identification of a new, selective small molecule binding site in this important antimalarial drug target will greatly improve its druggability. Our finding sets the stage for development of Plasmodium-specific FPPS/GGPPS inhibitors.
STAR Methods
Key Resource Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| P. falciparum Strains | ||
| W2 | Malaria Research and Reference Reagent Resource Center (MR4) | MRA-157 |
| Dd2attB | Malaria Research and Reference Reagent Resource Center (MR4) | MRA-843 |
| 019313R1 | This paper. | n/a |
| 019313R2 | This paper. | n/a |
| RL2-FPPS/GGPPS-GFP | This paper. | n/a |
| RL2-FPPS/GGPPS(S228T)-GFP | This paper. | n/a |
| CaM-FPPS/GGPPS-GFP | This paper. | n/a |
| CaM-FPPS/GGPPS(S228T)-GFP | This paper. | n/a |
| Bacterial Strains | ||
| Stellar Chemically Competent Cells | Clonetech | 636766 |
| One shot BL21 Star (DE3) Chemically Competent | ThermoFisher | C601003 |
| Chemicals | ||
| Malaria Box Compound library | Medicines for Malaria Venture | n/a |
| MMV019313 | ChemDiv | C498-0579 |
| ISOPENTENYL PYROPHOSPHATE, NH4+ SALT | Isoprenoids, ltd. | IPP001 |
| GERANYL PYROPHOSPHATE, NH4+ | Isoprenoids, ltd. | GPP002 |
| E,E-FARNESYL PYROPHOSPHATE, NH4+ | Isoprenoids, ltd. | FPP003 |
| Recombinant Proteins | ||
| PfFPPS/GGPPS | This paper. | n/a |
| PfFPPS/GGPPS(S228 T) | This paper. | n/a |
| HsFPPS | This paper. | n/a |
| HsGGPPS | This paper. | n/a |
| Recombinant plasmid DNA | ||
| pET28-PfFPPS/GGPPS | This paper. | n/a |
| pET28-PfFPPS/GGPPS(S228 T) | This paper. | n/a |
| pET28-HsGGPPS | This paper. | n/a |
| pET28-HsFPPS | This paper. | n/a |
| pLN(RL2)-PfFPPS/GGPPS-GFP | This paper. | n/a |
| pLN(RL2)-PfFPPS/GGPPS(S228 T)-GFP | This paper. | n/a |
| pLN(CaM)-PfFPPS/GGPPS-GFP | This paper. | n/a |
| pLN(CaM)-PfFPPS/GGPPS(S228 T)-GFP | This paper. | n/a |
| Antibodies | ||
| αGFP mouse monoclonal, JL-8 | Clonetech | 632381 |
| Critical Commercial Assays or Reagents | ||
| PPiLight Inorganic Pyrophosphate Assay | ThermoFisher (Lonza) | NC0665609 |
| Talon Metal Affinity Resin | ThermoFisher (Clonetech) | NC9484031 |
| Deposited Data | ||
| Whole genome sequencing data | This paper. NCBI SRA# SRP106479 | https://www.ncbi.nlm.nih.gov/sra |
| Software | ||
| BD Accuri C6 Software, version 1 | BD Biosciences | https://www.bdbiosciences.com/us/instruments/research/cell-analyzers/bd-accuri/m/1294932/features/software |
| Excel for Mac, version 15.33 | Microsoft Office | https://products.office.com/en-us/excel |
| Prism, version 7 | GraphPad | https://www.graphpad.com |
| Maestro, Small-molecule drug discovery suite, release 2017-1 | Schrödinger | https://www.schrodinger.com/suites/small-molecule-drug-discovery-suite |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ellen Yeh (ellenyeh@stanford.edu).
Experimental Model and Subject Details
P. falciparum in vitro cultures
Plasmodium falciparum W2 (MRA-157) and Dd2attB (MRA-843) were obtained from MR4. Parasites were grown in human erythrocytes (2% hematocrit, obtained from the Stanford Blood Center) in RPMI 1640 media supplemented with 0.25% Albumax II (GIBCO Life Technologies), 2 g/L sodium bicarbonate, 0.1 mM hypoxanthine, 25 mM HEPES (pH 7.4), and 50 μg/L gentamycin, at 37°C, 5% O2, and 5% CO2. For passage of drug-treated, IPP-rescued parasites, the media was supplemented with 5 μM drug and 200 μM IPP (Isoprenoids LC or NuChem). For comparison of growth between different treatment conditions, cultures were carried simultaneously and handled identically with respect to media changes and addition of blood cells
Method Details
Chemical handling
Malaria Box compounds were received as 10 mM DMSO stocks in 96-well plates and diluted three-fold manually in DMSO. Fosmidomycin was included in control wells. Two-fold serial dilutions of the 96-well plates were performed on Velocity11. Compound stocks stored in DMSO were diluted for growth assays. MMV019313 was purchased from ChemDiv Biotech.
qHTS screen for IPP chemical rescue
Growth assays were performed in 384-well clear bottom assay plates (E and K scientific, Santa Clara, CA). Drug (100–400 nL) was added directly to each well using PinTool (V&P Scientific) on a Sciclone ALH3000 (Caliper Sciences). Using the Titertek Multidrop 384, first 40 μL of growth media with and without 375 μM IPP was dispensed, followed by 10 μL ring-stage P. falciparum D10 parasites (parasitemia 0.8% in 10% hematocrit) into 384-well plates using the Titertek Multidrop 384. The final assay consisted of 50 μL ring-stage cultures at 0.8% parasitemia/2% hematocrit and drug concentrations from 0.01–26.7 μM ± 300 μM IPP. The plate was incubated at 37°C for 72 h. Parasites were lysed with 10 μL 5mM EDTA, 1.6% Triton-X, 20mM Tris-HCl and 0.16% Saponin containing 0.1% Sybr Green I (Invitrogen). The plates were then incubated at −80 °C for 20 min and thawed at room temperature overnight in the dark. Fluorescence was detected using Flexstation II-384. Compounds that showed IPP rescue of growth inhibition at 1 or more drug concentrations in the initial 384-well high-throughput screen were re-tested in a 96-well growth assay.
Growth inhibition assays to determine EC50 values
P. falciparum cultures (125 μL) were grown in 96-well plates containing serial dilution of drugs in triplicate. Media was supplemented with 200 μM IPP as indicated. Growth was initiated with ring-stage parasites at 1% parasitemia and 0.5% hematocrit. Plates were incubated for 72h. Growth was terminated by fixation with 1% formaldehyde and parasitized cells were stained with 50 nM YOYO-1 (Invitrogen). Parasitemia was determined by flow cytometry. Data were analyzed by BD C6 Accuri C-Sampler software and fitted to a sigmoidal dose-inhibition function (Inhibition=Bottom + (Top-Bottom)/(1+10^((LogIC50-[drug])*HillSlope) by GraphPad Prism.
P. falciparum mutagenesis and resistance selection
Late-stage parasites were purified using a SuperMACS II separator (Miltenyi Biotec) and incubated in complete medium with 8.3 – 2025 μM ethyl methanesulfonate (EMS, 6 concentrations total) for 2 hours. The concentrations of EMS used were selected by determining the EC50 in 72h parasite growth inhibition assays using fresh EMS as EMS solution is not stable. The highest concentration used for mutagenesis was equal to the EC50 in order to maximize the selection pressure. The mutagen was then serially diluted 1:3 to test lower concentrations that might give lower mutation rates and therefore a lower frequency of passenger mutations. Mutagenized parasites were washed and separated into wells of 10 mL total volume (approximately 10% parasitemia, 2 % HCT). MMV019313 drug selection was applied to one well for each mutagenesis condition at 600 nM (approximately EC75), while the other well was left untreated in order to serve as a control for whole genome sequencing. This concentration was chosen to maximize selection pressure for developing resistance in the IPP-rescuable target, while minimizing that for developing resistance in secondary targets. Parasites were fed daily for the first week and every 3 days thereafter. Each culture was split in half every 6 days in order to introduce fresh RBC. Drug pressure was maintained for 32 days, with no observable parasite growth. After 32 days of selection, half of the culture from each EMS condition was removed from drug pressure. In these cultures, parasites which showed resistance to MMV019313 by a standard drug assay were observable after 7 days at all EMS conditions used. The parasites treated with the two lowest concentrations of EMS were selected for whole genome sequencing.
Whole Genome Sequencing
Plasmodium falciparum strains were sequenced by Illumina sequencing as described previously (Straimer et al., 2012). Briefly, NEBNext DNA library reagents (NEB) and NEXTflex DNA barcode adapters (Bioo Scientific) were used to prepare PCR-free libraries (Kozarewa et al., 2009). Eight whole genome gDNA libraries were multiplexed and spiked with 8% PhiX control. Single-end sequencing was performed across two lanes on an Illumina HiSeq 2500 system. Data was analyzed using tools available in the Galaxy platform (Blankenberg et al., 2010; Giardine et al., 2005; Goecks et al., 2010). Sequencing reads were mapped against the P. falciparum 3D7v.10.0 reference genome using the Burrows-Wheeler Alignment tool (Li and Durbin, 2009). Sequencing data was visualized using Integrative Genomics Viewer (IGV) (Robinson et al., 2011; Thorvaldsdóttir et al., 2013). Variants were called using Freebayes (Garrison and Marth) and filtered for Quality >100 and Read Depth >30 using GATK tools (Van der Auwera et al., 2013). SnpEff was used to annotate the list of variants based on the P. falciparum 3D7v9.1 reference genome (Cingolani et al., 2012). Sequencing data have been deposited to the NCBI SRA (www.ncbi.nlm.nih.gov/sra;SRP106479).
P. falciparum Transfections
An E. coli codon optimized version of PfFPPS/GGPPS (PF3D7_1128400) was designed and synthesized by GeneWiz. Quick change mutagenesis was used to mutate GGPPS serine 228 to a threonine in the pUC vector provided by GeneWiz. These constructs were then moved into the pLN transfection plasmid designed for Bxb1 mycobacteriophage integrase system (Adjalley et al., 2010). The In-fusion cloning kit (Clontech) was used for all cloning. Restriction enzymes AvrII and BsiWI were used to linearize the pLN vector. GGPPS was designed to have a C-terminal GFP tag. All transgenes were driven with either the ribosomal L2 protein (RL2) promoter (PF3D7_1132700) or the calmodulin (CaM) promoter (PF3D7_1434200) as denoted.
Transfections were carried out as previously described (Spalding et al., 2010). Briefly, 400 μL fresh red blood cells were preloaded with 100 μg of both pINT, which carries the bacteriophage integrase, and pRL2, which carries the gene of interest and the blasticidin resistance cassette, using a BioRad Gene-Pulser Xcell Electroporator. Electroporation conditions were infinite resistance, 310 V, and 950 μF using a 2 mm cuvette. Preloaded red blood cells were combined with 2.5 mL ~20% schizont Dd2attB parasites and allowed to recover for 2 days before selection pressure was applied. Transfected parasites were selected with 2.5 μg/mL blasticidin are were detectable by thin smear within 15 days. Integration was confirmed by PCR and identity of the transgene was confirmed by sanger sequencing.
Immunoblots
Parasites expressing either ACPL-GFP or one of the FPPS/GGPPS constructs generated in this study were isolated by saponin lysis and resuspended in 1xNuPage LDS sample buffer (Invitrogen). Whole cell lysate was separated by SDS-PAGE using 4–12% Bis-Tris gels (Initrogen) and transferred to nitrocellulose using a Trans Turbo-blot (BioRad). Membranes were blocked with 3% BSA, probed with 1:5,000 monoclonal αGFP JL-8 (Clonetech) overnight, washed, then probed with 1:10,000 IRDye 680LT goat-anti-mouse. The Western was imaged using a Oddysey Imager (LiCor Biosciences).
Live microscopy
Infected red blood cells were treated with Hoescht to stain the nucleus. Single z-stack images were collected on an epifluorescence Nikon eclipse.
Quantative PCR
Quantitative PCR was performed as previously published (Yeh and Derisi, 2011). Briefly, parasites from 200 μL of culture were isolated by saponin lysis followed by PBS wash to remove extracellular DNA. DNA was purified using DNeasy Blood and Tissue kit (Qiagen). Primers were designed to target genes found on the apicoplast or nuclear genome: tufA (apicoplast) 5′-GATATTGATTCAGCTCCAGAAGAAA-3′ / 5′-ATATCCATTTGTGTGGCTCCTATAA-3′ and CHT1 (nuclear) 5′-TGTTTCCTTCAACCCCTTTT-3′ / 5′-TGTTTCCTTCAACCCCTTTT-3′. Reactions contained template DNA, 0.15 μM of each primer, and 0.75× LightCycler 480 SYBR Green I Master mix (Roche). PCR reactions were performed at 56°C primer annealing and 65°C template extension for 35 cycles on a Lightcycler 6500 (Roche). For each time point, the apicoplast: nuclear genome ratio of the fosmidomycin-treated positive control, chloramphenicol-treated negative control, or MMV019313-treated experiment were calculated relative to that of an untreated control collected at the same time.
Recombinant protein purification
Full length constructs of PfFPPS/GGPPS, HsFPPS, and HsGGPPS were cloned into pET28a with an n-terminal hexahistidine tag. The P. falciparum FPPS/GGPPS was codon optimized for expression in E. coli (GeneWiz). PfFPPS/GGPPS was mutagenized (S228T) using quick change mutagenesis. When expressed in E. coli, PfFPPS/GGPPS (wt and S228T) and HsFPPS were toxic. Cultures were supplemented with 0.4% glucose and grown to OD600 of 0.8–1 and induced with 0.5 mM IPTG. HsGGPPS was grown without supplementation to an OD600 of 0.8–1. All cultures were induced for 4 hours at 37 °C, then harvested. Cells were lysed in 20 mM HEPES pH 8.0, 150 mM NaCl, 2 mM MgCl2, and protease inhibitor cocktail using sonication. Cleared lysates were either mixed with Talon metal affinity resin (Clonetech) or purified over 5 ml HisTrap columns (GE Healthcare). His tagged protein was purified with a single step purification eluting with buffer with 300 mM imidazole. Proteins were dialyzed to remove imidazole and flash frozen.
In vitro FPPS/GGPPS assays
FPPS/GGPPS activity was measured by monitoring pyrophosphate release using the Lonza PPiLight kit under kinetic conditions. Drug and either 20 μg/ml PfFPPS/GGPPS, 40 μg/ml HsGGPPS, or 100 μg/ml HsFPPS protein were incubated for 30 min room temperature. The reaction was initiated by the addition of Lonza PPiLight kit reaction mix and substrates. Saturating substrate conditions were 100 μM GPP or FPP, and 200 μM IPP. Sub-saturation conditions were KM conditions as shown in Figure S4. Luciferase activity was monitored over time using a BioTek plate reader. Reaction rates were calculated from the linear portion of the raw luminescence over time curves (R2 >0.9). Data were fitted to a sigmoidal dose-inhibition function (Inhibition=Bottom + (Top-Bottom)/(1+10^((LogIC50-[drug])*HillSlope) by GraphPad Prism software.
MMV019313 ligand docking using Schrödinger Maestro
Using a solved PvFPPS/GGPPS structure (PDB: 3EZ3, zoledronate and IPP bound) as a receptor model protein preparation wizard was used to add back in missing hydrogens and side chains. All crystallographic waters were removed. Hydrogen bonds were calculated using Epik at pH 8 (±1). The protein structure was minimized using OPL3 force field. MMV019313 was docked using glide to receptor grids generated from the relevant crystallized small molecules.
Chemical synthesis
5-methyl-4-oxo-N-(4-(piperidin-1-yl)butyl)-4,5-dihydrothieno[3,2-c]quinoline-2-carboxamide (ZH-G)
To a stirring solution of 5 ( 0.0092 g, 35.5 μmol, 1 eq. ), EDAC·HCl (0.0071 g, 46.4 μmol, 1.3 eq.), and HOBt·H2O (0.0087 g, 45.4 μmol, 1.3 eq.), in DMF ( 2 mL) was added Et3N ( 0.0047 g, 46.6 μmol, 1.3 eq.) followed by 4-(piperidin-1-yl)butan-1-amine (0.0073 g, 47.0 μmol, 1.3 eq.). The resulting solution was stirred for 24 hours at RT, diluted in 20 mL of saturated sodium carbonate and extracted with EtOAc (3×30 mL). The organic layers were combined and washed with brine basified to pH 10.8 by NaOH (3 × 20 mL). The resulting organic layer was dried by sodium sulfate, and rotovapped to yield 14.4 mg of crude product. This was purified by trituration and filtration in EtOAc:Hex to yield the title compound ( 3.3 mg, 8.3 μmol, 23.4 % yield) 1H NMR (CD3OD, 500 MHz) δ: 1.50–1.57 (m, 2H), 1.65–1.71 (m, 8 H), 2.52–2.66 (m, 6H), 3.46 (t, 2H, J = 6.4 Hz), 3.82 (s, 3H), 7.40 (t, 1H, J = 7.4 Hz), 7.66–7.72 (m, 2H), 7.95 (d, 1H, J = 8.4 Hz), 8.15 (s, 3H)
5-methyl-4-oxo-N-(3-(piperidin-1-yl)propyl)-4,5-dihydrothieno[3,2-c]quinoline-2-carboxamide (ZH-I)
To a stirring solution of 5 ( 0.0092 g, 32.4 μmol, 1 eq. ), EDAC·HCl (0.0156 g, 81.4 μmol, 2.5 eq.), and HOBt·H2O (0.0091 g, 59.4 μmol, 1.8 eq.), in DMF ( 2 mL) was added Et3N ( 0.0080 g, 78.9 μmol, 2.4 eq.) followed by 3-(piperidin-1-yl)propan-1-amine (0.0073g, 51.3 μmol, 1.6 eq.). The resulting solution was stirred for 24 hours at RT, diluted in 20 mL of saturated sodium carbonate and extracted with EtOAc (3×30 mL). The organic layers were combined and washed with brine basified to pH 10.8 by NaOH (3 × 20 mL). The resulting organic layer was dried by sodium sulfate, and rotovapped to yield a crude product. This was purified by trituration and filtration in Et2O to yield the title compound ( 5.0 mg, 13.0 μmol, 40 % yield) 1H NMR (CD3OD, 500 MHz) δ: 1.56–1.65 (m, 2H), 1.77–1.84 (m, 4H), 1.85–1.92 (m, 2H), 2.50–2.69 (m, 6H), 3.63 (q, 2H, J = 5.0 Hz), 3.81 (s, 3H), 7.32 (t, 1 H, J = 7.7 Hz), 7.46 (d, 1H, J = 8.6 Hz), 7.60 (t, 1H, J = 7.7 Hz), 7.86 (d, 1H, J = 7.9 Hz), 8.14 (s, 1H), 8.97 (s, br, 1H)
N-(4-(azepan-1-yl)butyl)-5-methyl-4-oxo-4,5-dihydrothieno[3,2-c]quinoline-2-carboxamide (ZH-K)
To a stirring solution of 5 ( 0.0118 g, 45.5 μmol, 1.1 eq. ), EDAC·HCl (0.0081 g, 42.2 μmol, 1 eq.), and HOBt·H2O (0.0289 g, 189 μmol, 4.5 eq.), in DMF ( 2 mL) was added Et3N ( 0.0047 g, 46.6 μmol, 1.1 eq.) followed by 4-(azepan-1-yl)butan-1-amine (0.0075 g, 44.0 μmol, 1 eq.). The resulting solution was stirred for 18 hours at RT, diluted in 20 mL of saturated sodium carbonate and extracted with EtOAc (3×30 mL). The organic layers were combined and washed with brine basified to pH 10.8 by NaOH (3 × 20 mL). The resulting organic layer was dried by sodium sulfate, and rotovapped to yield a crude product. This was purified by trituration and filtration in Et2O to yield the title compound ( 7.0 mg, 17.0 μmol, 40 % yield) as white crystals. 1H NMR (CDCl3, 500 MHz) δ: 1.57–1.63 (m, 4H), 1.64–1.74 (m, 8H), 2.60 (t, 2H, J = 6.7 Hz), 2.75 (t, 4H, J = 5.0 Hz), 3.49 (q, 2H, J = 5.9 Hz), 3.77 (s, 3H), 7.28 (t, 1 H, J = 7.8 Hz), 7.42 (m, 2H), 7.57 (t, 1H, J = 7.8 Hz), 7.81 (d, 1H, J = 7.8 Hz), 8.07 (s, 1H)
Quantification and Statistical Analysis
Results are shown as the mean with error bars representing standard deviation. All assays were completed at least two independent times. Data were analyzed in GraphPad Prism using the appropriate method as noted in each experimental section.
Data Software and Availability
Raw whole genome sequencing files have been deposited at the NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra) under the accession number SRP106479. Results of the qHTS and the analysis of the whole genome sequencing are presented in the supplemental material.
Supplementary Material
Table S1 (related to Figure 1). qHTS IPP chemical rescue results for the Malaria Box.
Table S2 (related to Figure 2 and Table 1). Comparison of identified SNV between MMV019313-resistant populations and mutagenized controls.
Highlights.
A new non-bisphosphonate compound inhibits PfFPPS/GGPPS, a validated drug target.
Unlike bisphosphonates, MMV019313 is specific for PfFPPS/GGPPS over human homologs.
We reveal a novel mode-of-inhibition of this high-priority antimalarial drug target.
This study sets the stage for development of specific PfFPPS/GGPPS inhibitors.
Acknowledgments
We are grateful to Medicines for Malaria Ventures (MMV) for providing the Malaria Box compounds and making this valuable library freely available, as well as GlaxoSmithKline for their screening efforts that first identified MMV019313 (TCMDC-123889). We would like to thank Dr. Susmitha Suresh for performing drug screens, Dr. Felice Kelly for advice on chemical mutagenesis, Dr. James Dunford (University of Oxford) for advice in developing the in vitro enzyme activity assays, and Dr. Wei Zhu and Professor Eric Oldfield (University of Illinois, Urbana-Champaign) for providing BPH-703. Funding support for this project was generously provided by the Stanford Consortium for Innovation, Design, Evaluation and Action (C-IDEA), NIH 1K08AI097239 (EY), NIH 1DP5OD012119 (EY), the Burroughs Wellcome Fund Career Award for Medical Scientists (EY), the Burroughs Wellcome Fund Investigators in Pathogenesis of Infectious Disease (PATH) Award (ML), and the Stanford School of Medicine Dean’s Postdoctoral Fellowship (JEG). The authors declare no conflict of interest.
Footnotes
Author Contributions
JEG, ZH, LO, and EY conducted experiments. JEG, ZH, LO, ML, and EY were responsible for data analysis. JEG and EY designed experiments and wrote the paper. ML and EY supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjalley SH, Lee MCS, Fidock DA. A method for rapid genetic integration into Plasmodium falciparum utilizing mycobacteriophage Bxb1 integrase. Methods Mol Biol. 2010;634:87–100. doi: 10.1007/978-1-60761-652-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman EL, Painter HJ, Samra J, Carrasquilla M, Llinás M. Metabolomic Profiling of the Malaria Box Reveals Antimalarial Target Pathways. Antimicrob Agents Chemother. 2016;60:6635–6649. doi: 10.1128/AAC.01224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg-Johnson K, Hari SB, Ganesan SM, Lorenzi HA, Sauer RT, Niles JC, Yeh E. Small molecule inhibition of apicomplexan FtsH1 disrupts plastid biogenesis in human pathogens. Elife. 2017;6:4525. doi: 10.7554/eLife.29865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz JD, Wernimont AK, Dunford JE, Schapira M, Dong A, Zhao Y, Lew J, Russell RGG, Ebetino FH, Oppermann U, et al. Molecular characterization of a novel geranylgeranyl pyrophosphate synthase from Plasmodium parasites. J Biol Chem. 2011;286:3315–3322. doi: 10.1074/jbc.M109.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg D, Kuster Von G, Coraor N, Ananda G, Lazarus R, Mangan M, Nekrutenko A, Taylor J. Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol. 2010;Chapter 19(Unit19.10.1–Unit19.10.21) doi: 10.1002/0471142727.mb1910s89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JD, Merino EF, Brooks CF, Striepen B, Carlier PR, Cassera MB. Antiapicoplast and gametocytocidal screening to identify the mechanisms of action of compounds within the malaria box. Antimicrob Agents Chemother. 2014;58:811–819. doi: 10.1128/AAC.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti D, Da Silva T, Barger J, Paquette S, Patel H, Patterson S, Allen CM. Protein farnesyltransferase and protein prenylation in Plasmodium falciparum. J Biol Chem. 2002 doi: 10.1074/jbc.M202860200. [DOI] [PubMed] [Google Scholar]
- Chen SH, Lin SW, Lin SR, Liang PH, Yang JM. Moiety-linkage map reveals selective nonbisphosphonate inhibitors of human geranylgeranyl diphosphate synthase. J Chem Inf Model. 2013;53:2299–2311. doi: 10.1021/ci400227r. [DOI] [PubMed] [Google Scholar]
- Cingolani P, Patel VM, Coon M, Nguyen T, Land SJ, Ruden DM, Lu X. Using Drosophila melanogaster as a Model for Genotoxic Chemical Mutational Studies with a New Program, SnpSift. Front Genet. 2012;3:35. doi: 10.3389/fgene.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creek DJ, Chua HH, Cobbold SA, Nijagal B, MacRae JI, Dickerman BK, Gilson PR, Ralph SA, McConville MJ. Metabolomics-Based Screening of the Malaria Box Reveals both Novel and Established Mechanisms of Action. Antimicrob Agents Chemother. 2016;60:6650–6663. doi: 10.1128/AAC.01226-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers SCLM, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44:551–570. doi: 10.2165/00003088-200544060-00001. [DOI] [PubMed] [Google Scholar]
- Crowther GJ, Napuli AJ, Gilligan JH, Gagaring K, Borboa R, Francek C, Chen Z, Dagostino EF, Stockmyer JB, Wang Y, et al. Identification of inhibitors for putative malaria drug targets among novel antimalarial compounds. Mol Biochem Parasitol. 2011;175:21–29. doi: 10.1016/j.molbiopara.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol Lett. 2002;207:13–20. doi: 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- DeRisi JL. UCSF DeRisi Lab MMV Box Apicoplast Screening (EMBL-EBI) 2014. [Google Scholar]
- Dunford JE, Kwaasi AA, Rogers MJ, Barnett BL, Ebetino FH, Russell RGG, Oppermann U, Kavanagh KL. Structure-activity relationships among the nitrogen containing bisphosphonates in clinical use and other analogues: time-dependent inhibition of human farnesyl pyrophosphate synthase. J Med Chem. 2008;51:2187–2195. doi: 10.1021/jm7015733. [DOI] [PubMed] [Google Scholar]
- Eastman RT, White J, Hucke O, Bauer K, Yokoyama K, Nallan L, Chakrabarti D, Verlinde CLMJ, Gelb MH, Rathod PK, et al. Resistance to a protein farnesyltransferase inhibitor in Plasmodium falciparum. J Biol Chem. 2005;280:13554–13559. doi: 10.1074/jbc.M413556200. [DOI] [PubMed] [Google Scholar]
- Eastman RT, White J, Hucke O, Yokoyama K, Verlinde CLMJ, Hast MA, Beese LS, Gelb MH, Rathod PK, Van Voorhis WC. Resistance mutations at the lipid substrate binding site of Plasmodium falciparum protein farnesyltransferase. Mol Biochem Parasitol. 2007;152:66–71. doi: 10.1016/j.molbiopara.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JF, Lell B, Agnandji ST, Obiang RM, Bassat Q, Kremsner PG, Mordmüller B, Grobusch MP. Fosmidomycin as an antimalarial drug: a meta-analysis of clinical trials. Future Microbiol. 2015;10:1375–1390. doi: 10.2217/FMB.15.60. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, et al. Glide: a new approach for rapid, accurate docking and scoring. 1 Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Gabriel HB, Silva MF, Kimura EA, Wunderlich G, Katzin AM, Azevedo MF. Squalestatin is an inhibitor of carotenoid biosynthesis in Plasmodium falciparum. Antimicrob Agents Chemother. 2015;59:3180–3188. doi: 10.1128/AAC.04500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DVS, Kumar V, Hasan S, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. HttpsarxivorgabsV. 2012 [Google Scholar]
- Ghosh S, Chan JMW, Lea CR, Meints GA, Lewis JC, Tovian ZS, Flessner RM, Loftus TC, Bruchhaus I, Kendrick H, et al. Effects of bisphosphonates on the growth of Entamoeba histolytica and Plasmodium species in vitro and in vivo. J Med Chem. 2004;47:175–187. doi: 10.1021/jm030084x. [DOI] [PubMed] [Google Scholar]
- Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J, et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 2005;15:1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselberg JE, Zhang L, Elias JE, Yeh E. The Prenylated Proteome of Plasmodium falciparum Reveals Pathogen-specific Prenylation Activity and Drug Mechanism-of-action. Mol Cell Proteomics. 2017;16:S54–S64. doi: 10.1074/mcp.M116.064550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J, Nekrutenko A, Taylor J, Galaxy Team. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, et al. Chemical genetics of Plasmodium falciparum. Nature. 2010;465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: a new approach for rapid, accurate docking and scoring. 2 Enrichment factors in database screening. J Med Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- Jahnke W, Rondeau JM, Cotesta S, Marzinzik A, Pellé X, Geiser M, Strauss A, Götte M, Bitsch F, Hemmig R, et al. Allosteric non-bisphosphonate FPPS inhibitors identified by fragment-based discovery. Nat Chem Biol. 2010;6:660–666. doi: 10.1038/nchembio.421. [DOI] [PubMed] [Google Scholar]
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler HK, et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- Jordão FM, Gabriel HB, Alves JMP, Angeli CB, Bifano TD, Breda A, de Azevedo MF, Basso LA, Wunderlich G, Kimura EA, et al. Cloning and characterization of bifunctional enzyme farnesyl diphosphate/geranylgeranyl diphosphate synthase from Plasmodium falciparum. Malar J. 2013;12:184. doi: 10.1186/1475-2875-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordão FM, Saito AY, Miguel DC, de Jesus Peres V, Kimura EA, Katzin AM. In vitro and in vivo antiplasmodial activities of risedronate and its interference with protein prenylation in Plasmodium falciparum. Antimicrob Agents Chemother. 2011;55:2026–2031. doi: 10.1128/AAC.01820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RGG, Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USa. 2006;103:7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz J. SciBX. 2010. Going to bis-school. [Google Scholar]
- Kozarewa I, Ning Z, Quail MA, Sanders MJ, Berriman M, Turner DJ. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat Methods. 2009;6:291–295. doi: 10.1038/nmeth.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lell B, Ruangweerayut R, Wiesner J, Missinou MA, Schindler A, Baranek T, Hintz M, Hutchinson D, Jomaa H, Kremsner PG. Fosmidomycin, a novel chemotherapeutic agent for malaria. Antimicrob Agents Chemother. 2003;47:735–738. doi: 10.1128/AAC.47.2.735-738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu W, Ge H, Gao J, He Q, Su L, Xu J, Gu LQ, Huang ZS, Li D. Syntheses and characterization of non-bisphosphonate quinoline derivatives as new FPPS inhibitors. Biochim Biophys Acta. 2014;1840:1051–1062. doi: 10.1016/j.bbagen.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- Marzinzik AL, Amstutz R, Bold G, Bourgier E, Cotesta S, Glickman JF, Götte M, Henry C, Lehmann S, Hartwieg JCD, et al. Discovery of Novel Allosteric Non-Bisphosphonate Inhibitors of Farnesyl Pyrophosphate Synthase by Integrated Lead Finding. ChemMedChem. 2015;10:1884–1891. doi: 10.1002/cmdc.201500338. [DOI] [PubMed] [Google Scholar]
- Martin MB, Grimley JS, Lewis JC, Heath HT, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, et al. Bisphosphonates Inhibit the Growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum:3 A Potential Route to Chemotherapy. J Med Chem. 2001;44:909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- Nallan L, Bauer KD, Bendale P, Rivas K, Yokoyama K, Hornéy CP, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, et al. Protein farnesyltransferase inhibitors exhibit potent antimalarial activity. J Med Chem. 2005;48:3704–3713. doi: 10.1021/jm0491039. [DOI] [PubMed] [Google Scholar]
- Nkrumah LJ, Muhle RA, Moura PA, Ghosh P, Hatfull GF, Jacobs WR, Fidock DA. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3:615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No JH, de Macedo Dossin F, Zhang Y, Liu YL, Zhu W, Feng X, Yoo JA, Lee E, Wang K, Hui R, et al. Lipophilic analogs of zoledronate and risedronate inhibit Plasmodium geranylgeranyl diphosphate synthase (GGPPS) and exhibit potent antimalarial activity. Proc Natl Acad Sci USa. 2012;109:4058–4063. doi: 10.1073/pnas.1118215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto TD, Wilinski D, Assefa S, Keane TM, Sarry LR, Böhme U, Lemieux J, Barrell B, Pain A, Berriman M, et al. New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-Seq. Mol Microbiol. 2010;76:12–24. doi: 10.1111/j.1365-2958.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zielinski M, Magder A, Tsantrizos YS, Berghuis AM. Human farnesyl pyrophosphate synthase is allosterically inhibited by its own product. Nat Commun. 2017;8:14132. doi: 10.1038/ncomms14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul AS, Moreira CK, Elsworth B, Allred DR, Duraisingh MT. Extensive Shared Chemosensitivity between Malaria and Babesiosis Blood-Stage Parasites. Antimicrob Agents Chemother. 2016;60:5059–5063. doi: 10.1128/AAC.00928-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, Lehmann S, Ramage P, Rieffel S, Strauss A, et al. Structural Basis for the Exceptional in vivo Efficacy of Bisphosphonate Drugs. ChemMedChem. 2006;1:267–273. doi: 10.1002/cmdc.200500059. [DOI] [PubMed] [Google Scholar]
- Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, et al. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Zhang Y, No JH, Docampo R, Nussenzweig V, Oldfield E. Lipophilic bisphosphonates are potent inhibitors of Plasmodium liver-stage growth. Antimicrob Agents Chemother. 2010;54:2987–2993. doi: 10.1128/AAC.00198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia L, Varenna M, Casari S. Pharmacokinetic profile of bisphosphonates in the treatment of metabolic bone disorders. Clin Cases Miner Bone Metab. 2007;4:30–36. [PMC free article] [PubMed] [Google Scholar]
- Spalding MD, Allary M, Gallagher JR, Prigge ST. Validation of a modified method for Bxb1 mycobacteriophage integrase-mediated recombination in Plasmodium falciparum by localization of the H-protein of the glycine cleavage complex to the mitochondrion. Mol Biochem Parasitol. 2010;172:156–160. doi: 10.1016/j.molbiopara.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg T, Burrows JN, Kowalczyk P, McDonald S, Wells TNC, Willis P. The open access malaria box: a drug discovery catalyst for neglected diseases. PLoS ONE. 2013;8:e62906. doi: 10.1371/journal.pone.0062906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straimer J, Lee MCS, Lee AH, Zeitler B, Williams AE, Pearl JR, Zhang L, Rebar EJ, Gregory PD, Llinás M, et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nat Methods. 2012;9:993–998. doi: 10.1038/nmeth.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suazo KF, Schaber C, Palsuledesai CC, Odom John AR, Distefano MD. Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci Rep. 2016;6:38615. doi: 10.1038/srep38615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KB. Enzyme Kinetics and Mechanisms. Dordrecht: Kluwer Academic Publishers; 2004. Slow and Tight Inhibition; pp. 122–146. [Google Scholar]
- Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinformatics. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonhosolo R, D’Alexandri FL, de Rosso VV, Gazarini ML, Matsumura MY, Peres VJ, Merino EF, Carlton JM, Wunderlich G, Mercadante AZ, et al. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2009;284:9974–9985. doi: 10.1074/jbc.M807464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonhosolo R, D’Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN, Peres VJ, Kimura EA, Katzin AM. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem J. 2005;392:117–126. doi: 10.1042/BJ20050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T, Ishibashi K, Terakawa M, Nishiyama M, Itoh N, Noguchi H. Pharmacokinetics and metabolism of fosmidomycin, a new phosphonic acid, in rats and dogs. Eur J Drug Metab Pharmacokinet. 1982;7:59–64. doi: 10.1007/BF03189544. [DOI] [PubMed] [Google Scholar]
- Ullah I, Sharma R, Biagini GA, Horrocks P. A validated bioluminescence-based assay for the rapid determination of the initial rate of kill for discovery antimalarials. J Antimicrob Chemother. 2017;72:717–726. doi: 10.1093/jac/dkw449. [DOI] [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11, 10.1–10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis WC, Adams JH, Adelfio R, Ahyong V, Akabas MH, Alano P, Alday A, Alemán Resto Y, Alsibaee A, Alzualde A, et al. Open Source Drug Discovery with the Malaria Box Compound Collection for Neglected Diseases and Beyond. PLoS Pathog. 2016;12:e1005763. doi: 10.1371/journal.ppat.1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis WC, Rivas KL, Bendale P, Nallan L, Hornéy C, Barrett LK, Bauer KD, Smart BP, Ankala S, Hucke O, et al. Efficacy, pharmacokinetics, and metabolism of tetrahydroquinoline inhibitors of Plasmodium falciparum protein farnesyltransferase. Antimicrob Agents Chemother. 2007;51:3659–3671. doi: 10.1128/AAC.00246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Herrera Z, Ebert D, Baska K, Cho SH, Derisi JL, Yeh E. A chemical rescue screen identifies a Plasmodium falciparum apicoplast inhibitor targeting MEP isoprenoid precursor biosynthesis. Antimicrob Agents Chemother. 2015;59:356–364. doi: 10.1128/AAC.03342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Derisi JL. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 2011;9:e1001138. doi: 10.1371/journal.pbio.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Mizuguchi M, Ostermann A, Kusaka K, Niimura N, Schrader TE, Tanaka I. Protonation State and Hydration of Bisphosphonate Bound to Farnesyl Pyrophosphate Synthase. J Med Chem. 2015;58:7549–7556. doi: 10.1021/acs.jmedchem.5b01147. [DOI] [PubMed] [Google Scholar]
- Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry. 2011;50:3570–3577. doi: 10.1021/bi200113y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cao R, Yin F, Hudock MP, Guo RT, Krysiak K, Mukherjee S, Gao YG, Robinson H, Song Y, et al. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J Am Chem Soc. 2009;131:5153–5162. doi: 10.1021/ja808285e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu W, Liu YL, Wang H, Wang K, Li K, No JH, Ayong L, Gulati A, Pang R, et al. Chemo-Immunotherapeutic Anti-Malarials Targeting Isoprenoid Biosynthesis. ACS Med Chem Lett. 2013;4:423–427. doi: 10.1021/ml4000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 (related to Figure 1). qHTS IPP chemical rescue results for the Malaria Box.
Table S2 (related to Figure 2 and Table 1). Comparison of identified SNV between MMV019313-resistant populations and mutagenized controls.