Abstract
Influenza virus infections result in considerable morbidity and mortality both in the temperate and tropical world. Influenza surveillance over multiple years is important to determine the impact and epidemiology of influenza and to develop a national vaccine policy, especially in countries developing influenza vaccine manufacturing capacity, such as Vietnam. We conducted surveillance of influenza and influenza-like illness in Vietnam through the National Influenza Surveillance System during 2006–2010. At 15 sentinel sites, the first two patients presenting each weekday with influenza-like illness (ILI), defined as fever and cough and/or sore throat with illness onset within 3 days, were enrolled and throat specimens were collected and tested for influenza virus type and influenza A subtype by RT-PCR. De-identified demographic and provider reported subsequent hospitalization information was collected on each patient. Each site also collected information on the total number of patients with influenza-like illness evaluated per week. Of 29,804 enrolled patients presenting with influenza-like illness, 6516 (22%) were influenza positive. Of enrolled patients, 2737 (9.3%) were reported as subsequently hospitalized; of the 2737, 527 (19%) were influenza positive. Across all age groups with ILI, school-aged children had the highest percent of influenza infection (29%) and the highest percent of subsequent hospitalizations associated with influenza infection (28%). Influenza viruses co-circulated throughout most years in Vietnam during 2006–2010 and often reached peak levels multiple times during a year, when >20% of tests were influenza positive. Influenza is an important cause of all influenza-like illness and provider reported subsequent hospitalization among outpatients in Vietnam, especially among school-aged children. These findings may have important implications for influenza vaccine policy in Vietnam.
Keywords: Influenza, Southeast Asia, Influenza A (H1N1) subtype, Influenza A (H3N2) subtype, Influenza B, Pandemic influenza, Vietnam
1. Introduction
Influenza virus infection is associated with considerable morbidity and mortality globally, including in Southeast Asian nations like Vietnam [1–5]. Moreover, it has been proposed that Southeast Asia may be an important region for the evolution of influenza seasonal influenza A (H3N2) virus strains that subsequently spread globally [6,7]. Thus, influenza virologic surveillance in Southeast Asia is especially important in detecting virus strains that may need to be included in annual influenza vaccine development [6,7]. In countries such as Vietnam, national influenza vaccine production capacity is currently being developed, so that large scale national vaccination programs may soon become more feasible in the region [8]. Epidemiological, virological, and clinical data from influenza surveillance in these countries can provide important information for policy makers on the burden of seasonal influenza and help develop prevention and control policies throughout the region. A preliminary report of the first two years of data collection of influenza surveillance in Vietnam’s National Influenza Surveillance System (NISS), from 2006 to 2007, indicated that 19% of outpatients tested positive for influenza [9]. This report summarizes five years (2006–2010) of influenza surveillance among patients with influenza-like illness (ILI) seeking care at outpatient clinics in Vietnam, and describes the burden of influenza virus infection among those patients who were reported as subsequently hospitalized.
2. Methods and materials
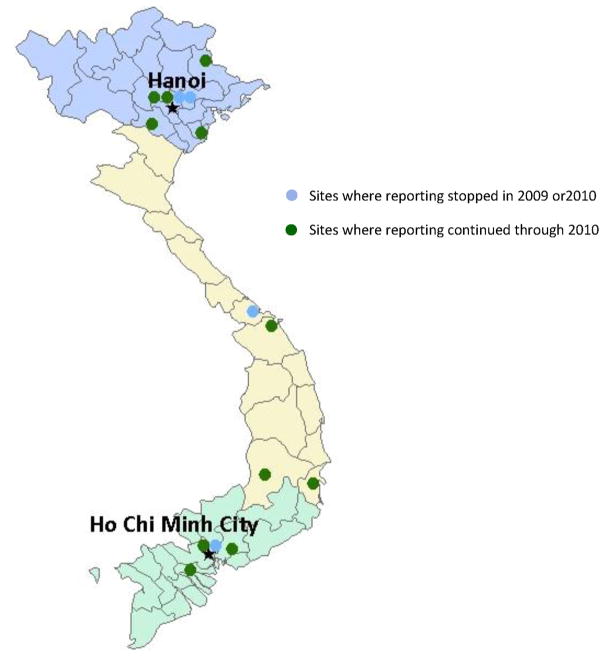
In 2006, Vietnam initiated national influenza surveillance with assistance from the World Health Organization (WHO) and the United States Centers for Disease Control and Prevention (CDC) through the creation of the National Influenza Surveillance System (NISS), as previously described [9]. NISS consists of a network of sentinel sites located in the four major regions of Vietnam. NISS is linked to regional public health laboratories, and administered by the National Institute of Hygiene and Epidemiology of the Ministry of Health [9] (Fig. 1). Sentinel sites include adult and pediatric outpatient clinics at the central, provincial, district hospitals, and the smaller stand-alone or polyclinic levels.
Fig. 1.
Fifteen initial and eleven currently operating sentinel sites, National Influenza Surveillance System, January 1, 2006–December 31, 2010.
Data collection was officially implemented at seven sentinel sites on January 1, 2006 and was expanded to 15 sites by July 2007, to include outpatient clinics at four central hospitals, two provincial hospitals, seven district hospitals, and two polyclinics (Fig. 1). Of the original 15 sites, seven sites were located in the Northern Region, four in the Southern Region, three in the Central Coastal, and one in the Central Highland Region. In 2009 and 2010, four surveillances sites discontinued data collection due to reduced funding: two central hospitals in the Northern Region, one district hospital in the Central Coastal Region, and one polyclinic in the Southern Region. Because of the relatively small numbers of patients at the Central Coastal and Central Highland Regions, for the purposes of these analyses, the two regions have been combined into the Central Regions.
At each participating surveillance sentinel site, the first two patients presenting to the designated outpatient clinic with ILI (defined as a measured temperature ≥38 °C and cough and/or sore throat) with onset of illness within 3 days were enrolled, and a throat swab was collected and placed into viral transport media. Specimens were stored at each surveillance site at 4 °C and sent twice a week to a regional laboratory, where specimens were stored at −70 °C until tested for influenza viruses each week. Specimens were tested for influenza A and B; influenza A viruses were further subtyped for H1, H3 and H5 by reverse transcription polymerase chain reaction (RT-PCR) using primers, probes, and reagents recommended by the CDC and the WHO, as previously described [9]. In May 2009, NISS also began testing for 2009 pandemic influenza A (H1N1) (2009 H1N1) using CDC recommended primers, probes, and techniques [10,11].
Demographic, clinical, and epidemiologic data were collected on every enrolled ILI patient, including information on provider reported subsequent clinical disposition; that is, whether the patient had a hospital admission – either referred or admission on site. This question did not specify either the time period for follow-up on subsequent hospitalization status or if the patient was simply referred for admission or actually admitted by the provider. In the Vietnamese government’s organized health care system, central hospitals are designed to evaluate and treat patients requiring the highest level of medical care and possible inpatient service, while provincial hospitals, district hospitals and polyclinics, are designed to treat patients requiring progressively less intense medical care (Nguyen H. Tuan, personal communication). Laboratory and epidemiologic data collected through NISS were entered into a Microsoft Access 2003 database and analyzed. Summary data were reported monthly to each site and to the Ministry of Health in Vietnam.
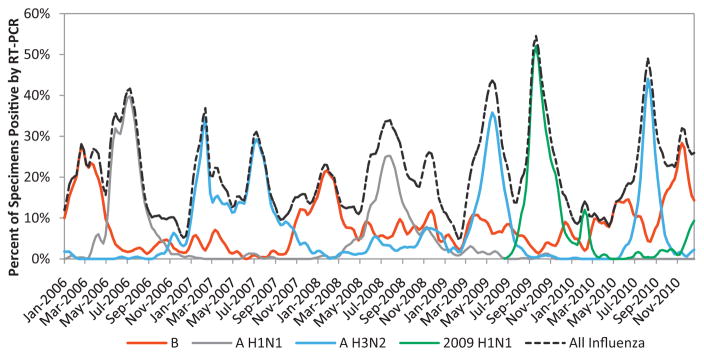
For this report, influenza activity by influenza virus type and influenza A virus subtype was determined from the weekly percent of enrolled ILI patients that tested positive among all tested. For descriptive purposes, we defined periods of peak influenza activity as the weeks where >20% of specimens tested positive for any influenza virus. Chi-square tests of proportions were used to assess statistical differences across groups when appropriate. For illustrative purposes, local polynomial linear regression was used in Fig. 2 to smooth the lines for the percent of influenza positive specimens by virus type and influenza A virus subtype. Descriptive analyses were performed using Stata 11 (College Station, TX).
Fig. 2.
Weekly percent of specimens testing positive for influenza by reverse transcription polymerase-chain reaction (RT-PCR), by influenza type and subtype—National Influenza Surveillance System, Vietnam, January 1, 2006–December 31, 2010. Percent of influenza positive specimens by type and subtype has been smoothed for illustrative purposes only in this figure. Smoothing was done through local polynomial linear regression of percent positive by week (.lpoly bw(1) n(200)).
3. Results
3.1. Influenza-like illness and influenza virus infection in Vietnam
During January 1, 2006 through December 31, 2010, 326,752 persons presented for care for ILI at outpatient clinics participating in NISS; 29,804 (9.1%) were enrolled and tested for influenza. Of enrolled patients, 14,556 (49%) were female and the overall median age was 12 years (range: 1 month–94 years); 18,306 (61%) were <15 years of age (Table 1). Of enrolled patients, 13,728 (46%) patients presented to district hospitals, and 12,991 (44%) presented to surveillance sites in the Northern Region.
Table 1.
Influenza testing results of patients presenting with influenza-like illnessa by gender, region, type of medical facility, and age group—National Influenza Surveillance System, Vietnam, January 1, 2006–December 31, 2010.
| No. of presenting with ILI tested for influenza | No. of influenza positive by RT-PCR (%) | No. of hospitalized (%)b | No. of influenza positive by RT-PCR of hospitalized (% of hospitalized) | |
|---|---|---|---|---|
| Gender | ||||
| Female | 14,556 | 3114 (21) | 1485 (9.7) | 281 (19) |
| Male | 15,248 | 3402 (22) | 1252 (8.6) | 246 (20) |
| Type of medical facility | ||||
| Central hospital | 7483 | 1753 (23) | 1375(18) | 206 (15) |
| Provincial hospital | 5020 | 1009 (20) | 55 (1.1) | 12 (22) |
| District hospital | 13,728 | 3000 (22) | 1296 (10) | 307 (24) |
| Polyclinic | 3573 | 754 (21) | 11 (0.3) | 2 (18) |
| Age groups | ||||
| 0–6 mos. | 314 | 32 (10) | 63 (20) | 3 (4.8) |
| 6 mos.–2 years | 3610 | 520 (14) | 528(15) | 53 (10) |
| 2–4 years | 6212 | 1247 (20) | 646 (10) | 108 (17) |
| 5–14 years | 7900 | 2330 (29) | 679 (8.7) | 188 (28) |
| 15–24 years | 3684 | 867 (24) | 280 (7.7) | 61 (22) |
| 25–64 years | 7195 | 1404 (20) | 479 (6.7) | 100 (21) |
| >64 years | 889 | 116 (13) | 62 (7.0) | 14 (23) |
| Regions | ||||
| Northern | 12,991 | 2733 (21) | 1560 (12) | 350 (22) |
| Central | 8760 | 2037 (23) | 189 (2.2) | 72 (38) |
| Southern | 8053 | 1746 (22) | 988 (12) | 105 (11) |
| Total | 29,804 | 6516 (22) | 2737 (9.3) | 527 (19) |
ILI = influenza-like illness: fever (≥38 °C) with onset of either cough or sore throat ≤3 days prior to presentation.
Information on hospitalization was not reported for 219 patients.
For the entire study period, 6516 (22%) of enrolled ILI patients tested positive for influenza viruses. A similar percentage of males (22%) and females (21%) tested positive for influenza viruses. The percentage of ILI patients that tested positive for influenza viruses was similar across the types of medical facilities at which patients presented, including central, district, and provincial hospitals, and polyclinics (20–23%), and across regions (21–23%). School-aged children aged 5–14 years were most likely to test positive for influenza viruses (29%), whereas the lowest proportion of influenza viruses were identified in children aged <6 months (10%) and persons aged >64 years (13%) (Table 1).
3.2. Subsequent hospitalization and influenza illness
Of ILI patients initially presenting to outpatient clinics in NISS, providers reported that 2737 (9.3%) were subsequently hospitalized. After adjustment for the type of medical facility where a sentinel site was designated, since more patients presented to central or district hospitals than provincial hospitals or polyclinics, we estimated that 6.6% of ILI patients who presented to outpatient clinics were subsequently hospitalized. Males had a marginally higher reported level of subsequent hospitalization than females (9.7% vs. 8.6%). Children <6 months of age were reported by providers as subsequently hospitalized more commonly than adults 25–64 years of age; 20% of children <6 months of age were reported as hospitalized versus 6.7% of adults aged 25–64 years (P < 0.001).
Of ILI patients who presented to outpatient clinics, 527 (19%) were influenza positive; when adjusting for the type of presenting medical facility, 21% of reported subsequently admitted ILI patients were influenza positive. The highest (28%) proportion of influenza infections among reported subsequently hospitalized patients was observed among school-aged children (5–14 years), followed by patients >64 years of age (23%), and lowest among children <6 months of age (4.8%).
Among enrolled influenza positive patients, there was no statistical difference in the percent of reported subsequent hospitalization across age groups (range 7–12%; P = 0.19). Provider reported subsequent hospitalization was highest (13%) among patients infected with seasonal, non-pandemic influenza A (H1N1) compared to influenza A (H3N2), influenza B, and 2009 H1N1 (6.4%, 7.4%, and 7.6%, respectively) (P < 0.001).
Differences were noted across regions with regard to ILI patients with a provider reported subsequent hospitalization, and for the proportion of influenza virus infections among subsequent hospitalizations. Providers in the Central Regions reported the lowest (2.2) percent of patients subsequently hospitalized, but the highest proportion of subsequent hospitalizations specifically associated with influenza virus infection (38%) (Table 1).
3.3. Influenza subtype by year, age group, and region
From 2006 to 2010, the annual percent of influenza positive specimens ranged from 18% in 2007 to 26% in 2009 (Table 2). During most years of surveillance, only one influenza A subtype tended to dominate circulation over the other (seasonal influenza A (H1N1) vs. H3N2), except in 2009, when influenza A (H3N2) circulated widely prior to the emergence and eventual dominance of 2009 H1N1. In 2006 and 2008, seasonal influenza A (H1N1) dominated circulation, whereas influenza A (H3N2) circulated only at low levels. On the other hand, in 2007 and 2010, influenza A (H3N2) dominated circulation, whereas seasonal influenza A (H1N1) circulated at low levels. Influenza B viruses circulated widely every year, regardless of influenza A subtype circulation, with peaks in 2006, 2008, and 2010. Testing for 2009 H1N1 began in May 2009; 9.4% of all sampled patients were 2009 H1N1 positive that year. In 2010, 2009 H1N1 continued to circulate, but at much lower levels, and influenza B and influenza A (H3N2) virus dominated circulation, while the seasonal influenza A (H1N1) virus was not detected since 2009 (Table 2).
Table 2.
Influenza type and subtype RT-PCR testing results for patients presenting with influenza-like illness by year and age group—National Influenza Surveillance System, Vietnam, January 1, 2006–December 31, 2010.
| ILI a patients tested for influenza (%)b | Influenza, positive (%)b | A/H1 positive (%) | A/H3 positive (%) | B positive (%) | 2009 H1N1 positive (%) | |
|---|---|---|---|---|---|---|
| Total | 29,804 | 6516 (22) | 1298 (4.4) | 2249 (7.6) | 2163 (7.3) | 785 (2.6) |
| Year | ||||||
| 2006 | 4624 | 947 (20) | 574 (12) | 56 (1.2) | 315 (6.8) | – |
| 2007 | 6467 | 1167 (18) | 16 (0.3) | 875 (14) | 272 (4.2) | – |
| 2008 | 6954 | 1486 (21) | 641 (9.2) | 221 (3.2) | 617 (8.9) | – |
| 2009 | 7357 | 1937 (26) | 67 (0.9) | 718 (9.8) | 454 (6.2) | 691 (9.4) |
| 2010 | 4402 | 979 (22) | 0 | 379 (8.6) | 505 (11) | 94 (2.1) |
| Age groupsb | ||||||
| 0–15 years | 18,026 | 4129 (23) | 775 (4.3) | 1384 (7.7) | 1478 (8.2) | 482 (2.7) |
| 15–24 years | 3681 | 867 (24) | 166 (4.5) | 259 (7.0) | 256 (7.0) | 183 (5.0) |
| 25–64 years | 7187 | 1404 (20) | 329 (4.6) | 557 (7.8) | 390 (5.4) | 120 (1.7) |
| >64 years | 889 | 116 (13) | 28 (3.2) | 49 (5.5) | 39 (4.4) | 0 |
| Region | ||||||
| Northern | 10,258 | 2733 (21) | 630 (4.9) | 1023 (7.9) | 820 (6.3) | 246 (1.9) |
| Central | 6723 | 2037 (23) | 429 (4.9) | 619 (7.1) | 686 (7.8) | 297 (3.4) |
| Southern | 6307 | 1746 (22) | 239 (3.0) | 607 (7.6) | 657 (8.2) | 242 (3.0) |
ILI = influenza-like illness: fever (≥38 °C) with onset of either cough or sore throat ≤3 days prior to presentation.
12 patients had co-infection with seasonal influenza A (H3N2) and B viruses, 2 patients had co-infection with seasonal influenza A (H1N1) and B viruses, and 7 influenza positive patients had no reported subtype information.
Prior to the emergence of 2009 H1N1, the percent of ILI patients positive for influenza A infection (both seasonal A(H1) and A(H3)), was similar across age groups, though patients >64 years of age had slightly lower levels of influenza A infection than other age groups. In contrast, influenza B virus infection was highest (8.2%) among those <15 years of age, while 2009 H1N1 infection was highest (5.0%) among young adults aged 15–24 years.
Across regions, although the proportion influenza positive tests were relatively similar overall, there were some regional differences by subtype. When compared to the other regions, the Southern region had a slightly lower proportion of influenza A (H1N1) positive subtypes, but a slightly higher proportion of influenza B positive strains (Table 2). Also, the Northern region had a slightly lower proportion of 2009 H1N1 positive subtypes compared to other regions (Table 2).
3.4. Influenza circulation by time, overall and by type and subtype
During 2006–2010, influenza viruses circulated throughout the entire year (Fig. 2). Influenza A and B viruses often co-circulated, though peaks in circulation between influenza A and B viruses, when they did occur, tended to occur at different times of the year. Influenza A viruses accounted for 3547 (62%) of all influenza positive ILI cases, compared with 2163 (38%) for influenza B (Table 3). Peaks in influenza A virus circulation often occurred during the months of May–October, although peaks in influenza A activity also occurred outside of these months (Fig. 2). For example, influenza A (H3N2) exhibited two distinct peaks in circulation in 2007, one during February–March and another May–October. Of all the seasonal influenza A viruses detected, 76% were detected during the months the Southern Hemisphere vaccine is typically available suggesting a seasonality to the circulation of influenza A viruses (Table 3). Influenza B viruses also often circulated throughout the year. When influenza B viruses did reach peak circulation, these peaks occurred during November–March, generally within the months the Northern Hemisphere influenza vaccine is typically available (Fig. 2). Of all the influenza B viruses detected, 59% were during months the Northern Hemisphere vaccine is available, though this pattern was not observed in each region. Only in the Central region were influenza B viruses detected at much higher levels in the November–May period, whereas influenza B viruses were detected at much more similar levels throughout the year in the Southern region and only 56% were detected in the November–May period in the Northern Region (Table 3). Of note, circulation of 2009 H1N1 was observed from August to December 2009 and peaked during the week of September 20, 2009, when 58% of patients presenting with ILI to sentinel sites tested positive for 2009 H1N1.
Table 3.
Influenza positive RT-PCR testing results by type, region, and overall for patients presenting with influenza-like illness at any time, during the months of northern hemispherea vaccine formulation availability, and during the months of southern hemispherea vaccine availability—National Influenza Surveillance System, Vietnam, January 1, 2006–December 31, 2010†.
| Region influenza type | Influenza positive RT-PCR testing from patients presenting with ILI
|
||
|---|---|---|---|
| Northern hemispherea vaccine formulation availability (November–April) (% of entire year) | Southern hemispherea vaccine formulation availability (May–October) (% of entire year) | Entire year (January–December) | |
| Northern region | 809 (33) | 1664 (67) | 2473 |
| A, all seasonal subtypes | 347 (21) | 1306 (79) | 1653 |
| B | 462 (56) | 358 (44) | 820 |
| Central region | 811 (47) | 1815 (53) | 2037 |
| A, all seasonal subtypes | 328 (31) | 720 (69) | 1048 |
| B | 483 (70) | 203 (30) | 686 |
| Southern region | 496 (33) | 1007 (67) | 1503 |
| A, all seasonal subtypes | 159 (19) | 687 (81) | 846 |
| B | 337 (51) | 320 (49) | 657 |
| All regions, A and B | 2116 (37) | 3594 (63) | 5710 |
| A, all seasonal subtypes | 834 (24) | 2713 (76) | 3547 |
| B | 1282 (59) | 881 (41) | 2163 |
Influenza-like illness, by World Health Organization (WHO): fever (≥38 °C) with either cough or sore throat, and onset of symptoms ≤3 days.
As designated by the World Health Organization, based on surveillance of circulating strains in each hemisphere.
Testing for influenza was done through polymerase-chain reaction on pharyngeal swabs, excludes those testing positive for 2009 H1N1. Two patients had co-infection with seasonal influenza A (H1N1) and B viruses, 12 patients had co-infection with influenza A (H3N2) and B viruses, eight were not subtyped, and one had no subtyping information available.
4. Discussion
During 2006–2010 in Vietnam, influenza represented an important cause of ILI among patients presenting for medical evaluation at outpatient clinics in NISS; 22% of sampled outpatients presenting with ILI had laboratory-confirmed influenza virus infection. During this period, influenza viruses circulated throughout the year and peaks in influenza activity occurred at different periods depending upon influenza virus type or subtype. Influenza virus infection was also an important cause of provider reported subsequent hospitalization for all ILI patients, but especially among school-aged children. Among all patients with ILI presenting to outpatient clinics in the surveillance system nearly 10% were reported as subsequently hospitalized; of which, nearly 20% tested positive for influenza. School-aged children, 5–14 years old, had the highest percentage of influenza virus infections among all age groups. School-aged children also had the highest proportion of influenza infection of ILI patients who initially presented to outpatient clinics and were reported by providers as subsequently hospitalized.
High influenza attack rates among school-aged children regionally and globally have been previously reported [12–14]. That provider reported subsequent hospitalization associated with ILI is highest among patients <6 months of age likely reflects the fact that children at this age are more susceptible to severe disease, likely because of the absence of pre-existing immunity to all types of infections [15] and clinical management of this patient population may tend to be more conservative. Nonetheless, the relatively low proportion of influenza infections among subsequently hospitalized infants may suggest that other pathogens, such as rhinoviruses, respiratory syncytial viruses (RSV), and enteroviruses, may also result in severe disease in this age group. For example, during two years of surveillance in Laos, enterovirus and rhinovirus infections were more common among those children <5 years of age than in older age groups [16]. Influenza virus infection is also often associated with more severe outcomes among the elderly [17,18]. Although we did observe high levels of subsequent hospitalization associated with influenza infection in patients >64 years of age, the overall difference in the proportion of influenza positive infections among subsequently hospitalized patients among age groups was not statistically significant. This finding likely reflects the fact that NISS enrolls patients from outpatient clinics and is not intended to include patients directly admitted to hospitals. It may be likely that the older, medically fragile populations >64 years of age may progress to severe infection more quickly than other age groups, necessitating early direct admission prior to outpatient evaluation and thereby missing the opportunity for enrollment in NISS.
Our findings provide an important contribution to the available literature on influenza surveillance and the burden of influenza in the region. In a 2008 review of published influenza studies in the region, influenza was reported to account for 11–26% of febrile illness and 6–14% of hospitalized cases of pneumonia [4]. The slightly higher (19%) percent of influenza infections among ILI patients subsequently hospitalized reported here likely reflects NISS’s unique study design, which captures influenza infection among persons presenting as outpatients, but is not intended to capture influenza infection among all hospitalized patients, as in other studies that have described the burden of influenza [4]. During two years of influenza surveillance in Laos, 29.5% of outpatients with ILI and 12.6% of patients hospitalized with pneumonia were influenza positive [16]. In hospital-based influenza surveillance of admitted patients with pneumonia in two Thai provinces during 2005–2008, Simmerman et al. reported that influenza infection was present in 10.4% of hospitalized pneumonia patients; of which, 52% were <15 years of age. These authors also reported an overall bimodal age distribution for the annual incidence of influenza related hospitalized pneumonia, with the highest rates among those <5 and >75 years of age. In a prior study conducted in Thailand during 2004–2005, 19.5% of inpatients with pneumonia were found to have influenza infection [19]. Similar to our findings on seasonality of influenza types and subtypes, Simmerman et al. also noted increases in influenza A circulation during the months of the rainy seasons and an additional peak in influenza A activity in 2007. In contrast to our study, no apparent seasonality to influenza B virus infections were found among hospitalized patients in Thailand [20]. This pattern has been consistently reported in Thailand [3,21].
This report is subject to several limitations. Our results may not be generalizable in Vietnam, since NISS oversamples certain populations, specifically patients evaluated in the Northern Region and most pediatric populations, yet under-samples those <6 months of age and >64 years of age. For instance, 61% of patients were <15 years of age in NISS, whereas, throughout Vietnam only 24% of the entire population are <15 years of age [22]. Also, children may be more likely to present for evaluation of ILI than other age groups, though a better understanding of the age distribution of ILI in the general population in Vietnam would help clarify this issue. Further, our estimation of the percent influenza positive among the very young and old age groups may be subject to error, since there were a low number of enrollees in these groups. In both groups, patients may be less likely to meet the case definition for ILI, since infants and the extremely old may be unable to verbally report subjective ILI symptoms, like sore throat. Also, there may be variation in the manner in which providers interpreted the survey question on whether a patient was subsequently hospitalized and providers may not have had information to accurately determine whether each patient was hospitalized. Further, we cannot account for the finding that hospital admission among patients presenting with ILI was higher among district than provincial hospitals, since provincial hospitals are designed to treat more severe disease. The threshold for hospital admission may vary by provider, site and even throughout Vietnam; thus, subsequent hospital admission may not be a generalizable measure of severity when comparing influenza severity and hospitalization across different settings and countries. Furthermore, data on the severity of influenza and detailed information on illness duration, use of antiviral medications, and use of extended resources, like mechanical ventilators, to manage influenza patients, are not collected by NISS in Vietnam.
In summary, the findings presented in this report have important implications for influenza vaccine and health policy in Vietnam and in Southeast Asia. Although year-round circulation of influenza viruses occurs in Vietnam, the NISS data suggest that the timing of influenza vaccination should consider targeting the prominent mid-year peaks of influenza A viruses (Southern Hemisphere vaccine formulation availability). Our findings that influenza virus infection represents an important cause of ILI and use of outpatient and inpatient services, especially among school-aged children, suggests that a national influenza vaccination program targeting school children could be considered [13,23–25]. While ILI is typically a self-limited mild disease, one ILI episode may also be associated with significant financial disruption to individuals and families, in terms of missed work or school, in the region [24–26]. In 2007, a household study conducted in a southern Chinese province estimated that one episode of influenza-like illness can result in a 20% loss of individual monthly income [26]. Despite the insights that multi-year ILI surveillance has provided, more data on the severity of influenza, health services utilization, and further data on the direct and indirect economic impact of influenza in Vietnam is warranted, including the collection of additional data on the burden of influenza among hospitalized patients with severe acute respiratory infection. These additional data will further inform decisions regarding prevention and control of influenza in Vietnam and in Southeast Asia.
Acknowledgments
We would like to acknowledge specifically Tung Nguyen for his extremely valuable contribution. We also acknowledge all of the field and staff of the National Influenza Surveillance System network.
Abbreviations
- ILI
influenza-like illness
- NISS
National Influenza Surveillance System
- RT-PCR
reverse transcription-polymerase chain reaction
References
- 1.Beckett CG, Kosasih H, Ma’roef C, Listiyaningsih E, Elyazar IR, Wuryadi S, et al. Influenza surveillance in Indonesia: 1999–2003. Clinical Infectious Diseases. 2004 Aug;39(4):443–9. doi: 10.1086/422314. [DOI] [PubMed] [Google Scholar]
- 2.Moura FE. Influenza in the tropics. Current Opinion in Investigational Drugs. 2010 Oct;23(5):415–20. doi: 10.1097/QCO.0b013e32833cc955. [DOI] [PubMed] [Google Scholar]
- 3.Simmerman JM, Lertiendumrong J, Dowell SF, Uyeki T, Olsen SJ, Chittagan-pitch M, et al. The cost of influenza in Thailand. Vaccine. 2006 May;24(20):4417–26. doi: 10.1016/j.vaccine.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 4.Simmerman JM, Uyeki TM. The burden of influenza in East and South-East Asia: a review of the English language literature. Influenza Other Respiratory Viruses. 2008 May;2(3):81–92. doi: 10.1111/j.1750-2659.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Medicine. 2006 Apr;3(4):e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008 May;453(7195):615–9. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine. 2008 Sep;26(Suppl 4):D31–4. doi: 10.1016/j.vaccine.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 8.BARDA grants help build global flu vaccine manufacturing capacity. 2010 Available from: http://www.hhs.gov/news/press/2010pres/09/20100930d.html [cited 21.06.11]
- 9.Nguyen HT, Dharan NJ, Le MT, Nguyen NB, Nguyen CT, Hoang DV, et al. National influenza surveillance in Vietnam, 2006–2007. Vaccine. 2009 Dec;28(2):398–402. doi: 10.1016/j.vaccine.2009.09.139. [DOI] [PubMed] [Google Scholar]
- 10.Awofeso N, Fennell M, Waliuzzaman Z, O’Connor C, Pittam D, Boonwaat L, et al. Influenza outbreak in a correctional facility. Australian and New Zealand Journal of Public Health. 2001 Oct;25(5):443–6. [PubMed] [Google Scholar]
- 11.Hien TT, Boni MF, Bryant JE, Ngan TT, Wolbers M, Nguyen TD, et al. Early pandemic influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: a clinical virological and epidemiological analysis. PLoS Medicine. 2010 May;7(5):e1000277. doi: 10.1371/journal.pmed.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savage RWM, Johnson I, Rea E, Lafreniere M, Rosella LC, Lam F, et al. Assessing secondary attack rates among household contacts at the beginning of the influenza A (H1N1) pandemic in Ontario, Canada, April–June 2009: a prospective, observational study. BMC Public Health. 2011 Apr;11:234. doi: 10.1186/1471-2458-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR - Recommendations and Reports. 2010 Aug;59(RR-8):1–62. [PubMed] [Google Scholar]
- 14.Cowling BJ, Chan KH, Fang VJ, Lau LL, So HC, Fung RO, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. New England Journal of Medicine. 2010 Jun;362(23):2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long SP, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases. 3. Churchill Livingstone; 2008. [Google Scholar]
- 16.Vongphrachanh P, Simmerman JM, Phonekeo D, Pansayavong V, Sisouk T, Ongkhamme S, et al. An early report from newly established laboratory-based influenza surveillance in Lao PDR. Influenza Other Respiratory Viruses. 2010 Mar;4(2):47–52. doi: 10.1111/j.1750-2659.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Journal of American Medical Association. 2003 Jan;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clinical Infectious Diseases. 2010 Mar;50(5):747–51. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waicharoen S, Thawatsupha P, Chittaganpitch M, Maneewong P, Thanadachakul T, Sawanpanyalert P. Influenza viruses circulating in Thailand in 2004 and 2005. Japanese Journal of Infectious Diseases. 2008 Jul;61(4):321–3. [PubMed] [Google Scholar]
- 20.Simmerman JM, Chittaganpitch M, Levy J, Chantra S, Maloney S, Uyeki T, et al. Incidence, seasonality and mortality associated with influenza pneumonia in Thailand: 2005–2008. PLoS ONE. 2009;4(11):e7776. doi: 10.1371/journal.pone.0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmerman JM, Thawatsupha P, Kingnate D, Fukuda K, Chaising A, Dowell SF. Influenza in Thailand: a case study for middle income countries. Vaccine. 2004 Nov;23(2):182–7. doi: 10.1016/j.vaccine.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 22.The 2009 Vietnam Population and Housing census: completed results. 2009 Available from: http://www.gso.gov.vn/defaulten.aspx?tabid=515&idmid=5&ItemID=10799 [cited 21.06.11]
- 23.Khazeni N, Hutton DW, Garber AM, Hupert N, Owens DK. Effectiveness and cost-effectiveness of vaccination against pandemic influenza (H1N1) 2009. Annals of Internal Medicine. 2009 Dec;151(12):829–39. doi: 10.1059/0003-4819-151-12-200912150-00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan AL, Shie HJ, Lee YJ, Lin SJ. The evaluation of free influenza vaccination in health care workers in a medical center in Taiwan. Pharmacy World and Science. 2008 Jan;30(1):39–43. doi: 10.1007/s11096-007-9137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KK, Li SC, Kwong KS, Chan TY, Lee VW, Lau JT. A study of the health and economic effects of influenza-like illness on the working population under different working environments of a large corporation in Hong Kong. Journal of Medical Economics. 2008;11(4):639–50. doi: 10.3111/13696990802533179. [DOI] [PubMed] [Google Scholar]
- 26.Guo RN, Zheng HZ, Li JS, Sun LM, Li LH, Lin JY, et al. A population-based study on incidence and economic burden of influenza-like illness in south China, 2007. Public Health. 2011 Jun;125(6):389–95. doi: 10.1016/j.puhe.2011.03.004. [DOI] [PubMed] [Google Scholar]