Abstract
Recent advancements toward the treatment of Crohn’s disease (CD) indicate great promise for long-term remission. CD patients suffer from a complex host of dysregulated interactions between their innate immune system and microbiome. The most predominant link to the onset of CD is a genetic mutation in the innate immune receptor nucleotide-binding oligomerization domain-containing 2 (NOD2). NOD2 responds to the presence of bacteria and stimulates the immune response. Mutations to NOD2 promote low diversity and dysbiosis in the microbiome, leading to impaired mucosal barrier function. Current treatments suppress the immune response rather than enhancing the function of this critical protein. New progress towards stabilizing NOD2 signaling through its interactions with chaperone proteins holds potential in the development of novel CD therapeutics.
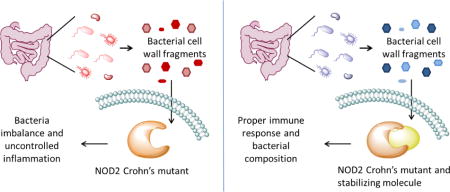
Graphical abstract
Introduction
Crohn’s disease (CD) is a debilitating, inflammatory bowel disorder that is proposed to arise from an atypical reaction to commensal bacteria. Traditional treatments for CD target reducing inflammation by suppressing the immune response. In instances of severe intestinal damage, antibiotics are also implemented to allow the intestinal tissue to heal. If medical treatments fail, surgical removal of the effected intestine is required to prevent potentially life-threatening complications [1]. Recent discoveries indicate that by decreasing the biodiversity of the microbiome and weakening the immune response, current therapies may be harmful [2]. A better approach for therapeutics may be to enhance microbial diversity and the immune response to avoid relapse.
Recognizing this issue, attention has turned to probing the interactions between the host and microbiome. Genetic predisposition is highly correlated to the onset of CD. Specifically, mutations to the innate immune receptor nucleotide-binding oligomerization domain-containing 2 (NOD2) are the strongest genetic factor in the advancement of CD and development of an aggressive phenotype [3]. NOD2 variants contribute to various aspects of the pathogenesis. CD patients with NOD2 mutations possess a distinct and compromised microbial composition that allows harmful bacteria to thrive [4]. In order to replenish the microbiome with a stable microbial composition, fecal transplant therapy has been explored with astounding success [5–7]. NOD2 mutations are also linked to low levels of mucosal defensins, resulting in a compromised mucosal barrier [8]. Defensin-based therapeutics could compensate for the decreased expression of the necessary anti-microbial peptides without eliminating commensal bacteria [9]. Recent efforts have demonstrated that the NOD2 mutants are unstable, and by enhancing their half-life through interactions with a chaperone protein, appropriate signaling was restored [10]. Use of a pharmacological chaperone to mimic this heightened function has the potential to directly target all mis-signaling events of NOD2 mutants. This review will highlight recent biochemical and basic science advancements towards new therapeutic targets that are based on enhancing the stability of the critical signaling protein, NOD2.
Role of Host Genetics in Disease Predisposition
The emergence of CD has rapidly increased worldwide, steadily augmenting both its prevalence and incidence. Each year approximately 20 new cases (per 100,000 people) are diagnosed in North America, 12 in Europe, and 5 in Asia and the Middle East. Higher incidences in developed countries may be attributed to better diagnostics that can differentiate CD from other irritable bowel disorders [11]. A growing number of environmental factors that are more frequent in developed countries are also linked to pathogenesis including smoking, diet, stress and appendectomy [12].
In addition to environmental influences, genetic predisposition is predominantly linked with increased CD susceptibility. Recent efforts have identified 140 distinct loci with genome-wide significant evidence for CD association [13]. When evaluating all of the single nucleotide polymorphisms associated with CD, NOD2 mutations stand out as statistically most significant [3]. The prevalent mutations include the point mutations R702W and G908R, as well as a frame shift mutation at residue 1007 [14]. At least one NOD2 mutation is present in 30–40% of CD cases compared with 6–7% present in non-diseased controls [15]. NOD2 CD mutations are the strongest genetic factor in determining the complexity of the disease and need for surgical intervention because they are associated with more severe phenotypes [3,15,16].
NOD2 is an intracellular sensor of bacterial cell wall fragments, present in intestinal epithelial cells. It directly binds to a component of the bacterial peptidoglycan called muramyl dipeptide (MDP) which then elicits an immune response through the NF-κB pathway [17,18]. Several genes that affect NOD2 NF-κB signaling and NOD2-induced IL-8 secretion are present in loci associated with CD risk, increasing the significance of NOD2′s central role in CD [19,20]. NOD2 is the most significant therapeutic target for CD based upon its statistical prevalence, severity of the disease phenotype, and interplay with other CD-associated proteins.
Microbiome Shifts in Crohn’s Disease Patients with NOD2 mutants
The microbiome comprises a vast number of diverse microorganisms and greatly influences the immune response. A hallmark of CD is a shift in an individual’s microbiome composition (Table 1). NOD2 mutations lead to an inability to properly regulate commensal bacteria resulting in decreased bacterial diversity and increased susceptibility to pathogenic bacteria. Individuals with irritable bowel disorders typically have a decrease in the commensal Firmicutes and Bacteroides and an increase in Proteobacteria, which includes common pathogens such as E. coli, Salmonella, Helicobacter, and Vibrio [4,21–29]. These changes are often exacerbated by antibiotic treatment in CD patients [1,4].
Table 1.
Shifts in the microbiota of Crohn’s disease patients compared to healthy individuals
| Bacteria | Shift | Significance | Ref |
|---|---|---|---|
| Proteobacteria | + | Include pathogenic strains Salmonella, Vibrio, and E.coli | [4,22,24- 26] |
| Fusobacteriaceae | + | Pathogenic genus of gram negative bacteria | [4] |
| B. Vulgatus | + | Has been demonstrated to increase inflammatory cytokines | [21,27] |
| Neisseriaceae | + | Pathogenic bacteria which can cause gonorrhea and meningitis | [4] |
| H. Hepaticus | + | Pathogenic bacteria | [23,25] |
| Bifidobacteriaceae | − | Help regulate pathogens and aid digestion | [4] |
| Bacteroides | − | Can regulate pathogenic invasion and aid digestion | [4,22,25] |
| Firmincutes | − | Most common flora in healthy individuals | [4,22,26] |
| F. Prausnitizii | − | Have been demonstrated to decrease IL-12 and IFN-γ production | [4,28] |
There is a symbiotic relationship between NOD2 and the microbiome, with NOD2 maintaining the proper balance of commensal bacteria and commensal bacteria stimulating the appropriate levels of NOD2. Germfree mice show decreased levels of NOD2; normal NOD2 gene expression can be restored through inoculation with commensal bacteria [24]. In contrast, NOD2 mutants promote an increased bacterial load and a decrease in diversity, which is associated with diminished bactericidal activity [25,26]. With many changes in the microbiome associated with CD, it is logical that treatments focus on restoring balance to the microbiome. Current treatments include probiotic supplementation [1] and fecal transplants [5–7] to restore the proper balance of commensal bacteria.
Increasing evidence supports a role of inheritance in the composition of the microbiome [30]. However, the abnormal microbiome of CD patients is distinct from family members [31] where the correct composition is directly altered by an improper immune response. Although typically stable, alterations to the microbiome can rapidly occur in response to environmental changes, making relapses inevitable [32,33]. Therefore the long term impact of fecal transplant therapy is of limited use when CD NOD2 mutants result in a reversion of the microbiome to its diseased state. Many long-term successful fecal transplants have been for invasive pathogens not permanent genetic mutations [34]. Therefore, fecal transplants must be coupled with treatments that continue to maintain a proper immune response.
Role of NOD2 in the Impaired Function of the Mucosal Barrier
The gastric mucosal barrier is an integral part of the body’s natural defense against invading pathogens. Permeability of the mucosal layer indicates that the mucosal immune response has been compromised [35]. The mucosal layer of CD patients is often damaged, allowing for pathogen penetration through the epithelium and leading to severe symptoms [36]. To protect the integrity of the mucosal layer, anti-microbial peptides, such as α- and β-defensins, are released extracellularly from epithelial cells. Defensins are effective against a range of pathogens including Gram-positive and Gram-negative bacteria, mycobacteria, and fungi [37]. Defensin levels are significantly reduced in CD patients, with patients harboring a NOD2 mutation showing a greater decrease [38].
Reduced expression of α-defensins predisposes patients to a complicated phenotype of CD, common to NOD2 mutations [38]. NOD2 can up-regulate expression of α-defensins mainly through induction of the NF-κB pathway, but the mutants have diminished activation [8]. Additionally NOD2 mutants interfere with release of α-defensins because they cannot activate ATG16L, a protein involved in packaging and secretion (Figure 1) [39,40]. NOD2 mutations are also linked to low levels of β-defensins. Activation of NOD2 with MDP induces β-defensins and in contrast, overexpression of NOD2 mutant 1007fs, the most frequent NOD2 variant, results in defective induction [41,42]. Additionally the CD mutants have dysregulated β-defensins induction with Vitamin D, a critical component of NOD2 induced β-defensin expression [43].
Figure 1. NOD2 promotes the expression and secretion of α-defensins in Paneth cells.
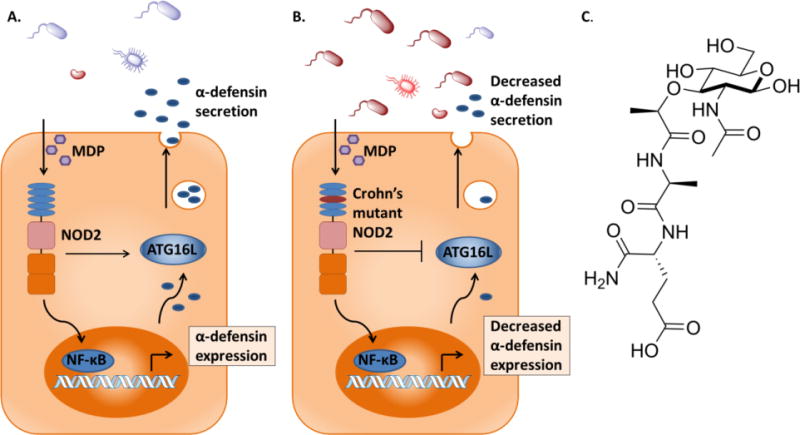
A. NOD2 activates the NF-κB pathway upon induction with MDP, resulting in the expression of α-defensins. NOD2 interacts with ATG16L, which leads to the secretion of α-defensins. Commensal bacteria remain distant from Paneth cells. B. CD mutant NOD2 has reduced activation of α-defensin expression. It is unable to recruit ATG16L, decreasing secretion of α-defensins. Harmful bacteria can invade the mucosal layer with decreased protection. C. The structure of NOD2-activating ligand MDP.
Defensin-like drugs have successfully been developed as antibacterial treatments for various applications [37]. Prominent members of the microbiome are resilient to antimicrobial peptides; thus the healthy microbiome is not destroyed by introducing defensin-like drugs [9]. Defensin-like drugs have the benefit of having low molecular weights, resistance to proteolysis and potent anti-microbial activity. However, their production costs, potential toxicity and low stability in vivo make them less attractive as a potential CD therapeutic [37].
Interactions with Chaperone Proteins Stabilize NOD2
In the crowded environment of the cell, efficient folding of newly synthesized proteins can be compromised resulting in misfolding and aggregation. To overcome these challenges and ensure protein homeostasis, a variety of molecular chaperones assist in proper folding. Chaperone proteins improve the stability of proteins by enhancing folding energetics [44]. NOD2 recruits the chaperone proteins heat-shock protein 70 (HSP70) and heat-shock protein 90 to confer stability and dissociation from either result in increased degradation [10,45].
In comparison to the wild-type, the NOD2 CD mutants have displayed significant in vivo instability. Interestingly, by overexpressing HSP70, the NOD2 CD mutants have restored stability and correct inflammatory responses [10]. Interactions between HSP70 and NOD2 are critical for proper function as there are also CD mutations of HSP70 [46]. Modulating the interactions between NOD2 and HSP70 becomes a potential target for the inflammatory response brought about by poor signaling. Variation of HSP70 levels has already proven to be successful in reducing inflammation through the innate immune system following brain injury [47,48].
Commensal bacteria have also been shown to regulate HSP70 levels. Bacteroidetes and Firmicutes secrete n-butyrate, which regulates gene expression in intestinal macrophages [49] and when administered at physiological concentrations increases levels of HSP70, inducing an anti-inflammatory effect [50–52]. Thus, it appears that commensal bacteria are potentially utilizing butyrate as a means to induce HSP70 expression to stabilize NOD2 in order to regulate the microbiome and prevent the spread of pathogenic bacteria.
Enhancing the function of NOD2 was previously targeted through small molecules that are derivatives of its ligand, MDP (Figure 1C) [53,54]. The success of these derivatives could be further enhanced by small molecules that mimic HSP70 stabilization (Table 2). Pharmacological chaperones are small molecule mimics of chaperones proteins that bind to a protein, directly affecting its stability. They have successfully been applied to various proteins that lead to a disease state due to instability (e.g. G protein–coupled receptors, neurotransmitter receptors, and glycosidases [55,56]). We propose that the next trend in CD therapeutics will be the implemention of pharmacological chaperones to stabilize the NOD2 mutants.
Table 2. Proposed mechanism of correcting Crohn’s mutant function.
WT NOD2 has appropriate NF-κB signaling upon induction with MDP and enhanced stability. These effects are amplified when HSP70 is overexpressed. The NOD2 CD mutants would similarly benefit to have enhanced stability and NF-κB signaling with overexpressed HSP70 [10].
| NOD2 Isoform | Bacterial Cell Wall Ligand | HSP70 | Effects |
|---|---|---|---|
| Wild-type | + | − | Increased stability and NF-κB activation |
| Wild-type | − | + | Increased stability |
| Wild-type | + | + | Increased stability and enhanced NF-κB activation |
| CD mutant | + | − | Increased stability and decreased NF-κB activation |
| CD mutant | − | + | Increased stability |
| CD mutant | + | + | Increased stability and enhanced NF-κB activation |
Conclusions and Outlook
The CD mutants of NOD2 have more widespread influence on the development of CD than any other factor. The NOD2 mutants contribute to pathogenesis by promoting microbial dysbiosis and reducing defensin levels. Potential therapeutics such as fecal transplants could modify the microbiome to a healthy composition. However, the rebounding effect of the highly dynamic microbiome necessitates a solution that addresses the causes of microbiome imbalance. It is evident that a biochemical approach is necessary to correct the response of the permanent genetic mutations that influence CD. Defensin-like drugs could compensate for the negative effects of aberrated signaling but would only mask the underlying issues of the improper microbiome. A superior approach is to enhance the stability and correct the mis-signaling of CD NOD2 mutants through pharmacological chaperones. Manipulating the stability of this central protein would broadly target each of the mechanisms in which it contributes to the pathogenesis of CD.
Highlights.
NOD2 mutations are strongly linked to Crohn’s disease and its most severe phenotype
Host microbiome of NOD2 variant patients promotes harmful bacteria and dysbiosis
Decreased mucosal defensin expression and secretion correlated to NOD2 mutations
Instability and mis-signaling of NOD2 mutants can be corrected through chaperones
Acknowledgments
Financial support provided by the Delaware COBRE program (NIGMS 1 P30 GM110758 and 1 P20 GM104316-01A1) and the Chemistry-Biology Interface predoctoral training program (NIGMS 5T32 GM 08550-15), supported by grants from the National Institutes of Health. C.L.G. is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trusts. We thank Brian Bahnson for critical reading of this manuscript.
References
- 1.Lahad A, Weiss B. Current therapy of pediatric Crohn’s disease. World journal of gastrointestinal pathophysiology. 2015;6:33–42. doi: 10.4291/wjgp.v6.i2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics Associated With Increased Risk of New-Onset Crohn’s Disease But Not Ulcerative Colitis: A Meta-Analysis. American Journal of Gastroenterology. 2014;109:1728–1738. doi: 10.1038/ajg.2014.246. [DOI] [PubMed] [Google Scholar]
- 3*.Cleynen I, Gonzalez JR, Figueroa C, Franke A, McGovern D, Bortlik M, Crusius BJA, Vecchi M, Artieda M, Szczypiorska M, et al. Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62:1556–1565. doi: 10.1136/gutjnl-2011-300777. This paper correlated the known single nucleotide polymorphisms associated with Crohn’s to the severity and complexity of the disease course. NOD2 was identified as being the most predictive factor in the association with complications, need for surgical intervention, and severity of the disease. [DOI] [PubMed] [Google Scholar]
- 4**.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren BY, Schwager E, Knights D, Song SJ, Yassour M, et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host & Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. This study was the largest microbiome study in pediatric Crohn’s disease. By collecting samples from multiple gastrointestinal locations prior to treatment in new-onset Crohn’s disease, the authors identified that the rectal mucosal-associated microbiome offers unique potential for convenient and early diagnosis of CD. It was conclusively determined that antibiotic use amplifies the microbial dysbiosis associated with CD, calling for a change in the treatment of the disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F-M, Wang H-G, Wang M, Cui B-T, Fan Z-N, Ji G-Z. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World Journal of Gastroenterology. 2013;19:7213–7216. doi: 10.3748/wjg.v19.i41.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suskind DL, Brittnacher MJ, Wahbeh G, Shaffer ML, Hayden HS, Qin X, Singh N, Damman CJ, Hager KR, Nielson H, et al. Fecal Microbial Transplant Effect on Clinical Outcomes and Fecal Microbiome in Active Crohn’s Disease. Inflammatory Bowel Diseases. 2015;21:556–563. doi: 10.1097/MIB.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, Huang G, Liu Z, Wu P, Fan Z, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51–58. doi: 10.1111/jgh.12727. [DOI] [PubMed] [Google Scholar]
- 8.Tan G, Zeng B, Zhi F-C. Regulation of human enteric alpha-defensins by NOD2 in the Paneth cell lineage. European Journal of Cell Biology. 2015;94:60–66. doi: 10.1016/j.ejcb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 9**.Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 2015;347:170–175. doi: 10.1126/science.1260580. This study was the first to probe the mechanism of how commensal bacteria are distinguished from pathogenic bacteria by our anti-microbial peptides. Bacteroides thetaiotaomicron, a prominent commensal bacterium, are resistant to anti-microbial peptides during inflammation because of a modified LPS outer coat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Mohanan V, Grimes CL. The Molecular Chaperone HSP70 Binds to and Stabilizes NOD2, an Important Protein Involved in Crohn Disease. Journal of Biological Chemistry. 2014;289:18987–18998. doi: 10.1074/jbc.M114.557686. Using the tetracycline-regulated expression system to control NOD2 levels, the authors identified heat-shock protein 70 as a novel interacting protein with NOD2. Overexpression of HSP70 resulted in an increased half-life of both WT and Crohn’s mutant NOD2. Most importantly, HSP70 was able to rescue the Crohn’s mutants response to bacterial ligand, MDP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Ananthakrishnan AN. Environmental Risk Factors for Inflammatory Bowel Diseases: A Review. Digestive Diseases and Sciences. 2015;60:290–298. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 15.Liu JZ, Anderson CA. Genetic studies of Crohn’s disease: Past, present and future. Best Practice & Research in Clinical Gastroenterology. 2014;28:373–386. doi: 10.1016/j.bpg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Lobos M, Arostegui JI, Sans M, Tassies D, Plaza S, Delgado S, Lacy AM, Pique JM, Yague J, Panes J. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Annals of Surgery. 2005;242:693–700. doi: 10.1097/01.sla.0000186173.14696.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimes CL, Ariyananda LD, Melnyk JE, O’Shea EK. The Innate Immune Protein Nod2 Binds Directly to MDP, a Bacterial Cell Wall Fragment. Journal of the American Chemical Society. 2012;134:13535–13537. doi: 10.1021/ja303883c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nature Reviews Immunology. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 19*.Warner N, Burberry A, Franchi L, Kim YG, McDonald C, Sartor MA, Nunez G. A Genome-Wide siRNA Screen Reveals Positive and Negative Regulators of the NOD2 and NF-kappa B Signaling Pathways. Science Signaling. 2013;6:11. doi: 10.1126/scisignal.2003305. The authors identified regulators of the NOD2 signaling pathway using a genome-wide siRNA screening. Several of the identified genes were associated with Crohn’s disease risk. This supports a more significant role of NOD2 and its signaling pathway in the pathogenesis of Crohn’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente JC, Ursell LK, Parfrey LW, Knight R. A Genome-wide Small Interfering RNA (siRNA) Screen Reveals Nuclear Factor-kappa B (NF-kappa B)-independent Regulators of NOD2-induced Interleukin-8 (IL-8) Secretion. Journal of Biological Chemistry. 2014;289:28213–28224. doi: 10.1074/jbc.M114.574756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemente JC, Ursell LK, Parfrey LW, Knight R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dicksved J, Halfvarson J, Rosenquist M, Jarnerot G, Tysk C, Apajalahti J, Engstrand L, Jansson JK. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008;2:716–727. doi: 10.1038/ismej.2008.37. [DOI] [PubMed] [Google Scholar]
- 23.Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petnicki-Ocwieja T, Hrncir T, Liu Y-J, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang HL, Tyler AD, van Sommeren S, Imhann F, Stempak JM, et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Medicine. 2014;6 doi: 10.1186/s13073-014-0107-1. The authors identify a correlation between Crohn’s variants of NOD2 and the increase of pathogenic Enterobateriaceae. This supports the hypothesis that NOD2 regulates the composition of the microbiome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondot S, Barreau F, Al Nabhani Z, Dussaillant M, Le Roux K, Doré J, Leclerc M, Hugot J-P, Lepage P. Altered gut microbiota composition in immune-impaired Nod2−/− mice. Gut. 2011 doi: 10.1136/gutjnl-2011-300478. [DOI] [PubMed] [Google Scholar]
- 27.Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW. Inflammation Drives Dysbiosis and Bacterial Invasion in Murine Models of Ileal Crohn’s Disease. Plos One. 2012;7 doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial Sensor Nod2 Prevents Inflammation of the Small Intestine by Restricting the Expansion of the Commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faith JJ, Colombel JF, Gordon JI. Identifying strains that contribute to complex diseases through the study of microbial inheritance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:633–640. doi: 10.1073/pnas.1418781112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 32.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The Long-Term Stability of the Human Gut Microbiota. Science. 2013;341:44–+. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. Host lifestyle affects human microbiota on daily timescales. Genome Biology. 2014;15 doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smits LP, Bouter KEC, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic Potential of Fecal Microbiota Transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability - a new target for disease prevention and therapy. Bmc Gastroenterology. 2014;14:25. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders DSA. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn’s disease. Journal of Clinical Pathology. 2005;58:568–572. doi: 10.1136/jcp.2004.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarczak J, Kosciuczuk EM, Lisowski P, Strzalkowska N, Jozwik A, Horbanczuk J, Krzyzewski J, Zwierzchowski L, Bagnicka E. Defensins: Natural component of human innate immunity. Human Immunology. 2013;74:1069–1079. doi: 10.1016/j.humimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nature Reviews Immunology. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology. 2010;139:1630–1641. 1641 e 1631–1632. doi: 10.1053/j.gastro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. Journal of Biological Chemistry. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 42.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. [PubMed] [Google Scholar]
- 43.Wang T-T, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, et al. Direct and Indirect Induction by 1,25-Dihydroxyvitamin D-3 of the NOD2/CARD15-Defensin beta 2 Innate Immune Pathway Defective in Crohn Disease. Journal of Biological Chemistry. 2010;285:2237–2241. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular Chaperone Functions in Protein Folding and Proteostasis. In: Kornberg RD, editor. Annual Review of Biochemistry, Vol 82. Annual Reviews; 2013. pp. 323–355. [DOI] [PubMed] [Google Scholar]
- 45.Lee K-H, Biswas A, Liu Y-J, Kobayashi KS. Proteasomal Degradation of Nod2 Protein Mediates Tolerance to Bacterial Cell Wall Components. Journal of Biological Chemistry. 2012;287 doi: 10.1074/jbc.M112.410027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Ren JA, Gu GS, Wang GF, Wu XW, Yan DS, Liu S, Li JS. Crohn’s disease and polymorphism of heat shock protein gene HSP70-2 in the Chinese population. Journal of Gastroenterology and Hepatology. 2013;28:814–818. doi: 10.1111/jgh.12163. [DOI] [PubMed] [Google Scholar]
- 47.Kim N, Kim JY, Yenari MA. Anti-inflammatory properties and pharmacological induction of Hsp70 after brain injury. Inflammopharmacology. 2012;20:177–185. doi: 10.1007/s10787-011-0115-3. [DOI] [PubMed] [Google Scholar]
- 48.Kacimi R, Yenari MA. Pharmacologic Heat Shock Protein 70 Induction Confers Cytoprotection against Inflammation in Gliovascular Cells. Glia. 2015;63:1200–1212. doi: 10.1002/glia.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Chang PV, Hao LM, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. The authors demonstrate that n-butyrate, a short chain fatty acid produced by commensal bacteria, is able to down regulate pro-inflammatory cytokines through histone deacetylase inhibition. This supports the notion that proper balance of the microbiota is important in regulating inflammation associated with Crohn’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.GarciaBermejo L, Vilaboa NE, Perez C, Galan A, DeBlas E, Aller P. Modulation of heat-shock protein 70 (HSP70) gene expression by sodium butyrate in U-937 promonocytic cells: Relationships with differentiation and apoptosis. Experimental Cell Research. 1997;236:268–274. doi: 10.1006/excr.1997.3725. [DOI] [PubMed] [Google Scholar]
- 51.Malago JJ, Koninkx J, Tooten PCJ, van Liere EA, van Dijk JE. Anti-inflammatory properties of heat shock protein 70 and butyrate on Salmonella-induced interleukin-8 secretion in enterocyte-like Caco-2 cells. Clinical and Experimental Immunology. 2005;141:62–71. doi: 10.1111/j.1365-2249.2005.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimoto T, Imaeda H, Takahashi K, Kasumi E, Bamba S, Fujiyama Y, Andoh A. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. Journal of Gastroenterology and Hepatology. 2013;28:613–619. doi: 10.1111/jgh.12073. [DOI] [PubMed] [Google Scholar]
- 53.Rubino SJ, Magalhaes JG, Philpott D, Bahr GM, Blanot D, Girardin SE. Identification of a synthetic muramyl peptide derivative with enhanced Nod2 stimulatory capacity. Innate Immunity. 2013;19:493–503. doi: 10.1177/1753425912471691. [DOI] [PubMed] [Google Scholar]
- 54.Melnyk JE, Mohanan V, Schaefer AK, Hou C-W, Grimes CL. Peptidoglycan Modifications Tune the Stability and Function of the Innate Immune Receptor Nod2. Journal of the American Chemical Society. 2015;137:6987–6990. doi: 10.1021/jacs.5b01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makley LN, Gestwicki JE. Expanding the Number of ‘Druggable’ Targets: Non-Enzymes and Protein-Protein Interactions. Chemical Biology & Drug Design. 2013;81:22–32. doi: 10.1111/cbdd.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Annual Review of Biochemistry. Annual Reviews; 2009. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency; pp. 959–991. [DOI] [PubMed] [Google Scholar]


