Abstract
Objective
Little information is available on the relationship between the clinical course of ulcerative colitis (UC) and the outcomes of pregnancy and delivery in pregnant Japanese women. The aim of this retrospective study was to determine the factors that influence pregnancy and childbirth in middle-aged UC patients.
Methods
We studied 53 pregnancies in 45 pregnant women with UC who delivered at our department. They included 41 pregnancies that started while in UC remission and 12 pregnancies that started in the UC active phase. The following factors were evaluated: 1) the clinical course of UC; 2) the frequency and details of abnormal pregnancy/abnormal delivery; and 3) the course of pregnancy/delivery. We compared the clinical features, course of UC, and details of treatment between women with a normal pregnancy/delivery and those with an abnormal delivery.
Results
A comparison of the remission and acute groups showed lower clinical activity indices (CAIs) during pregnancy in the remission group and significantly higher rates of recurrence/exacerbation in the active group (75%) than in the remission group (7.3%). The respective CAIs in the first, second, and third trimesters were 3 and 6, 3 and 5, and 3 and 4, in the remission and active groups, respectively. Live infants were delivered in 51 (96%) pregnancies, with 7 (17%) abnormal pregnancies in the remission group and 4 (33.3%) in the active group (p>0.05). Abnormal delivery occurred in 16 of 53 (30.1%) pregnancies, and the rate was higher in the remission group than in the active group (p>0.05). In both groups, the most common abnormal event during pregnancy was delivery of low-birth-weight infants. Delivery was normal in 37 cases and abnormal in 16 cases. A multivariate analysis showed that a shorter UC disease duration (odds ratio=1.16) and higher CAI in the first trimester (odds ratio=1.49) were associated with an increased risk of abnormal pregnancy.
Conclusion
Our findings demonstrated that the clinical course of UC, as evaluated by the CAI, during pregnancy influenced the outcome of pregnancy and delivery.
Keywords: ulcerative colitis, pregnancy, maintenance of remission, childbirth, clinical course [clinical activity index (CAI); Lichtiger index]
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease classified as an intractable disease by the Japanese Ministry of Health, Labour and Welfare (1). UC has marked influence on the social life of patients, and among women of childbearing age, the influence of this disease on pregnancy and childbirth can lead to various complications (e.g., exacerbation of UC and difficulties with treatment). In addition, the results of various laboratory tests are different before and during pregnancy. In pregnant women with UC, invasive investigations, such as colonoscopy, are usually avoided, and the disease status is mainly evaluated based on the clinical symptoms.
The aim of the present retrospective study was to define the correlation between the clinical state of UC based on the Lichtiger index [clinical activity index (CAI)] (2) and the outcomes of pregnancy/delivery of 53 pregnancies in 45 UC patients who delivered at our hospital.
Materials and Methods
We studied 53 pregnancies in 45 women with UC who delivered at our department between January 2008 and March 2016. We targeted patients who were visiting regularly from pregnancy to childbirth. In 41 pregnancies starting when UC was in remission (remission group) and 12 pregnancies starting in the active phase of UC (active group), the following factors were evaluated: 1) the clinical features and clinical course of UC; 2) the frequency and details of abnormal pregnancy/abnormal delivery; and 3) the course of pregnancy/delivery. In addition, we also compared the clinical features and details of treatment of 37 cases of normal pregnancy and delivery (normal group) and 16 cases of abnormal delivery (abnormal group).
The observation period extended from one year before pregnancy to the time of delivery. The clinical disease activity of UC was evaluated by the CAI (2). This index assesses the following items: the frequency of diarrhea (number of daily stools, score 0-4), nocturnal diarrhea (yes/no), visible blood (score 0-3), fecal incontinence (yes/no), abdominal pain or cramping (score 0-3), general well-being (score 0-5), abdominal tenderness (score 0-3), and need for antidiarrhea drugs (yes/no), with a higher score representing more severe disease, e.g., CAI ≤4 reflecting remission and CAI ≥5 for at least 1 month representing active UC. Patients considered in remission for one year before pregnancy were assigned to the remission group, while those who had not achieved remission within one year before pregnancy were assigned to the active group.
Among the patients in remission, if the CAI increased to ≥5 and the increase persisted for at least 1 month with the addition of steroid therapy or steroid dose escalation and administration of biological drugs being required, the patient was considered to have recurrence. In pregnant women with active UC, when the addition of steroid therapy/steroid dose escalation/administration of biological drugs was required during pregnancy, the patient was considered to have exacerbation. The clinical activity of UC was evaluated according to the total CAI determined at hospital visits and classified as mild, moderate, and severe for CAI <6, 6 to <10, and ≥10, respectively.
In this study, abnormal pregnancy was defined as any pregnancy-related event causing illness in the mother or fetus. Abnormal delivery was defined as any of the following: abortion, threatened abortion, premature delivery, threatened premature delivery, stillbirth, placental abnormality, hydatidiform mole, delivery of a low-birth-weight infant, and congenital defect. The first, second, and third trimester of pregnancy were defined as the period from the onset to week 16, from week 17 to week 28, and from week 29 to delivery, respectively.
Statistical analyses
Data are expressed as number of cases and median (minimum-maximum). Comparisons between two groups were performed by the Wilcoxon test, and the difference was considered significant at p<0.05. All statistical analyses were conducted using the software program called JMP Statistical Discovery (SAS, Version 11, SAS Institute Japan, Tokyo, Japan).
Ethical considerations
The study protocol was reviewed and approved by the Committee on Ethics at the Tokyo Women's Medical University. Written informed consent was obtained from all patients before endoscopic procedures. Patients agreed to participate in this study after being informed of the study purpose and the nature of the procedures involved. The study was conducted with strict adherence to the Declaration of Helsinki.
Results
Clinical course of UC
A significant difference in the clinical course was observed between the remission group and active group (p<0.05). During pregnancy, the mean CAI was 3 (1-4) in the remission group and 5.6 (3-9) in the active group. There were no significant differences in treatment between the two groups before pregnancy (Table 1). Regarding recurrence/exacerbation, 3 recurrences were noted (7.3%) in the remission group and 9 exacerbations (75%) in the active group, showing a significant difference in the frequency of recurrence/exacerbation (p=0.001). Among the 12 patients with recurrence/exacerbation, oral drugs were self-discontinued in 2 cases in the remission group (66%) and 1 case in the active group (11%). Regarding the CAI during pregnancy, the respective CAIs in the first, second, and third trimesters in the remission and active groups were 3 (2-6) and 6 (2-9) (p=0.001), 3 (2-8) and 5 (3-14) (p=0.0148), and 3 (2-9) and 4 (2-13) (p=0.013), respectively, with the differences being significant (Table 2). Also shown are graphs of the fluctuation of the CAI for relaxed pregnancy and active-stage pregnancy (Fig. 1, 2).
Table 1.
Clinical Characteristics of the Remission Group and the Active Group.
| Remission group n=41 |
Active group n=12 |
p value | |
|---|---|---|---|
| Age of onset (year) * | 25 (12-38) | 25 (18-34) | NS |
| Duration of UC (year)* | 7 (1-27) | 5 (0.5-18) | NS |
| Age at pregnancy (year)* | 32 (16-42) | 34 (20-41) | NS |
| CAI in pregnancy* | 3 (1-4) | 5 (3-9) | 0.0001 |
| Type: Total colitis/left-sided colitis/proctitis | 13/12/16 | 5/4/3 | NS |
| Medications before pregnancy, yes / no | 36/5 | 11/1 | NS |
| Oral 5-ASA/enema/suppository | 33/3/5 | 10/4/3 | NS |
| Oral steroids/enema/suppository | 4/4/8 | 3/4/4 | NS |
| Apheresis therapy | 0 | 0 | NS |
| Immunosuppressants | 1 | 0 | NS |
| Biologics (tacrolimus) | 0 | 0 | NS |
Data are number of patients or *median (minimum-maximum). CAI: clinical activity index, 5-ASA: 5-nitrosalicylic acid
Table 2.
Clinical Characteristics and Course of UC in the Remission and Active Groups.
| Remission group n=41 |
Active group n=12 |
p value | |
|---|---|---|---|
| Maintenance of remission, n, recurrence/exacerbation, n (%) | 37, 3 (7.3) | 2, 9 (75) | <0.001 |
| Time of recurrence/exacerbation: 1st, 2nd, 3rd trimester, n (%) | 2 (67),1 (33), 0 (0) | 4 (44), 4 (44), 1 (12) | |
| Recurrence and exacerbation, n/total (%) | 2/3 (66) | 1/9 (11) | NS |
| Recurrence of 1st/2nd/3rd trimester, n | 3/0/0 | 6/3/0 | NS |
| Continuation of drug therapy, n, yes/no | 36/5 | 11/1 | NS |
| CAI score | |||
| at first trimester | 3 (2-6) | 6 (2-9) | <0.0001 |
| at second trimester | 3 (2-8) | 5 (3-14) | <0.0148 |
| at third trimester | 3 (2-9) | 4 (2-13) | <0.013 |
Abortion due to ectopic pregnancy occurred at week 8 of gestation in one case from the remission group and at week 9 in one case from the active group. These two cases were excluded from evaluation of the maintenance of remission, recurrence/exacerbation, recurrence rate, and CAI in the second and third trimesters of pregnancy. CAI: clinical activity index
Figure 1.
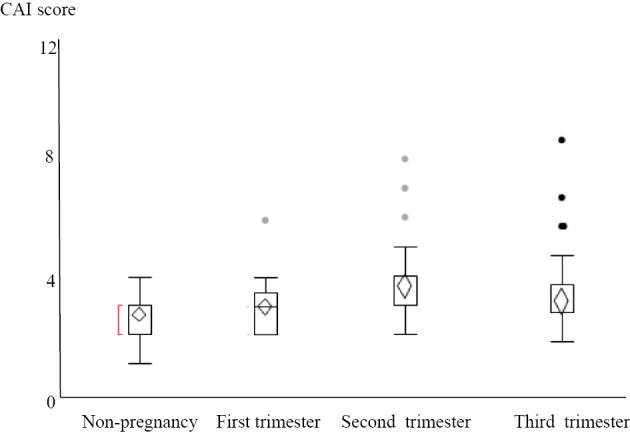
Clinical characteristics and course of UC in the remission groups. UC: ulcerative colitis
Figure 2.
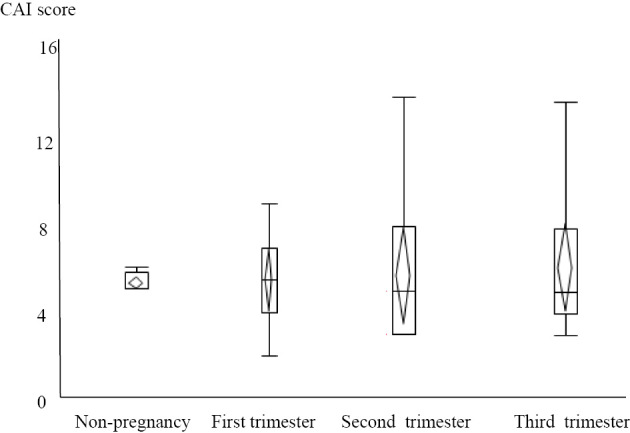
Clinical characteristics and course of UC in the active groups. UC: ulcerative colitis
Abnormal pregnancy/abnormal delivery
Among the 53 pregnancies, spontaneous abortion occurred at week 8 of gestation in 1 case from the remission group, and ectopic pregnancy was detected at week 9 of gestation in 1 case from the active group. Except for these two cases, live infants were delivered in all other pregnancies (51/53, 96%). Abnormal pregnancy was observed in 7 cases (17%) in the remission group and 4 cases (33.3%) in the active group, with the difference not being significant. There was no trend toward a specific type of abnormal pregnancy. Abnormal delivery occurred in 16 of 53 pregnancies (30.1%), including 8 of 41 pregnancies (19.6%) in the remission group and 8 of 12 pregnancies (66.6%) in the active group, with the frequency of abnormal delivery being significantly higher in the active group (p=0.0018). In both groups, the most frequent abnormal event was delivery of a low-birth-weight infant (Table 3).
Table 3.
Frequency of Abnormal Pregnancy and Abnormal Delivery in 53 Pregnancies.
| Remission group n=41 |
Active group n=12 |
p value | |
|---|---|---|---|
| Abnormal pregnancy | 7 (17%) | 4 (33.3%) | NS |
| Hyperemesis gravidarum | 3 (7.3%) | 1 (8.3%) | NS |
| Ectopic pregnancy | 1 (2.5%) | 0 | |
| Pregnancy induced hypertension | 3 (7.3%) | 1 (8.3%) | NS |
| Hydatidiform mole | 1 (2.5%) | 0 | |
| Insufficient amniotic fluid volume | 1 (2.5%) | 0 | |
| Congenital anomaly (cardiac disease) | 1 (2.5%) | 0 | |
| Abnormal delivery | 8 (19.6%) | 8 (66.6%) | 0.0018 |
| Abortion | 1 (2.5%) | 0 | |
| Threatened abortion | 0 | 0 | |
| Premature delivery | 2 (5%) | 2 (18.2%) | NS |
| Threatened premature delivery | 0 | 0 | |
| Stillbirth | 0 | 0 | |
| Placental abnormality | 1 (2.5%) | 2 (18.2%) | NS |
| Low birth weight infant | 5 (12.5%) | 4 (36.4%) | NS |
| Birth defect | 2 (5%) | 0 | |
| Hydatidiform mole | 1 (2.5%) | 0 |
One or more events per patient were noted in some cases.
Course of pregnancy/delivery
Delivery was normal in 37 (70%) pregnancies (normal group), while abnormal delivery occurred in 16 (30%) pregnancies (abnormal group). The comparison of the clinical characteristics of patients of the two groups showed that the number of pregnancies starting in the active phase of UC was higher (p=0.025), age of onset of UC was older (p=0.03), and duration of UC was shorter (p=0.015) in the abnormal group than in the normal group. There was no marked difference in the treatment of UC between the two groups. A multivariate analysis using the stepwise procedure to identify the clinical features associated with abnormal pregnancy selected two factors: duration between the diagnosis of UC and pregnancy and the CAI in the first trimester of pregnancy. A shorter duration of UC was associated with abnormal pregnancy (odds ratio: 1.16 per year), as was a higher CAI in the first trimester (odds ratio: 1.49 per point) (Table 4).
Table 4.
Clinical Characteristics and Course of UC in the Normal Pregnancy and Delivery Group and the Abnormal Group.
| Univariate analysis | Multivariate analysis |
||||
|---|---|---|---|---|---|
| Normal group n=37 |
Abnormal group n=16 |
p value | Odds ratio | ||
| Pregnancy commenced at remission /active phase | 33 / 4 | 8 / 8 | 0.02 | ||
| Remission throughout pregnancy, yes/no* | 28 / 9 | 8 / 6 | NS | ||
| Age of onset (year) | 25 (12-38) | 27.5 (14-34) | 0.03 | ||
| Duration of UC (year)¶ | 7 (2-27) | 5.1 (0.5-15) | 0.03 | 1.16 | |
| Age at pregnancy (year) | 32 (16-42) | 32 (20-40) | NS | ||
| CAI in the first trimester¶ | 3 (2-9) | 4 (2-9) | 0.01 | 1.49 | |
| CAI in the second trimester | 4 (2-8) | 4 (2-14) | NS | ||
| CAI in the third trimester | 3 (2-9) | 3.5 (2-13) | NS | ||
| Type of disease pancolitis/left-sided/proctitis | 15 / 9 / 13 | 3 / 7 / 6 | NS | ||
| Details of treatment during pregnancy | |||||
| Medication, Yes / No | 33 / 4 | 13 / 3 | NS | ||
| oral 5-ASA / enema / suppository | 30 / 1 / 3 | 11 / 2 / 2 | NS | ||
| oral steroid / enema / suppository | 5 / 3 / 6 | 1 / 1 / 3 | NS | ||
| Apheresis therapy | 2 | 1 | NS | ||
| Immunosuppressants | 1 | 0 | NS | ||
| Biologics (tacrolimus) | 0 | 0 | NS | ||
*Abortion occurred in two cases in the abnormal group. These two cases were excluded from evaluation of maintenance of remission during pregnancy.
¶Included in the logistic analysis as explanatory variables.
UC: ulcerative colitis, CAI: clinical activity index, 5-ASA: 5-nitrosalicylic acid
As mentioned above, two abortions occurred. Of the remaining 51 pregnancies, 41 were delivered vaginally and 10 by Caesarean section.
Discussion
The effect of pregnancy on UC is generally considered to be mild (3). Recurrence of UC during pregnancy is reported in about one third of patients, and a meta-analysis showed that the frequency of such recurrence was similar to that in non-pregnant patients; the reported annual recurrence rate is 32% for non-pregnant UC patients and 34% for pregnant UC patients (4,5). In the present study, the relapse rate of UC during pregnancy was 7.5% in the remission group and 82% in the active group. The relapse rate among female patients 18 to 45 years of age who visited our hospital between January 1, 2009, and December 31, 2012, and who had been followed for more than 1 year was 18.7%.
In our data, there were no significant differences in the relapse rate between non-pregnant and pregnant subjects. Some studies (6,7) have shown that the UC disease activity is higher when pregnancy starts in the active phase of UC than when pregnancy commences in the remission phase, suggesting that it is desirable to become pregnant when the disease is in remission. However, these studies did not include detailed evaluation of the clinical activity of UC at the onset of pregnancy and the clinical course of UC during pregnancy. In the present study, the CAI was used for the evaluation of disease activity during pregnancy, when it is relatively difficult to perform invasive investigations. The recurrence/exacerbation rate was significantly lower in the remission group than in the active group (patients with CAI ≤4 maintained for 1 year before pregnancy). We also observed no marked differences in the clinical features between the remission and active groups (Table 1). However, a comparison of the frequency of recurrence/exacerbation during pregnancy between the groups showed that recurrence/exacerbation was significantly more frequent in the active group than in the remission group (Table 2). Based on the CAIs, recurrence/exacerbation occurred during the first and second trimesters of pregnancy in both groups. Especially in the active group, the CAI score increased (recurrence) in the first and second trimesters, and CAI did not improve even in the third trimester (Table 2). These results indicate deterioration of the clinical state of UC during pregnancy in the active group, further suggesting that becoming pregnant during the remission phase of UC is desirable.
UC recurrence was also noted in three patients in the remission group. Of these three pregnancies, medications for UC were discontinued due to recurrence in two cases. Medications for UC were discontinued after the detection of pregnancy in one case in the active group. Treatment of UC is sometimes discontinued after the detection of pregnancy, as seen in this study. One reason for the discontinuation of UC therapy is hyperemesis gravidarum (morning sickness) in the first trimester. If a patient cannot tolerate oral medications due to severe hyperemesis gravidarum, local treatment or a reduction of the number of oral doses should be considered. Another cause of the discontinuation of oral drugs for UC is patients' concern about the effects on the fetus, with some patients deciding to discontinue oral drugs for UC themselves. It is important to explain the effects of drugs on the fetus to pregnant women with UC. One report suggested that when the benefits from controlling disease activity outweigh the risks of abnormal pregnancy and fetal toxicity, treatment for UC should be actively administered (5). The safety of azathioprine and anti-tumor necrosis factor (TNF) therapy in pregnant women was evaluated in the European Crohn's and Colitis Organization (ECCO) guideline (5), and studies performed in Japan have also reported the safety of UC medication (8,9). Obstetricians, gastroenterologists, and physicians should understand the concerns of pregnant women regarding the possible effects of these drugs on the fetus and should try to be actively involved in the treatment of pregnant women with UC (10).
The incidence rates of abnormal pregnancy and delivery, such as premature delivery and delivery of a low-birth-weight infant, are reportedly slightly higher in pregnant women with UC than in those without UC (11). Indeed, premature delivery and delivery of a low-birth-weight infant were relatively frequent in this study. We believe that one cause of this increased incidence rate of low-birth-weight infants may be the relatively light body weight of pregnant women. In the 51 pregnancies, the mean body mass index (BMI) of the mothers was 22.8 kg/m2 at the time of the 42 deliveries for which BMI data were available. Delivery of a low-birth-weight infant is frequent in pregnant women with UC, probably because the mother's body weight is usually lighter than that of pregnant women without UC. In addition, it was reported that delivery of a low-birth-weight infant is related to maternal anemia and poor nutrition (12).
A multivariate analysis of the clinical features of the normal (pregnancy/delivery) group and the abnormal group showed a higher risk of abnormal pregnancy in pregnant patients with a shorter duration of UC and higher CAI in the first trimester. Physicians should give this information to the patients at the time of the diagnosis. We believe that the increased risk of abnormal pregnancy in patients with a short duration of UC may be related to the poor overall understanding of the disease and poor compliance with treatment. Therefore, better patient education is necessary. The higher CAI during early pregnancy in this study was probably related to recurrence or exacerbation associated with the discontinuation of medications for UC. Accordingly, active treatment is required, even after the detection of pregnancy (5).
This study is a retrospective study of patients who were able to continue hospital visits throughout the entire gestation period. Therefore, there is a possibility of selection bias. This is a limitation associated with the present study.
Conclusion
Factors that may influence the clinical course of UC during pregnancy and the outcomes of pregnancy and delivery were evaluated in pregnant women with UC. These results showed a higher frequency of recurrence/exacerbation when pregnancy started in the active phase of UC (CAI ≥5 for at least 1 month) than when pregnancy started in the remission phase of UC (CAI ≤4). Recurrence/exacerbation of UC during pregnancy was not associated with improvement in the clinical state of UC (CAI) until after delivery. The frequency of abnormal pregnancy/delivery was higher in pregnancies that commenced in the active phase of UC than in those during remission. The risk of abnormal pregnancy was higher in patients with a shorter duration of UC and higher CAI in the first trimester than in others. Therefore, the clinical course of UC (CAI) during pregnancy seems to influence the outcome of pregnancy and delivery.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Professor Satoshi Kusuda (Maternal and Perinatal Center, Tokyo Women's Medical University) and Assistant Professor Satoru Shimizu (Research Division, Tokyo Women's Medical University Medical Research Institute) for their excellent support and clinical advice throughout the study.
References
- 1. Suzuki Y. 2015 Report of a research project for action for specified intractable diseases supported by Health and Labour Sciences Research Grants “A study of refractory inflammatory bowel disease.” [Google Scholar]
- 2. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 330: 1841-1845, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Bortoli A, Pedersen N, Duricova D, et al. Pregnancy outcome in inflammatory bowel disease: prospective European case-control ECCO-EpiCom study, 2003-2006. Aliment Pharmacol Ther 38: 328, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Alstead EM. Inflammatory bowel disease in pregnancy. Postgrad Med J 78: 23-26, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van der Woude CJ, Ardizzone S, Bengtson MB, et al. ; European Crohn's and Colitis Organization The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 9: 107-124, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Abhyankar A, Ham M, Moss AC, et al. Meta-analysis: the impact of disease activity at conception on disease activity during pregnancy in patients with inflammatory bowel disease. Aliment Pharmacol Ther 38: 460-466, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen OH, Andreasson B, Bondesen S, et al. Pregnancy in ulcerative colitis. Scand J Gastroenterol 18: 735-742, 1983. [DOI] [PubMed] [Google Scholar]
- 8. Komoto S, Motoya S, Nishiwaki Y, et al. Pregnancy outcome in women with inflammatory bowel disease treated with anti-tumor necrosis factor and/or thiopurine therapy: a multicenter study from Japan. Intest Res 14: 139-145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naganuma M, Kunisaki R, Yoshimura N, et al. Conception and pregnancy outcome in women with inflammatory bowel disease: a multicentre study from Japan. J Crohns Colitis 5: 317-323, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Julsgaard M1, N›rgaard M, Hvas CL, et al. Self-reported adherence to medical treatment prior to and during pregnancy among women with ulcerative colitis. Inflammatory Bowel Dis 17: 1573-1580, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Stephansson O, Larsson H, Pedersen L, et al. Congenital abnormalities and other birth outcomes in children born to women with ulcerative colitis in Denmark and Sweden. Inflamm Bowel Dis 17: 795-801, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Lin HC, Chiu CC, Chen SF, et al. Ulcerative colitis and pregnancy outcomes in an Asian population. Am J Gastroenterol 105: 387-394, 2010. [DOI] [PubMed] [Google Scholar]


