Abstract
We herein report a 55-year-old woman who presented with erythema and bilateral hilar lymphadenopathy 4 months prior to the detection of pancreatic lesions on an ultrasound. A skin biopsy showed evidence of sarcoidosis. The largest lesion in the tail of the pancreas was hypoechoic on endoscopic ultrasonography (EUS). The lesion was initially iso-enhanced on contrast enhanced-EUS (CE-EUS) but subsequently became hypoenhanced. The lesion revealed heterogeneous components of both soft and hard tissue on EUS elastography. She was ultimately diagnosed with pancreatic sarcoidosis based on the presence of noncaseating granulomas seen on pancreatic tissue retrieved through an EUS-guided fine needle aspiration biopsy.
Keywords: pancreas, sarcoidosis, endoscopic ultrasonography (EUS), contrast enhanced-EUS, EUS elastography
Introduction
Sarcoidosis is a multiorgan disease characterized by the presence of noncaseating granulomas that mainly affects the lymph nodes, lung, heart, liver, spleen, eyes and kidney (1,2). The pancreas is rarely affected, with a frequency of pancreatic sarcoidosis of 1.8% in Japan according to an autopsy study (1). Therefore, the radiological appearance of pancreatic sarcoidosis has not been well described. Indeed, only a few reports have described the imaging characteristics of pancreatic sarcoidosis on computed tomography (CT) or magnetic resonance imaging (MRI) (3-5), and only one report has described the findings on endoscopic ultrasonography (EUS) (6).
We herein report a case of biopsy-proven pancreatic sarcoidosis in which the findings on EUS, contrast enhanced-EUS (CE-EUS) and EUS elastography are described.
Case Report
A 55-year old woman presented for the evaluation of a pancreatic lesion detected on ultrasonography (US) during the evaluation of diabetes and dyslipidemia. Four months prior to the detection of the pancreatic lesion, she was diagnosed with sarcoidosis on the basis of bilateral hilar lymphadenopathy, cutaneous erythema and a skin biopsy showing noncaseating granulomas. The cutaneous erythema resolved prior to the admission. Specific symptoms such as abdominal pain were absent. She was not anemic or jaundiced. There was no palpable abdominal mass, nor did she have any history of alcohol or tobacco use.
Laboratory tests showed elevated levels of γ-globulin (1.54 g/dL), lysozyme (17.2 μg/mL) and angiotensin-converting enzyme (ACE; 37.0 IU/L). Levels of carcinoembryonic antigen (CEA; 2.5 ng/mL), carbohydrate antigen 19-9 (CA19-9; 6.0 IU/mL), DUPAN-2 (≤ 25 IU/mL) and s-pancreas-1 antigen (Span-1; 5.4 IU/mL) were normal, as were serum amylase (37 IU/L) and lipase (48 IU/L). A tuberculin skin test was negative.
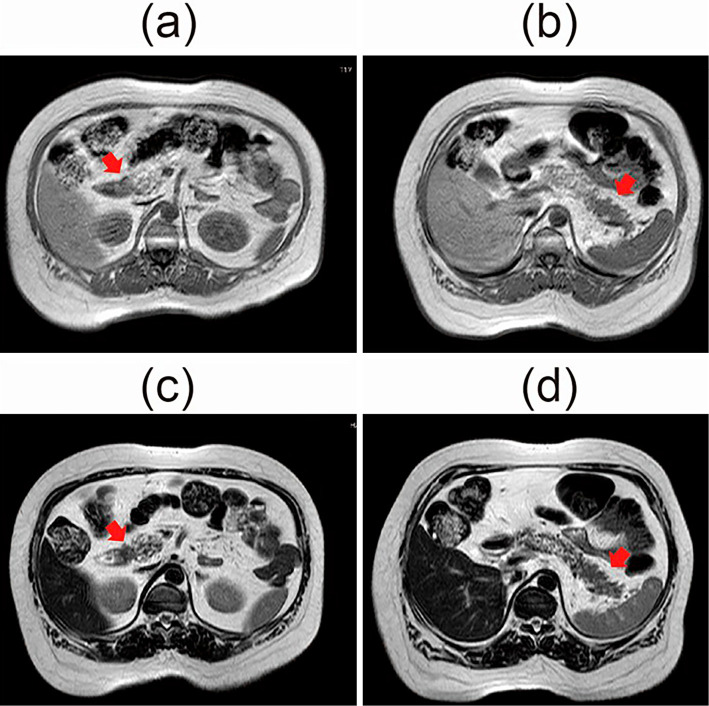
The pancreatic lesion was not visualized on repeat US and was not detected on plain CT (Fig. 1). Contrast-enhanced CT was not performed because of contrast media allergy. MRI revealed the presence of pancreatic lesions in the pancreatic head and tail. These pancreatic tumors showed slightly low intensity on T1-weighted images and slightly high intensity on T2-weighted images compared to the pancreatic parenchyma and liver (Fig. 2). In addition, positron emission tomography-CT (PET-CT) revealed several accumulated lesions in the head of the pancreas (1.0-cm in size) and in the tail of pancreas (4.0-cm in size) [head: standard uptake value (SUV)max=7.2, tail: SUVmax=13.7] (Fig. 3). The main pancreatic duct (MPD) was not dilated on endoscopic retrograde pancreatography (ERP) or magnetic resonance cholangiopancreatography (MRCP), although the pancreatic duct of the tail was not well visualized.
Figure 1.

Plain computed tomography (CT) could not visualize the pancreatic lesions.
Figure 2.
Magnetic resonance imaging (MRI) revealed the presence of pancreatic lesions. These pancreatic lesions showed slightly low intensity on T1-weighted images (a: head, b: tail) and slightly high intensity on T2-weighted images (c: head, d: tail) compared to the pancreatic parenchyma and the liver.
Figure 3.

Positron emission tomography-CT (PET-CT) revealed accumulated lesions in the head and tail of the pancreas (head: SUVmax=7.2, tail: SUVmax=13.7). SUV: standard uptake value
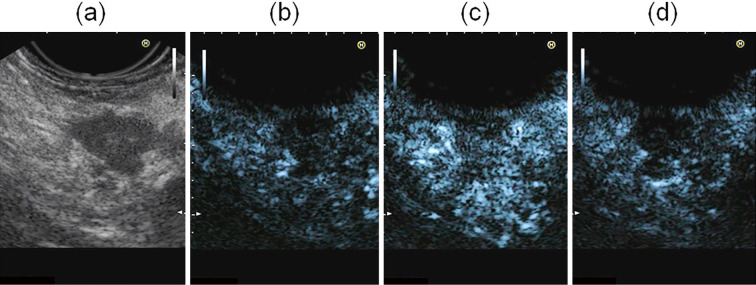
Conventional EUS (EG3870UTK; Pentax Japan, Tokyo, Japan) combined with Preirus (Hitachi Medical Systems, Tokyo, Japan) revealed several 1-cm lesions in the pancreatic head and a 4-cm lesion in the pancreatic tail that was observed on positron emission tomography (PET)-CT (Fig. 4). The lesions were mosaic echoic but mainly hypoechoic, and the border between the tumor and parenchyma of the pancreas was clearly distinguishable. Pancreatic ducts were not dilated throughout the pancreas. We performed enhanced EUS and EUS elastography, focusing only on the lesion in the tail of pancreas because the lesions in the head showed identical features on MRI. Subsequently, ultrasound contrast agent (Sonazoid; Daiichi-Sankyo, Tokyo, Japan) was injected intravenously. At five seconds post-injection, the lesions demonstrated iso-enhancement, and the borders of the lesions became indistinguishable from pancreatic parenchyma. Subsequently, at 30 seconds post-injection, the lesions became hypoenhanced compared to pancreatic parenchyma (Fig. 5). EUS elastography demonstrated the heterogeneity of the lesions ranging from soft tissue to hard tissue. The proportion of hard tissue was greater than that of soft tissue in the heterogenetic lesion (Fig. 6).
Figure 4.
Conventional endoscopic ultrasonography (EUS) demonstrated several 1-cm lesions in the pancreatic head (a) and a 4-cm lesion in the pancreatic tail (b). The lesions were mosaic echoic but mainly hypoechoic, and the border between the tumor and parenchyma of the pancreas was clearly distinguishable.
Figure 5.
Conventional endoscopic ultrasonography (EUS) visualized a hypoechoic lesion at the pancreatic tail: B mode (a) and contrast harmonic mode before the infusion of the ultrasound contrast agent (b). After the infusion of the ultrasound contrast agent, EUS visualized iso-enhancement relative to the surrounding pancreatic parenchyma (c: 5 s.) followed by hypoenhancement compared to the surrounding pancreatic parenchyma (d: 30 s.).
Figure 6.
Endoscopic ultrasonography (EUS) visualized at the pancreatic tail. Heterogeneous coloration of soft and hard tissue is shown.
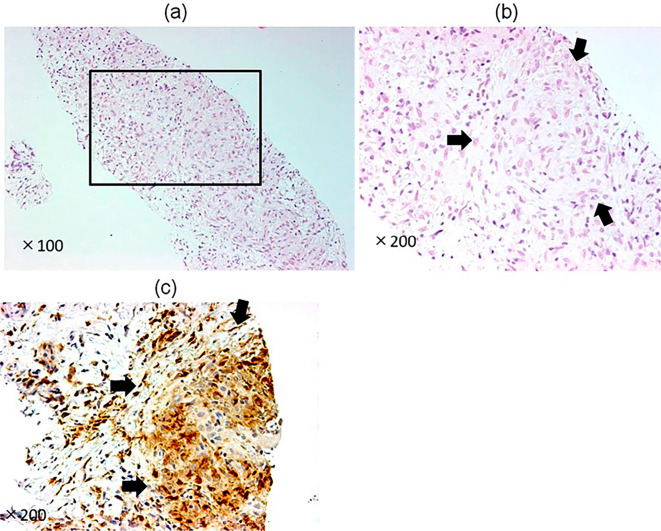
An EUS-guided fine needle aspiration biopsy (EUS-FNAB) using a disposable 22-gauge needle (EZ-Shot 2™; Olympus, Tokyo, Japan) was performed for a 4-cm lesion in the pancreatic tail. An EUS-FNAB was not performed for the head lesions because the head lesions were assumed to be identical to the tail lesion. Histology revealed noncaseating granulomas positive for CD68 on immunostaining and surrounded by collagen fibers (Fig. 7). There was histological evidence of noncaseating granulomas, and she was negative for infectious disease, we therefore speculated that the pancreas lesions were consistent with pancreatic sarcoidosis. The patient was followed for three years after discharge using MRI; no steroids were prescribed. The pancreatic lesions became invisible on both T1- and T2-weighted images, and the pancreas itself returned to normal size.
Figure 7.
(a, b) Hematoxylin and Eosin staining of the pancreatic tumor in the pancreatic tail retrieved by endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) (a: ×100, b: ×200). Noncaseating granulomas and collagen fiber surrounding noncaseating granulomas are shown. (c) The noncaseating granulomas were positive for CD68 on immunostaining.
Discussion
In sarcoidosis, a multi-organ disease characterized by the presence of noncaseating granulomas, the involvement of the pancreas is rare. In an autopsy study the prevalence of pancreatic sarcoidosis was 2.1% among patients with known sarcoidosis and 1.3% among those found to have sarcoidosis post-mortem (1). Until now, there has been no report of the appearances of pancreatic sarcoidosis on CE-EUS and EUS elastography.
Previous reports have indicated that pancreatic sarcoidosis detected on contrast-enhanced CT shows lower density than pancreatic parenchyma (3). In patients without a history of pancreatitis, plain CT shows diffuse pancreatic calcifications (7). The appearances on non-contrast-enhanced MRI are of slightly increased signal intensity on T2-weighted images and slightly decreased signal intensity on T1-weighted images, and lesions are hypointense compared to the pancreatic parenchyma during the arterial phase, becoming iso-intense during the portal phase or delayed phase post-gadolinium (4). In our case, although plain CT could not detect pancreatic lesions, they were consistent with pancreatic sarcoidosis on MRI. Why CT could not detect the tumor in our case is unclear. Nonetheless, the diagnosis should preferably be confirmed by a biopsy, since laboratory tests, radiographic features and nuclear imaging are not always helpful in the diagnosis of pancreatic sarcoidosis.
The pancreatic lesions in our patient were mainly hypoechoic on EUS, which is similar to the appearance of pancreatic cancer. Findings on CE-EUS were also similar to pancreatic cancer (8,9). There have been no reports to date describing the findings of pancreatic sarcoidosis on CE-EUS. EUS imaging of pancreatic sarcoidosis may distinguish this entity from mild pancreatic inflammation (6). Pancreatic sarcoidosis may resemble chronic pancreatitis in the presence of fibrotic tissue. Pancreatic blood flow also decreases as pancreatic fibrosis progresses (10).
The findings on EUS elastography were also similar to the appearance of PC, which appears large blue on the elastogram (11). Heterogeneous elastograms ranging from soft tissue to hard tissue may indicate the presence of variable amounts of collagen fiber surrounding the noncaseating granulomas.
We performed EUS-FNA to confirm the diagnosis and rule out pancreatic cancer to determine the treatment strategy. The size of the retrieved specimen is crucial and may be problematic when it is too small to show characteristic findings for the disease, i.e., noncaseating granulomas for sarcoidosis. Although challenging, noncaseating granulomas need to be distinguished from immune reaction caused by Hodgkin's disease, non-Hodgkin's lymphoma and carcinoma, including pancreatic cancer (12-14). In this regard, other clinical features that support the diagnosis are helpful, i.e., a history of sarcoidosis of the skin or absence of infectious disease. Autoimmune pancreatitis and chronic pancreatitis are other differential diagnoses for focal swelling of the pancreas. However, there have been no reports showing an association between noncaseating granulomas and autoimmune pancreatitis or chronic pancreatitis.
In summary, this is the first report of the appearances of pancreatic sarcoidosis on EUS, CE-EUS and EUS elastography. These points differ from previous reports on pancreatic sarcoidosis. In addition, EUS-FNA plays a cardinal role in the diagnosis, although the definitive diagnosis of pancreatic sarcoidosis remains a challenge when extra-pancreatic manifestations are not present.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to thank Dr. Venessa Pattullo (Sydney Medical School, University of Sydney) and Dr. Gavin Pattullo (Royal North Shore Hospital, University of Sydney, Australia) for English assistance in the preparation of the manuscript.
References
- 1. Iwai K, Tachibana T, Hosoda Y, Matsui Y. Sarcoidosis autopsies in Japan. Sarcoidosis 5: 60-65, 1988. [PubMed] [Google Scholar]
- 2. Schauer RJ, Völker U, Kreuzmayr A. An unorthodox pancreatic lesion in a young man presenting with jaundice. Gastroenterology 141: 1563, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Bonhomme A, Dhadamus A, De Bie P, Van Hoe L, Baert AL. Pancreatic involvement in systemic sarcoidosis: CT findings. J Belge Radiol 80: 116-117, 1997. [PubMed] [Google Scholar]
- 4. Baroni RH, Pedrosa I, Tavernaraki E, Goldsmith J, Rofsky NM. Pancreatic sarcoidosis: MRI features. J Magn Reson Imaging 20: 889-893, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics 31: 993-1015, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Romboli E, Campana D, Piscitelli L, et al. . Pancreatic involvement in systemic sarcoidosis. A case report. Dig Liver Dis 36: 222-227, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Folz SJ, Johnson CD, Swensen SJ. Abdominal manifestations of sarcoidosis in CT studies. J Comput Assist Tomogr 19: 573-579, 1995. [DOI] [PubMed] [Google Scholar]
- 8. Yasuda K, Mukai H, Nakajima M. Endoscopic ultrasonography diagnosis of pancreatic cancer. Gastrointest Endosc Clin N Am 5: 699-712, 1995. [PubMed] [Google Scholar]
- 9. Kitano M, Kudo M, Yamao K, et al. . Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol 107: 303-310, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Azemoto N, Kumagi T, Yokota T, et al. . Utility of contrast-enhanced transabdominal ultrasonography to diagnose early chronic pancreatitis. Biomed Res Int 2015: 393124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Itokawa F, Itoi T, Sofui A, et al. . EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol 46: 843-853, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Bacal D, Hoshal VL, Schaldenbrand JD, Lampman RM. Sarcoidosis of the pancreas: case report and review of the literature. Am Surg 66: 675-678, 2000. [PubMed] [Google Scholar]
- 13. Lee YN. Tissue diagnosis for carcinoma of the pancreas and periampullary structures. Cancer 49: 1035-1039, 1982. [DOI] [PubMed] [Google Scholar]
- 14. Brickner H. Sarcoid reactions in malignant tumors. Cancer Treat Rev 13: 147-156, 1986. [DOI] [PubMed] [Google Scholar]