Abstract
Sepsis caused by a Capnocytophaga canis infection has only been rarely reported. A 67-year-old female with a past medical history of splenectomy was admitted to our hospital with fever and general malaise. She had been bitten by a cat. She showed disseminated intravascular coagulation and multi-organ failure because of severe sepsis. On blood culture, characteristic gram-negative fusiform rods were detected; therefore, a Capnocytophaga species infection was suspected. A nucleotide sequence analysis revealed the species to be C. canis, which was newly identified in 2016. C. canis may have low virulence in humans; however, C. canis with oxidase activity may cause severe zoonotic infection.
Keywords: Capnocytophaga canis, Capnocytophaga canimorsus, sepsis, oxidase activity
Introduction
The genus Capnocytophaga consists of gram-negative rod-shaped bacteria that reside in the oral cavities of humans and domestic animals. Capnocytophaga formerly comprised eight species (1,2). Of these, six species (C. gingivalis, C. granulosa, C. haemolytica, C. leadbetteri, C. ochracea, and C. sputigena) inhabit the human oral cavity and commonly cause dental infections. The other two species (C. canimorsus and C. cynodegmi) inhabit the oral cavities of dogs and cats and sometimes cause local wound infection and systemic infection in humans after a dog or cat bite. Recently, C. canis has been added (3). C. canis has been isolated from the oral cavity of healthy dogs and is assumed to have low pathogenicity in humans (3).
Sepsis caused by C. canis infection has only been rarely reported. We herein report the successful treatment of C. canis-related severe sepsis.
Case Report
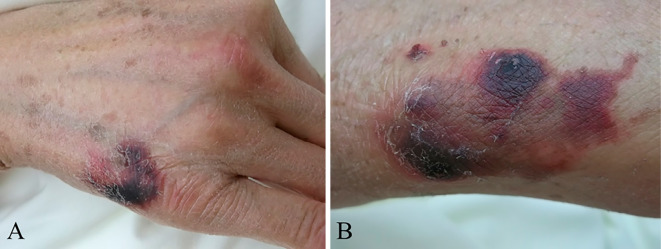
A 67-year-old woman was admitted to our hospital with general malaise and fever for 3 days starting the day after being bitten by a cat on both her hands. She had a medical history of idiopathic portal hypertension and had undergone a splenectomy for esophageal and pharyngeal varices at 44 years of age. She had no pneumococcal and meningococcal vaccination. On admission, she was awake and oriented. Her vital signs were as follows: temperature, 39.8°C; blood pressure, 96/52 mmHg; pulse rate, 99 beats/min; respiratory rate, 20 breaths/min; and percutaneous oxygen saturation (SpO2), 96% on room air. The findings of a general examination were unremarkable except for oozing from her lips and cat bite wounds on the back of her right hand and on her left wrist. The cat bite wounds were crusted and dark purple, and had no redness and swelling (Fig. 1). The axillary and cervical lymph nodes were not swollen and there were no signs of lymphadenitis suggesting Bartonella infection. The rabies vaccination history of the cat was unknown, but no abnormal behavior was observed. Laboratory data at admission revealed leukocytosis with a left shift, thrombocytopenia, and liver and renal dysfunction (Table 1), indicating disseminated intravascular coagulation (DIC) and multi-organ failure because of bacterial infection. No source of bacterial infection was identified on whole-body computed tomography scan and echocardiography. After entering the emergency ward, dopamine was administered because she went into septic shock (systolic blood pressure, 60-70 mmHg). Antibiotics were administered after obtaining blood cultures. Since the cat bite wounds were in the healing process, as an empiric therapy for sepsis of unknown origin, we selected broad-spectrum antibiotics (panipenem/betamipron, PAPM/BP, 4 g/day), which are effective in treating pathogens associated with animal bites (including Pasteurella multocida, Streptococcus species, Staphylococcus species and Capnocytophaga species). The wounds were small and no sign of infection was noted, so human tetanus immune globulin was not administered.
Figure 1.
Small scars with scabs on the back of right hand (A) and left wrist (B).
Table 1.
Laboratory Findings on Admission.
| Normal range | Normal range | ||||||
|---|---|---|---|---|---|---|---|
| Albumin | 2.3 | g/dL | 3.8-5.3 | WBC | 270 | ×102/μL | 40-85 |
| ALP | 1,086 | IU/L | 100-350 | MYELO | 4 | % | |
| AST | 47 | IU/L | 5-40 | META | 23 | % | |
| ALT | 26 | IU/L | 4-40 | BAND | 41 | % | |
| T-Bil | 4.7 | mg/dL | 0.3-1.2 | SEG | 30 | % | |
| LYMP | 2 | % | |||||
| BUN | 45.4 | mg/dL | 8-22 | RBC | 370 | ×104/μL | 370-510 |
| Cre | 0.95 | mg/dL | 0.4-0.8 | Hb | 11.7 | g/dL | 11-15 |
| Na | 133 | mEq/L | 135-148 | PLT | 0.4 | ×104/μL | 13-35 |
| K | 2.9 | mEq/L | 3.5-5.0 | ||||
| FDP | 13.4 | µg/mL | 0-10 | ||||
| CRP | 30.65 | mg/dL | 0.0-0.3 | Fibrinogen | 378 | mg/dL | 180-400 |
| PCT | 61.85 | ng/mL | 0.0-0.5 | PT-INR | 1.28 |
ALP: alkaline phosphatase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, T-bil: total bilirubin, BUN: blood urea nitrogen, Cre: creatinine, CRP: C-reactive protein, PCT: procalcitonin, WBC: white blood cell count, MYELO: myelocyte, META: metamyelocyte, BAND: band neutrophil, SEG:segmented neutrophil, LYMP: lymphocyte, RBC: red blood cell count, Hb: hemoglobin, PLT: platelet count, FDP: fibrin degradation product, PT-INR: prothrombin time-international normalized ratio
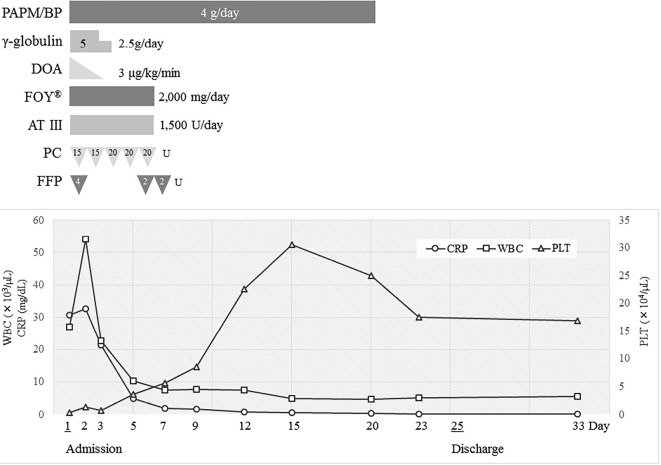
She responded well to the treatment; her fever and hypotension improved quickly, and her C-reactive protein level decreased. Her condition gradually improved, and she was discharged on day 25 after admission. The antibiotics had been administered continuously until her white blood cell count and C-reactive protein level had normalized (for 3 weeks). We did not de-escalate the antibiotics, because it took 21 days to identify the bacteria. Her clinical course is shown in Fig. 2.
Figure 2.
The clinical course of this case. PAPM/BP: panipenem/betamipron, DOA: dopamine, FOY®: gabexate mesilate, AT III: antithrombin III, PC: platelet concentrate, FFP: fresh frozen plasma, WBC: white blood cell count, CRP: C-reactive protein, PLT: platelet count
Bacterial identification
On day 2, gram-negative rods were recognized in a smear of anaerobic blood culture fluid (Fig. 3). Capnocytophaga species were suspected because of the characteristic morphological features of fusiform appearance of the bacteria and her history of cat bites. The isolates were oxidase activity-positive and catalase activity-positive, suggesting that the bacteria were animal-derived Capnocytophaga species, such as C. canimorsus and C. cynodegmi (4). Based on the results of tests performed using ID Test HN-20 Rapid Kit (Nissui Pharmaceutical, Tokyo, Japan), the bacteria were presumed to be C. canimorsus on day 21. To confirm the bacteria, isolates were sent to the National Institute of Infectious Diseases in Japan. Comparative sequence analyses conducted at this institution revealed that the 16S rRNA and gyrB gene sequence identities were 96.9% and 75.5% for the C. canimorsus-type strain (ATCC 35979) and were 96.9% and 77.6% for the C. cynodegmi-type strain (ATCC 49044), respectively, on Basic Local Alignment Search Tool (BLAST) search (5). As of 2014, no known bacteria had a 16S rRNA sequence identity exceeding 98.7-99% (6), which is the threshold value above which bacteria are considered to be of the same species. The closest related bacteria were C. canimorsus and C. cynodegmi according to the results of the 16S rRNA sequence matching.
Figure 3.
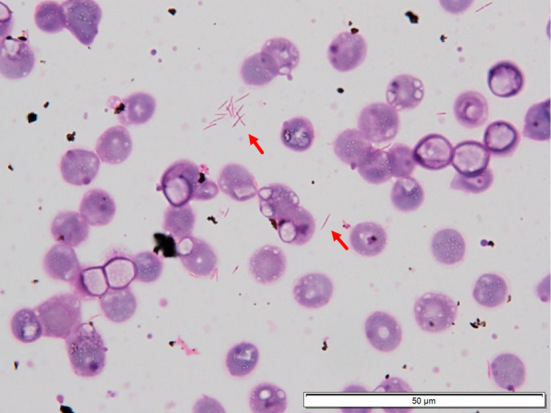
A blood culture smear with gram staining showing gram-negative fusiform-shaped rods (arrow).
In 2015, a novel species C. canis was reported (3). Thereafter, we performed the BLAST search again using the database of whole-genome shotgun contigs. The sequence identities between our strain and the C. canis-type strain (LMG29146) for 16S rRNA and the gyrB gene were 99.8% and 99.5%, respectively. As the identities were extremely high, our strain was therefore identified as C. canis.
Discussion
Capnocytophaga species are facultative anaerobic, fusiform, fastidious gram-negative rods. They grow slowly (2-7 days) on blood or chocolate agar in 10% CO2. C. canimorsus and C. cynodegmi can cause zoonotic infection ranging from local infection to severe sepsis, and fatal cases are mainly associated with C. canimorsus (1). The mortality rate of C. canimorsus infection has been estimated at 26% (7). As C. canis can also cause severe sepsis, it may have the mortality rate comparable to that of C. canimorsus.
C. canis is a novel species of the genus Capnocytophaga, and it was identified in the oral cavity of healthy dogs. Although the clinical features of C. canis infection have not yet been reported, experimental results suggest that C. canis is less virulent than C. canimorsus in humans (3). Most strains of C. canimorsus have a cytochrome-oxidase activity and the ability to deglycosylate human IgM, and these characteristics are related to its pathogenicity in humans. The detailed mechanism of oxidase activity is still unknown, but the fact that C. canimorsus strains without oxidase activity could not grow in human serum suggests that oxidase activity is responsible for pathogenicity of C. canimorsus (3). On the other hand, almost all strains of C. canis are oxidase-negative (97.1%) and cannot deglycosylate human IgM (97.1%) (3). An isolate from a patient reportedly suspected of having sepsis caused by C. canimorsus because of cat scratches in 2013 (8) was also identified as C. canis through the same analysis as that in our report (Suzuki et al., manuscript in preparation). Both this strain and our strain were oxidase positive. In addition, a strain of C. canis with oxidase activity isolated from cat bite wounds was reported (9). The rare C. canis strain with oxidase activity is a dangerous pathogen for humans and can cause severe sepsis similar to that caused by C. canimorsus.
On the host side, the risk factors for severe infection of C. canimorsus are splenectomy, alcoholic addiction (liver disease caused by alcohol abuse), and immunosuppression (1,7). Our case had undergone a splenectomy and the past case of C. canis infection (8) was a heavy drinker, so the risk factor of C. canis infection may be similar to that of C. canimorsus.
Our case is the first clinical report of severe sepsis caused by C. canis. However, C. canis can be mistakenly identified as C. canimorsus with catalase and oxidase activity tests and simple bacterial identification kits that are based on biochemical characteristics, and therefore, some cases of C. canis infection may have been reported as C. canimorsus infection previously (8). We recommend identifying bacteria by genetic procedures, if Capnocytophaga species infection is suspected. The clinical features of C. canis infection will be revealed by collecting a large number of C. canis infection cases.
In drug susceptibility tests, the C. canis strain in this case was broadly susceptible to many antibiotics, including β-lactam antibiotics, except trimethoprim-sulfamethoxazole (Table 2), and the drug susceptibility was similar to that of previously reported C. canimorsus strains (7). Amoxicillin-clavulanic acid (Augmentin) recommended for C. canimorsus (10) is also effective for C. canis as prophylactic administration and treatment following dog and cat bites.
Table 2.
Drug Susceptibility.
| Classification | generic name | Abbreviation | Sensitivity |
|---|---|---|---|
| Penicillin | ampicillin | ABPC | S |
| amoxicillin / clavulanate | AMPC/CVA | S | |
| Cephem | ceftriaxone | CTRX | S |
| cefotaxime | CTX | S | |
| Carbapenem | imipenem / cilastatin | IPM/CS | S |
| meropenem | MEPM | S | |
| Macrolide | azithromycin | AZM | S |
| Tetracycline | tetracycline | TC | S |
| Fluoroquinolone | ofloxacin | OFLX | S |
| ciprofloxacin | CPFX | S | |
| Chloramphenicol | chloramphenicol | CP | S |
| Antitubercular | rifampicin | RFP | S |
| ST | sulfamethoxazole / trimethoprim | ST | R |
Defined as "susceptible : S", "intermediate : I" or "resistance : R" based on the Clinical and Laboratory Standards Institute M100-S23 (criteria for Haemophilus influenzae).
In conclusion, we herein reported a rare case of C. canis-related severe sepsis. The possible presence of C. canis should therefore be considered along with C. canimorsus and C. cynodegmi in patients presenting with infection after cat and dog bites or cat scratches.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Gaastra W, Lipman LJ. Capnocytophaga canimorsus. Vet Microbiol 140: 339-346, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Frandsen EV, Poulsen K, Könönen E, Kilian M. Diversity of Capnocytophaga species in children and description of Capnocytophaga leadbetteri sp. nov. and Capnocytophaga genospecies AHN8471. Int J Syst Evol Microbiol 58: 324-336, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Renzi F, Dol M, Raymackers A, Manfredi P, Cornelis GR. Only a subset of C. canimorsus strains is dangerous for humans. Emerg Microbes Infect 4: e48, 2015. Corrigendum in: 5: e29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner DJ, Hollis DG, Fanning GR, Weaver RE. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J Clin Microbiol 27: 231-235, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki M, Kimura M, Imaoka K, Yamada A. Prevalence of Capnocytophaga canimorsus and Capnocytophaga cynodegmi in dogs and cats determined by using a newly established species-specific PCR. Vet Microbiol 144: 172-176, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 33: 152-155, 2006. [Google Scholar]
- 7. Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 34: 1271-1280, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Yamamoto U, Kunita M, Mohri M. Shock following a cat scratch. BMJ Case Rep: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zangenah S, Abbasi N, Andersson AF, Bergman P. Whole genome sequencing identifies a novel species of the genus Capnocytophaga isolated from dog and cat bite wounds in humans. Sci Rep 6: 22919, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lloret A, Egberink H, Addie D, et al. Capnocytophaga canimorsus infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 15: 588-590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]