Abstract
Background
Enterococcus faecium, especially vancomycin-resistant E. faecium (VREfm), is a major concern for patients with hematologic diseases. Exposure to antibiotics including fluoroquinolone, which is used as a routine prophylaxis for patients with hematologic (MH) diseases, has been reported to be a risk factor for infection with vancomycin-resistant eneterocci. We compared the characteristics of E. faecium isolates according to their vancomycin susceptibility and patient group (MH vs non-MH patients).
Methods
A total of 120 E. faecium bacteremic isolates (84 from MH and 36 from non-MH patients) were collected consecutively, and their characteristics (susceptibility, multilocus sequence type [MLST], Tn1546 type, and the presence of virulence genes and plasmids) were determined.
Results
Among the vancomycin-susceptible E. faecium (VSEfm) isolates, resistance to ampicillin (97.6% vs 61.1%) and high-level gentamicin (71.4% vs 38.9%) was significantly higher in isolates from MH patients than in those from non-MH patients. Notably, hyl, esp, and pEF1071 were present only in isolates with ampicillin resistance. Among the VREfm isolates, ST230 (33.3%) and ST17 (26.2%) were predominant in MH patients, while ST17 (61.1%) was predominant in non-MH patients. Plasmid pLG1 was more prevalent in E. faecium isolates from MH patients than in those from non-MH patients, regardless of vancomycin resistance. Transposon analysis revealed five types across all VREfm isolates.
Conclusions
The antimicrobial resistance profiles and molecular characteristics of E. faecium isolates differed according to the underlying diseases of patients within the same hospital. We hypothesize that the prophylactic use of fluoroquinolone might have an effect on these differences.
Keywords: Ampicillin, Fluoroquinolone, Enterococcus, Multilocus sequence typing, pEF1071, pLG1, ST230
INTRODUCTION
Enterococci have emerged as major nosocomial pathogens that cause healthcare-associated infections as well as prolonged colonization in patients with comorbidities [1]. They are the third most common cause of bacteremia at the Catholic Blood and Marrow Transplantation (BMT) Center of Seoul St. Mary's Hospital, Seoul, Korea. Additionally, vancomycin-resistant enterococci (VRE) account for 28% of all enterococcal bacteremia in hematologic [MH] patients [2,3] compared with approximately 15% in non-MH patients in this hospital. Recent history of antibiotic use, especially fluoroquinolone or broad-spectrum beta-lactam agents, has been reported as the major risk factor for VRE infection [4,5]. Fluoroquinolone is commonly used as a prophylaxis during chemotherapy or stem cell transplantation (SCT) for MH patients.
One of the clinical concerns regarding enterococcal infection stems from its intrinsic resistance to broad-spectrum antibiotics, which can lead to a delay in appropriate antimicrobial therapy. Other important issues regarding VRE include the poor outcomes of infected patients and the possibility of horizontal transfer of vancomycin resistance [6,7]. Vancomycin-resistant Enterococcus faecium (VREfm) has recently been recognized as an endemic nosocomial pathogen in many hospitals worldwide [8,9].
As only a limited number of reports have investigated the molecular characteristics of E. faecium bacteremic isolates according to different host factors and antibiotic resistance, we compared the molecular characteristics of (1) vancomycin-susceptible E. faecium (VSEfm) and VREfm bacteremic isolates in general, (2) VSEfm from MH and non-MH patients, and (3) VREfm from MH and non-MH patients.
METHODS
1. Bacterial isolates
A total of 120 E. faecium bacteremic isolates were collected from January 2012 to December 2013 at Seoul St. Mary's Hospital. An equal number of VSEfm and VREfm isolates were consecutively obtained from adult MH and non-MH patients: 42 VSEfm and 42 VREfm from MH patients, and 18 VSEfm and 18 VREfm from non-MH patients. For this study, E. faecium bacteremia was defined as isolation of E. faecium species from one or more blood cultures using an automated blood culture system (Bactec FX, Becton Dickinson, Sparks, MD, USA) [10]. If E. faecium was repeatedly isolated from a single patient, only the first bacteremic isolate was used for molecular analysis. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital at the Catholic University of Korea with an informed consent waiver because the study utilized previously collected bacterial isolates without any individualized patient information (IRB number: KC14SISI0696).
2. Antimicrobial susceptibility test
The susceptibility to ampicillin, vancomycin, teicoplanin, tigecycline, linezolid, and quinupristin/dalfopristin and high-level resistance to gentamicin (HLGR) and streptomycin were evaluated using the Vitek AST-P600 and Vitek II systems (bioMérieux, Hazelwood, MO, USA), according to the CLSI guidelines [11]. The presence of vanA and vanB was determined using the Seeplex VRE ACE Detection kit (Seegene, Seoul, Korea).
3. Molecular typing, plasmid analysis, and virulence gene profiling
Multilocus sequence typing (MLST) of E. faecium isolates was conducted as previously described, based on seven housekeeping genes (adk, atpA, ddl, gdh, gyd, purk, and pstS) [12]. Different sequences at a given locus were assigned an allele number based on the E. faecium MLST database (http://efaecium.mlst.net), and each unique combination of alleles (the allelic profile) was designated as an ST.
Eight enterococcal plasmids (pIP501, pRE25, PEF1071, pRI, pRUM, pEF418, pMG1, and pLG1) were sequenced by PCR-based typing as previously described [7,13]. The presence of the virulence genes hyl (glucoside hydrolase), cylA (cytolysin), gelE (gelatinase), esp (enterococcal surface protein), acm (adhesin of collagen from E. faecium), scm (second collagen adhesion of E. faecium, fms10), sgrA (serine-glutamate repeat containing protein A), ecbA (E. faecium collagen binding protein, fms18), asa1 and agg (aggregation substances), pilA and pilB (pilus-like structures), fms11, fms14, and fms15 (E. faecium surface proteins) was evaluated by PCR as previously described [13,14].
4. Structural analysis of Tn1546 elements
For the structural analysis of Tn1546 elements, overlapping internal regions of Tn1546 were amplified using PCR as previously described [15]. Representative isolates in each ST exhibiting PCR fragments longer or shorter than those of the BM4147 prototype vanA gene cluster 7 were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). Purified PCR products were directly sequenced using an ABI Prism 3700 DNA Sequencer (Applied Biosystems, Foster City, CA, USA). The acquired nucleotide sequences were analyzed using the BLASTN tool from the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
5. Statistical analysis
Statistical analysis was performed using SPSS software version 24.0 (SPSS Korea, Seoul, Korea). Chi-square analysis and Student's t-test were used to compare categorical variables and continuous variables, respectively. If any of the cells of a contingency table were below five, Fisher's exact test was used to compare categorical variables. Mann-Whitney U test was used to compare nonparametric continuous variables. A two-tailed P value≤0.05 was considered statistically significant.
RESULTS
1. Antimicrobial resistance
The results of resistance to eight antibiotics are listed in Table 1. Ampicillin resistance was observed in 86.7% (52 of 60) of VSEfm isolates and was higher in VSEfm from MH patients than in VSEfm from non-MH patients (97.6% vs 61.1%, P=0.001). HLGR was also higher in VSEfm from MH patients than in VSEfm from non-MH patients (71.4% vs 38.9%, P=0.023). The resistance rate for the other antibiotics was similar in E. faecium isolates from MH and non-MH patients. All 60 VREfm isolates carried the vanA gene.
Table 1. In vitro resistance rate of Enterococcus faecium bacteremic isolates.
| VSEfm (N=60) | VREfm (N=60) | |||||
|---|---|---|---|---|---|---|
| MH (N=42) | Non-MH (N=18) | P | MH (N=42) | Non-MH (N = 18) | P | |
| Ampicillin | 41 (97.6%) | 11 (61.1%) | 0.001 | 42 (100%) | 18 (100%) | - |
| High level gentamicin resistance | 30 (71.4%) | 7 (38.9%) | 0.023 | 26 (61.9%) | 11 (61.1%) | > 0.999 |
| High level streptomycin resistance | 10 (23.8%) | 3 (16.7%) | 0.736 | 5 (11.9%) | 5 (27.8%) | 0.256 |
| Vancomycin | 0 (0%) | 0 (0%) | - | 42 (100%) | 18 (100%) | - |
| Teicoplanin | 0 (0%) | 0 (0%) | - | 41 (97.6%) | 18 (100%) | 0.303 |
| Linezolid | 0 (0%) | 0 (0%) | - | 2 (4.8%)* | 0 (0%) | > 0.999 |
| Quinupristin-dalfopristin | 1 (2.4%)† | 0 (0%) | > 0.999 | 1 (2.4%)† | 1 (5.6%)† | 0.514 |
| Tigecycline | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - |
Data are presented as n (%).
*The minimal inhibitory concentrations (MICs) for two linezolid non-susceptible isolates were ≥8 mg/dL; †The MICs for three quinupristin-dalfopristin non-susceptible isolates were 2 mg/dL.
Abbreviations: MH, hematologic patients; non-MH, non-hematologic patients; VREfm, vancomycin-resistant E. faecium; VSEfm, vancomycin-susceptible E. faecium.
2. MLST
The predominant ST among the 120 E. faecium isolates tested was ST17 (36.7%, 44 of 120), followed by ST230 (19.2%, 23 of 120), ST192 (14.2%, 17 of 120), ST78 (6.7%, 8 of 120), and ST262 (4.2%, 5 of 120). These five major clones represented 80.8% (97 of 120) of E. faecium bacteremic isolates. Table 2 shows the MLST results segregated by vancomycin resistance and a comparison between MH and non-MH patients.
Table 2. Distribution of multilocus sequence types of Enterococcus faecium bacteremic isolates and Tn1546 element characteristics.
| STs* (N) | VSEfm (N=60) | VREfm (N=60) | ||||
|---|---|---|---|---|---|---|
| Multilocus Sequence Typing, N (%)† | Multilocus Sequence Typing, N (%): Tn1546 type (N)† | |||||
| MH (N=42) | Non-MH (N=18) | P | MH (N=42) | Non-MH (N=18) | P | |
| ST17 (44) | 18 (42.9%) | 4 (22.2%) | 0.155 | 11 (26.2%): I (1), II (3), IV (2), V (5) | 11 (61.1%): I (4), II (5), IV (2) | 0.010 |
| ST230 (23) | 6 (14.3%) | 2 (11.1%) | > 0.999 | 14 (33.3%): II (10), IV (4) | 1 (5.6%): II (1) | 0.025 |
| ST192 (17) | 6 (14.3%) | 3 (16.7%) | > 0.999 | 6 (14.3%): II (2), IV (4) | 2 (11.1%): II (1), V (1) | >0.999 |
| ST78 (8) | 4 (9.5%) | 1 (5.6%) | > 0.999 | 2 (4.8%): II (1), III (1) | 1 (5.6%): I (1) | > 0.999 |
| ST262 (5) | 1 (2.4%) | 0 (0%) | > 0.999 | 4 (9.5%): I (1), II (1), IV (2) | 0 (0%) | 0.306 |
| ST18 (4) | 3 (7.1%) | 0 (0%) | 0.547 | 0 (0%) | 1 (5.6%): IV (1) | 0.300 |
| ST812 (4) | 1 (2.4%) | 3 (16.7%) | 0.077 | 0 (0%) | 0 (0%) | - |
| ST64 (2) | 1 (2.4%) | 0 (0%) | > 0.999 | 1 (2.4%): II (1) | 0 (0%) | > 0.999 |
Data are presented as n (%).
*Other STs not presented in the table are singletons (ST66, ST80, ST117, ST178, ST202, ST203, ST233, ST389, ST7850, ST994, ST995, ST996, and ST997); †Chi-square analysis was used to compare categorical variables. If any of the cells of a contingency table are below five, Fisher's exact test was used.
Abbreviations: MH, hematologic patients; non-MH, non-hematologic patients; VSEfm, vancomycin-susceptible E. faecium; VREfm, vancomycin-resistant E. faecium; Tn, transposon.
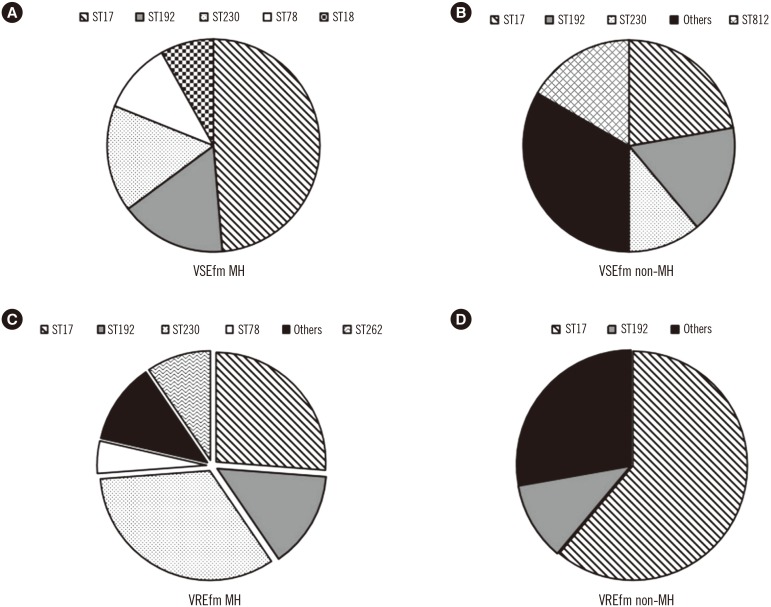
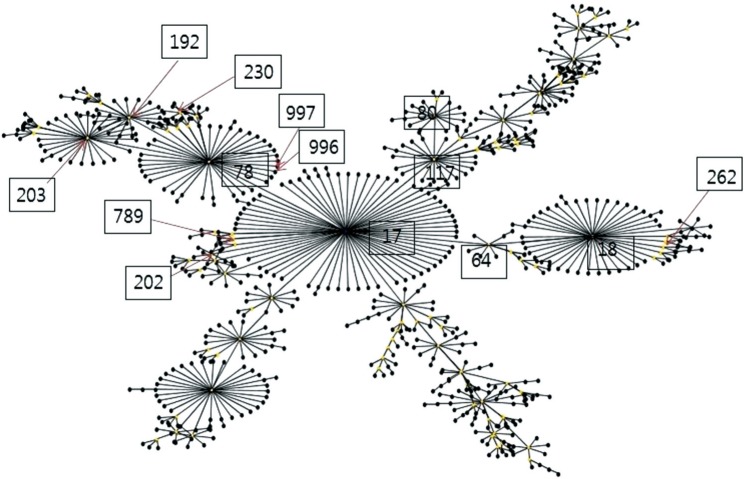
No significant differences were observed in the overall distribution of STs between VSEfm and VREfm. However, the ST distribution of VSEfm and VREfm could be distinguished according to the patient group. Among the 42 VSEfm isolates from MH patients (VSEfm MH), ST17 was predominant (42.9%), followed by ST192 and ST230 (14.3%) (Fig. 1A). In comparison, no predominant clone was observed among the 18 VSEfm isolated from non-MH patients (VSEfm non-MH); identified STs included ST17 (22.2%), ST192, and ST812 (16.7%) (Fig. 1B). ST230 was the most common (33.3%) ST among the VREfm isolates from MH patients (VREfm MH), followed by ST17 (26.2%) (Fig. 1C), while ST17 was predominant (61.1%) in VREfm isolates from non-MH patients (VREfm non-MH) (Fig. 1D). A population snapshot obtained using eBURST analysis (http://eburst.mlst.net) is shown in Fig. 2.
Fig. 1. Distribution of VSEfm and VREfm sequence types in MH and non-MH patients. (A) STs of VSEfm isolated from MH patients. (B) STs of VSEfm isolated from non-MH patients. (C) STs of VREfm isolated from MH patients. (D) STs of VREfm isolated from non-MH patients.
“Others” comprises single individual STs.
Abbreviations: MH, hematologic patients; non-MH, non-hematologic patients; ST, sequence type; VREfm, vancomycin-resistant E. faecium; VSEfm, vancomycin-susceptible E. faecium.
Fig. 2. Population snapshot by eBURST analysis (http://eburst.mlst.net) showing clusters of linked and unlinked sequence types (STs) identified in this study. The boxed numbers represent sequence types.
3. Prevalence of the virulence genes and plasmids
Of the 15 virulence genes tested, several were rarely identified or not identified in the 120 E. faecium isolates; these included cylA (n=1), gelE (n=0), asa1 (n=1), and agg (n=0). In contrast, acm, which encodes a factor related to adhesion of collagen, was detected in all bacteremic isolates (n=120). The distribution of virulence factors differed between VSEfm and VREfm; hyl (91.7% vs 76.7%, P=0.024) and sgrA (100% vs 85.0%, P=0.003) were more frequent in VREfm than in VSEfm (Table 3). In addition, hyl (85.7% vs 55.6%, P=0.011), esp (83.3% vs 55.6%, P=0.023), and sgrA (92.9% vs 66.7%, P=0.016) were more frequent in VSEfm MH than in VSEfm non-MH.
Table 3. Virulence factors and plasmids observed in E. faecium isolates.
| VSEfm (N = 60) | VREfm (N = 60) | P | |
|---|---|---|---|
| Virulence factor* | |||
| hyl | 45 (76.7%) | 55 (91.7%) | 0.024 |
| esp | 45 (75.0%) | 52 (86.7%) | 0.104 |
| scm | 45 (75.0%) | 46 (76.7%) | > 0.999 |
| sgrA | 51 (85.0%) | 60 (100%) | 0.003 |
| ecbA | 34 (56.7%) | 43 (71.7%) | 0.087 |
| pilA | 41 (68.3%) | 39 (65.0%) | 0.699 |
| pilB | 40 (66.7%) | 41 (68.3%) | 0.845 |
| fms11 | 49 (81.7%) | 51 (85.0%) | 0.654 |
| fms14 | 23 (38.3%) | 19 (31.7%) | 0.444 |
| fms15 | 40 (66.7%) | 49 (81.7%) | 0.061 |
| Plasmid | |||
| pIP501 | 3 (5.0%) | 6 (10.0%) | 0.491 |
| pRE25 | 48 (80.0%) | 52 (86.7%) | 0.327 |
| PEF1071 | 42 (70.0%) | 54 (90.0%) | 0.006 |
| pRI | 50 (83.3%) | 56 (93.3%) | 0.153 |
| pRUM | 12 (20.0%) | 8 (13.3%) | 0.327 |
| pEF418 | 45 (75.0%) | 42 (70.0%) | 0.540 |
| pMG1 | 8 (13.3%) | 16 (26.7%) | 0.068 |
| pLG1 | 44 (73.3%) | 50 (83.3%) | 0.184 |
*Of the 15 virulence genes tested, several virulence genes were rarely identified or not found in the 120 E. faecium isolates; cylA (n=1), gelE (n=0), asa1 (n=1), agg (n=0). acm was found in all of the isolates tested in this study (n=120).
Abbreviations: VSEfm, vancomycin-susceptible E. faecium; VREfm, vancomycin-resistant E. faecium.
Furthermore, we compared the virulence factors correlated with resistance to antibiotics other than vancomycin. Hyl (90.2%, vs 0%, P<0.001), esp (86.6% vs 0%, P<0.001), sgrA (97.3% vs 25.0%, P<0.001), ecbA (67.9% vs 12.5%, P=0.003), scm (78.6% vs 37.5%, P=0.020), pilB (71.4% vs 12.5%, P=0.002), and fms15 (78.6% vs 12.5%, P<0.001) were more frequently detected in ampicillin-resistant E. faecium than in ampicillin-susceptible E. faecium. Additionally, hyl (93.2% vs 68.9%, P<0.001), esp (87.8% vs 68.9%, P=0.011), sgr (98.6% vs 82.2%, P=0.002), ecbA (71.6% vs 51.1%, P=0.024), and pilB (74.3% vs 55.6%, P=0.034) were more frequent in E. faecium with HLGR than in E. faecium without HLGR.
Similar to the virulence factors, the plasmids harboring rep genes exhibited different patterns according to antimicrobial resistance and/or patient group. pEF1071 was more frequent in VREfm than in VSEfm (90.0% vs 70.0%, P=0.006; Table 3). In addition, the prevalence of pEF1071 was higher among ampicillin-resistant E. faecium isolates (85.7%), whereas it was not found in any of the ampicillin-susceptible E. faecium isolates. Furthermore, E. faecium harboring pEF1071 more frequently harbored hyl (93.8% vs 45.8%, P<0.001), esp (91.7% vs 37.5%, P<0.001), sgrA (99.0% vs 66.7%, P<0.001), ecbA (71.9% vs 33.3%, P<0.001), pilB (76.0% vs 33.3%, P<0.001), and fms15 (80.2% vs 50.0%, P=0.002).
pLG1 was more frequent in ampicillin-resistant E. faecium isolates than in ampicillin-susceptible isolates (81.3% vs 37.5%, P=0.012). pLG1 was also more prevalent in both VSEfm (81.0% vs 55.6%, P=0.041) and VREfm (90.5% vs 66.7%, P=0.023) from MH patients than those from non-MH patients. This prevalence was significantly related to the presence of esp, sgrA, pilB, and fms15; E. faecium harboring pLG1 also harbored esp (85.1% vs 65.4%, P=0.024), sgrA (95.7 vs 80.8%, P=0.022), pilB (73.4 vs 46.2%, P=0.009), and fms15 (79.8% vs 53.8%, P=0.007) more frequently than did E. faecium without pLG1.
Taken together, the common virulence factors esp and sgrA were more prevalent in E. faecium isolates from MH patients, ampicillin-resistant isolates, and isolates harboring pEF1071 or pLG1. In addition, of the seven virulence factors prevalent in ampicillin-resistant isolates, six were also more prevalent among isolates harboring pEF1071, as pEF1071 was found only in ampicillin-resistant isolates.
4. Structural analysis of Tn1546
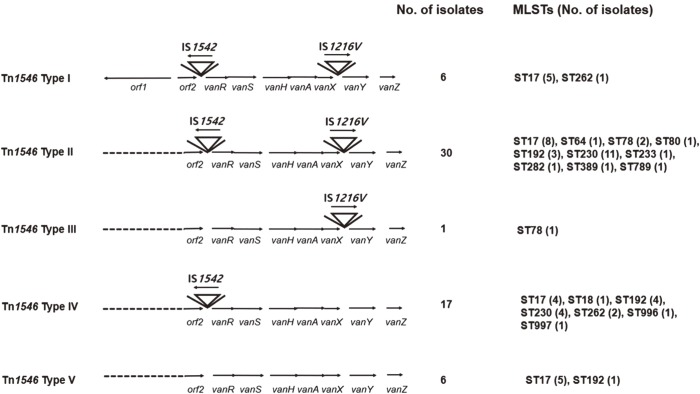
Result of the structural analysis of the main Tn1546 type for VREfm is presented in Table 2 and Fig. 3. None of the isolates harbored the prototype Tn1546. The isolates were classified into five (I–V) main Tn types according to the presence of orf1 and insertion of IS1542 and/or IS1216. orf1 was detected in most (54 of 60, 90%) of the isolates. Insertion of IS1542 in the orf2-vanR region was observed in 85.7% (36 of 42) of VREfm MH and 94.4% (17 of 18) of VREfm non-MH, respectively. Insertion of IS1216V in the vanX–vanY intergenic region was found in 57.1% (24 of 42) of VREfm MH and 72.2% (13 of 18) of VREfm non-MH, and most of these isolates also harbored an IS1542 insertion. In other words, 88.3% (53 of 60) and 61.7% (37 of 60) of all the VREfm isolates had IS1542 and IS1216V insertions, respectively. Type II was the most common, accounting for 50% (30 of 60) of all VREfm isolates. Tn types exhibited a variable pattern of distribution, which was difficult to characterize according to ST or host specificity.
Fig. 3. Genetic maps of Tn1546 in vancomycin-resistant Enterococcus faecium bacteremic isolates. The positions of genes and open reading frames and the direction of transcription are marked by arrows. Inverted triangles represent insertion sequence (IS) elements. Dotted lines indicate deletions.
DISCUSSION
In this study, we compared the molecular characteristics of E. faecium bacteremic isolates according to host factors (MH vs non-MH patients). Seoul St. Mary's Hospital is a 1,300-bed, university-affiliated, tertiary care center in Seoul, South Korea; approximately 230 beds were allocated to MH patients. The Catholic BMT Center performs over 500 SCTs annually. Oral ciprofloxacin (500 mg twice daily) was used as a routine prophylaxis during chemotherapy or SCT throughout the study period. The initial empirical treatment for neutropenic fever in patients with hematological malignancy includes anti-pseudomonal cephalosporin (ceftazidime or cefepime) and/or an aminoglycoside (isepamicin), excluding the initial use of glycopeptides [16,17]. The medical illness severity of MH patients and prolonged antibiotic use might be related to the relatively higher rate of ampicillin-resistant E. faecium and VREfm isolates in MH patients than in non-MH patients.
We found that ampicillin resistance is much higher in VSEfm MH than in VSEfm non-MH (97.6% vs 61.1%). We also found that ampicillin-resistant isolates harbored several virulence genes more frequently than ampicillin-susceptible isolates; hyl and esp were detected only in ampicillin-resistant isolates. Interestingly, there were also significant differences in ampicillin resistance rate among the non-MH isolates according to the type of ward: 94.1% in general ward (GW), 83.3% in intensive care unit (ICU), and 42.9% in emergency department (ER), respectively (P= 0.011). Similar trends were also observed for the prevalence of hyl (88.2% in GW, 83.3% in ICU, and 42.9% in ER, P=0.038) and esp (82.4% in GW, 75.0% in ICU, and 42.9% in ER, P=0.093). These differences may be due to previous exposure to antibiotics or to the bloodstream infection developed in a nosocomial or community setting. However, the scope of this study did not include investigating antibiotic exposure in the non-MH group.
pEF1071 was not detected in any of the ampicillin-susceptible isolates, although the result should be interpreted bearing in mind that the number of these isolates was low (n=7). pEF1071 encodes enterocins 1071A and 1071B [18]; as enterocins possess antimicrobial activity against closely related species, a producer strain would have a selective advantage over other strains in the same ecological niche [19].
It is interesting that hyl, esp, and sgrA were the virulence factors most frequently identified in isolates from MH patients, ampicillin-resistant isolates, and isolates harboring pEF1071. esp is known to be associated with resistance to ampicillin, imipenem, and ciprofloxacin [20,21]. A recent study observed high prevalence and persistence of ampicillin-resistant E. faecium colonization in patients receiving levofloxacin prophylaxis [22]. We hypothesize that the relatively high ampicillin resistance rate in E. faecium from MH patients in our hospital might be associated with ciprofloxacin prophylaxis administered to the MH patients. Further study is needed to determine whether there is any link between ampicillin resistance, virulence factors, and pEF1071.
Regardless of patient group, hyl and sgrA were more frequent in VREfm than in VSEfm. This might be due to co-localization of vanA and hylEfm on the same plasmid [23]. sgrA, along with other virulence factors (esp, hyl, acm, scm, ecbA, pilA, and pilB), is known to play an important role in the emergence of ST78 VREfm in nosocomial infections [24]. Considering that hyl, esp, and sgrA are related to colonization of the gastrointestinal tract, primary surface attachment, and adhesion [25,26,27], these virulence factors could play a role in the development of enterococcal bacteremia originating from the gastrointestinal tracts of MH patients who frequently suffer from severe gut mucositis during prolonged, severe neutropenia. It is noteworthy that the prevalence of hyl and esp among the VREfm MH isolates increased from 49% to 88% and 68% to 86%, respectively, compared with a previous report from this BMT center [15]. esp plays a significant role in the prevalence of VREfm [27,28].
In terms of plasmid replicon typing, the majority of E. faecium isolates from MH patients harbored pLG1, which is a newly sequenced, 280-kb, conjugative plasmid encoding VanA-type glycopeptide resistance, macrolide resistance, carbon uptake-utilization genes, and putative virulence genes including hyl and a pilin gene cluster [23]. Further study is needed to investigate the role of pLG1 in the acquisition and transmission of vancomycin resistance in E. faecium.
In this study, we also analyzed the molecular epidemiology of E. faecium bacteremic isolates. Most E. faecium isolates (80.8%) belonged to clonal complex 17 (CC17) and comprised various STs, including ST17, ST230, ST192, ST78, and ST262. Previous studies have shown that CC17 can be resolved into three different lineages, originating from ST17, ST18, and ST78. While lineages 17 and 18 were predominant from 1990 to 2004, lineage 78 has become predominant since 2005 [29,30]. However, ST17 remained the most prevalent ST while ST230, a single locus variant of ST78, was predominant among MH patients at this hospital. In addition, five types of Tn1546 were identified, of which two (II and IV) accounted for >70% of the VREfm isolates. This finding indicates that both clonal spread and horizontal transfer played a role in the spread of vancomycin resistance within a hospital. In addition, diverse STs harbored Tn1546 types II and IV. In cases of common STs, ST17 harbored Tn1546 types I, II, IV, and V, and ST230 contained Tn1546 types II and IV.
We wish to emphasize three findings. First, the rate of ampicillin resistance was higher in VSEfm MH than in VSEfm non-MH, and hyl, esp, and pEF1071 were detected only in isolates with ampicillin resistance. Based on these findings, we have decided to limit administration of ciprofloxacin prophylaxis to a select group of patients. Second, the prevalence of pLG1 was higher in E. faecium MH than in E. faecium non-MH, regardless of vancomycin resistance. Third, both clonal and horizontal transfers contributed to the transmission of VRE. Further study is, therefore, needed to investigate the genetic link between antimicrobial resistance and virulence factors.
In conclusion, antimicrobial resistance profiles and molecular characteristics, including the distribution of STs, virulence genes, and plasmids, were different and associated with the underlying diseases of patients within the same hospital. We presume that the prophylactic use of fluoroquinolone might have affected the antimicrobial resistance profiles and molecular characteristics of E. faecium and have thus decided to use fluoroquinolone more stringently in MH patients.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea in 2014 (2014R 1A1A3051975). We wish to thank Prof. Wee Gyo Lee for the generous gift of strain BM4147. This work was also supported by a grant from the Korean Health Technology R&D project through the Korea Health Industry Development Institute (grant number: HI16C0443).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: The authors declare that they have no conflicts of interest related to this study.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Cho SY, Lee DG, Choi SM, Kwon JC, Kim SH, Choi JK, et al. Impact of vancomycin resistance on mortality in neutropenic patients with enterococcal bloodstream infection: a retrospective study. BMC Infect Dis. 2013;13:504. doi: 10.1186/1471-2334-13-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon JC, Kim SH, Choi JK, Cho SY, Park YJ, Park SH, et al. Epidemiology and clinical features of bloodstream infections in hematology wards: one year experience at the Catholic Blood and Marrow Transplantation Center. Infect Chemother. 2013;45:51–61. doi: 10.3947/ic.2013.45.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med. 2001;135:484–492. doi: 10.7326/0003-4819-135-7-200110020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Peel T, Cheng AC, Spelman T, Huysmans M, Spelman D. Differing risk factors for vancomycin-resistant and vancomycin-sensitive enterococcal bacteraemia. Clin Microbiol Infect. 2012;18:388–394. doi: 10.1111/j.1469-0691.2011.03591.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis. 2001;1:314–325. doi: 10.1016/S1473-3099(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 7.Rosvoll TC, Pedersen T, Sletvold H, Johnsen PJ, Sollid JE, Simonsen GS, et al. PCR-based plasmid typing in Enterococcus faecium strains reveals widely distributed pRE25-, pRUM-, pIP501- and pHTbeta-related replicons associated with glycopeptide resistance and stabilizing toxin-antitoxin systems. FEMS Immunol Med Microbiol. 2010;58:254–268. doi: 10.1111/j.1574-695X.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 8.Cattoir V, Leclercq R. Twenty-five years of shared life with vancomycin-resistant enterococci: is it time to divorce? J Antimicrob Chemother. 2013;68:731–742. doi: 10.1093/jac/dks469. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis G, Cataldo MA, De Waure C, Venturiello S, La Torre G, Cauda R, et al. Infection control and prevention measures to reduce the spread of vancomycin-resistant enterococci in hospitalized patients: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:1185–1192. doi: 10.1093/jac/dkt525. [DOI] [PubMed] [Google Scholar]
- 10.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 11.CLSI. Performance standards for antimicrobial susceptibility testing. 24th ed. CLSI supplement, M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute; 2014. [Google Scholar]
- 12.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, et al. Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol. 2002;40:1963–1971. doi: 10.1128/JCM.40.6.1963-1971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosvoll TC, Lindstad BL, Lunde TM, Hegstad K, Aasnaes B, Hammerum AM, et al. Increased high-level gentamicin resistance in invasive Enterococcus faecium is associated with aac(6′)Ie-aph(2′′)Ia-encoding transferable megaplasmids hosted by major hospital-adapted lineages. FEMS Immunol Med Microbiol. 2012;66:166–176. doi: 10.1111/j.1574-695X.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 14.Vankerckhoven V, van Autgaerden T, Vael C, Lammens C, Chapelle S, Rossi R, et al. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J Clin Microbiol. 2004;42:4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Park C, Choi SM, Lee DG, Kim SH, Kwon JC, et al. Molecular epidemiology of vancomycin-resistant Enterococcus faecium bloodstream infections among patients with neutropenia over a 6-year period in South Korea. Microb Drug Resist. 2011;17:59–65. doi: 10.1089/mdr.2010.0091. [DOI] [PubMed] [Google Scholar]
- 16.Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011;26:220–252. doi: 10.3904/kjim.2011.26.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissinger F, Auner HW, Bertz H, Buchheidt D, Cornely OA, Egerer G, et al. Antimicrobial therapy of febrile complications after high-dose chemotherapy and autologous hematopoietic stem cell transplantation--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2012;91:1161–1174. doi: 10.1007/s00277-012-1456-8. [DOI] [PubMed] [Google Scholar]
- 18.Balla E, Dicks LM, Du Toit M, van der Merwe MJ, Holzapfel WH. Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE1071. Appl Environ Microbiol. 2000;66:1298–1304. doi: 10.1128/aem.66.4.1298-1304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balla E, Dicks LM. Molecular analysis of the gene cluster involved in the production and secretion of enterocins 1071A and 1071B and of the genes responsible for the replication and transfer of plasmid pEF1071. Int J Food Microbiol. 2005;99:33–45. doi: 10.1016/j.ijfoodmicro.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Dupre I, Zanetti S, Schito AM, Fadda G, Sechi LA. Incidence of virulence determinants in clinical Enteorococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy) J Med Microbiol. 2003;52:491–498. doi: 10.1099/jmm.0.05038-0. [DOI] [PubMed] [Google Scholar]
- 21.Billstrom H, Ludn B, Sullivan A, Nord CE. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int J Antimicrob Agents. 2008;32:374–377. doi: 10.1016/j.ijantimicag.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Díaz AM, Cuartero C, Rodríguez JD, Lozano S, Alonso JM, Rodríguez-Domínguez M, et al. The rise of ampicillin-resistant Enterococcus faecium high-risk clones as a frequent intestinal colonizer in oncohaematological neutropenic patients on levofloxacin prophylaxis: a risk for bacteraemia? Clin Microbiol Infect. 2016;22:59.e1–59.e8. doi: 10.1016/j.cmi.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Laverde Gomez JA, van Schaik W, Freitas AR, Coque TM, Weaver KE, Francia MV, et al. A multiresistance megaplasmid pLG1 bearing a hylEfm genomic island in hospital Enterococcus faecium isolates. Int J Med Microbiol. 2011;301:165–175. doi: 10.1016/j.ijmm.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Jiang Y, Guo L, Ye L, Ma Y, Luo Y. Prevalence of diverse clones of vancomycin-resistant Enterococcus faecium ST78 in a Chinese hospital. Microb Drug Resist. 2016;22:294–300. doi: 10.1089/mdr.2015.0069. [DOI] [PubMed] [Google Scholar]
- 25.Rice LB, Lakticová V, Carias LL, Rudin S, Hutton R, Marshall SH. Transferable capacity for gastrointestinal colonization in Enterococcus faecium in a mouse model. J Infect Dis. 2009;199:342–349. doi: 10.1086/595986. [DOI] [PubMed] [Google Scholar]
- 26.van Wamel WJ, Hendrickx AP, Bonten MJ, Top J, Posthuma G, Willems RJ. Growth condition-dependent Esp expression by Enterococcus faecium affects initial adherence and biofilm formation. Infect Immun. 2007;75:924–931. doi: 10.1128/IAI.00941-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sava IG, Heikens E, Huebner J. Pathogenesis and immunity in enterococcal infections. Clin Microbiol Infect. 2010;16:533–540. doi: 10.1111/j.1469-0691.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- 28.Hendrickx AP, van Schaik W, Willems RJ. The cell wall architecture of Enterococcus faecium: from resistance to pathogenesis. Future Microbiol. 2013;8:993–1010. doi: 10.2217/fmb.13.66. [DOI] [PubMed] [Google Scholar]
- 29.Willems RJ, Top J, van Schaik W, Leavis H, Bonten M, Sirén J, et al. Restricted gene flow among hospital subpopulations of Enterococcus faecium. MBio. 2012;3:e00151–e00112. doi: 10.1128/mBio.00151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galloway-Peña JR, Nallapareddy SR, Arias CA, Eliopoulos GM, Murray BE. Analysis of clonality and antibiotic resistance among early clinical isolates of Enterococcus faecium in the United States. J Infect Dis. 2009;200:1566–1573. doi: 10.1086/644790. [DOI] [PMC free article] [PubMed] [Google Scholar]