Abstract
Among five C57BL/6 mice fed a high-fat diet (HFD) for 12 weeks, one mouse showed a body weight (BW) similar to normal diet (ND)-fed mice. We compared obesity-related parameters of three groups (ND-fed mice, one HFD-fed normal-weight mouse, and HFD-fed overweight mice), including visceral fat weight, serum levels of total cholesterol (TC), glucose, and aminotransferases (AST and ALT), adipocyte size, percentage of crown-like structures, severity of hepatic steatosis, and number of inflammatory foci. Compared to ND-fed mice, the HFD-fed normal-weight mouse exhibited a similar visceral fat weight, similar serum levels of glucose and aminotransferases, and a similar percentage of crown-like structures. On the other hand, the serum TC level, adipocyte size, and hepatic steatosis severity of the HFD-fed normal-weight mouse were intermediate between those of ND-fed mice and HFD-fed overweight mice. Interestingly, the number of hepatic inflammatory foci in the HFD-fed normal-weight mouse was remarkably increased compared with those in HFD-fed overweight mice. These results suggest that having BW or serum ALT levels within normal ranges may not guarantee absence of hepatic inflammation and that the HFD-fed normal-weight mouse can be used as an animal model for the study of liver inflammation, particularly in patients with normal BWs and/or serum ALT values.
Keywords: body mass index, calorie dense food, hepatic disease, inflammation, obesity
Obesity is known as a major risk factor for several diseases, including chronic kidney disease1, type 2 diabetes2, and cardiovascular diseases3. Furthermore, it plays an important role in inducing a state of chronic, systemic, low-grade inflammation that increases both circulating and adipose tissue (AT) levels of inflammatory cytokines (e.g., interleukin-6 and tumor necrosis factor-α)2, 4. In addition to genetic factors, environmental factors, such as a sedentary lifestyle and increased intake of calorie-dense foods (e.g., fat-rich foods), are closely associated with obesity. Intake of a high-fat diet (HFD) induces obesity in both humans and animal models. High levels of dietary fat correlate with body weight (BW) and fat mass gain, while reduction of dietary fat results in their loss. Generally, body mass index is used as a screening tool for identification of overweight individuals5, 6.
The intake of a HFD induces fatty acid uptake by the liver, which leads to liver inflammation and injury. Obesity-induced liver inflammation and injury is closely associated with the development of liver fibrosis and cirrhosis and hepatocellular carcinoma7, 8. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are widely used as a serum enzyme biomarker of liver injury, and their levels have been shown to increase with BW gain in both humans9 and rodents10. Of the two enzymes, ALT is a more specific indicator of liver injury because of its greater concentration in the liver compared with other tissues and is confined to the cytoplasm. On the other hand, the heart has the highest concentration of AST, followed by the liver, skeletal muscles, kidneys, brain, pancreas, and erythrocyte. AST also has two different isoenzyme forms, a mitochondrial and a cytoplasmic form11, 12. Furthermore, ALT has been shown to be the most sensitive biomarker for the presence of hepatic steatosis in both men and women13, 14 and is associated with increased abdominal weight and obesity9, 15.
Despite multiple obesity studies, there are limited data available on the obesity-related parameters of HFD-fed normal-weight mice. In the present study, we compared obesity-related parameters between a mouse that maintained a normal weight after 12 weeks of HFD feeding and normal diet (ND)-fed mice or HFD-fed overweight mice.
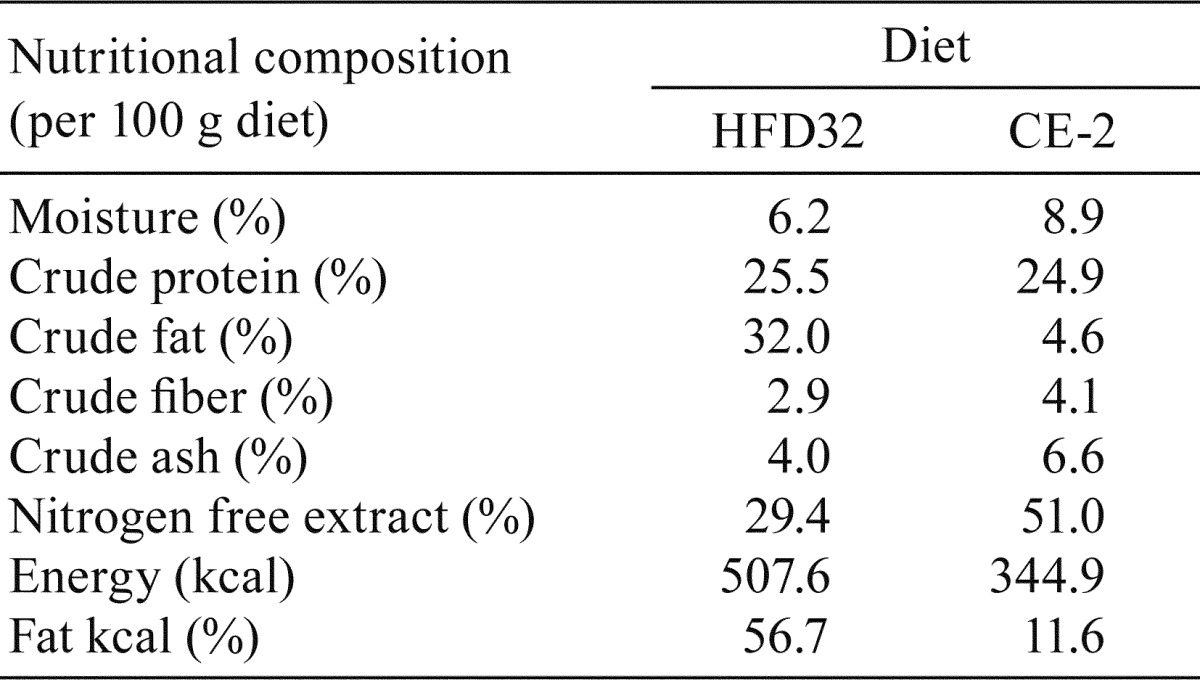
Specific-pathogen-free (SPF) male C57BL/6J mice aged 5 weeks were purchased from Japan SLC (Shizuoka, Japan) and were bred under SPF conditions. Mice were housed with a maximum of 5 mice per cage and were maintained in a temperature-controlled room (22°C) with a 12-h light-dark cycle. They were fed a ND (CE-2, CLEA Japan, Inc., Tokyo, Japan) and tap water ad libitum for a week. The 6-week-old mice were split into two groups, and each group was fed either an ND or HFD (HFD32, CLEA Japan) and tap water ad libitum for 12 weeks. The nutritional compositions of the HFD32 and CE-2 are presented in Table 1. HFD32 and CE-2 were stored at –20°C and room temperature, respectively. HFD32 was changed every day, and CE-2 was changed twice a week. All diets were used within the manufacturer’s recommended expiry period. Animal studies were performed in accordance with the Guidelines for Animal Experiments established by the Ministry of Health, Labour and Welfare of Japan and by the National Cerebral and Cardiovascular Center Research Institute.
Table 1. Nutritional Compositions of HFD32 and CE-2.
After 12 weeks of HFD feeding, blood samples from all mice were collected from the caudal vena cava. Blood was collected in BD Microtainer® Blood Collection tubes (BD, Franklin Lakes, NJ, USA), and serum samples were obtained by centrifuging the tubes for five minutes at 10,000 g. Total cholesterol (TC) in the serum samples was measured using the Cholesterol E-Test Wako (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The level of serum glucose was measured using a glucose assay kit (Sigma-Aldrich, St. Louis, MO, USA). AST and ALT were measured using a transaminase assay kit (Transaminase C II-test, Wako) according to the manufacturer’s instructions.
Visceral epididymal adipose and liver tissues were removed and fixed in 10% buffered formalin. After routine processing by paraffin embedding, tissue sectioning, and slide mounting, sections were strained with hematoxylin and eosin (HE) or immunostained with anti-F4/80 antibody (monoclonal antibody; Abcam, Cambridge, UK). Adipocyte diameters were determined using the ImageJ software16. Percentages of crown-like structures were measured as previously described17. Micro- and macrovesicular steatosis severity were determined at 50–100× magnification and expressed as percentages of the total surface area. Micro- and macrovesicular steatosis were differentiated from each other by whether centrally placed nuclei were visible in the foamy hepatocytes (micro) or not (macro). In addition, the number of inflammatory foci was counted in five fields viewed for each sample and expressed as the average number of inflammatory foci per field (view size 2.2 mm2).
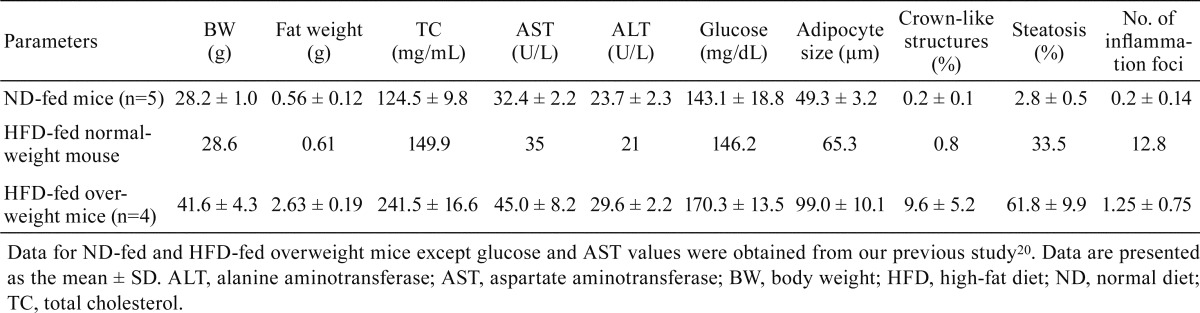
Intake of a HFD induces obesity and BW gain in humans5, 6 and animals10, 18, 19. However, among the five mice fed a HFD for 12 weeks, one mouse showed a BW similar to the ND-fed mice (Table 2). We compared obesity-related parameters of all three groups (ND-fed mice, the one HFD-fed normal-weight mouse, and HFD-fed overweight mice), including visceral fat weight, serum levels of TC, glucose, and aminotransferases (AST and ALT), adipocyte size, percentage of crown-like structures, severity of hepatic steatosis, and number of inflammatory foci. Visceral fat weight, serum levels of glucose and aminotransferases, and the percentage of crown-like structures of the normal-weight mouse after long-term HFD feeding were similar to those of the ND-fed mice. Serum TC level, adipocyte size, and hepatic steatosis severity of the HFD-fed normal-weight mouse were higher than those of ND-fed mice but lower than those of HFD-fed overweight mice (Table 2). A significant relationship between dietary fat intake and serum TC levels has been observed in humans5, 6 and rodents18, 19, but patients with severe cirrhosis have shown decreased serum levels of TC21.
Table 2. Obesity-related Parameters in the ND-fed and HFD-fed Mice.
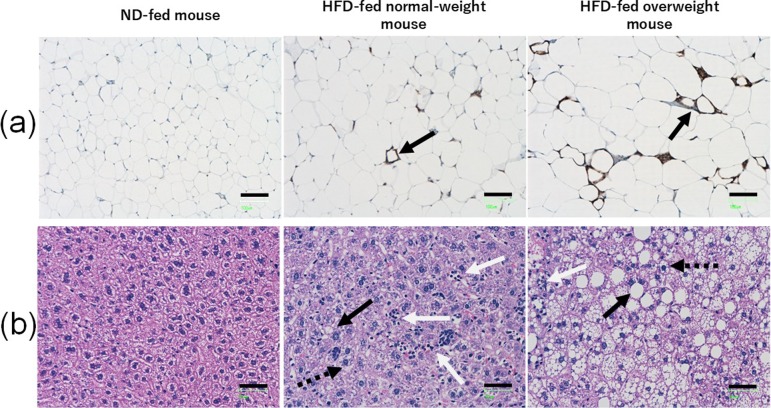
Several studies have suggested a high positive correlation among adipocyte size, adipose tissue macrophage (ATM) infiltration, and adipocyte death in obese mice and humans17, 22,23,24. Adipocyte death and ATM infiltration increase the occurrence of crown-like structures. Furthermore, infiltrating ATMs in crown-like structures stimulate the secretion of inflammatory-related cytokines such as tumor necrosis factor α and interleukin-617, 22, 24, 25 and the development of hepatic steatosis22, 23. In the present study, the percentage of crown-like structures decreased among groups in the following order: HFD-fed overweight mice > HFD-fed normal-weight mouse > ND-fed mice (Table 2 and Fig. 1a). This supports the previous findings that HFD feeding leads to increased damage or apoptosis in adipose tissues.
Fig. 1.
(a) Representative photomicrographs of F4/80-stained adipose tissue cross sections. The black arrows show typical crown-like structures. Scale bar, 100 μm. (b) Representative pictures of HE-stained liver cross sections. The black arrows show typical macrovesicular steatosis, the dotted black arrows show typical microvesicular steatosis, and the white arrows show inflammatory foci. Scale bar, 50 μm. ND, normal diet; HFD, high-fat diet.
Intake of a HFD induces structural alterations of the liver, including micro- and macrovesicular steatosis. Hepatic steatosis is associated with severe liver diseases (e.g., non-alcoholic steatohepatitis [NASH] and fibrosis)26,27,28. Steatosis and lobular and portal inflammation have been found to be significantly higher in patients with definite NASH compared with those without NASH26. Significant correlation has been reported between microvesicular steatosis and NASH and fibrosis in human patients and between macrovesicular steatosis and NASH and fibrosis in HFD-fed mice. In our study, the severity of hepatic steatosis decreased in the following order: HFD-fed overweight mice > HFD-fed normal-weight mouse > ND-fed mice (Table 2 and Fig. 1b). These results show that intake of a HFD is associated with an increased risk of hepatic steatosis.
On the other hand, the number of hepatic inflammatory foci in the HFD-fed normal-weight mouse was remarkably increased compared with the numbers in the ND-fed mice and HFD-fed overweight mice (Table 2 and Fig. 1b). Serum ALT levels are broadly used as a sensitive biomarker of liver damage or injury9, 11, 12,13,14. However, several studies have suggested that ALT levels may lack correlation with the severity of a patient’s liver disease (advanced fibrosis and NASH)10, 29,30,31. Bridging fibrosis and cirrhosis have been identified in patients with normal ALT values31, and NASH has been found in more than 50% of patients with normal ALT values30. However, another study demonstrated that ALT levels are higher in patients with definite NASH than in those without and higher in patients with mild/moderate and bridging fibrosis than in those with cirrhosis26.
In our study, the serum ALT levels were similar between the one HFD-fed normal-weight mouse and ND-fed mice, but the hepatic inflammatory foci in the HFD-fed normal-weight mouse were remarkably increased compared with those in the ND-fed mice and HFD-fed overweight mice (Table 2 and Fig. 1b). Although it is not clear why the number of inflammation foci was increased in the HFD-fed normal-weight mouse, several studies have reported that mice showing acute or severe hepatic inflammation either do not become obese or show a small weight loss32, 33. Therefore, increased hepatic inflammation in the HFD-fed normal-weight mouse may be associated with the reduction in BW gain. These results indicate that a normal BW or serum ALT value may not guarantee absence of hepatic inflammation and that the HFD-fed normal-weight mouse may potentially be used as an animal model for the study of liver inflammation in patients with normal BWs and/or serum ALT values.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP16K21212 and JP17K01386.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Gotoh K, Inoue M, Masaki T, Chiba S, Shiraishi K, Shimasaki T, Matsuoka K, Ando H, Fujiwara K, Fukunaga N, Aoki K, Nawata T, Katsuragi I, Kakuma T, Seike M, and Yoshimatsu H. Obesity-related chronic kidney disease is associated with spleen-derived IL-10. Nephrol Dial Transplant. 28: 1120–1130. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, and Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 121: 2111–2117. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, and Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 53: 1925–1932. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Chawla A, Nguyen KD, and Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 11: 738–749. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hariri N, and Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 23: 270–299. 2010. [DOI] [PubMed] [Google Scholar]
- 6.Hooper L, Abdelhamid A, Moore HJ, Douthwaite W, Skeaff CM, and Summerbell CD. Effect of reducing total fat intake on body weight: systematic review and meta-analysis of randomised controlled trials and cohort studies. BMJ. 345: e7666 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 20: 2515–2532. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun B, and Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 56: 704–713. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, and Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 39: 754–763. 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, and Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 46: 212–223. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, and Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 3: 17 2009. [PMC free article] [PubMed] [Google Scholar]
- 12.Kew MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. 355: 591–592. 2000. [DOI] [PubMed] [Google Scholar]
- 13.Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K, Murase K, Kadota T, Murata I, and Kohno S. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 17: 1098–1105. 2002. [DOI] [PubMed] [Google Scholar]
- 14.Sattar N, Forrest E, and Preiss D. Non-alcoholic fatty liver disease. BMJ. 349: g4596 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss RS, Barlow SE, and Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 136: 727–733. 2000. [PubMed] [Google Scholar]
- 16.Parlee SD, Lentz SI, Mori H, and MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 537: 93–122. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, and Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 49: 1562–1568. 2008. [DOI] [PubMed] [Google Scholar]
- 18.Gallou-Kabani C, Vigé A, Gross MS, Rabès JP, Boileau C, Larue-Achagiotis C, Tomé D, Jais JP, and Junien C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring). 15: 1996–2005. 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hwang LL, Wang CH, Li TL, Chang SD, Lin LC, Chen CP, Chen CT, Liang KC, Ho IK, Yang WS, and Chiou LC. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity (Silver Spring). 18: 463–469. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Toita R, Kawano T, Murata M, and Kang JH. Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials. 110: 81–88. 2016. [DOI] [PubMed] [Google Scholar]
- 21.Ghadir MR, Riahin AA, Havaspour A, Nooranipour M, and Habibinejad AA. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat Mon. 10: 285–288. 2010. [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, and Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 285: 3428–3438. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd , DeFuria J, Jick Z, Greenberg AS, and Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 56: 2910–2918. 2007. [DOI] [PubMed] [Google Scholar]
- 24.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, and Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 46: 2347–2355. 2005. [DOI] [PubMed] [Google Scholar]
- 25.Coenen KR, Gruen ML, Chait A, and Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 56: 564–573. 2007. [DOI] [PubMed] [Google Scholar]
- 26.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV. NASH Clinical Research Network Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 52: 913–924. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder P, Liang W, Wielinga PY, Verschuren L, Toet K, Havekes LM, van den Hoek AM, and Kleemann R. Macrovesicular steatosis is associated with development of lobular inflammation and fibrosis in diet-induced non-alcoholic steatohepatitis (NASH). Inflamm Cell Signal. 2: e804 2015. [Google Scholar]
- 28.Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Unalp-Arida A, Wilson LA, Chalasani N. NASH Clinical Research Network (NASH CRN) Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 55: 654–659. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, and Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 94: 2467–2474. 1999. [DOI] [PubMed] [Google Scholar]
- 30.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, and Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 48: 792–798. 2008. [DOI] [PubMed] [Google Scholar]
- 31.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, and Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 37: 1286–1292. 2003. [DOI] [PubMed] [Google Scholar]
- 32.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, and Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 6: 19 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, and Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 10: e0127991 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]