Abstract
Minimal change disease (MCD) can be experimentally induced in rats, but spontaneous cases have not been reported. Herein, we present a case of MCD in rats that resembled the phenotypes of human MCD. A 9-week-old male Sprague Dawley rat developed continuous albuminuria for 2 weeks and was sacrificed at 11 weeks of age. Histological testing revealed no glomerular or renal tubular abnormalities on light microscopy. Immunofluorescence revealed absence of immunoglobulin G or immunoglobulin M deposition in the glomerulus. Extensive foot process effacement of glomerular podocytes was observed by electron microscopy, with rearrangement of the actin cytoskeleton at the base of the fused foot processes. The rat did not show desmin-positive podocytes, and the nephrin showed a normal liner pattern of distribution along the glomerular capillary loop throughout the glomeruli. These pathological characteristics corresponded to those of human MCD, and the glomerular lesion was considered a rare case of rat MCD.
Keywords: kidney, minimal change disease, nephrin, podocyte, rat
Minimal change disease (MCD) is a steroid-sensitive idiopathic glomerulopathy that is common in young children1, 2. The clinical manifestation is characterized by highly selective proteinuria comprised largely of albumin rather than larger molecules such as immunoglobulins (Igs), without altered serum component levels1, 3, 4. On light microscopy, no glomerular abnormality is seen, and tubular or interstitial pathologic lesions are not prominent features in MCD. Immunofluorescence studies have reported that Igs were absent from the glomeruli1, 5. The only consistent pathologic characteristic in MCD is the change in glomerular podocytes seen as extensive foot process effacement on electron microscopy. Other changes in the podocytes include hypertrophy of the cells, microvillous transformation, and formation of vacuoles1, 6. The glomerular basement membrane (GBM), mesangial cells, and the endothelial cells remain normal. In rats, puromycin aminonucleoside nephrosis (PAN) could be used as a model of MCD7,8,9, but spontaneous cases have not been reported to our knowledge. In this report, we describe a typical case of spontaneous MCD in a Sprague Dawley (SD) rat.
An 8-week-old male Crl:CD (SD) rat was purchased from Charles River Laboratories Japan (Kanagawa, Japan). This animal was used in a study we conducted to collect background data for urinary biomarkers in SD rats (n=40). The study was approved by the Ethics Review Committee for Animal Experimentation of Daiichi Sankyo Co., Ltd. (Tokyo, Japan) and performed in accordance with the guidelines of the Animal Care and Use Committee of Daiichi Sankyo Co., Ltd. The animal was housed in an individual cage in an animal study room with a controlled temperature of 20 to 26°C, humidity of 30 to 70%, and a 12-h light (150 to 300 lux) and 12-h dark cycle. A certified pellet diet (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water were provided to the animal ad libitum. At 9 and 11 weeks of age, the rat was placed in a metabolic cage, and 24-h urine samples were collected, which were stored at −80°C until analyses were performed. Kidney injury marker-1 (KIM-1), albumin, osteopontin, neutrophil gelatinase-associated lipocalin (NGAL), and clusterin were measured using a Kidney Injury Panel 1 Rat Kit (Meso Scale Diagnostics, LLC., Rockville, MD, USA) and Rat Clusterin Kit (Meso Scale Diagnostics, LLC) according to the manufacturer’s protocol. Creatinine (CRE), alkaline phosphatase (ALP), lactate dehydrogenase (LD), γ-glutamyltransferase (γGT), glucose, urea nitrogen (UN), calcium (Ca), inorganic phosphorus (IP), sodium (Na), potassium (K), and chlorine (Cl) were also measured by TBA-2000FR chemistry analyzer (Toshiba Medical Systems Corporation, Tochigi, Japan). All urinary parameters were calculated and described in terms of the creatinine ratio.
The rat was euthanized by exsanguination under isoflurane anesthesia. At necropsy, no significant gross lesions were found in any organs including the kidneys.
The kidneys were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE), periodic acid-Schiff (PAS), periodic acid methenamine silver (PAM), and Masson’s trichrome staining.
For immunofluorescence or immunohistochemical analysis of the kidney, following incubation of the sections with 4% Block AceTM (Snow Brand Products Co., Ltd., Sapporo, Japan) or Protein Block Serum (Dako, Agilent Biotechnology, Inc., Santa Clara, CA, USA), dewaxed sections were incubated with the antibodies summarized in Table 1. FITC-conjugated anti-rat IgG and IgM were detected by direct immunofluorescence and others were detected by indirect methods. For anti-desmin antibody, immunohistochemical staining was performed using the immunoenzyme polymer method. Peroxidase-conjugated anti-mouse IgG (Histofine Simple Stain Rat MAX-PO (M), Nichirei Biosciences Inc., Tokyo, Japan) was used as the secondary antibody. After immunoreaction, the section was stained with diaminobenzidine and counterstained with Mayer’s hematoxylin. For anti-nephrin antibody, Alexa Fluor® 488-preadsorbed goat anti-mouse IgG H&L (Abcam, Cambridge, UK) was used as the secondary antibody. Fluorescence was analyzed using a BZ-X700 microscope (Keyence Corporation, Osaka, Japan).
Table 1. Top Five Organ-specific MicroRNAs (miRNAs) in Cynomolgus Monkeys Identified by Next-generation Sequencing (NGS).
Portions of the formalin-fixed tissue specimens from the kidney sample were cut into cubes of 1 mm3, refixed in 2.5% glutaraldehyde, and postfixed in 1% OsO4 for 2 h. These specimens were then dehydrated through ascending grades of alcohol and embedded in epoxy resin. Ultrathin sections were double-stained with uranyl acetate and lead citrate and examined using an H-7500 transmission electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan) at 80 kV.
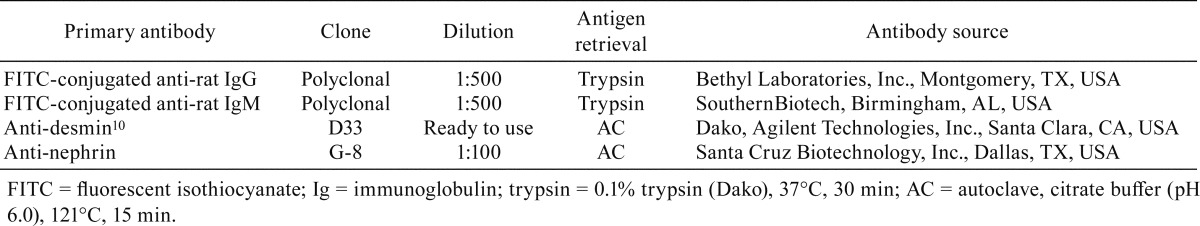
Moderate albuminuria was apparent from 9 weeks of age, and the urinary albumin level further increased at 11 weeks of age (Fig. 1B; 85.9 and 246.3 mg/g·CRE at 9 and 11 weeks of age, respectively). At 11 weeks of age, the level was approximately 16 times higher than the normal average value of SD rats at our laboratory (14.9 ± 5.5 mg/g·CRE; n=39). Other urinary biomarkers, such as KIM-1, osteopontin, NGAL, and clusterin, and urinary biochemical values did not show significant changes at both 9 and 11 weeks of age (osteopontin was below the limit of quantitation).
Fig. 1.
Moderate albuminuria was apparent from 9 weeks of age, and the urinary albumin level further increased at 11 weeks of age in the male Sprague Dawley (SD) rat showing minimal change disease (b; red plots represent the values of the present rat). Kidney injury marker-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and clusterin does not show significant changes. Black plots represent the background values of untreated SD rats at our laboratory (n=39).
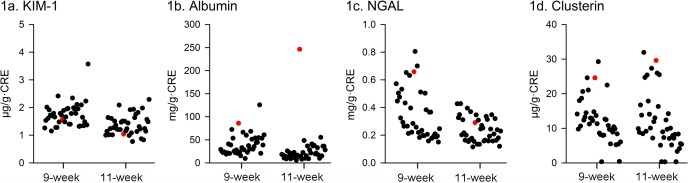
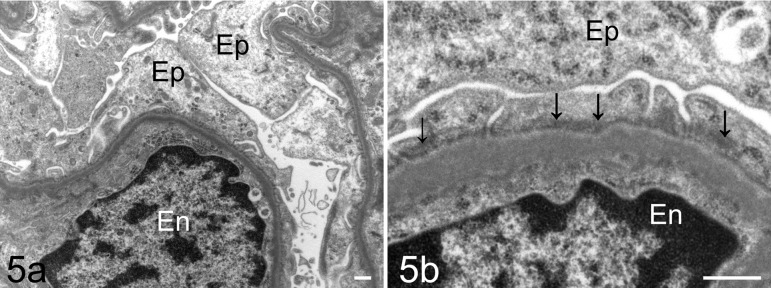
Light microscopy showed no abnormalities in the kidney, including the glomeruli, on HE (Fig. 2), PAS (Fig. 3), PAM, and Masson’s trichrome staining. On immunofluorescence staining, IgG and IgM were not observed in the glomeruli. Desmin-positive podocytes were also not observed. Nephrin showed a liner pattern of distribution along the glomerular capillary loop in the entire glomeruli without a discontinuous or punctuated pattern (Fig. 4). Electron microscopy revealed extensive foot process effacement of the glomerular podocytes (Fig. 5A). The slit pore, which is situated between the foot processes, was narrowed, and the formation of surface microvilli was observed. Rearrangement on the actin cytoskeleton was prominent at the base of fused foot processes (Fig. 5B). The GBM, mesangial cells and matrix, and endothelial cells showed normal morphology.
Fig. 2.
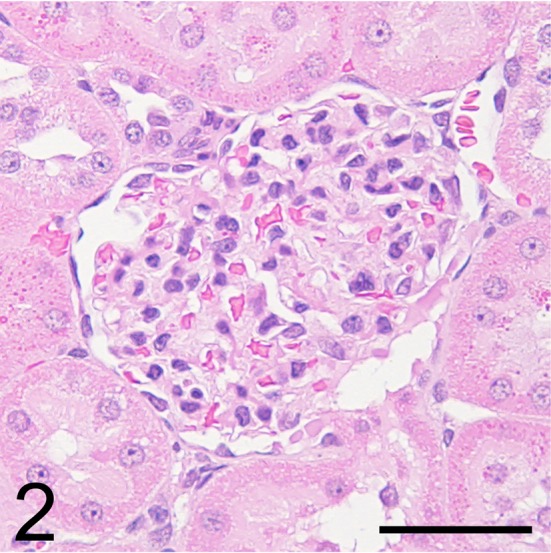
Light micrograph of the glomerulus of the male Sprague Dawley rat showing minimal change disease. This rat did not show glomerular changes. Hematoxylin and eosin (HE) stain. Bar = 50 μm.
Fig. 3.
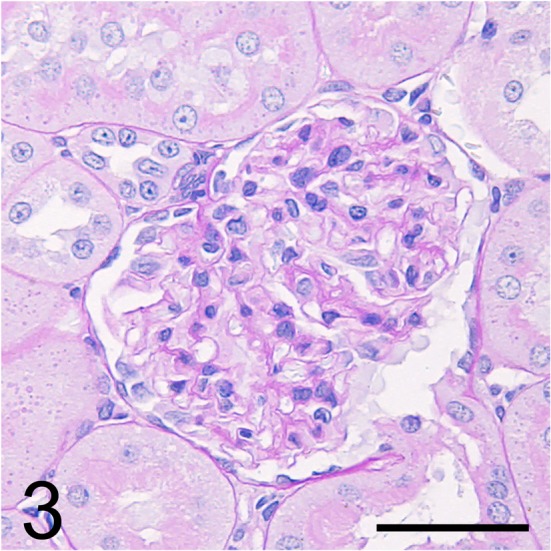
Serial section of Fig. 1 stained with periodic acid-Schiff. Bar = 50 μm.
Fig. 4.
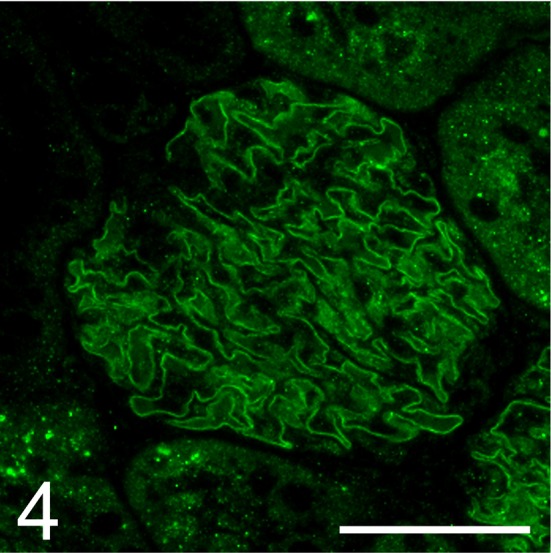
Expression of nephrin in the glomerulus of the male Sprague Dawley rat with minimal change disease. This rat showed a normal linear pattern of distribution along the glomerular capillary loop. Immunofluorescence. Bar = 50 μm.
Fig. 5.
Electron micrograph of a glomerulus of the male Sprague Dawley rat with minimal change disease. This rat showed extensive foot process effacement of the podocytes (a; Bar = 50 nm). The slit pore, which is situated between the foot processes, is narrowed, and rearrangement of the actin cytoskeleton (arrows) is observed in fused foot processes in the podocyte (b; Bar = 50 nm). Ep, glomerular epithelial cell (podocyte); En, endothelial cell.
Chronic progressive nephropathy of the rats is an important differential diagnosis for this case. The rat commonly develops chronic progressive nephropathy at an early stage from 2 months of age, which is characterized by an increased mesangium and thickened basement membrane of the glomerular capillary loops and Bowman’s capsules11, 12. The International Harmonization of Nomenclature Diagnosis Criteria (INHAND) describes several types of glomerular lesions, such as mesangioproliferative glomerulopathy and glomerulosclerosis, which are easily detected by light microscope13. The histopathological characterization of this case was consistent with that of human MCD and resembled rat PAN. (1) Continuous albuminuria, (2) no abnormalities in light microscopy, and (3) extensive foot process effacement of glomerular podocytes in electron microscopy were the primary characteristics of this SD rat case.
The most commonly used model of MCD is the PAN model in rats, which is induced by a single administration of puromycin aminonucleoside (PA)7,8,9. Glomerular podocytes are the primary target of PA-induced injury, and thus, the PAN rat model reveals podocyte injury such as foot process effacement, narrowing of the slit pore, absence of the slit diaphragm, and rearrangement of the cytoskeleton at the base of fused foot processes by electron microscopy, all of which result in proteinuria8, 14. Although these rats develop proteinuria and exhibit the classic morphological features, their manifestations differ from human patients with MCD in certain ways. Indeed, their proteinuria might be nonselective and only partially steroid-sensitive9. This suggests that the PAN is comprised of a combination of steroid-sensitive and steroid-resistant pathways. In addition, accompanied by the development of podocyte injury, segmental mesangial cell proliferation and expansion of the mesangial matrix, adhesion of the glomerular capillary loop to the Bowman’s capsule, and segmental collapse of the glomerular tuft are occasionally observed by light microscopy8. PAN rats with these advanced lesions at the glomerulus might progress to steroid-resistant focal segmental glomerulosclerosis (FSGS), as seen in a repeated administration model of PA7, 8. Although the susceptibility of the present study’s SD rat to steroids was unclear, no morphological changes were able to be detected by light microscopy, which indicated that the present case resembled human MCD rather than an advanced glomerular lesion leading to FSGS.
Nephrin is the central component of the slit diaphragm that plays a crucial role in the glomerular filtration barrier. Decreased nephrin levels have a significant impact on the development of proteinuria, which is also reported in PAN rats15, 16. However, despite the absence of a slit diaphragm, nephrin expression is retained in human MCD17, 18. This is one of the remarkable differences between human MCD and rat PAN. The SD rat in this report showed retention of nephrin expression, suggesting that this spontaneous case was much closer to human MCD as opposed to rat PAN. Anti-nephrin antibodies are known to have two types, one directed against the intracellular (C-terminal) domain and one directed against the extracellular (N-terminal) domain. Patrakka et al. reported that both types of antibodies showed retained expression of nephrin in human MCD18. The anti-nephrin antibody (G-8) used in this study was directed against the extracellular (N-terminal) domain of nephrin and did not show absence of nephrin as in human MCD. Therefore, further investigation using a number of spontaneous MCD cases in rats is necessary to clarify the behavior of nephrin expression in rat MCD.
Desmin is known as a conventional marker of podocyte injury in rodents, and it is reported to be upregulated in many cases of glomerular disease including rat PAN19, 20. In humans, desmin expression in podocytes is also upregulated in glomerular disease, such as membranous nephropathy, but is not upregulated in MCD21. In the present case, desmin expression in podocytes was not observed, as in human MCD, which also supports that our case being much closer to human MCD rather than rat PAN.
In contrast to urinary albumin, the urinary KIM-1, osteopontin, NGAL, and the clusterin levels, which are renal tubule injury markers, did not show any significant changes in this study. Tonomura et al. reported that, unlike KIM-1 and clusterin, urinary NGAL increased along with elevated albumin without renal tubule injury in rat PAN22. In their case, it was suggested that the increase in urinary NGAL level was caused by a decrease in megalin, a receptor of NGAL that is highly expressed in the brush border of the proximal tubule. Administration of PAN to rats causes a decrease in megalin expression in the proximal tubule23; therefore, the elevation of urinary NGAL might reflect functional changes in the proximal tubule in rat PAN. Although a small number of relapses are reported in steroid-resistant cases, human MCD usually shows a favorable prognosis, including spontaneous remission1, 24. Rat PAN induced by a single administration of PA is also reversible and is associated with the absence of proteinuria and histological recovery of the glomerular lesions at 28 days and 18 weeks after administration, respectively7, 25. In toxicological studies, the development of albuminuria that is not accompanied by any changes on light microscopy is rarely observed in untreated control rats (data not shown). The frequency of glomerular lesions in SD rats, similar to that in human MCD, is unclear, though a certain proportion of these lesions may be misrecognized in toxicological studies. In cases of rat albuminuria without histopathological changes on light microscopy, human MCD-like disease should be included in the differential diagnosis of rat glomerular lesions.
Acknowledgments
The authors would like to thank Ms. Rumiko Ishida (Daiichi Sankyo Co., Ltd.), Ms. Keiko Okado (Daiichi Sankyo RD Novare Co., Ltd.), and Ms. Sanae Takada (Daiichi Sankyo Co., Ltd.) for their excellent support in the pathological examination and their assistance in the collection of specimens.
Footnotes
Disclosure of Potential Conflicts of Interest: None.
References
- 1.Olson JL. The nephrotic syndrome and minimal change disease. In: Heptinstall’s Pathology of the Kidney. 6th ed. JC Jennette, JL Olson, MM Schwartz, and FG Silva (eds). Lippincott Williams & Wilkins, Philadelphia. 125–154. 2007. [Google Scholar]
- 2.Nair R, Bell JM, and Walker PD. Renal biopsy in patients aged 80 years and older. Am J Kidney Dis. 44: 618–626. 2004. [PubMed] [Google Scholar]
- 3.Bazzi C, Petrini C, Rizza V, Arrigo G, Beltrame A, and D’Amico G. Characterization of proteinuria in primary glomerulonephritides: urinary polymers of albumin. Am J Kidney Dis. 30: 404–412. 1997. [DOI] [PubMed] [Google Scholar]
- 4.White RH, Glasgow EF, and Mills RJ. Clinicopathological study of nephrotic syndrome in childhood. Lancet. 295: 1353–1359. 1970. [DOI] [PubMed] [Google Scholar]
- 5.Hopper J, Jr, Ryan P, Lee JC, and Rosenau W. Lipoid nephrosis in 31 adult patients: renal biopsy study by light, electron, and fluorescence microscopy with experience in treatment. Medicine (Baltimore). 49: 321–341. 1970. [PubMed] [Google Scholar]
- 6.Rivera A, Magliato S, and Meleg-Smith S. Value of electron microscopy in the diagnosis of childhood nephrotic syndrome. Ultrastruct Pathol. 25: 313–320. 2001. [DOI] [PubMed] [Google Scholar]
- 7.Diamond JR, and Karnovsky MJ. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 122: 481–487. 1986. [PMC free article] [PubMed] [Google Scholar]
- 8.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, and Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 296: F213–F229. 2009. [DOI] [PubMed] [Google Scholar]
- 9.Chugh SS, Clement LC, and Macé C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis. 59: 284–292. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa S, Nagaike M, and Ozaki K. Databases for technical aspects of immunohistochemistry. J Toxicol Pathol. 30: 79–107. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan KNM, Hard GC, and Alden CL. Kidney. In: Haschek and Rousseaux’s Handbook of Toxicologic Pathology. 3rd ed. WM Haschek, CG Rousseaux, and MA Wallig (eds). Academic Press in an Imprint of Elsevier, London. 1667–1774. 2013. [Google Scholar]
- 12.Simms HS, and Berg BN. Longevity and the onset of lesions in male rats. J Gerontol. 12: 244–252. 1957. [DOI] [PubMed] [Google Scholar]
- 13.Frazier KS, Seely JC, Hard GC, Betton G, Burnett R, Nakatsuji S, Nishikawa A, Durchfeld-Meyer B, and Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 40(Suppl): 14S–86S. 2012. [DOI] [PubMed] [Google Scholar]
- 14.Inokuchi S, Shirato I, Kobayashi N, Koide H, Tomino Y, and Sakai T. Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney Int. 50: 1278–1287. 1996. [DOI] [PubMed] [Google Scholar]
- 15.Patrakka J, and Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med. 13: 396–403. 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami H, Kamiie J, Yasuno K, Kobayashi R, Aihara N, and Shirota K. Dynamics of absolute amount of nephrin in a single podocyte in puromycin aminonucleoside nephrosis rats calculated by quantitative glomerular proteomics approach with selected reaction monitoring mode. Nephrol Dial Transplant. 27: 1324–1330. 2012. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani SR, Finn LS, Kowalewska J, McDonald RA, and Eddy AA. Expression of nephrin in acquired forms of nephrotic syndrome in childhood. Pediatr Nephrol. 19: 300–305. 2004. [DOI] [PubMed] [Google Scholar]
- 18.Patrakka J, Ruotsalainen V, Ketola I, Holmberg C, Heikinheimo M, Tryggvason K, and Jalanko H. Expression of nephrin in pediatric kidney diseases. J Am Soc Nephrol. 12: 289–296. 2001. [DOI] [PubMed] [Google Scholar]
- 19.Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, and Couser WG. Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo. Expression of antiadhesive proteins and cytoskeletal changes. Lab Invest. 67: 486–497. 1992. [PubMed] [Google Scholar]
- 20.Kakimoto T, Okada K, Hirohashi Y, Relator R, Kawai M, Iguchi T, Fujitaka K, Nishio M, Kato T, Fukunari A, and Utsumi H. Automated image analysis of a glomerular injury marker desmin in spontaneously diabetic Torii rats treated with losartan. J Endocrinol. 222: 43–51. 2014. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama M, Sugiyama H, Sada K, Kobayashi M, Maeshima Y, Yamasaki Y, and Makino H. Desmin as a marker of proteinuria in early stages of membranous nephropathy in elderly patients. Clin Nephrol. 68: 73–80. 2007. [DOI] [PubMed] [Google Scholar]
- 22.Tonomura Y, Tsuchiya N, Torii M, and Uehara T. Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology. 273: 53–59. 2010. [DOI] [PubMed] [Google Scholar]
- 23.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, and Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 71: 504–513. 2007. [DOI] [PubMed] [Google Scholar]
- 24.Bohlin AB. Clinical course and renal function in minimal change nephrotic syndrome. Acta Paediatr Scand. 73: 631–636. 1984. [DOI] [PubMed] [Google Scholar]
- 25.Olson JL, Rennke HG, and Venkatachalam MA. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab Invest. 44: 271–279. 1981. [PubMed] [Google Scholar]