Abstract
Objective
Pathologically increased activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the associated Ca2+-leak from the sarcoplasmic reticulum are recognized to be important novel pharmacotherapeutic targets in heart failure and cardiac arrhythmias. However, CaMKII-inhibitory compounds for therapeutic use are still lacking. We now report on the cellular and molecular effects of a novel pyrimidine-based CaMKII inhibitor developed towards clinical use.
Methods and results
Our findings demonstrate that AS105 is a high-affinity ATP-competitive CaMKII-inhibitor that by its mode of action is also effective against autophosphorylated CaMKII (in contrast to the commonly used allosteric CaMKII-inhibitor KN-93). In isolated atrial cardiomyocytes from human donors and ventricular myocytes from CaMKIIδC-overexpressing mice with heart failure, AS105 effectively reduced diastolic SR Ca2+ leak by 38% to 65% as measured by Ca2+-sparks or tetracaine-sensitive shift in [Ca2+]i. Consistent with this, we found that AS105 suppressed arrhythmogenic spontaneous cardiomyocyte Ca2+-release (by 53%). Also, the ability of the SR to accumulate Ca2+ was enhanced by AS105, as indicated by improved post-rest potentiation of Ca2+-transient amplitudes and increased SR Ca2+-content in the murine cells. Accordingly, these cells had improved systolic Ca2+-transient amplitudes and contractility during basal stimulation. Importantly, CaMKII inhibition did not compromise systolic fractional Ca2+-release, diastolic SR Ca2+-reuptake via SERCA2a or Ca2+-extrusion via NCX.
Conclusion
AS105 is a novel, highly potent ATP-competitive CaMKII inhibitor. In vitro, it effectively reduced SR Ca2+-leak, thus improving SR Ca2+-accumulation and reducing cellular arrhythmogenic correlates, without negatively influencing excitation-contraction coupling. These findings further validate CaMKII as a key target in cardiovascular disease, implicated by genetic, allosteric inhibitors, and pseudo-substrate inhibitors.
Keywords: CaMKII, Ca2+ handling, heart failure
Introduction
Heart failure is an important healthcare burden with debilitating consequences for the affected patients and high socioeconomic impact. Recent therapeutic advances for heart failure have been mainly in the field of device-based therapy, with the exception of Sacubitril, a neprilysin inhibitor approved by the FDA in 2015 in combination with valsartan. Despite these limited advances, the morbidity and mortality amongst heart failure patients remains high. In addition to pump failure, heart failure patients die from cardiac arrhythmias (sudden cardiac death) and both atrial and ventricular arrhythmias can worsen the progression of heart failure. Thus, additional strategies for therapeutic intervention in heart failure and cardiac arrhythmias are still needed.
In heart failure, neurohumoral and cytokine activation acutely serve compensatory purposes, but such chronic compensation leads to cardiac remodeling that further worsens the condition, resulting in a vicious cycle of increasingly impaired contractility and eventually myocardial failure. Pharmacotherapies such as beta blockers or ACE-inhibitors/AT2-antagonists intervene in this vicious cycle by antagonizing the compensatory stimuli.
Importantly, dysregulation of cardiac excitation-contraction couplings have been identified to be central to heart failure and cardiac arrhythmias (as reviewed in (1,2)). Specifically, diastolic Ca2+-leak from the sarcoplasmic reticulum (SR) through the ryanodine receptor (RyR2) is regarded as critically contributing to impaired cardiomyocyte contractility by depleting SR Ca2+-content, making less Ca2+ available for contraction (3–5). Furthermore, SR Ca2+-leak appears to be a mechanism for triggering atrial (6–8) as well as ventricular (9–11) arrhythmogenesis.
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine protein kinase that is of special interest in this cardiac pathophysiology. CaMKII is an important regulator of cardiac excitation-contraction coupling and Ca2+-homeostasis (as reviewed in (1,12)) by regulating the function of L-type Ca2+-channels, SR Ca2+-ATPase (SERCA2a, via phosphorylation of phospholamban (PLB) at Thr-17), and RyR2 (via phosphorylation at Ser-2814). CaMKII can furthermore activate pathways involved in hypertrophy, inflammation, and apoptosis (as reviewed in (13)). In heart failure (5,14,15) and atrial fibrillation (7,8), CaMKII is overactivated by stimuli that elevate Ca2+ and reactive oxygen species. Importantly, CaMKII-dependent RyR2 Ser-2814 hyper-phosphorylation has been demonstrated to induce SR Ca2+-leak (4,16,17). Accordingly, direct genetic or pharmacological inhibition of CaMKII as well as other disruptions of CaMKII-dependent pathways have shown promise for treating heart failure or cardiac arrhythmias (18–20). Consequently, CaMKII has become increasingly recognized as a consensus target for pharmacological intervention in heart disease. However, compounds suitable for use in humans are still lacking (21).
AS105 (Fig. 1A) is a novel CaMKII-inhibitor being advanced by Allosteros Therapeutics following computational optimization of pyrimidine-based CaMKIIδ inhibitors (21,22). We have explored the therapeutic potential of AS105 using isolated murine ventricular and human atrial cardiomyocytes. The field has largely relied on a tool inhibitor, KN-93, which is allosteric and non-ATP competitive, but is of low potency and inhibits multiple ion channels and other off-targets21. And this study also aims to evaluate the utility of an ATP-competitive inhibitor for cardiovascular studies of CaMKII action.
Figure 1. Enzymatic Characterization of AS105.
(A) Structure of AS105. (B): AS105 inhibits CaMKIIδ with low nanomolar affinity: We used a continuous spectrophotometric assay to follow the phosphorylation of a peptide substrate with recombinantly-expressed and purified CaMKIIδ at increasing AS105 concentrations. The IC50 is determined using standard non-linear regression analysis. (C): AS105 inhibition of CaMKIIδ is ATP-competitive: CaMKIIδ kinase activity is measured while varying the concentration of ATP at different AS105 concentrations. The inhibitory pattern indicates a competitive mode of inhibition against ATP. (D): Inhibition of CaMKIIδ activity by AS105 is independent of kinase autophosphorylation: Prior to the kinase activity assay CaMKIIδ was subjected to an autophosphorylation reaction in the presence of Ca2+/CaM and ATP (CaMKII-P) or to a control reaction lacking ATP (CaMKII) for 5 minutes at room temperature. The enzyme was then assayed for activity as normal in the presence of increasing concentrations of AS105 (pink symbols) or KN-93 (green symbols) to calculate inhibitor IC50s.
Mice with transgenic overexpression of CaMKIIδC develop severe heart failure associated with 50% reduction of twitch contractility and systolic Ca2+-transient amplitude due to over 50% reduction of SR Ca2+-content (16), which in turn has been attributed to about 2- to 3-fold higher SR Ca2+-leak (16,23) in these mice. Expression levels of SERCA2a, PLB and RyR2 are reduced in CaMKIIδC-TG hearts, while the expression of NCX is increased (16), in line with the heart failure phenotype.
Methods
CaMKIIδ purification and enzyme activity assays
Hexahistidine tagged human CaMKIIδ was expressed in High Five™ insect cells using a baculovirus expression system (Thermo Fisher Scientific, Waltham, MA, USA). Purification of the CaMKIIδ holoenzyme was accomplished by Ni-NTA affinity chromatography using a HisTrap HP column (GE Healthcare Life Sciences, Marlborough, MA, USA) followed by HiPrep 26/60 Sephacryl S-300 (GE Healthcare Life Sciences, Marlborough, MA, USA) size exclusion chromatography. The purified enzyme was stored at −80 °C in 25 mM Tris HCl pH 8, 300 mM KCl, 30 % glycerol (v/v), and 2 mM TCEP. The purity of the final preparation was >95% as assessed by SDS-PAGE and Coomassie Blue staining.
CaMKIIδ phosphotransferase activity was monitored using a continuous spectrophotometric assay as described previously (24). In this assay, the phosphorylation of a peptide substrate by CaMKIIδ is enzymatically coupled to the oxidation of NADH to NAD+, which produces a decrease in absorbance at 340 nm. The reactions were carried out in a final volume of 150 µl in 100 mM Tris HCl pH 7.5, 150 mM KCl, 0.2 mM CaCl2, 10 mM MgCl2, 0.1 mM ATP, 1% DMSO, 1 mM phosphoenolpyruvate, 0.28 mM NADH, 89 units/ml pyruvate kinase, 124 units/ml lactate dehydrogenase (Sigma-Aldrich), 150 µM autocamtide-3 (KKALHRQETVDAL; Biomer Technology), 60 nM CaM (EMD Millipore), and 5–10 nM CaMKIIδ. The enzyme concentration is expressed in terms of the concentration of kinase subunits and not holoenzymes. Reactions were initiated by the addition of CaM and ATP to the mix and the decrease in absorbance at 340 nm was monitored at 30 °C using a microplate spectrophotometer, SpectraMax Plus (Molecular Devices, Sunnyvale, CA, USA).
Autophosphorylation of CaMKIIδ was accomplished by incubating 37.5 nM CaMKIIδ with 450 nM CaM, 0.2 mM ATP in a buffer containing 80 nM Tris pH 7.4, 140 mM KCl, 10 mM Mg2+, and 0.5 mM Ca2+. A control reaction containing all the above components except ATP was processed in parallel. After 5 min at room temperature 20 µl of each reaction was assayed for kinase activity in the presence of increasing concentrations of AS105 or KN-93. The final components of the kinase activity assay (150 µl final volume) were 5 nM CaMKIIδ, 60 nM CaM, 150 µM autocamtide-3, 0.13 mM ATP, 92 mM Tris pH 7.4, 159 mM KCl, 11 mM Mg2+, 0.57 mM Ca2+, and 1% DMSO. In addition, 20 µl of each reaction were also identically assayed for kinase activity in the presence of 3 mM EGTA to verify the presence or absence of autophosphorylation.
Steady state initial velocity data were calculated from the slopes of the A340 curves and standard non-linear regression analysis was used to determine kinetic parameters using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). The inhibition constant Ki was calculated using the equation Ki=IC50/(1+[ATP]/Km). Using Michaelis-Menten analyses the Km for ATP at the described experimental conditions was experimentally determined to be KmATP=49 µM.
Sources of cardiac tissue
CaMKIIδC-overexpressing mice (25) and the respective wildtype (WT) mice were bred at the central animal research facilities of the University Medical Center Göttingen and the University Medical Center Regensburg. All investigations conformed to Directive 2010/63/EU of the European Parliament and the NIH Guide for the Care and Use of Laboratory Animals. Right atrial appendages from human patients were obtained during coronary artery bypass surgery in patients in sinus rhythm without higher degree valve dysfunction. Written consent had been given by the donors and approval had been granted by the local ethics committee. Clinical parameters of the donors are displayed in Suppl. table 1.
Isolation of murine cardiomyocytes
Isolation of cardiomyocytes was performed as previously described (17). Briefly, CaMKIIδC-overexpressing mice or the respective wildtype mice (C57BL6-J strain) were anaesthetized with isoflurane. After death by cervical dislocation, hearts were quickly excised, mounted on a Langendorff perfusion apparatus and retrogradely perfused with nominally Ca2+-free solution containing (in mM) 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 10 HEPES, 30 taurine, 10 BDM, 5.5 glucose, 0.032 phenol-red for 4 min at 37°C (pH 7.4). Then, 7.5 mg/ml liberase™ (Roche diagnostics, Mannheim, Germany), trypsin 0.6%, and 0.125 mM CaCl2 were added to the perfusion solution. Perfusion was continued for 3–4 min until the heart became flaccid. Ventricular tissue was collected in perfusion buffer supplemented with 5% bovine calf serum, cut into small pieces, and dispersed by repeatedly pipetting until no solid cardiac tissue was left. Ca2+-reintroduction was performed by stepwise increasing [Ca2+] from 0.1 to 1.7 mM. For measurements, cells were freshly plated onto superfusion chambers, which had been coated with laminin.
Isolation of human cardiomyocytes
Cardiomyocytes were isolated from human right atrial samples using the chunk-isolation technique. Samples were rinsed, cut into small pieces and incubated in a spinner flask with a solution consisting of (in mM) 100 NaCl, 10 KCl, 5 MgCl2, 1.2 KH2PO4, 50 taurine, 5 MOPS, 10 BDM, 20 glucose (pH 7.2 at 37°C). After 10 min, CaCl2 was added for a concentration of 0.02 mM. For the first digestion step, this solution was supplemented with 0.775 mg/ml collagenase type I (370 U/ml; Worthington) and also 0.4 mg/ml protease type XXIV (Sigma-Aldrich). After 45 min, the supernatant was discarded and solution containing collagenase used and the tissue was repeatedly aggregated by pipetting in addition to stirring until sufficient numbers of free cells appeared in the solution. Then, stopping solution was added to final levels of bovine calf serum (BCS) 2% and BDM to 20 mM and the supernatant with cells was centrifuged. Cells were then resuspended in storage medium containing (in mM) 30 KCl, 10 KH2PO4, 1 MgCl2, 10 HEPES, 11 glucose, 20 taurine, 70 glutamic acid, 20 BDM, 2% BCS (pH 7.4 with KOH). Cells were plated onto superfusion chambers coated with laminin and left to settle for 1 h.
Incubation with AS105
2 µM AS105 (or vehicle in control groups: “Ctrl”) was included in the loading buffers for Ca2+-dyes for per-incubation and in the respective superfusion solutions.
Experimental solutions
For experiments using murine cardiomyocytes, normal Tyrode’s solution was used consisting of (in mM) 140 NaCl, 4 KCl, 5 HEPES, 1 MgCl2, 10 glucose, 2 CaCl2 (pH 7.4 with NaOH). For experiments with human myocytes, the experimental solution consisted of (mM) 4 KCl, 136 NaCl, 1.6 MgCl2, 10 HEPES, 0.33 NaH2PO4, 4 NaHCO3, 10 glucose, 2 CaCl2 (pH 7.4 with NaOH). Loading buffers for Ca2+-fluorescent dye consisted of the respective experimental solution with Pluronic F-127 0.2 mg/ml in addition to the fluorescent dye. All experiments were performed at room temperature.
Investigation of EC-coupling
Ca2+-epifluorescence and sarcomere length measurements were performed using an epifluorescence setup (IonOptix Corp, Milton, USA) mounted to a Nikon TE2000U microscope (17). Myocytes were loaded with Fluo-4 AM (10 µM) for 15 min. Excitation was at 480±15 nm and F/F0 of the fluorescence signal was calculated. Cells were transilluminated by red light (>650 nm) and cardiomyocyte contractility (fractional shortening) was measured in parallel to epifluorescence using a sarcomere length detection system (IonOptix Corp, Milton, USA). Cardiomyocytes were field-stimulated at 1 Hz until steady-state was achieved. Post-rest potentiation of Ca2+-transient amplitude was investigated following 10 s of stimulation cessation to assess the SRs ability to accumulate Ca2+. SR Ca2+-content was estimated by rapid application of 10 mM caffeine after basal stimulation. Furthermore, the time constant τ (“tau”) of the monoexponential fit of the caffeine-induced transient was calculated to estimate NCX-function. To precisely assess SERCA2a function, tauSERCA2a was calculated from the tau of the electrically stimulated transients and tau of the caffeine-induced transients of the same cell as (26).
Tetracaine experiments
Tetracaine-experiments to measure SR Ca2+-leak were performed according to the method of Shannon et al. (27). Na+- and Ca2+-free bath solution was prepared by replacing Na+ in NT with Li+. Tetracaine 1 mM was added to this solution to prepare the tetracaine solution. The fractional shift in diastolic fluorescence upon tetracaine (an allosteric blocker of ryanodine receptors) under 0Na+/0Ca2+ conditions was measured, followed by caffeine application to calculate leak-load-relationship. [Ca2+]-values were calculated based on the previously reported diastolic Ca2+-concentration of 96 nM for the CaMKIIδC-TG mouse (16), assuming a Kd for Fluo-4 of 1100 nM in cardiomyocytes (28) using the equation (29).
Ca2+-spark measurements
Cardiomyocytes were incubated with 10 µM Fluo-3 AM for 15 min and experiments were started after washing out the loading buffer for 5 min with experimental solution. Fluorescence measurements for Ca2+-sparks were performed with a laser scanning confocal microscope (LSM 5 Pascal, Zeiss, Germany). Fluo-3 was excited at 488 nm and emitted fluorescence was collected through a 505 nm long-pass emission filter. Fluorescence images were recorded in line-scan mode with 512 pixels per line (width of each scan line 38.4 µm), pixel time 0.64 µs. Ca2+-sparks were detected and quantified using the ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD) plugin Sparkmaster (30) with visual confirmation of sparks detected. Ca2+-spark frequency (CaSpF) was calculated from this and normalized to scanned myocyte width and scanning interval. Ca2+-spark size (CaSpS, calculated as: amplitude * width * duration) was added for all sparks within a cell to calculate the SR Ca2+-leak for this cell. Only cells displaying Ca2+-sparks were included in the statistics. In addition to Ca2+-sparks, we also compared the occurrence of spontaneous global intracellular Ca2+-release events (“SCaEs”, (8,31)) in these cells in order to evaluate cellular antiarrhythmic effects of AS105. For measurements in wildtype murine cardiomyocytes, recordings were done using a Zeiss LSM 700 (Zeiss, Germany), width of each line 35.5 µm, and sparks evaluated as described above.
Patch clamp measurement of L-type Ca2+-currents
Patch-clamp experiments were performed using ventricular cardiomyocytes isolated from CaMKIIδC-TG mice using an EPC-10 amplifier and Patchmaster software (HEKA, Germany). The L-type Ca2+-current (ICa,L) was recorded at room temperature (21 °C) using the ruptured-patch whole-cell-patch clamp technique in voltage-clamp mode. After rupture, 2 minutes was allowed for equilibration of intracellular solution and cytosol before starting recordings. Fast and slow capacitance as well as series resistance were compensated for using the built in functions of Patchmaster. Pipettes were pulled to resistances of 2–3 MΩ and filled with Na+- and K+-free intracellular solution consisting of (in mM) 90 Cs-methanesulfonate, 20 CsCl, 10 HEPES, 4 MgATP, 0.4 Tris-GTP, 3 CaCl2, and 10 EGTA (pH 7.2 at 21 °C). Myocytes were superfused with K+-free external solution consisting of (in nM) 120 tetraethyl ammonium chloride, 10 CsCl, 10 HEPES, 10 glucose, 1 MgCl2 and 2 CaCl2 (pH 7.4 at 21 °C). From a holding potential of −80 mV, cells were briefly depolarized to −40 mV for 50 ms to ensure inactivation of Na+-currents, then clamped to test potentials between −30 mV and +40 mV for 200 ms in 10-mV steps, increasing in intervals of 1 s to establish current-voltage (IV) relationship. Measured currents were normalized to membrane capacitance (pA/pF).
Results
Characterization of AS105, an inhibitor of CaMKIIδ
The small molecule inhibitor AS105 (Fig. 1A) was identified during optimization of pyrimidine-based CaMKIIδ inhibitors (21,22). We synthesized AS105 and characterized its inhibitory properties using recombinantly expressed and purified CaMKIIδ, the CaMKII isoform that is predominantly expressed in cardiac tissue (32). In vitro, CaMKIIδ phosphorylation assays using peptide substrates revealed that AS105 is quite potent, with a measured IC50 of 8 nM (Fig. 1B). Computational modeling using crystal structures of CaMKIIδ predicts AS105 docks in the ATP binding pocket, a deep cleft located between the kinase N-terminal and C-terminal lobes. Drug binding would thus be expected to block ATP from binding at this site. To validate this kinetically, we carried out ATP competition experiments by measuring the inhibitory capacity of AS105 at increasing ATP concentrations. As predicted by our computational modeling, these kinetic analyses showed that AS105 is competitive against ATP (Fig. 1C). Given this information, the inhibition constant (Ki) of AS105 for CaMKIIδ was calculated, Ki = 3 nM, suggesting that even at the intracellular millimolar ATP concentrations present in cardiomyocytes, AS105 would be an effective inhibitor of CaMKIIδ.
One of the unique properties of CaMKII family of proteins is their ability to become “autonomous” or Ca2+-independent after autophosphorylation on Thr287 (Thr286 in CaMKIIα) (reviewed in (33)). Phosphorylation at this site prevents the enzyme from reverting to its initial autoinhibited state and renders the kinase constitutively active regardless of Ca2+/CaM levels in the cell, until dephosphorylated by phosphatases (reviewed in (33)). Oxidation, GlcNAcylation and S-nitrosylation, are additional recently described CaMKIIδ post-translational modifications that functionally mimic the effects of autophosphorylation [34–36].
The widely used CaMKII tool inhibitor KN-93 is sensitive to the autophosphorylation status of CaMKII, inhibiting only the unphosphorylated form of the kinase (37). KN-93 is thought to interfere with the process CaMKII activation and it is classified as a “non ATP-competitive” or “allosteric” inhibitor. To further explore AS105 mechanism of action, we compared the inhibitory properties of AS105 and KN-93 towards unphosphorylated and autophosphorylated CaMKIIδ (Fig. 1D). Prior to the kinase activity assay we subjected CaMKIIδ to an autophosphorylation reaction in the presence of Ca2+/CaM and ATP (CaMKII-P) or to a control reaction lacking ATP (CaMKII) for 5 minutes at room temperature. We then obtained inhibitor IC50s by assaying the autophosphorylation or control reactions for activity as normal in the presence of increasing concentrations of AS105 or KN-93. As autophosphorylated CaMKII is Ca2+/CaM independent, the Ca2+ chelator EGTA was included in the CaMKII-P kinase activity reactions to ensure only autophosphorylated kinase is being followed. For CaMKII-P reactions, inclusion of EGTA in the kinase assays did not alter the IC50s calculated from them (Fig. 1D). Control reactions that lacked ATP in the autophosphorylation reaction and that were subsequently assayed for kinase activity in the presence of EGTA showed no measurable activity, verifying that no autophosphorylation had occurred and that, as previously demonstrated, CaMKII needs Ca2+/CaM for activity unless autophosphorylation on Thr287 has occurred (Fig. 1D).
The results shown in Fig. 1D reveal that, in agreement with previous observations, KN-93 is unable to inhibit CaMKIIδ that has been subjected to autophosphorylation prior to kinase activity measurements, i.e. it blocks activation of the kinase but does not block catalytic activity. In contrast, AS105 inhibits unphosphorylated and autophosphorylated kinase equally well (IC50s of 8 and 7 nM respectively); it is not sensitive to the autophosphorylation state of the enzyme. In the absence of autophosphorylation, KN-93 inhibits CaMKIIδ with an IC50 value of 1.4 µM. Taken together, the high potency of AS105 (as demonstrated in vitro), coupled with its potential to inhibit multiple forms of CaMKIIδ, make it a promising lead for optimization for clinical use.
Functional experiments
Effects of AS105 (2 µM) on isolated cardiomyocyte Ca2+-handling and SR Ca2+-leak were compared against vehicle as described below.
AS105 effects on excitation-contraction coupling in murine cardiomyocytes
Effects of AS105 on excitation-contraction coupling were investigated in ventricular cardiomyocytes from CaMKIIδC-overexpressing mice. We found that Ca2+-transient amplitudes (mean data and original Ca2+-transients in Fig. 2A) and, consequently, systolic cardiomyocyte contractility (mean data and original single cell twitches in Fig. 2B) were significantly improved by AS105 during basal electrical stimulation. (Ca2+-transients amplitude: AS105: 3.44±0.06 F/F0 vs Ctrl: 3.23±0.06 F/F0, n=94 vs n=92, p<0.05; twitch amplitude: AS105: 4.66±0.19% fractional cell shortening vs Ctrl: 4.04±0.17%, n=93 vs n=92, p<0.05.) Underlying Ca2+-transients, SR Ca2+-content was also improved upon AS105 treatment, as assessed by the amplitudes of caffeine-induced Ca2+-transients (illustrative original registrations and mean values in Fig. 2C, caffeine-induced transient amplitude AS105: 5.49±0.19 F/F0 vs Ctrl: 4.88±0.14 F/F0, n=78 vs n=66, p<0.05). We found that fractional release of Ca2+ from the SR during basal stimulation (calculated by comparing the amplitude of electrical- to caffeine-induced transients) was not altered by AS105, suggesting that the improvements of Ca2+-transient can be attributed to the improved SR Ca2+-loading instead of just a larger fraction of stored Ca2+ being released from the SR. Ca2+-released from the SR during systole is recovered via SERCA2a. SERCA2a-function was not altered by AS105 (Fig. 2E, AS105: tauSERCA2a 0.330±0.009 s vs Ctrl: 0.339±0.009 s, n=78 vs n=66). (For cells without caffeine-data, SERCA2a-function was assessed by time to 80% decay of electrically evoked transients (rt80%). This also was not influenced by AS105 (data not shown).) The function of NCX was assessed during caffeine-application. AS105 did not influence NCX activity (Fig. 2F, AS105: tau 4.76±0.45 s vs Ctrl: 4.67±0.47 s, n=78 vs. n=66). Thus, we could exclude that the improvement of SR Ca2+-loading upon AS105 were due to enhancing SR Ca2+-reuptake (i.e. activation of SERCA2a) or reducing cardiomyocyte Ca2+-extrusion (i.e. inhibition of NCX).
Figure 2. Effect of AS105 on Excitation Contraction Coupling.
(A&B): Ca2+-transients and contractility are improved by AS105 during basal electrical stimulation: Ca2+-transients (Fluo-4 F/F0) and contractions (fractional sarcomere length shortening) were recorded in isolated cardiomyocytes from mice with heart failure due to CaMKIIδC-overexpression, as illustrated by original registrations (A). Mean values (B) show improved Ca2+-transient amplitudes and contractility upon AS105 during basal 1 Hz stimulation. n= indicates cells/mice. (C&D): AS105 improves SR Ca2+-content: SR Ca2+-content was measured by measuring caffeine-induced Ca2+-release. As shown by original registrations and mean values (C), AS105 improved SR Ca2+-content. Caffeine experiments also allowed to investigate systolic fractional SR Ca2+-release during basal stimulation, which was not altered by AS105 (D). n= indicates cells/mice. (E&F): AS105 does not impair diastolic SERCA2a or NCX activity: SERCA2a function was calculated from decay constants of electrically stimulated and caffeine-induced Ca2+-transients (E). This showed that SERCA2a activity was not altered by AS105. NCX-function was measured during caffeine exposure and was not affected by AS105 (F). n= indicates cells/mice.
AS105 effects on SR Ca2+-leak
Thus, we assessed post-rest potentiation of Ca2+-transient amplitudes during 10 s of stimulation cessation in the murine cardiomyocytes as an integrative measure of the ability of the SR to accumulate Ca2+. We found clearly enhanced post-rest-potentiation in AS105 treated cardiomyocytes, as illustrated in original registrations and mean values in Fig. 3A (AS105: 33.9±1.9% increase of the transient F/F0 after the pause vs Ctrl: 26.9±1.5%, n=39 vs. n=46, p<0.05), indicating an improved ability to accumulate Ca2+ in the SR, which explains the increased SR Ca2+-content under basal stimulation.
Figure 3. AS105 effects on SR Ca2+-leak.
(A): AS105 improves SR Ca2+-accumulation: Post-rest increase of Ca2+-transient amplitude was investigated to assess the ability of the SR to accumulate Ca2+, which was enhanced by AS105. n= indicates cells/mice. (B–D): AS105 reduces SR Ca2+-leak in ventricular murine cardiomyocytes: As illustrated in original recordings (B), tetracaine-sensitive shift of [Ca2+]i was measured to assess SR Ca2+-leak in ventricular cardiomyocytes from mice with heart failure due to CaMKIIδC-overexpression. AS105 reduced SR Ca2+-leak (C) by strongly attenuating SR leak/load-relationship (D). n= indicates cells/mice. (E–H): AS105 reduces SR Ca2+-leak in human atrial cardiomyocytes: Effects of AS105 on SR Ca2+-leak in human atrial cardiomyocytes were assessed by measuring Ca2+-sparks (E). (Original line scan recordings 20% contrast enhanced for better reproduction.) AS105 reduced both the size of individual Ca2+-sparks (F) as well as the frequency of their occurrence (G), resulting in a pronounced reduction of SR Ca2+-leak (H). n= indicates sparks/patients (F) or cells/patients (G&H). (I): AS105 suppresses arrhythmogenic cellular events: The occurrence of arrhythmogenic spontaneous global Ca2+-release events (original recording (I)) in human atrial cardiomyocytes was assessed during confocal line scans. AS105 suppressed the occurrence of these events by ~50%. n= indicates cells/patients.
We assessed AS105 effects on SR Ca2+-leak first using tetracaine experiments (27) in ventricular cardiomyocytes from CaMKIIδC-overexpressing mice. As illustrated in Fig. 3B, the tetracaine-induced diastolic [Ca2+]-shift was clearly lower in AS105-treated cells (Fig. 3C), indicating lower SR Ca2+-leak. ([Ca2+]-shift upon AS105: 16.46±1.58 nM vs. 23.25±2.20 nM, n=28 vs. n=24, p<0.05. For raw fluorescence signal AS105: 16.05±1.57% reduction of fluorescence vs. Ctrl: 22.81±2.21%, n=28 vs. n=24, p<0.05.) Importantly, AS105 effected a smaller SR Ca2+-leak despite the higher SR Ca2+-loading in the AS105-treated cells, as the leak/load-relationship was reduced by AS105 by ~65% (Fig. 3D, AS105: 1.59±0.42% vs. Ctrl: 4.55±1.18%, n=14 vs. n=15, p<0.05; for raw fluorescence AS105: 2.63±0.44% vs. Ctrl: 6.05±1.21%, n=14 vs. n=15, p<0.05).
To validate our key finding that AS105 suppresses SR Ca2+-leak for human cells and to do so using another method for assessing Ca2+-leak, we measured Ca2+-sparks in right atrial cardiomyocytes from human donors. As shown in Fig. 3E, we found that AS105 reduced both the size of individual Ca2+-sparks (Fig. 3F, AS105: 329.6±26.4 nF/F0*m*s vs Ctrl: 454.0±34.5 nF/F0*m*s, n=293 vs n=359 sparks, p<0.05) as well as the frequency of Ca2+-spark occurrence (Fig. 3G, AS105: 1.89±0.17 sparks/100µm/s vs Ctrl: 2.44±0.21 sparks/100µm/s, n=59 vs n=62 cells, p<0.05). This results in a ~44% reduction of total SR Ca2+-leak per cell in the human atrial cardiomyocytes (Fig. 3H, AS105: 6.24±1.18 mF/F0 vs. Ctrl: 11.07±2.08 mF/F0, n=59 vs. n=62, p<0.05).
Importantly, associated with its effects on SR Ca2+-leak, AS105 also effectively suppressed arrhythmogenic spontaneous global Ca2+-release events (“SCaEs”) in these human cardiomyocytes (shown in Fig. 3I), which were reduced by 51% (AS105: 0.082±0.011 events/cell/s vs Ctrl: 0.168±0.019 events/cell/s, n=255 vs n=262 cells, p<0.05). The reduced SCAEs resulted from fewer cardiomyocytes with any events and a reduced frequency of events in arrhythmic cells. Only 24.3% of cardiomyocytes showed any events with AS105 as compared to 35.5% of cells under control conditions (p<0.05), while AS105 reduced the frequency of events in these arrhythmogenic cells to 0.34±0.03 events/(arrhythmic cell)/s as compared to 0.47±0.04 in Ctrl (p<0.05). AS105 thus appears to both reduce the size of potential arrhythmia triggers (the number of cells capable to trigger arrhythmias) as well as the frequency at which these triggers arise.
AS105 effects on the L-type Ca2+-current
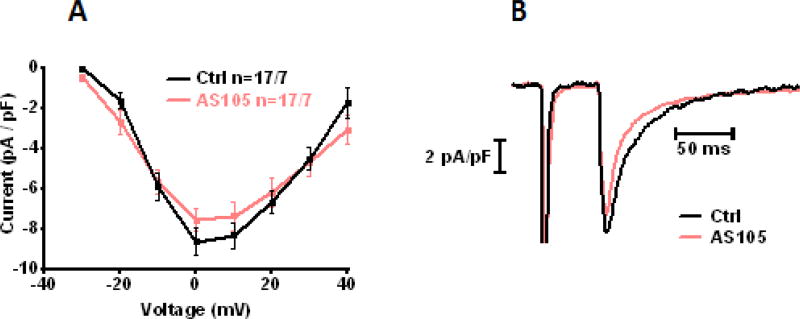
We tested whether the effects of AS105 on Ca2+-transient amplitude could be due to enhancing the L-type Ca2+-current (thus increasing the Ca2+-influx into the myocyte) by performing patch clamp experiments in the CaMKIIδC-TG cardiomyocytes. As shown in Fig. 4, we found that AS105 did not significantly affect the L-type Ca2+-current (peak current at 0 mV: AS105: −7.55±0.56 pA/pF vs Ctrl: −8.64±0.69 pA/pF, n=17 vs n=17 cells).
Figure 4. AS105 effects on L-type Ca2+-current.
(A&B): AS105 does not affect L-type Ca2+-current: IV-relationship of the L-type Ca2+-current in CaMKIIδC-TG cardiomyocytes was assessed by patch clamp recordings in voltage-clamp mode and was not affected by AS105 (A), as illustrated in original recordings (B). n= indicates cells/mice.
AS105 effects in wildtype cardiomyocytes
To assess whether the positive inotropic effects of AS105 in the CaMKIIδC-TG cells can indeed be attributed to the reduction of the pathologically high SR Ca2+-leak in these cells, we investigated the effects of the compound on SR Ca2+-leak and excitation-contraction coupling in cardiomyocytes from the corresponding wildtype mice, which also show some basal Ca2+-leak (16).
As shown in Suppl. Fig. 1A&B, AS105 could, to a slight but significant degree, further suppress the already low SR Ca2+-leak also in these healthy cells (AS105: 4.22±0.81 mF/F0 vs Ctrl: 2.15±0.34 mF/F0, n=50 vs n=53 cells, p<0.05). This minor reduction of the normal low SR Ca2+-leak, however, could not further increase SR Ca2+-content in these cells (Suppl. Fig. 1C: 4.73±0.16 F/F0 vs Ctrl: 5.01±0.33 F/F0, n=13 vs n=15). As fractional systolic SR Ca2+-release was not affected by AS105 (Suppl. Fig. 1D: AS105: 69.22±3.87% vs Ctrl: 73.58±2.39%, n=13 vs n=15), AS105 in these healthy cardiomyocytes (in contrast to the heart failing CaMKIIδC-TG cells) did not increase the amplitude of systolic Ca2+-transients (Suppl. Fig. 1E: AS105: 3.26±0.09 F/F0 vs Ctrl: 3.43±0.11F/F0, n=68 vs n=69 cells) or the resulting cellular contractility (Suppl. Fig. 1F: 3.88±0.28% systolic fractional cell shortening vs Ctrl: 4.22±0.28%, n=68 vs n=69).
While F/F0 cannot be compared between WT and CaMKIIδC-TG, since diastolic Ca2+-levels are different between these genotypes, Ca2+-transient amplitudes have been calculated based on these diastolic Ca2+-levels (16). Performing this analysis for our current data confirms normal systolic Ca2+-transient amplitudes in our WT, but severely diminished ones in the CaMKIIδC-TG cardiomyocytes (TG: 257.6±6.0 nmol/L vs WT: 481.0±9.1 nmol/L, n= 69 vs n=92, p<0.05) due to much lesser SR Ca2+-content in the CaMKIIδC-TG cells (caffeine-induced transients TG: 450.4±18.8 nmol/L vs. WT: 2119.9±574.2 nmol/L n=15 vs n=66). Thus, the ability of AS105 to improve SR Ca2+-content and systolic Ca2+-release could depend on the presence of pathologically high SR Ca2+-leak with consecutively diminished SR Ca2+-loading, while it is not effective in this regard under physiologic conditions.
Discussion
Inhibition of CaMKII has been suggested as a novel therapeutic approach in heart failure and cardiac arrhythmias. Increased CaMKII activity in these conditions has been linked to disturbances in excitation-contraction coupling, especially increased Ca2+-leak from the SR. While currently available CaMKII-inhibitory compounds are not suitable for clinical use, new small molecule CaMKII-inhibitors are under development that could fulfill that promise (21). We now report on AS105, a novel small molecule designed to be an ATP-competitive CaMKII-inhibitor that is one of a series of new inhibitors being developed. Our investigations confirmed that indeed, the CaMKII-inhibition by AS105 occurs via ATP-competition. We could also demonstrate that in this manner, unlike the often-used KN-93, AS105 is equally effective against phosphorylated and unphosphorylated CaMKII. This is of relevance in so far, as recent research suggests important roles for other modes of CaMKII activation besides the canonical Ca2+/CaM dependent pathway, i.e. by oxidation (34) (at Met-281/282) and by nitrosylation (36) (presumably at Cys-290). Our investigations showed AS105 to have high potency with an in vitro IC50 in the low nanomolar range.
We further investigated the effects of AS105 on isolated cardiomyocytes to delineate some of the roles of CaMKII on dysregulation of Ca2+-homeostasis. The first focus of our investigations was on AS105 effects (2 µM) on SR Ca2+-leak, which we investigated in both ventricular cardiomyocytes from CaMKIIδC-overexpressing mice with heart failure as well as in human atrial cardiomyocytes. Both methodologies employed by us to measure SR Ca2+-leak, tetracaine-sensitive Ca2+-leak and Ca2+-spark evaluation, indicate that AS105 effectively reduced SR Ca2+-leak – by 44 % (in the atrial cardiomyocytes) to 65 % (in the failing ventricular cells). Mechanistically, we could show that this was directly due to reduction of RyR2 “leakiness”, as we found leak/load relationship to be improved upon AS105. Diastolic Ca2+-leak from the SR is regarded as an arrhythmogenic mechanism both in ventricular tachyarrhythmias (9–11) as well as in atrial fibrillation (6–8) by inducing spontaneous electrical activity (i.e. providing an arrhythmogenic trigger due to delayed afterdepolarizations (8)). We could show that for AS105, the reduction of diastolic Ca2+-leak was indeed associated with a reduction of cellular arrhythmogenic correlates, indicating that AS105 might have antiarrhythmic effects. Ca2+-leak from the SR has been shown to deplete SR Ca2+-content (3–5), leading to lower amounts of Ca2+ being available for systolic release and impaired contractility. Our results indicate that, indeed, the reduction of SR Ca2+-leak by AS105 resulted in an enhanced capability to accumulate Ca2+ in the SR: In the failing ventricular cardiomyocytes, AS105 significantly improved post-rest-potentiation of Ca2+-transient amplitudes, a measure for the ability of the SR to store and release Ca2+ that been found to be severely impaired in heart failure (38). Accordingly, AS105 improved SR Ca2+-loading in these cells and thus also enhanced systolic Ca2+-release and systolic contractility of these cardiomyocytes upon basal electrical stimulation. In cardiomyocytes from healthy WT mice with low SR Ca2+-leak, however, AS105 did not affect systolic Ca2+-transients or cellular contractility. (This is in line with previous findings using another CaMKII-inhibitor also in healthy cardiomyocytes, where a reduction of SR Ca2+-leak did not increase SR Ca2+-content (39). In addition, we could show that the even lower SR Ca2+-leak in CaMKIIδ-knockout mice also did not affect SR Ca2+-load (17). It is possible that a certain threshold exists below which SR Ca2+-leak does not affect SR Ca2+-content e.g. due compensation by SR Ca2+-reuptake via SERCA2a. As such, the positive inotropic effects of AS105 appear to depend on it being able to lower pathologic SR Ca2+-leak (such as in failing hearts or in atrial fibrillation), while the compound has no direct positive inotropic effect. Thus, AS105 could also have therapeutic potential in the context of heart failure. Noteworthy, while the reduction of diastolic Ca2+-leak suggests a decreased Ca2+-sensitivity of the RyR2, this seems to be overcome by the increased Ca2+-content such that on balance, systolic fractional Ca2+-release is not different. From our measurements of L-type Ca2+-currents, we can also exclude potential effects of AS105 on this current, which might have affected SR Ca2+-loading or systolic Ca2+-transient amplitudes. Of note, CaMKII inhibition by AS105 had no discernible negative effects on cardiomyocyte excitation-contraction coupling in our experiments. Importantly, while SR Ca2+-reuptake could theoretically be compromised by CaMKII-inhibitors due to interference with regulation SERCA2a by PLB, AS105 in the concentration investigated by us had no effect on diastolic Ca2+-transient decay and calculated SERCA2a function. This is in line with previous findings by us showing that even upon 87% reduction of total CaMKII activity due to ablation of CaMKIIδ, frequency dependent acceleration of relaxation, an important physiologic adaptive mechanism, is not impaired (17). However, other results from this mouse model of reduced CaMKII-activity suggest that under specific pathologic conditions such as acidosis, reduction of CaMKII might have effects – a caveat towards CaMKII-inhibitors in general that will have to be closely monitored during further investigations leading to clinical use of such drugs.
Supplementary Material
Highlights.
AS105 is a novel, pyrimidine-based ATP-competitive CaMKII-inhibitor.
It is in this manner also effective against autophosphorylated CaMKII (as opposed to KN-93).
AS105 does not negatively affect basal excitation-contraction-coupling in cardiomyocytes.
In cardiomyocytes from CaMKIIδC-overexpressing mice with heart failure, AS105 reduces SR Ca2+-leak, thus improving SR Ca2+-accumulation, leading to improved systolic function.
AS105 also reduces SR Ca2+-leak in human atrial cardiomyocytes, suppressing arrhythmogenic single cell Ca2+-events.
Acknowledgments
We would like to acknowledge the expertise technical assistance of Thomas Sowa, Felicia Radtke, and Timo Schulte. We thank Erin Bradley for help with the 3D-modeling.
Sources of Funding
SN receives funding from a Regensburg University ReForM B research grant and Deutsches Zentrum für Herz-Kreislauf-Forschung (DZHK B 15-014 Extern). JM is funded through Deutsche Forschungsgemeinschaft grants MA 1982/7-1/WA2539/5-1 (to LSM and Stefan Wagner). SL is funded by a Max Weber scholarship (Elitenetzwerk Bayern), a scholarship from the Regensburg University, and a research grant of the German Cardiac Society (DGK). LSM receives funding from the Deutsche Forschungsgemeinschaft (MA 1981/5-1 and MA 1982/7-1). HS and PP receive funding from NIH Grant R43HL131089.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
HS and PP are employees of Allosteros Therapeutics, Inc. a biotechnology company developing CaMKII inhibitors for cardiovascular indications.
References
- 1.Neef S, Maier LS. Novel aspects of excitation-contraction coupling in heart failure. Basic Res Cardiol. 2013 Jul;108(4):360. doi: 10.1007/s00395-013-0360-2. [DOI] [PubMed] [Google Scholar]
- 2.Mustroph J, Neef S, Maier LS. CaMKII as a target for arrhythmia suppression. Pharmacol Ther. 2016 Oct 11; doi: 10.1016/j.pharmthera.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010 Oct 29;107(9):1150–61. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 4.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012 May 25;110(11):1474–83. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer TH, Eiringhaus J, Dybkova N, Förster A, Herting J, Kleinwächter A, et al. Ca(2+) /calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca(2+) leak in human ischaemic and dilated cardiomyopathy. Eur J Heart Fail. 2014 Dec;16(12):1292–300. doi: 10.1002/ejhf.163. [DOI] [PubMed] [Google Scholar]
- 6.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009 Jul;119(7):1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010 Apr 2;106(6):1134–44. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 8.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012 May 1;125(17):2059–70. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010 Dec 21;122(25):2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, et al. Ca(2+)-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation. 2011 May 24;123(20):2192–203. doi: 10.1161/CIRCULATIONAHA.110.016683. [DOI] [PubMed] [Google Scholar]
- 11.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009 Nov;2(6):664–75. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulman H, Anderson ME. Ca2+/Calmodulin-dependent Protein Kinase II in Heart Failure. Drug Discov Today Dis Mech. 2010;7(2):e117–22. doi: 10.1016/j.ddmec.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreusser MM, Backs J. Integrated mechanisms of CaMKII-dependent ventricular remodeling. Front Pharmacol. 2014;5:36. doi: 10.3389/fphar.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999 Apr 2;84(6):713–21. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 15.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005 Dec 9;97(12):1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 16.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003 May 2;92(8):904–11. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 17.Neef S, Sag CM, Daut M, Bäumer H, Grefe C, El-Armouche A, et al. While systolic cardiomyocyte function is preserved, diastolic myocyte function and recovery from acidosis are impaired in CaMKIIδ-KO mice. J Mol Cell Cardiol. 2013 Jun;59:107–16. doi: 10.1016/j.yjmcc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreusser MM, Lehmann LH, Wolf N, Keranov S, Jungmann A, Gröne H-J, et al. Inducible cardiomyocyte-specific deletion of CaM kinase II protects from pressure overload-induced heart failure. Basic Res Cardiol. 2016 Nov;111(6):65. doi: 10.1007/s00395-016-0581-2. [DOI] [PubMed] [Google Scholar]
- 19.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2342–7. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Zhu W-Z, Joiner M, Zhang R, Oddis CV, Hou Y, et al. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol Heart Circ Physiol. 2006 Dec 291;(6):H3065–3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 21.Pellicena P, Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Front Pharmacol. 2014;5:21. doi: 10.3389/fphar.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavunkel B, Xu Y-J, Goyal B, Lim D, Lu Q, Chen Z, et al. Pyrimidine-based inhibitors of CaMKIIdelta. Bioorg Med Chem Lett. 2008 Apr 1;18(7):2404–8. doi: 10.1016/j.bmcl.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 23.Sag CM, Mallwitz A, Wagner S, Hartmann N, Schotola H, Fischer TH, et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J Mol Cell Cardiol. 2014 Nov;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Chao LH, Pellicena P, Deindl S, Barclay LA, Schulman H, Kuriyan J. Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol. 2010 Mar;17(3):264–72. doi: 10.1038/nsmb.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003 May 2;92(8):912–9. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 26.Primessnig U, Schönleitner P, Höll A, Pfeiffer S, Bracic T, Rau T, et al. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur J Heart Fail. 2016 Aug;18(8):987–97. doi: 10.1002/ejhf.524. [DOI] [PubMed] [Google Scholar]
- 27.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002 Oct 4;91(7):594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 28.Ljubojević S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskämper J. In situ calibration of nucleoplasmic versus cytoplasmic Ca2+ concentration in adult cardiomyocytes. Biophys J. 2011 May 18;100(10):2356–66. doi: 10.1016/j.bpj.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999 Nov 26;85(11):1009–19. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 30.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293(3):C1073–1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 31.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014 Jan 14;129(2):145–56. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edman CF, Schulman H. Identification and characterization of delta B-CaM kinase and delta C-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta. 1994 Mar 10;1221(1):89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 33.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002 Jun 15;364(Pt 3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson JR, Joiner MA, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008 May 2;133(3):462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013 Oct 17;502(7471):372–6. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM. S-Nitrosylation Induces Both Autonomous Activation and Inhibition of Calcium/Calmodulin-dependent Protein Kinase II δ. J Biol Chem. 2015 Oct 16;290(42):25646–56. doi: 10.1074/jbc.M115.650234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vest RS, O’Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post-insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem. 2010 Jul 2;285(27):20675–82. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieske B, Sütterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996 Aug 1;98(3):764–76. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neef S, Mann C, Zwenger A, Dybkova N, Maier LS. Reduction of SR Ca(2+) leak and arrhythmogenic cellular correlates by SMP-114, a novel CaMKII inhibitor with oral bioavailability. Basic Res Cardiol. 2017 Jul;112(4):45. doi: 10.1007/s00395-017-0637-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.