Abstract
Methamphetamine (METH) is a highly addictive drug, but no pharmacological treatment is yet available for METH use disorders. Similar to METH, the wake-promoting drug (R)- modafinil (R-MOD) binds to the dopamine transporter (DAT). Unlike METH, R-MOD is not a substrate for transport by DAT and has low abuse potential. We tested the hypothesis that the atypical DAT inhibitor R-MOD and compounds that are derived from Modafinil would decrease METH intake by reducing the actions of METH at the DAT. We tested the effects of systemic injections of R-MOD and four novel Modafinil-derived ligands with increased DAT affinity (JJC8-016, JJC8-088, JJC8-089, and JJC8-091) on intravenous (i.v.) METH self-administration in rats that were allowed short access (ShA; 1 h) or long access (LgA; 6 h) to the drug. ShA rats exhibited stable METH intake over sessions, whereas LgA rats exhibited an escalation of drug intake. R-MOD decreased METH self-administration in ShA and LgA rats (in the 1st hour only). JJC8-091, and JJC8-016 decreased METH self-administration in both ShA and LgA rats. JJC8- 089 decreased METH self-administration in LgA rats only, whereas JJC8-088 had no effect on METH self-administration in either ShA or LgA rats. These findings support the potential of atypical DAT inhibitors for the treatment of METH use disorders and suggest several novel compounds as candidate drugs.
Keywords: Methamphetamine, Addiction, Drug Dependence, Operant Intravenous Selfadministration, Dopamine Transporter (DAT)
Introduction
Methamphetamine (METH) blocks the reuptake of dopamine by binding to the dopamine transporter (DAT) and causes dopamine release through the reversal of DAT transport, effluxing DA into the synapse (Sulzer et al, 2005). The effects of smoked or injected METH are experienced rapidly and include euphoria and alertness. METH has a long half-life compared with other psychostimulants (e.g., cocaine), which facilitates the high blood levels that are achieved during binge episodes and contributes to its high abuse potential (Scott et al, 2007). Chronic METH abuse can lead to psychosis, malnutrition, cardiovascular complications, and other medical and psychiatric symptoms, particularly during withdrawal (Scott et al, 2007). METH addiction is characterized by compulsive METH seeking and taking that includes the inability to control drug intake. Although behavioral treatments exist for METH use disorders, these have varied effectiveness (Ballester et al, 2017). Currently, there are no FDA-approved pharmacological treatments for METH addiction, and clinical trials have not yet found a compound with clear efficacy (Brackins et al, 2011; Fulde & Forster, 2015; Morley et al, 2017). Given the high rate of METH abuse worldwide (Ballester et al, 2017), an effective treatment for METH addiction is urgently needed.
(±)-Modafinil (Provigil®) is an FDA-approved drug that is used to treat narcolepsy and shift-work sleep disorder by promoting wakefulness (Wisor et al, 2006). Modafinil's mechanism of action is complex, but its main pharmacological effect is achieved through DAT binding, resulting in the inhibition of dopamine reuptake (Mereu et al, 2013). DAT mutagenesis studies showed that the binding affinities of modafinil and all of the analogues reported herein, are significantly and negatively impacted by a Y156F DAT mutation that precludes the formation of a H-bonding gate between Y156 and D79 (Cao et al, 2016). Indeed, R-MOD and the analogues reported herein appear to exhibit an “atypical binding mode”, preferentially binding an inward occluded conformation of the DAT (Loland et al, 2012; Cao et al, 2016). In general, these atypical DAT inhibitors have behavioral profiles that are quite different from typical DAT inhibitors (e.g., cocaine). Typical DAT inhibitors, such as cocaine, have high abuse potential because of their ability to induce dramatic and rapid elevations of extracellular dopamine. Atypical DAT inhibitors, including modafinil, perhaps because of their unique binding mode at the DAT (Wang et al, 2015a; Schmitt & Reith, 2011), can have a slower onset and promote a longer-lasting increase in extracellular dopamine (Reith et al, 2015), which likely contribute to their unique behavioral profiles.
Reichel and See (2010, 2012) reported that modafinil decreased METH seeking in a rat model of relapse in tests of context-induced, cue-induced, and METH-primed reinstatement, and a high dose of modafinil reduced METH self-administration. Clinical studies indicated that modafinil may reduce the severity of METH withdrawal and sleep disturbances (McGregor et al, 2008) and may reduce METH use among modafinil-compliant individuals (Shearer et al, 2009; Anderson et al, 2012). In a small sample, modafinil reduced, albeit nonsignificantly, ratings of METH-induced euphoria and craving and shifted choice behavior away from METH toward a monetary reinforcement alternative (De La Garza et al, 2010). Critically, clinical evidence also suggests that modafinil has low abuse potential and is not an amphetamine-like compound (Vosburg et al, 2010; Jasinski, 2000).
Despite its potential for the treatment of METH use disorders, the effects of modafinil and its analogues on compulsive-like METH intake in animal models have not yet been evaluated. The present study investigated the effects of the increased DAT affinity (R)-(-)- enantiomer of modafinil (R-MOD; ∼3-times higher affinity for the DAT than (S)-(+)-MOD; Wang et al, 2015b) and four novel analogues with varying DAT affinities (Figure 1); R-MOD, Ki = 3260 nM; JJC8-016, Ki = 116 nM; JJC8-088, Ki = 2.53 nM; JJC8-089, Ki = 16.7 nM; JJC8-091, Ki = 289 nM (Cao et al, 2016; Zhang et al, 2017) in a rat model of METH addiction. Herein, rats were given short access (ShA; 1 h daily sessions) or long access (LgA; 6 h daily sessions) to METH to model controlled and stable vs. escalated METH intake (Whitfield et al, 2015), respectively. We hypothesized that these novel atypical DAT inhibitors would decrease compulsive-like METH intake by reducing the ability of METH to bind the DAT.
Figure 1.
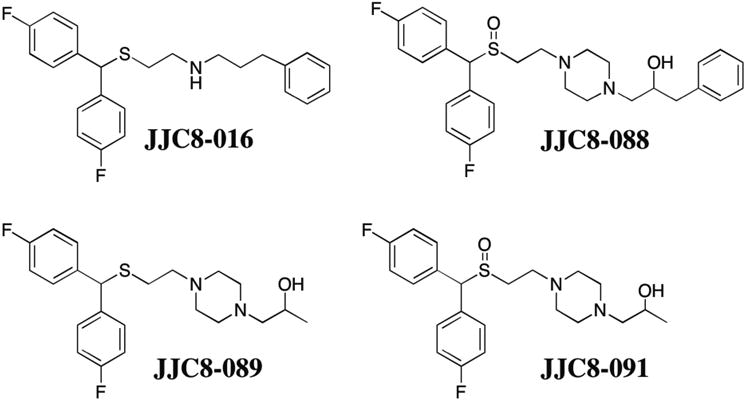
Chemical structures of the R-MOD analogues JJC8-016, JJC8-088, JJC8-089, and JJC8-091.
Materials and Methods
Animals
For METH self-administration and behavioral pharmacological testing, adult male Wistar rats (N = 101) were purchased from Charles River (Kingston, NY, USA). The initial body weights of the animals were 225-275 g. The rats were group-housed (2-3 per cage) in standard plastic cages with sani-chip bedding and maintained under a reverse 12 h/12 h light/dark cycle (lights on at 8:00 PM) at 21°C ± 2°C. The rats had free access to food and water throughout the experiment, except during operant self-administration sessions. All self-administration and behavioral pharmacological testing studies were performed according to protocols approved by the Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program.
Pharmacokinetic studies were conducted in male Wistar rats (6-8 weeks old; weighing 200-250 g) that were obtained from Harlan Laboratories (Indianapolis, IN, USA). The rats were maintained in a controlled environment and had ad libitum access to food and water. All of the pharmacokinetic studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
All procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition).
Surgical procedure
The rats were implanted with chronic indwelling i.v. catheters (0.64 mm inner diameter, 1.19 mm outer diameter; Dow Corning, Midland, MI, USA) in the right external jugular vein as described previously (Whitfield et al, 2015). The rats were allowed to recover for at least 5 days before behavioral testing.
METH self-administration
The i.v. self-administration procedures were conducted as previously reported (Whitfield et al, 2015). The rats were trained to self-administer 0.05 mg/kg/infusion METH during daily 1-h sessions under a fixed-ratio 1 (FR1) schedule of reinforcement (i.e., each lever press resulted in a drug infusion). METH self-administration sessions were conducted in standard operant chambers (32 cm × 25.5 cm × 25.5 cm; Med Associates, St. Albans, VT, USA) that were housed inside light- and sound-attenuating cubicles. At the beginning of each session, two retractable levers mounted to the right wall were extended into the chamber. Responses on the right lever resulted in the delivery of an infusion of 0.05 mg/kg METH in a volume of 0.1 ml, infused over 2.3 s. A stimulus light located directly above the active lever was illuminated for 20 s at the onset of each infusion, during which responses had no programmed consequences (timeout period). Responses on the left (inactive) lever were recorded but had no programmed consequences. The session ended with retraction of the levers from the chamber.
The rats were trained in 5 sessions per week, to self-administer METH for 10-15 sessions. The rats were then divided into ShA and LgA groups and given an additional 10 sessions of METH self-administration (FR1) under ShA (1 h) or LgA (6 h) conditions to produce an escalation of METH intake in LgA rats (Kitamura et al, 2006) before behavioral pharmacological testing began.
Behavioral pharmacological testing
R-MOD ([R]-2-[(diphenylmethyl)sulfinyl]acetamide), JJC8-016 (N-[2-([bis(4- fluorophenyl)methyl]thio)ethyl]-3-phenylpropan-1-amine), JJC8-088 (1-[4-(2-[(bis[4- fluorophenyl]methyl)sulfinyl]ethyl)piperazin-1-yl]-3-phenylpropan-2-ol), JJC8-089 (1-[4-(2- [(bis[4-fluorophenyl]methyl)thio]ethyl)piperazin-1-yl]propan-2-ol), and JJC8-091 (1-[4-(2-[(bis[4-fluorophenyl]methyl)sulfinyl]ethyl)piperazin-1-yl]propan-2-ol) were dissolved in 10% dimethyl sulfoxide and 15% Tween 80, and diluted with sterile saline. R-MOD, JJC8-016, JJC8-JJC8-089, and JJC8-091 were synthesized according to previously published procedures (Cao et al, 2016; Okunola-Bakare et al, 2014).
Different cohorts of LgA and ShA rats were used to test the effects of each drug on METH self-administration. The rats in each group received an intraperitoneal (i.p.) injection of R-MOD (0, and 100 mg/kg), JJC8-016 (0, 10, and 30 mg/kg), JJC8-088 (0, 3, 10, and 30 mg/kg), JJC8-089 (0, 1, 3, 10, and 30 mg/kg), or JJC8-091 (0, 10, 30, and 56 mg/kg). The pretreatment time for R-MOD, JJC8-016, JJC8-089, and JJC8-091 was 1 h. The pretreatment time for JJC8-088 was 30 min to account for its shorter half-life, as reported in mice (Cao et al, 2016). After receiving the treatment, ShA and LgA rats were placed in the operant chambers and tested under an FR1 schedule of reinforcement. For each drug tested, the order of doses was assigned randomly using a within-subjects Latin-square design. Each dose tested was separated by at least one day without any treatment or self-administration, followed by a regular FR1 self-administration session without any treatment, followed by at least one more day without any treatment or self-administration. In some cases, rats were tested with two different drugs; five ShA rats tested with R-MOD were also tested with JJC8-016, three ShA rats tested with R-MOD were also tested with JJC8-089, three ShA rats tested with JJC8-016 were also tested with JJC8-089, and eight LgA rats tested with R-MOD were also tested with JJC8-016. The different drug treatments were separated by at least a week, and rats were given a re-baseline session before testing.
Rat liver microsomal stability assay
Phase I metabolic stability assays for all of the compounds were conducted in rat liver microsomes as previously described (Cao et al, 2016; Kumar et al, 2016; Supplemental Material).
Pharmacokinetic evaluation of JJC8-088, JJC8-089, and JJC8-091 in rats
The compounds were administered as a single i.p. dose of 10 mg/kg. All of the solutions were freshly prepared on the day of the experiment in 10% dimethylsulfoxide, 10% Tween 80, and diluted with sterile saline. Thirty and 120 min after administration, the animals (n = 3 per time point) were euthanized with CO2. Blood samples were collected in heparinized microtubes by cardiac puncture, and brain tissue was dissected and immediately flash frozen (-80°C). Plasma was prepared by centrifugation immediately after collecting the blood samples. All of the samples were stored at -80°C until analyzed by LC/MS/MS. Pharmacokinetic evaluation of JJC8-016 was not conducted in the rat, but both pharmacokinetic and metabolism studies were previously conducted in the mouse (see Supplemental Material).
Bioanalysis of JJC8-088, JJC8-089, and JJC8-091
For the quantification of analytes in plasma and brain tissues, extraction was performed using protein precipitation (Rais et al, 2014, 2015) and subsequently processed for analysis by LC/MS/MS (Supplemental Material).
Off-target in vitro screening
JJC8-088, JJC8-89, and JJC8-091 were screened for binding to 69 receptors, transporters, and enzymes at concentrations of 100 nM and 10 μM. These data were previously published for R-MOD and JJC8-016 (Zhang et al, 2017). For details of the in vitro screening experiments, please refer to Supplemental Information.
Effects of R-modafinil and analogues on saccharin self-administration and locomotion
A separate group of rats was trained to self-administer saccharin in operant chambers equipped with infrared beams for measuring locomotion. For details of the saccharin self-administration and locomotion experiment, please refer to Supplemental Information.
Data analysis
The data is presented as the mean and standard error of the mean. The level of significance for all of the statistical tests was α = 0.05. For METH self-administration in ShA rats and the 1st h of self-administration in LgA rats, escalation of METH intake and behavioral pharmacological tests were analyzed using two-way analysis of variance (ANOVA), with group (ShA vs. LgA 1st h) as the between-subjects factor and session or dose as the within-subjects factor. A one-way repeated-measures ANOVA was used to assess the escalation of METH intake and the effects of test drugs in the LgA rats for the entire 6 h self-administration session. For R- MOD, a paired-sample t-test was used to compare the single dose of R-MOD to vehicle. The Holms-Sidak test was used for post hoc analyses as appropriate. GraphPad Prism 7.01 software was used for the statistical analysis.
Results
Escalation of METH self-administration
We used five cohorts of rats and combined their data for analysis of the 10 days of METH self-administration (Figure 2). The two-way ANOVA comparing ShA and LgA (1st h) rats revealed a significant group × session interaction (F9,801 = 16.3, p < 0.0001). LgA rats escalated their drug intake in the 1st h, with intake significantly increased during the fourth session (p < 0.001), and further increase through sessions 5-10 (p < 0.0001) compared to their first session. ShA rats allowed limited (1 h) access to METH self-administration exhibited stable drug intake over 1 h sessions (Figure 2A). Furthermore, LgA rats self-administered significantly more METH in the 1st h than ShA rats did in 1 h, during sessions 6-10 (p < 0.0001). For the entire 6 h session in the LgA rats, a main effect of session (F9,405 = 23.5, p < 0.0001) was observed, with LgA rats demonstrating significantly increased METH self-administration during the third session (p < 0.001) and further escalating their METH intake during sessions 4-10 (p < 0.0001).
Figure 2.
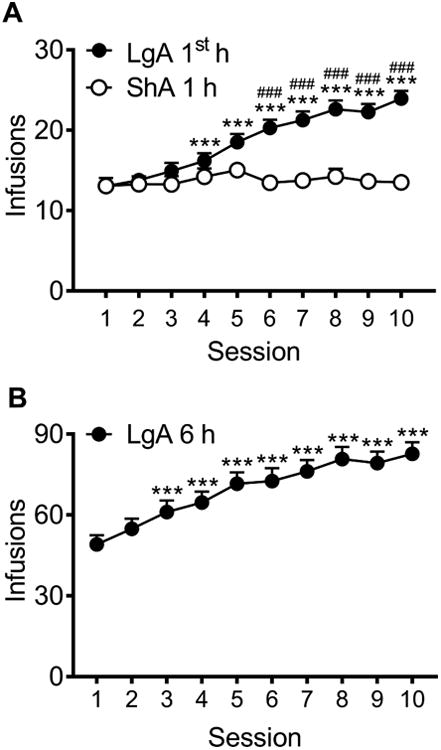
Escalation of METH intake in LgA but not ShA rats. Combined data from five cohorts of rats. Rats in each cohort were given either ShA (1 h; n = 37 total rats) or LgA (6 h; n = 46 total rats) to METH. In the 1st h of the LgA session, LgA rats demonstrated an escalation of METH intake over sessions, and self-administered significantly more METH in 1 h compared with ShA rats (A). LgA rats demonstrated an escalation of METH intake over 6-h sessions (B). ###p < 0.001, LgA 1st h compared with ShA; ***p < 0.001, compared with session 1.
Effects of R-MOD on METH self-administration
LgA rats exhibited higher METH intake compared with ShA rats in the first hour (group effect: F1,19 = 5.0, p < 0.05), and R-MOD (100 mg/kg) significantly decreased drug intake in the first hour of METH self-administration, regardless of group (treatment effect: F1,19 = 11.0, p < 0.01; Figure 3A, B). The paired-sample t-test indicated that the decrease in METH intake that was caused by R-MOD was nonsignificant (t11 = 2.1, p = 0.058) when considering the entire 6 h session in LgA rats (Figure 3C).
Figure 3.
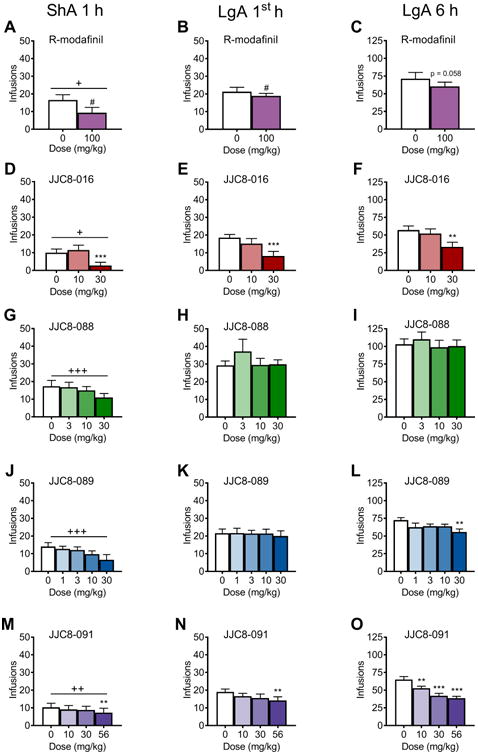
Number of METH infusions after treatment with R-MOD and its analogues. R-MOD at a dose of 100 mg/kg decreased the number of infusions in the ShA 1 h group (A) and LgA group in the 1st hour (B). R-MOD decreased the total number of METH infusions in the LgA group over 6 h but not significantly (p = 0.058) (C). (D, F) JJC8-016 at a dose of 30 mg/kg decreased the number of infusions in the ShA 1 h group (D) and LgA group in the 1st hour (E) and in the 6 h session (F). (G-I) JJC8-088 did not affect METH intake in the ShA 1 h group (G) or in the LgA group in the 1st hour (H) or in the 6 h session (I). JJC8-089 at a dose of 30 mg/kg decreased the number of infusions in the ShA 1 h group and in the LgA group in the 6 h session. JJC8-091 at a dose of 56 mg/kg decreased the number of infusions in the ShA 1 h group and in the LgA group in the 1st hour. JJC8-091 at doses of 10, 30, and 56 mg/kg decreased the number of infusions in the LgA group in the 6 h session. +p < 0.05, ++p < 0.01, +++p < 0.001 main effect of group (ShA 1 h vs. LgA 1st h); #p < 0.05, main effect of dose; **p < 0.01, ***p < 0.001, different from vehicle.
Effects of JJC8-016 on METH self-administration
A group effect (F1,20 = 5.4, p < 0.05) indicated that in this cohort of rats, LgA rats had significantly higher METH intake compared with ShA rats during the first hour of self-administration. The highest dose of JJC8-016 (30 mg/kg) significantly decreased METH self-administration in both ShA and LgA rats (treatment effect: F2,40 = 11.9, p < 0.0001; post hoc analysis: p < 0.0001; Figure 3D, E). For the entire 6 h session in LgA rats, JJC8-016 (30 mg/kg) significantly decreased METH intake compared with vehicle (treatment effect: F2,22 = 9.1, p < 01; post hoc analysis: p < 0.01; Figure 3F).
Effects of JJC8-088 on METH self-administration
In this cohort, the LgA rats exhibited higher METH intake in the first hour of self-administration than ShA rats (group effect: F1,17 = 16.8, p < 0.001). However, JJC8-088 did not significantly change METH intake in LgA or ShA rats in the first hour of self-administration (treatment effect: F3,51 = 2.1, p = 0.11; Figure 3G, H). Treatment with JJC8-088 did not affect METH intake in the entire 6 h session in LgA rats (treatment effect: F3,27 = 0.8, p = 0.49; Figure 3I).
Effects of JJC8-089 on METH self-administration
In the first hour of METH self-administration, we only observed a group effect (F1, 17 = 17.0, p < 0.001; Figure 3J, K), indicating that the LgA rats in this cohort exhibited an increase in METH intake compared with ShA rats. For the 6 h session in LgA rats, 30 mg/kg JJC8-089 significantly decreased METH intake (treatment effect: F4,36 = 3.0, p < 0.05; Figure 3L; post hoc test, p < 0.01).
Effects of JJC8-091 on METH self-administration
The LgA rats in this cohort self-administered significantly more METH in the first hour compared with ShA rats (group effect: F1,18 = 8.8, p < 0.01; Figure 3M, N). The highest dose of JJC8-091 (56 mg/kg) decreased METH intake in both LgA and ShA rats (treatment effect: F3,54 = 3.4, p < 0.05; post hoc test, p < 0.01). For the 6 h session in LgA rats, JJC8-091 significantly decreased METH intake in the LgA rats (treatment effect: F3,27 = 18.8, p < 0.0001; Figure 3O). The post hoc comparisons indicated a significant reduction of METH intake at doses of 10 mg/kg (p < 0.01), 30 mg/kg (p < 0.0001), and 56 mg/kg (p < 0.0001).
Rat liver microsomal stability
JJC8-016, JJC8-088, JJC8-089, and JJC8-091 were tested for susceptibility to CYP- dependent metabolism in rat liver microsomes and compared with R-MOD (Figure 4). JJC8-016 and JJC8-089 were the least metabolically stable, with half-lives of 9 and 14 min, respectively, suggesting the potential for rapid clearance. In contrast, JJC8-088 and R-MOD were moderately stable, with half-lives of 39 and 55 min, respectively. The rat liver microsome data for JJC8-088 contrasts with our previous report in mouse liver microsomes, in which JJC8-088 was rapidly metabolized (t1/2 = 12.9 min; Cao et al, 2016), underscoring species differences in metabolism. Notably, JJC8-091 was the most metabolically stable analogue, with a calculated half-life of 126 min.
Figure 4.
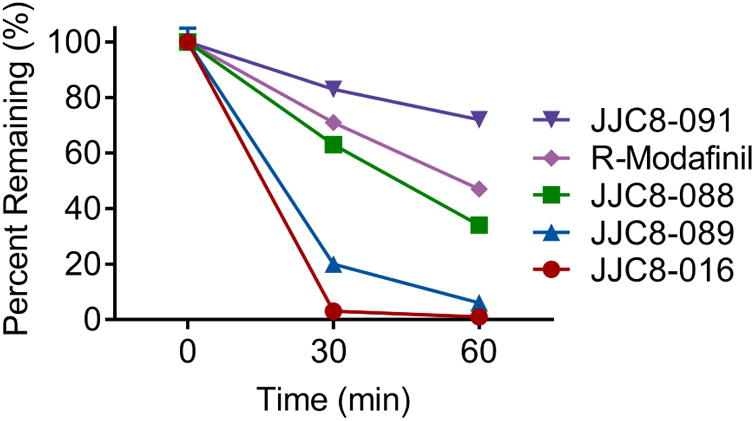
Phase I metabolic stability of R-MOD, JJC8-016, JJC8-088, JJC8-091, and JJC8-089 in rat liver microsomes. Compounds showed varying degrees of metabolic stability in rat liver microsomes fortified with NADPH. JJC8-089 and JJC8-016 both showed rapid metabolism. In contrast, JJC8-088 was moderately stable and similar to modafinil in its metabolic profile. JJC8- 091 showed the highest stability to phase I oxidation.
Plasma and brain levels of JJC8-088, JJC8-089, and JJC8-091 in rats
The three most metabolically stable compounds (JJC8-088, JJC8-089, and JJC8-091) were then tested for plasma and brain exposure following i.p. administration. Figure 5 shows plasma and brain exposure at 30 min and 2 h post-administration. JJC8-089 had the highest brain levels (7.6 ± 0.3 nmol/g and 3.6 ± 0.7 nmol/g at 30 min and 2 h post-administration, respectively). Interestingly, brain levels were 8-9 fold higher than plasma levels (Figure 5A). JJC8-091 presented moderate brain penetration (0.45 ± 0.18 nmol/g and 0.30 ± 0.015 nmol/g at 30 min and 2 h post-administration, respectively; Figure 5B). In contrast, JJC8-088 presented low levels in both brain and plasma (Figure 5C). Pharmacokinetic evaluation of JJC8-016 was not conducted in the rat, but it was previously conducted in the mouse (see Supplemental Material). Both pharmacokinetic and metabolism data indicated good brain penetration for JJC8- 016 (see Supplemental Material).
Figure 5.
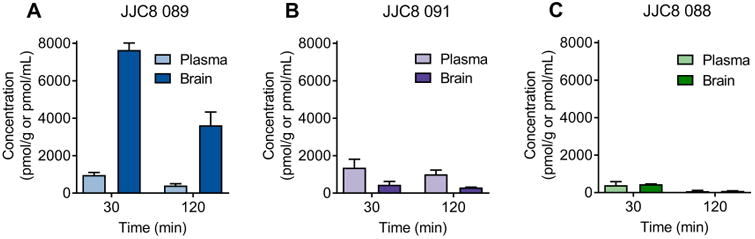
JJC8-089 and JJC8-091 presented higher brain concentrations following systemic administration compared with JJC8-088. Rats received i.p. injections of 10 mg/kg JJC8-089 (A), JJC8-091 (B), and JJC8-088 (C). Plasma and brains were extracted 30 and 120 min later, and analytes were quantified by LC/MS/MS. JJC8-089 presented the best brain-to-plasma ratios (7.9 and 8.8, respectively) at both the 30 and 120 min time points (n = 3/group).
Discussion
Extended access to METH in rats has been shown to cause an escalation of METH intake, compulsive-like responding for METH (Whitfield et al, 2015), and deficits in brain reward function during METH withdrawal (Jang et al, 2013). These changes reflect important features of METH addiction and provide evidence of the validity of the extended access model of compulsive-like METH self-administration (Koob, 2017). Consistent with previous reports (Whitfield et al, 2015; Kitamura et al, 2006), we found that rats that were allowed 6 h access (LgA) to i.v. METH self-administration escalated their drug intake, whereas rats that were allowed 1 h access (ShA) exhibited stable intake over sessions. This escalation of METH intake in LgA rats was evident in the first hour of access as well as in their overall intake. Acute treatment with the R-MOD analogues JJC8-016 and JJC8-091 dose-dependently decreased METH self-administration in both ShA and LgA rats, as did a single dose of R-MOD. JJC8-016 was the most effective in reducing METH intake in ShA rats and first-hour intake in LgA rats, whereas JJC8-091 was the most effective over the entire 6 h session in LgA rats, likely because of its superior metabolic stability (Figure 4). JJC8-089 decreased METH self-administration only in LgA rats and over the entire 6 h session, at the highest dose. JJC8-088 did not significantly reduce METH self-administration in either group.
ShA rats were generally more sensitive to the effects of R-MOD and its analogues compared with LgA rats. In ShA rats, R-MOD, JJC8-089, and JJC8-016 induced a >50% reduction of METH intake (Supplemental Figure S2). LgA rats appeared to be more resistant to treatment, and only JJC8-016 induced a >50% reduction of self-administration at the 1 h time point (Supplemental Figure S2). These findings are consistent with the hypothesis that the escalation of drug self-administration reflects the development of less flexible, compulsive-like drug self-administration (Koob, 2017). Over the 6 h session, JJC8-089, JJC8-091, and JJC8-016 produced a moderate but significant (22-41%) decrease in METH intake in LgA rats (Supplemental Figure S2), and JJC8-091 was effective within a lower dose range (10-56 mg/kg) compared with the other compounds tested.
Following R-MOD administration, ShA and LgA rats exhibited a reduction of METH self-administration at 1 h. However, this effect was transient, and METH intake did not significantly decrease over the entire 6 h test in LgA rats. Consistent with this finding, Reichel and See (2012) reported that only a very high-dose of (±)-modafinil (300 mg/kg) significantly decreased METH self-administration. However, modafinil was more effective in blocking the reinstatement of METH seeking that was induced by exposure to a priming dose of METH or a cue or context that was associated with METH (Reichel & See, 2012). These preclinical data suggest that modafinil may more effectively prevent relapse than reduce METH self-administration.
The ability of JJC8-016 and JJC8-091 to attenuate METH self-administration in ShA and LgA rats was long-lasting (i.e., effective over the 6 h session). Additional promising preclinical results were recently reported for JJC8-016, showing that it effectively inhibited cocaine-induced locomotion, cocaine self-administration, and cocaine-induced reinstatement, without affecting locomotor activity or intracranial self-stimulation (Zhang et al, 2017). The time-course of efficacy of JJC8-089 appears to be somewhat more complex than JJC8-016 and JJC8-091. JJC8-089 significantly attenuated METH self-administration in LgA rats in the 6 h session but not in the 1st h or in the ShA rats. The reasons for these results are not apparent and may be complicated by the finding that although JJC8-089 had very high brain penetration (Figure 5) it was rapidly metabolized (Figure 4). Moreover, off-target actions of JJC8-089 (e.g., at sigma-1 receptors; and see Supplemental Tables S2 and S3) may also play a role. Both JJC8-089 and JJC8-016 have been reported to have high affinities for sigma-1 receptors (Cao et al, 2016), which have been implicated in the reinforcing effects of abused drugs (for review, see Katz et al, 2016). Indeed, a dual DAT-sigma-1 mechanism for blocking both cocaine and METH self-administration has been reported (Hiranita et al, 2011, 2014, 2017). The classic atypical DAT inhibitor JHW 007 (benztropine-derived, not modafinil-derived) has been shown to effectively block cocaine and METH self-administration in rats (Hiranita et al, 2014, 2017). The off-target binding profile of both classes of drug are different and likely important for explaining the mechanism of action their effects (see for review, Avelar et al, 2017). One potentially relevant point of overlap is that the sigma-1/DAT binding ratio (0.24) for JJC8-089 (attenuated METH self-administration in LgA rats in the 6 h session) is equivalent to that of JHW 007 (0.20-0.23; Cao et al, 2016; Hiranita et al, 2017). JHW 007 does not support self-administration behavior, even in rats with a cocaine self-administration history (Hiranita et al, 2009). JHW 007 has also been found to attenuate many actions of psychostimulants in rodent models including but not limited to drug self-administration. Importantly, JHW 007 caused a downward dose-response curve shift in cocaine self-administration, indicating a decrease in reinforcement efficacy rather than changes in drug potency (Hiranita et al, 2009). These findings are consistent with the decrease in FR1 self-administration of METH reported by Ferragud et al (2014) and with the present results demonstrating that modafinil-derived compounds also attenuate METH self-administration. Similarly, one of the compounds that we presently report as attenuating METH self-administration (JJC8-016) was recently demonstrated to produce a downward dose-response curve shift on cocaine self-administration (Zhang et al, 2017). Together, these results suggest that atypical DAT inhibitors do not enhance psychostimulant reinforcing effects, but rather decrease their reinforcing efficacy. Follow-up studies to extend these findings will assess the effects of the most promising compounds on progressive ratio, extinction, reinstatement, punishment, demand-curve and dose-response-curve functions.
JJC8-088 did not significantly alter METH self-administration in ShA or LgA rats at either 1 or 6 h. Although this compound has high DAT affinity, selectivity, and metabolic stability in rat liver microsomes (Figure 4), the most parsimonious explanation for its lack of efficacy appears to be that JJC8-088 has very poor brain penetration (Figure 5A). Indeed, very little JJC8-088 was detected in the brain at 30 min and it was nearly undetectable by 120 min. Nevertheless, we cannot rule out that JJC8-088's relatively low affinity for sigma1 receptors (Ki=336 nM) relative to its DAT affinity (sigma1/DAT= >100; Cao et al, 2016) may also contribute to this compound's lack of effect on METH self-administration in either the ShA or LgA rats. In addition, recently, the differential effects of JHW007 and R-MOD on D2 autoreceptor neurotransmission have been reported (Avelar et al, 2017) that may also contribute to the effectiveness (or lack thereof) of these atypical DAT inhibitors in models of psychostimulant abuse. Overall, these results are consistent with our overarching hypothesis that although all atypical DAT inhibitors, by definition, bind to DAT, they bind in a preferred conformation that differs from cocaine, and so it is the nature of binding rather than affinity that predicts their effects in vivo (i.e., DAT binding affinity alone cannot predict the behavioral profile of an atypical DAT inhibitor, as summarized in Supplemental Figure S2). Importantly, although R-MOD and the analogues reported herein are all DAT inhibitors, the behavioral effects observed in these METH self-administration models are not simply related to DAT binding, as off target actions and differences in pharmacokinetic properties clearly contribute to each individual compound's efficacy in the ShA and LgA METH self-administering rats.
Consistent with previous studies (Wang et al, 2015b), R-MOD did not alter the self-administration of sweetened water or locomotion (Supplemental Figure S3). However, Zhang et al (2017) reported that 10-30 mg/kg R-MOD increased locomotion and enhanced brain stimulation reward. These findings indicate that the R-MOD-induced reduction of METH intake is likely not attributable to sedative effects or motor impairments that can affect operant responding. Importantly, JJC8-016, JJC8-089, and JJC8-091 were all effective in reducing compulsive-like METH self-administration without causing significant locomotor effects, as previously reported for JJC8-016 tested on cocaine self-administration (Zhang et al, 2017), suggesting that these compounds are effective in curbing METH maintained behavior without causing significant motor effects. In addition, inactive-lever responding was not significantly altered by any of the compounds, in either group (see Supplemental Table S7). It should be noted that JJC8-016, JJC8-089, and JJC8-091, which all effectively decreased METH intake, also significantly reduced saccharin self-administration (Supplemental Figure S3). JJC8-016 was recently reported to reduce sucrose self-administration in rats (Zhang et al, 2017). A possible explanation for this effect includes a decrease in appetite (i.e., anorexigenic effect). Furthermore, we cannot exclude the possible actions of JJC8-016, JJC8-089, and JJC8-091 (Supplementary Tables S2-6) at sites other than the DAT (i.e., off-target effects) that may decrease saccharin intake. Different from the other compounds, JJC8-088 increased saccharin drinking and locomotion (Figure S3), although the mechanisms are unclear at this point. Future studies will investigate the mechanisms that underlie the unique behavioral profiles of these atypical DAT inhibitors.
Although modafinil has been reported to engage glutamatergic and other mechanisms, there is so far no evidence that modafinil and the analogues tested in the present study bind directly to any of the glutamate receptors (Supplemental Material), suggesting an indirect effect. There are many possible contributors to the effect profile of these drugs including GABA, norepinephrine, serotonin, orexin, and histamine, all of which contribute to the mediation of sleep-wake regulation, and have been proposed to be involved in the cognitive-enhancing effects of modafinil (see for review, Mereu et al, 2013). Histamine signaling is a particularly interesting mechanism, and possibly a direct mechanism of action, given that histamine binding was observed for some of the present compounds in our off-target assays (Supplemental Material).
In summary, JJC8-016, JJC8-091, JJC8-089, and to a lesser degree R-MOD significantly decreased compulsive-like METH self-administration, in rats. These effects did not appear to be a consequence of sedation or motor impairment. None of the compounds that effectively reduced METH self-administration exerted stimulant effects. Although the pharmacological mechanisms of action of atypical DAT inhibitors are complex and not fully understood, the present results support the further testing of this class of compounds for the treatment of METH use disorders. R-MOD is approved for human use, and it may be repurposed and tested in adequately powered studies in individuals with METH use disorders. Indeed, R-MOD may be more effective than racemic modafinil in certain patient populations (Kampman et al, 2015). Moreover, several of the R-MOD analogues (JJC8-091, JJC8-089, and JJC8-016) significantly reduced METH selfadministration in LgA rats over the 6 h session, thus supporting the further investigation of these atypical DAT inhibitors as pharmacotherapeutics. Further modifications of our drug design to improve their metabolic and pharmacokinetic properties may facilitate the clinical translation of these findings. Preclinical studies can provide additional information about the mechanisms by which atypical DAT inhibitors decrease METH intake, and these analogues will be useful pharmacological tools in this endeavor.
Supplementary Material
Highlights.
There is currently no available pharmacological treatment for METH use disorder
Rats allowed extended access to METH escalate their drug intake
R-Modafinil and several modafinil analogues reduced escalated METH intake
Atypical DAT inhibitors have potential for treating METH use disorders
Acknowledgments
The National Institute on Drug Abuse Intramural Research Program supported this work. The authors thank Michael Arends for editorial assistance.
Footnotes
Disclosure/Conflicts of interest: The authors have no financial conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10:305–314. doi: 10.1080/17512433.2017.1268916. [DOI] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse a literature review for the clinician. J Pharm Pract. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Fulde GW, Forster SL. The impact of amphetamine-type stimulants on emergency services. Curr Opin Psychiatry. 2015;28:275–279. doi: 10.1097/YCO.0000000000000171. [DOI] [PubMed] [Google Scholar]
- Morley KC, Cornish JL, Faingold A, Wood K, Haber PS. Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Expert Opin Investig Drugs. 2017;26:563–578. doi: 10.1080/13543784.2017.1313229. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Dement WC, Aimone L, Williams M, Bozyczko-Coyne D. Armodafinil, the R-enantiomer of modafinil: wake-promoting effects and pharmacokinetic profile in the rat. Pharmacol Biochem Behav. 2006;85:492–499. doi: 10.1016/j.pbb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS. Novel and High Affinity 2-[(Diphenylmethyl) sulfinyl] acetamide (Modafinil) Analogues as Atypical Dopamine Transporter Inhibitors. J Med Chem. 2016;59:10676–10691. doi: 10.1021/acs.jmedchem.6b01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, et al. R-MOD (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72:405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015a;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PloS one. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, et al. Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend. 2015;147:1–19. doi: 10.1016/j.drugalcdep.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol. 2012;15:919–929. doi: 10.1017/S1461145711000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, Van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106:233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol (Oxf) 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Wang XF, Bi GH, He Y, Yang HJ, Gao JT, Okunola-Bakare OM, et al. R-Modafinil Attenuates Nicotine-Taking and Nicotine-Seeking Behavior in Alcohol-Preferring Rats. Neuropsychopharmacology. 2015b;40:1762–1771. doi: 10.1038/npp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Yang HJ, He Y, Xue G, Cao J, et al. The Novel Modafinil Analog, JJC8-016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.41. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Schlosburg JE, Wee S, Gould A, George O, Grant Y, et al. κ opioid receptors in the nucleus accumbens shell mediate escalation of methamphetamine intake. J Neurosci. 2015;35:4296–4305. doi: 10.1523/JNEUROSCI.1978-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, Shi L, Newman AH. Elucidation of structural elements for selectivity across monoamine transporters: novel 2- [(diphenylmethyl) sulfinyl] acetamide (modafinil) analogues. J Med Chem. 2014;57:1000–1013. doi: 10.1021/jm401754x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Bonifazi A, Ellenberger MP, Keck TM, Pommier E, Rais R, et al. Highly Selective Dopamine D3 Receptor (D3R) Antagonists and Partial Agonists Based on Eticlopride and the D3R Crystal Structure: New Leads for Opioid Dependence Treatment. J Med Chem. 2016;59:7634–7650. doi: 10.1021/acs.jmedchem.6b00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais R, Rojas C, Wozniak K, Wu Y, Zhao M, Tsukamoto T, et al. Bioanalytical method for evaluating the pharmacokinetics of the GCP-II inhibitor 2-phosphonomethyl pentanedioic acid (2-PMPA) J Pharm Biomed Anal. 2014;88:162–169. doi: 10.1016/j.jpba.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais R, Wozniak K, Wu Y, Niwa M, Stathis M, Alt J, et al. Selective CNS Uptake of the GCP-II Inhibitor 2-PMPA following Intranasal Administration. PloS one. 2015;10:e0131861. doi: 10.1371/journal.pone.0131861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology (Berl) 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology (Berl) 2017;234:1315–1332. doi: 10.1007/s00213-016-4484-6. [DOI] [PubMed] [Google Scholar]
- Katz JL, Hong WC, Hiranita T, Su TP. A role for sigma receptors in stimulant self-administration and addiction. Behav Pharmacol. 2016;27:100–115. doi: 10.1097/FBP.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, et al. Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and σ receptors. J Pharmacol Exp Ther. 2011;339:662–677. doi: 10.1124/jpet.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Kohut SJ, Soto PL, Tanda G, Kopajtic TA, Katz JL. Preclinical efficacy of N-substituted benztropine analogs as antagonists of methamphetamine self-administration in rats. J Pharmacol Exp Ther. 2014;348:174–191. doi: 10.1124/jpet.113.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Hong WC, Kopajtic T, Katz JL. Sigma Receptor Effects of N-Substituted Benztropine Analogs: Implications for Antagonism of Cocaine Self Administration. J Pharmacol Exp Ther. 2017 doi: 10.1124/jpet.117.241109. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelar AJ, Cao J, Newman AH, Beckstead MJ. Atypical dopamine transporter inhibitors R-modafinil and JHW 007 differentially affect D2 autoreceptor neurotransmission and the firing rate of midbrain dopamine neurons. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.06.016. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of Reinforcing Effects of Benztropine Analogs and Their Effects on Cocaine Self-Administration in Rats: Comparisons with Monoamine Uptake Inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragud A, Velazquez-Sánchez C, Canales JJ. Modulation of methamphetamine's locomotor stimulation and self-administration by JHW 007, an atypical dopamine reuptake blocker. Eur J Pharmacol. 2014;15:73–79. doi: 10.1016/j.ejphar.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, O'Brien CP. A Double Blind, Placebo Controlled Trial of Modafinil for the Treatment of Cocaine Dependence without Co-Morbid Alcohol Dependence. Drug Alcohol Depend. 2015;155:105–110. doi: 10.1016/j.drugalcdep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


