Abstract
Many functions of the hippocampus are affected by prenatal alcohol exposure (PAE). In particular, dysregulation of the stress response is especially important because individuals with PAE carry increased risks for exposure to stressful environments throughout life. Little is known, though, about how adolescent stress in the context of PAE-related stress system dysregulation may further alter hippocampal development. Here we investigate the short- and long-term effects of adolescent chronic mild stress (CMS) on mRNA expression of stress-related mineralocorticoid (MR), glucocorticoid (GR), and type 1 CRH (CRHR1) receptors in the dorsal and ventral hippocampal formation of PAE and control rats. Our results indicate that PAE affects the expression of stress-related receptors in the hippocampus; however, PAE effects were more prominent during adolescence, as MR and CRHR1 mRNA expression were altered in both male and female PAE animals, with GR mRNA expression alterations observed only in PAE female. In adulthood, the effects of PAE were restricted to alterations in CRHR1 mRNA expression in females, while there were no effects in males. In contrast, the effects of adolescent CMS were more pronounced in adulthood, long after stress exposure termination. Importantly, PAE animals were less responsive to adolescent CMS, with effects only on CRHR1 in PAE animals compared to the altered MR, GR and CRHR1 mRNA expression observed in controls. Together, our results show that PAE and adolescent CMS induce dynamic alterations in the expression of stress-related receptors in the hippocampal formation that manifest differently depending on the age and sex of the animal.
Keywords: prenatal alcohol exposure, chronic mild stress, mineralocorticoid receptor, glucocorticoid receptor, type 1 CRH receptor
Introduction
Clinical and experimental studies have clearly demonstrated that alcohol consumption during pregnancy alters fetal brain development and can induce a wide range of cognitive, neurobehavioral, and physiological deficits (Drew and Kane, 2014; Hellemans et al., 2010; Riley et al., 2011; Schneider et al., 2011; Valenzuela et al., 2012; Weinberg et al., 2008). Although the structure and function of many brain regions are negatively affected by prenatal alcohol exposure (PAE), the hippocampus is particularly sensitive to the effects of PAE (Fontaine et al., 2016; Gil-Mohapel et al., 2010; Valenzuela et al., 2012). For example, several clinical studies indicate that PAE is associated with reduced hippocampal volume (Coles, et al., 2011; Donald et al., 2016; Gross et al., in press; Riikonen et al., 1999, Willoughby et al., 2008). Preclinical studies have considerably extended the clinical findings demonstrating that PAE results in hypertrophy of mossy fibers (West et al., 1981), reduced numbers of neurons (Burke et al., 2015) and dendritic spines (Abel et al., 1983), altered neural activity (Raineki et al., 2014), impaired long-term potentiation (Christie et al., 2005; Pattern et al., 2013; Sutherland et al., 1997) and reduced adult neurogenesis (Gil-Mohapel et al., 2010; Redila et al., 2006; Sliwowska et al., 2010; Uban et al., 2010) in the hippocampus.
The hippocampus is a unique brain area as its structure is constantly adapting in response to environmental stimuli (McEwen, 1999). This plasticity is critical for the proper function of the hippocampus, playing a key role in a wide range of functions including learning and memory as well as emotional and stress regulation (Fanselow and Dong, 2010). This hippocampal plasticity is mediated, at least in part, by stress-related receptors (Maras and Baram, 2012; McEwen et al., 2015). Hippocampal neurons express high levels of the type 1 CRH receptor (CRHR1), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR), which are major regulators of hippocampal development, structure, and function (Maras and Baram, 2012, McEwen, 2012; McEwen et al., 2015). PAE has been shown to reduce CRHR1 mRNA expression in the amygdala, medial prefrontal cortex (mPFC), and pituitary in adulthood (Glavas et al., 2007; Raineki et al., 2016). Hippocampal CRHR1 expression following PAE has only been assessed in adolescent mice, and the data indicate that PAE reduces CRHR1 protein levels (Caldwell et al., 2015). Conversely, several studies have demonstrated that adult PAE male rats do not show changes in hippocampal MR and GR expression (Glavas et al., 2007; Kim et al., 1999; Uban et al., 2013). However, PAE adult females showed reduced hippocampal MR mRNA expression (Sliwowska et al., 2008; Uban et al., 2013). Moreover, PAE has been shown to reduce MR and to elevate GR protein levels in the nuclear fraction of hippocampal neurons in adolescent male mice, whereas levels of both receptors were not altered in the cytosolic fraction (Caldwell et al., 2014). Studies investigating PAE effects on MR, GR, and CRHR1 expression in the hippocampal formation have focused exclusively in the dorsal hippocampus (Glavas et al., 2007; Kim et al., 1999; Uban et al., 2013) or used the entire hippocampus (Caldwell et al., 2014, 2015). Importantly, the dorsal and ventral components of the hippocampal formation have different functions: the dorsal hippocampus is primarily involved in cognitive functions while the ventral hippocampus is more essential for stress and emotional regulation (Fanselow and Dong, 2010). Here, we expand this literature by evaluating the effects of PAE on the expression of MR, GR and CRHR1 in different subfields of the dorsal and ventral hippocampus.
Cortical and limbic areas, including the hippocampus, undergo extensive functional and structural reorganization during adolescence (Crews et al., 2007; Eiland and Romeo, 2013; Hueston et al., 2017; McCormick and Mathews, 2010; Spear, 2000). These maturation processes occur in parallel with many changes in neuroendocrine function (Crews et al., 2007; Ojeda et al., 2006), including the unique hyperresponsivity of the hypothalamic-pituitary-adrenal (HPA) axis to both acute and chronic stressors (Doremus-Fitzwater et al., 2009; Eiland and Romeo, 2013; Green et al., 2016; Hollis et al., 2013; McCormick and Mathews, 2010; Romeo et al., 2004, 2006). The combination of extensive brain maturation and hyperresponsivity to stressors makes adolescence a period of increased vulnerability to adverse environmental stimuli. This is particularly relevant for individuals exposed to alcohol during gestation, as both human and rodent studies have demonstrated that PAE is associated with increased HPA activation and/or a delayed return to basal levels when faced with a wide range of stressful stimuli (Jacobson et al., 1999; Lee and Rivier, 1996; Nelson et al., 1986; Taylor et al., 1982; Weinberg et al., 2008). Notably, clinical data indicate that individuals prenatally exposed to alcohol are, in general, at a higher risk of being exposed to a more stressful environment throughout the lifespan (O’Connor and Kasari, 2000; O’Connor and Paley, 2006; Streissguth et al., 2004). Moreover, similar to PAE, exposure to chronic stress during adolescence can also lead to short- and long-term alterations in the expression of stress-related receptors in the hippocampus (Iredale et al., 1996; Isgor et al., 2004; Li et al., 2015; Sterlemann et al., 2008; Veenit et al., 2014). Nevertheless, little is known about how this higher risk for stress exposure affects ongoing hippocampal development during adolescence in the context of higher PAE-induced hyperresponsivity. To fill this gap in the literature, here we evaluate the short- and long-term effects of adolescent stress on stress-related receptor expression in PAE animals by performing a comprehensive assessment of CRHR1, MR and GR mRNA expression in specific subfields of the dorsal and ventral hippocampal formation.
Methods
Animals and Breeding
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories (St. Constant, Canada). Rats were pair-housed by sex and maintained at a constant temperature (21 ± 1°C) and on a 12 h light-dark cycle (lights on at 0700 h) with ad libitum access to water and standard lab chow (Harlan, Canada). After a 10-day acclimation period, male and female pairs were placed together for breeding. Vaginal smears were taken each morning, and the presence of sperm was used as an indicator of pregnancy (gestation day 1; G1). All experiments were performed in accordance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals, Canadian Council on Animal Care guidelines, and were approved by the University of British Columbia Animal Care Committee.
Prenatal Alcohol Exposure
On G1, females were single-housed and randomly assigned to one of three treatment groups: Prenatal Alcohol Exposure (PAE), pair-feeding, or control. Dams in the PAE group were offered ad libitum liquid ethanol diet with 36% ethanol-derived calories (Weinberg-Keiver High Protein Control Diet #710109, Experimental Diet # 710324, Dyets Inc., Bethlehem, PA). The liquid ethanol diet was introduced gradually over the first 3 days with bottles containing: G1 - 66% control diet, 34% ethanol diet; G2 - 34% control diet, 66% ethanol diet; G3-21 - 100% ethanol diet. This diet is formulated to provide adequate nutrition to pregnant rats regardless of ethanol intake (Lan et al., 2006). Blood alcohol levels were measured from tail blood samples collected on G15 approximately 4-6 h after lights off using Pointe Scientific Inc. Alcohol Reagent Set (Lincoln Park, USA; Workman et al., 2015). Alcohol-consuming dams showed an average of 118.20 ± 8.11 mg/dl. Pair-fed dams were offered a liquid control diet with maltose-dextrin isocalorically substituted for ethanol, in an amount matched to the consumption of an alcohol-fed partner (g/Kg body weight/day of gestation). The control dams were offered ad libitum access to a pelleted form of the liquid control diet. All animals had ad libitum access to water, and those in the PAE and pair-fed groups were provided with fresh diet daily within 1 h prior to lights off to maintain the normal HPA circadian rhythm (Gallo and Weinberg, 1981; Krieger, 1974). Experimental diets were continued through G21. Beginning on G22, all animals were offered ad libitum access to standard laboratory chow and water, which they received throughout lactation. Pregnant dams were left undisturbed except for cage changing (G1, G7, and G14) and weighing (G1, G7, G14, and G21). On the day of birth (postnatal day 1, PN1) the litters were culled to 12 pups with an attempt to preserve an equal number of males and females per litter. Dams and pups were cage changed and weighed on PN1, PN8, PN15, and PN22. Dam and pup body weight data were published in Raineki et al., 2016. On PN22 pups were weaned and group-housed by litter and sex.
Effects of adolescent CMS on stress-related receptors in PAE and ad libitum-fed control offspring is presented in the Results section below, whereas similar to Raineki et al., 2017, specific pair-feeding effects are analyzed and presented separately in the Supplementary Materials. The adverse effects of alcohol per se on a vast number of outcomes, including hippocampal structure and function, are well established. Moreover, while the pair-fed group is used to account for the decreased food intake associated with chronic alcohol consumption, it can not separate alcohol effects from those of undernutrition, as it can never account for the nutritional effects associated with alcohol consumption, including changes in absorption and utilization of nutrients (Weinberg, 1984) and satiety (Lin et al., 1998). Moreover, pair-feeding is in many aspects a confounded “control” condition and in many ways is a treatment group in itself, as it leads to an abnormal feeding pattern: because pair-fed animals receive less food than they would consume if allowed to eat ad libitum, they consume their entire day’s ration within a few hours, and are thus essentially food deprived until the next feeding (Gallo and Weinberg, 1981; Weinberg 1984). This abnormal feeding pattern introduces a prenatal stress component, which in itself can have long-term impacts on the development of offspring neurobiological systems, including the neuroendocrine axis (Vieau et al., 2007).
Adolescent Chronic Mild Stress (CMS)
One male and one female from each litter were randomly assigned to either the CMS or the non-CMS condition and were pair-housed with another animal of the same sex and prenatal group. Animals in the CMS condition were subjected to 10 consecutive days of chronic, unpredictable, mild stressors. To account for the sexually dimorphic time of pubertal onset (McCormick and Mathews, 2010; Vetter-O’Hagen and Spear, 2012), males and females were exposed to CMS at ages consistent with puberty onset – PN31-41 for females, PN 37-47 for males. On each CMS day, animals received two different stressors at random times: one in the morning (between 0800 and 1200 h) and one in the afternoon (between 1300 and 1800 h), with a minimum of 2 h between stressors. On day 1 of CMS and the day immediately following the end of CMS, within 2 h of lights on, basal blood samples were obtained from all animals (including those in the non-CMS group) via tail nick. After tail nick, all animals were weighed and placed in a new home cage. Pre- and post-CMS blood sample and body weight data were published in Raineki et al., 2016. Except for blood sampling and routine husbandry, animals in the non-CMS condition were left undisturbed during this period. CMS and non-CMS animals were housed in different colony rooms so that non-CMS animals were not exposed to the disturbance and stress odors of the CMS animals (Mackay-Sim and Laing, 1980). The order and type of stressor was randomized, but all animals received the same number of exposures to each stressor over the 10-day period. Stressors included: 1) Platform: exposure to an elevated Plexiglas platform (20 × 20 cm) mounted on 90 cm high post for 10 min; 2) Cage tilt: the home cage was tilted at a 30° angle for 2 h; 3) Novel cage: exposure to a novel cage without food and water, with a small amount of novel bedding for 1 h; 4) Soiled cage: exposure to a soiled cage of another animal of the same sex for 1 h; 5) Restraint: restraint in PVC tubes (tube size varied to ensure proper restraint/immobility of each animal) for 30 min; 6) Social isolation without food and water: overnight isolation in a smaller cage (28 × 17 cm with 12.5 cm high) for 12 h; and 7) Empty water bottle: animals given their empty water bottles for 1 h following the social isolation/food and water deprivation period.
Behavioral Exposure and Brain Extraction
All animals were tested on the open field before and after CMS exposure. For the post-CMS test, half the animals were tested in adolescence (short-term effects of CMS) and the remaining animals were tested in adulthood (long-term effects of CMS). Following post-CMS open field tests, all animals were exposed to the FST (habituated for 15 min and then tested for 5 min the following day). Pre- and post-CMS behavioral data were published in Raineki et al., 2016. Animals were decapitated 30-min after the end of day 2 FST testing and brains were collected, quickly frozen on dry ice and stored at -80°C.
Neural assessment of CRHR1, MR, and GR by in situ hybridization
Probes
CRHR1 (1.3kb fragment cloned into Bluescript SK plasmid) was provided by Dr. Victor Viau (source: Dr. Cyntia Donaldson, Perrin et al., 1993). Rat MR (550bp fragment in Bluescript SK) and GR (456bp fragment in pGem4) were provided by Dr. James Herman. All probes were transcribed using 35S-UTP (Perkin-Elmer, Waltham, MA) and the Promega Riboprobe System (Promega Corp., Madison, WI) with polymerase T7 for CRHR1 and GR antisense probes, and T3 for MR antisense probe. Probes were purified using Micro Bio-Spin 30 Columns (Bio-Rad, CA, USA) and 0.1 M DTT was added to prevent oxidation.
In situ hybridization
Brains were sectioned coronally (20 μm) using a cryostat (-16°C) and stored at -80°C. Thawed sections were fixed in formalin for 30 min and pre-hybridized as follows: 1 × PBS for 10 min twice, proteinase K (0.1μg/L; 37°C) for 9 min, 0.1 M triethanolamine-hydrochloride (TEA) for 10 min, 0.1 M TEA with 0.25% acetic anhydride for 10 min, 2 × SSC for 10 min twice, dehydrated by a graded series of ethanol, chloroform, and 100% ethanol and air-dried. Probes were applied at 1 × 106 cpm/slide in 75% Hybridization buffer (75% formamide, 3 × SSC, 1× Denhardt’s solution, 200 μg/mL yeast tRNA, 50 mM sodium phosphate buffer (pH 7.4), 10% dextran sulphate, 10 mM DTT) and covered with HybriSlips (Sigma-Aldrich, ON). Sections were incubated overnight at 55°C in chambers humidified with 75% formamide. HybriSlips were removed and slides were rinsed as follows: 2 × SSC twice for 20 min, 2 × SSC for 30 min, 50 μg/L RNAse A solution (37°C) for 60 min, 2 × SSC with 0.01 M DTT for 10 min, 1 × SSC for 10 min, 0.5 × SSC with 0.01 M DTT for 10 min, 0.1 × SSC with 0.01 M DTT (60°C) for 60 min, 0.1 × SSC for 5 min. Sections were dehydrated by a graded series of ethanol and air dried overnight. The hybridized slides were then exposed to Kodak BioMax MR film, and developed using Kodak GBX developer and fixer.
Densitometric analysis
The autoradiograph films were scanned and analyzed with ImageJ 1.48v (National Institutes of Health, USA). The left and right subregions of the dorsal (CA1, CA3, and DG) and ventral hippocampal formation (CA1, CA3, DG, and ventral subiculum) were traced free-hand in two sections per animal to determine mean grey density levels. Corrected grey levels were obtained by subtracting the background level (corpus callosum) from each of the four measurements. Left and right levels in each measured area were averaged together for analysis.
Statistical analysis
All data are expressed as mean ± SEM and were analyzed by two-way ANOVA (prenatal treatment and CMS as factors). When significant interactions between prenatal treatment and CMS were detected, ANOVAs were followed by Newman-Keuls post hoc tests. Sex was not used as a factor in the ANOVAs because females and males were exposed to CMS during different ages (PN31-41 and PN 37-47 respectively). Age was also not used as factor because in situ hybridizations for brains collected during adolescence and adulthood were run separately. Additionally, we utilized planned comparisons (Student’s t-tests) to test the a priori hypotheses that: 1) PAE will alter animals’ receptor expression compared to controls; and 2) CMS will differentially alter animals’ receptor expression. In all cases, differences were considered significant when p≤0.05. Outliers were identified and removed using the Robust regression and Outlier removal (ROUT) method with Q=0.05.
Results
Mineralocorticoid receptor mRNA expression
Dorsal hippocampal formation
In adolescent males, PAE decreased MR mRNA expression in the CA1, CA3, and DG, independently of CMS exposure [Figure 1A,E,I; significant main effects of prenatal treatment for CA1 (F(1,28)=9.63, p=0.004), CA3 (F(1,26)=13.99, p=0.0009), and DG (F(1,28)=4.85, p=0.04)]. Neither PAE nor CMS affected MR mRNA expression in the dorsal hippocampal formation of adolescent females or in adult males or females (Figure 1).
Figure 1.
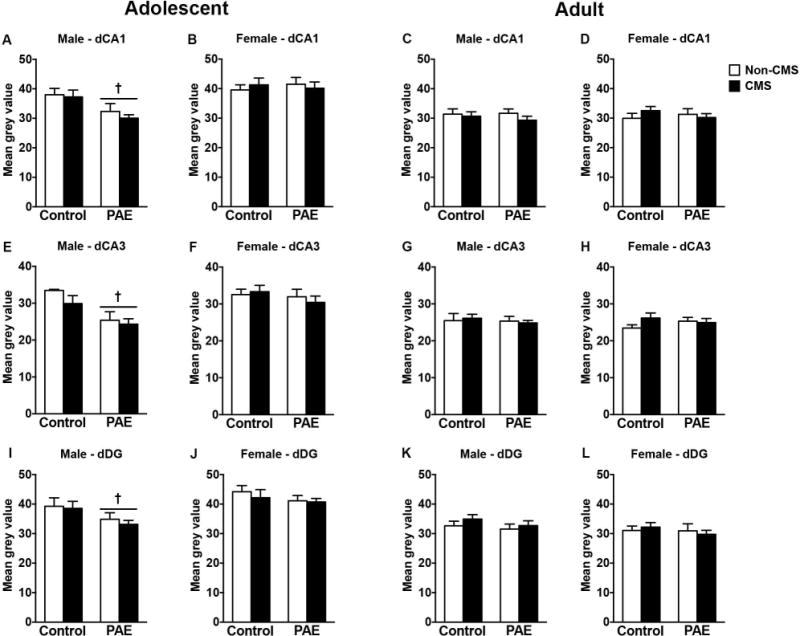
Short- and long-term effects of adolescent CMS on dorsal hippocampus MR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of MR mRNA expression in the CA1 (A-D), CA3 (E-H), and DG (I-L). † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals (n = 6-10 for all groups).
Ventral hippocampal formation
In adolescent males, CMS decreased MR mRNA expression in the ventral subiculum in control but not PAE rats, while no changes were observed in CA1, CA3, or DG [Figure 2A,E,I,M; a priori analysis for ventral subiculum comparing control non-CMS to control CMS (p=0.04)]. In adolescent females, however, CMS increased MR mRNA expression only in the CA1 of controls [Figure 2B; a priori analysis for CA1 comparing control non-CMS to control CMS (p=0.04)]. Moreover, adolescent PAE non-CMS females showed increased MR mRNA expression in the ventral subiculum compared to control non-CMS females [Figure 2N; a priori analysis for ventral subiculum comparing PAE non-CMS to PAE CMS (p=0.02)]. Neither PAE nor CMS affected MR mRNA expression in the ventral CA3 or DG of adolescent females (Figure 2F,J).
Figure 2.
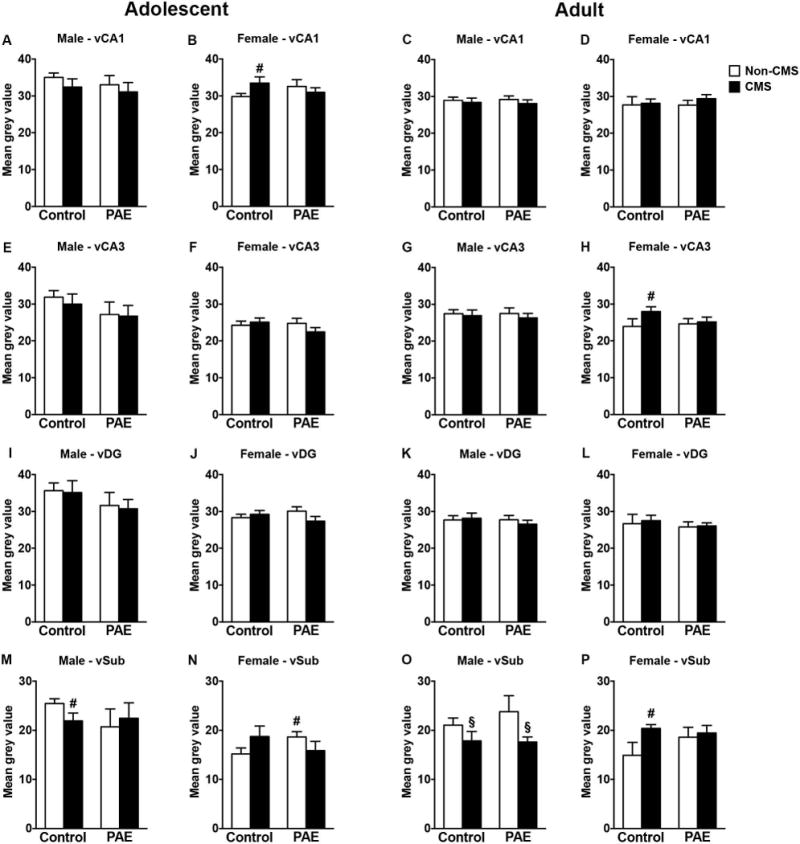
Short- and long-term effects of adolescent CMS on ventral hippocampus MR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of MR mRNA expression in the CA1 (A-D), CA3 (E-H), DG (I-L), and ventral subiculum (M-P). § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for B, H, M, and P, # indicates that control CMS is different from control non-CMS based on a priori comparisons; for N, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons (n = 4-10 for all groups).
In adulthood, adolescent CMS reduced MR mRNA expression in the ventral subiculum of males independently of prenatal treatment [Figure 2O; significant main effect of CMS for ventral subiculum (F(1,30)=5.46, p=0.03)]. However, neither PAE nor CMS affected MR mRNA expression in the ventral CA1, CA3 and DG of adult males (Figure 2 C,G,K). In adult females, adolescent CMS increased MR mRNA expression in the CA3 and ventral subiculum of control but not PAE animals [Figure 2H,P; a priori analysis comparing control non-CMS to control CMS for CA3 (p=0.05) and ventral subiculum (p=0.01)]. Neither PAE nor CMS affected MR mRNA expression in the ventral CA1 or DG of adult females (Figure 2D,L).
Glucocorticoid receptor mRNA expression
Dorsal hippocampal formation
In adolescent males, CMS decreased GR mRNA expression in CA1 only in control rats, but no changes were observed in CA3 or DG [Figure 3A,E,I; a priori analysis for CA1 comparing control non-CMS to control CMS (p=0.05)]. In adolescent females, CMS increased GR mRNA expression in CA1, CA3, and DG in both groups independently of prenatal exposure (Figure 3B,F,J). Moreover, PAE reduced GR mRNA expression in CA1 and DG independently of CMS exposure [significant main effects of CMS for CA1 (F(1,28)=7.78, p=0.009), CA3 (F(1,28)=7.22, p=0.01), and DG (F(1,28)=8.77, p=0.006) and significant main effects of prenatal treatment for CA1 (F(1,28)=10.04, p=0.003), and DG (F(1,28)=8.63, p=0.006)].
Figure 3.
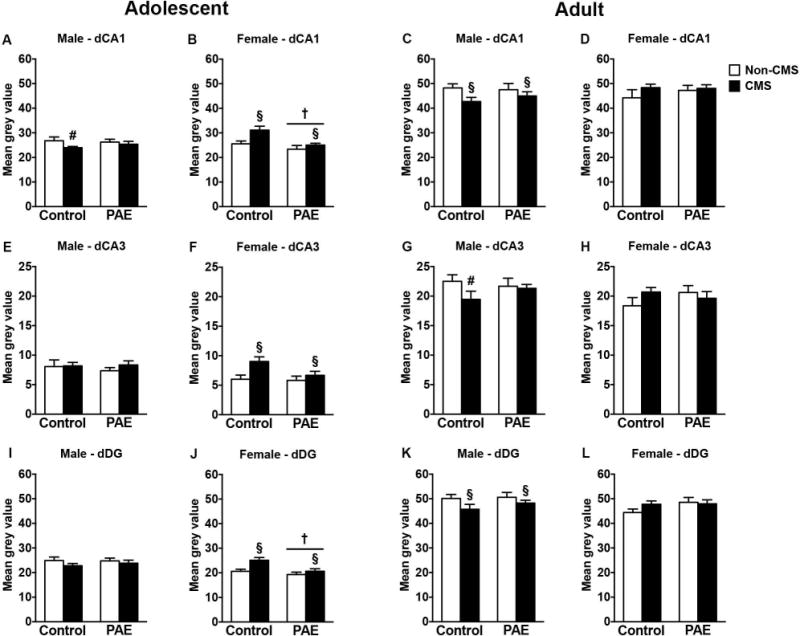
Short- and long-term effects of adolescent CMS on dorsal hippocampus GR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of GR mRNA expression in the CA1 (A-D), CA3 (E-H), and DG (I-L). † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for A and G, # indicates that control CMS is different from control non-CMS based on a priori comparisons (n = 6-10 for all groups).
In adult males, CMS during adolescence reduced GR mRNA expression in the CA1 and DG in both groups and in CA3 only in control animals [Figure 3C,G,K; significant main effects of CMS for CA1 (F(1,30)=4.47, p=0.04), and DG (F(1,30)=3.97, p=0.05); a priori analysis for CA3 comparing control non-CMS to control CMS (p=0.05)]. Neither PAE nor CMS affected GR mRNA expression in the dorsal CA1, CA3, or DG of adult females (Figure 3D,H,L).
Ventral hippocampal formation
Neither PAE nor CMS affected GR mRNA expression in the ventral hippocampal formation of adolescent males (Figure 4A,E,I,M). However, in adolescent females, PAE increased GR mRNA expression in the CA1 independently of CMS exposure, but no changes were observed in CA3, DG, or ventral subiculum [Figure 4B,F,J,N; a priori analysis for CA1 comparing control non-CMS to PAE non-CMS (p=0.04)].
Figure 4.
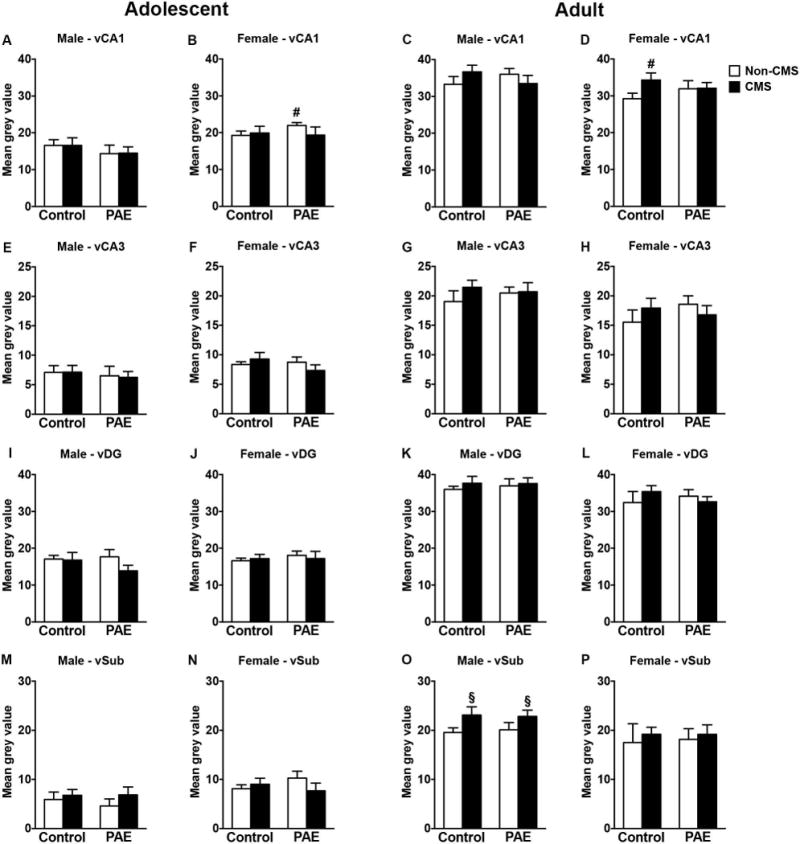
Short- and long-term effects of adolescent CMS on ventral hippocampus GR mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of GR mRNA expression in the CA1 (A-D), CA3 (E-H), DG (I-L), and ventral subiculum (M-P). § indicates a significant main effect of CMS exposure, where all animals exposed to CMS are different from animals not exposed to CMS; for B, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons; for D, # indicates that control CMS is different from control non-CMS based on a priori comparisons; (n = 4-10 for all groups).
In adulthood, adolescent CMS increased GR mRNA expression in the ventral subiculum of males independently of prenatal treatment [Figure 4O; significant main effect of CMS for ventral subiculum (F(1,30)=5.23, p=0.03)]. However, neither PAE nor CMS affected GR mRNA expression in the ventral CA1, CA3 or DG of adult males (Figure 4C,G,K). In adult females, adolescent CMS increased GR mRNA expression in the CA1 only in controls, but no changes were observed in CA3, DG, or ventral subiculum [Figure 4D,H,L,P; a priori analysis for CA1 comparing control non-CMS to control CMS (p=0.04)].
Type 1 CRH receptors mRNA expression
Dorsal hippocampal formation
In adolescent males, PAE decreased CRHR1 mRNA expression in the CA1 independently of CMS exposure [Figure 5A; significant main effect of prenatal treatment for CA1 (F(1,28)=7.51, p=0.01)]. No changes were observed in CRHR1 mRNA expression in the CA3 and DG of adolescent males (Figure 5E,I). Additionally, neither PAE nor CMS affected CRHR1 mRNA expression in the dorsal hippocampal formation of adolescent females (Figure 5B,F,J).
Figure 5.
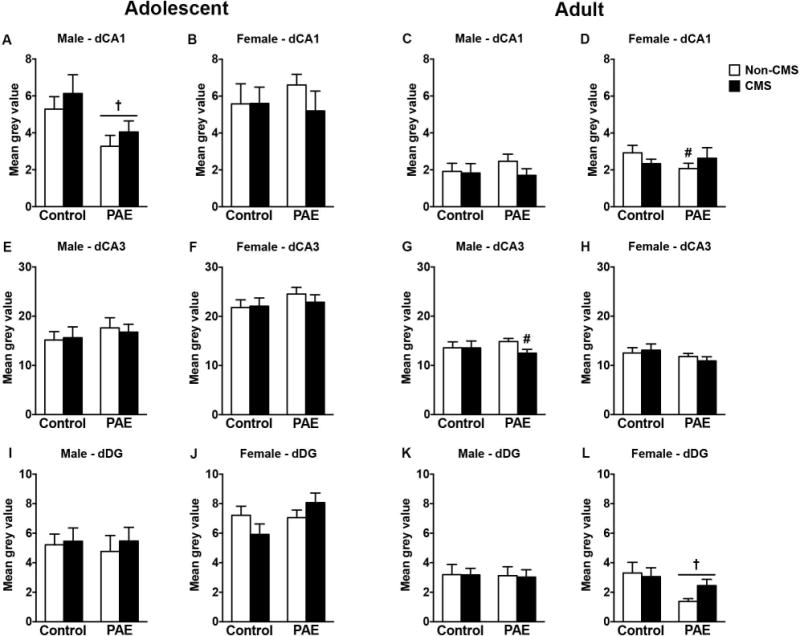
Short- and long-term effects of adolescent CMS on dorsal hippocampus CRHR1 mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of CRHR1 mRNA expression in the CA1 (A-D), CA3 (E-H), and DG (I-L). † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; for D, # indicates that PAE non-CMS is different from control non-CMS based on a priori comparisons; for G, # indicates that PAE CMS is different from PAE non-CMS based on a priori comparisons (n = 6-10 for all groups).
In adult males, CMS decreased CRHR1 mRNA expression only in the CA3 of PAE rats, while no changes were observed in CA1 or DG [Figure 5C,G,K; a priori analysis for CA3 comparing PAE non-CMS to PAE CMS (p=0.02)]. In adult females, PAE reduced CRHR1 mRNA receptors in the CA1 and DG. In the CA1 the effect was only observed in the non-CMS animals and in the DG the effect was independent of CMS exposure [Figure 5D,L; a priori analysis for CA1 comparing control non-CMS to PAE non-CMS (p=0.05); significant main effect of prenatal treatment for DG (F(1,28)=5.75, p=0.02)]. No changes were observed in CRHR1 mRNA expression in CA3 of adult females (Figure 5H).
Ventral hippocampal formation
In adolescent males, PAE decreased CRHR1 mRNA expression in CA1 independently of CMS exposure [Figure 6A; significant main effect of prenatal treatment for CA1 (F(1,27)=12.01, p=0.001)]. Moreover, CMS decreased CRHR1 mRNA expression in CA3 of PAE adolescent male rats [Figure 6E; significant interaction between prenatal treatment and CMS for CA3 (F(1,27)=7.19, p=0.01)]. Neither PAE nor CMS affected CRHR1 mRNA expression in DG or ventral subiculum of adolescent males (Figure 6I,M). In adolescent females, PAE decreased CRHR1 mRNA expression in DG independently of CMS exposure [Figure 6J; significant main effect of prenatal treatment for CA1 (F(1,28)=4.18, p=0.05)]. However, neither PAE nor CMS affected CRHR1 mRNA expression in CA1, CA3, or ventral subiculum of adolescent females (Figure 6B,F,N).
Figure 6.
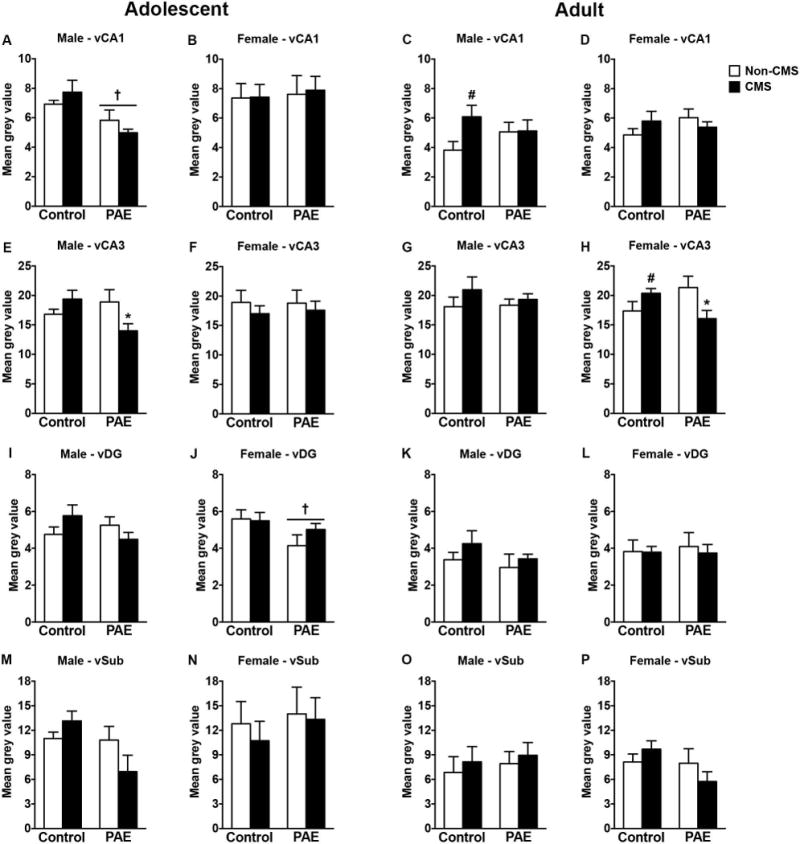
Short- and long-term effects of adolescent CMS on ventral hippocampus CRHR1 mRNA expression in control and PAE rats. Bars represent the mean ± SEM (mean gray value) of CRHR1 mRNA expression in the CA1 (A-D), CA3 (E-H), DG (I-L), and ventral subiculum (M-P). † indicates a significant main effect of prenatal treatment, where all PAE animals are different from control animals; for E and H, * indicates that PAE CMS is different from PAE non-CMS; for C and H, # indicates that control CMS is different from control non-CMS based on a priori comparisons (n = 3-10 for all groups).
In adult males, CMS during adolescence increased CRHR1 mRNA expression only in CA1 of control animals [Figure 6C; a priori analysis for CA1 comparing control non-CMS to control CMS (p=0.02)]. Neither PAE nor CMS affected CRHR1 mRNA expression in the CA3, DG, or ventral subiculum of adult males (Figure 6G,K,O). In adult females, adolescent CMS increased CRHR1 mRNA receptors in CA3 of controls and reduced CRHR1 mRNA receptors in CA3 of PAE animals [Figure 6H; significant interaction between prenatal treatment and CMS for CA3 (F(1,28)=8.23, p=0.008); a priori analysis for CA3 comparing control non-CMS to control CMS (p=0.04)]. Neither PAE nor CMS affected CRHR1 mRNA expression in CA1, DG, or ventral subiculum of adult females (Figure 6D,L,P).
Discussion
Our ontogenetic approach investigating how PAE affects stress-related receptor mRNA expression in the hippocampus over the course of development revealed that the effects of PAE are more apparent during adolescence and decline by adulthood. Indeed, in adolescence, PAE resulted in altered MR, GR, and CRHR1 mRNA expression in several subfields of the dorsal and ventral hippocampal formation in a sexually dimorphic manner (Table 1). However, in adulthood, PAE only altered CRHR1 mRNA expression in the dorsal hippocampus of females. In contrast, the effects of adolescent CMS on stress-related receptor mRNA expression in the hippocampus showed the opposite pattern; that is, the effects of adolescent CMS on hippocampal expression of stress-related receptors were more pronounced in adulthood, long after the termination of the stress exposure. PAE animals also appeared to be less responsive to the effect of adolescent CMS, as CMS uniquely affected only CRHR1 in PAE animals compared to the altered MR, GR and CRHR1 mRNA expression observed in controls.
Table 1.
Summary of the short- and long-term effects of PAE and adolescent CMS on MR, GR, and CRHR1 mRNA expression in different subfields of the dorsal and ventral hippocampus
| Adolescent
| ||||||||
|---|---|---|---|---|---|---|---|---|
| PAE effects | CMS all groups | CMS control | CMS PAE | |||||
|
| ||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| MR | ↓dCA1 ↓dCA3 ↓dDG |
↑vSub | – | – | ↓vSub | ↑vCA1 | – | – |
|
| ||||||||
| GR | – | ↓dCA1 ↓dDG ↑vCA1 |
– | ↑dCA1 ↑dCA3 ↑dDG |
↓dCA1 | – | – | – |
|
| ||||||||
| CRHR1 | ↓dCA1 ↓vCA1 |
↓vDG | – | – | – | – | ↓vCA3 | – |
|
Adulthood | ||||||||
| PAE effects | CMS all groups | CMS control | CMS PAE | |||||
|
|
||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
|
| ||||||||
| MR | – | – | ↓vSub | – | – | ↑vCA3 ↑vSub |
– | – |
|
| ||||||||
| GR | – | – | ↓dCA1 ↓dDG ↑vSub |
– | ↓dCA3 | ↑vCA1 | – | – |
|
| ||||||||
| CRHR1 | – | ↓dCA1 ↓dDG |
– | – | ↑vCA1 | ↑vCA3 | ↓dCA3 | ↓vCA3 |
PAE effects on stress-related receptors in the hippocampus are transient
Studies investigating the long-term effect of PAE on stress-related receptors expression in the dorsal hippocampus have demonstrated that, in adult males, hippocampal MR and GR expression is not altered (Glavas et al., 2007; Kim et al., 1999; Uban et al., 2013); however, in adult females, PAE has been shown to reduce hippocampal MR mRNA expression (Sliwowska et al., 2008; Uban et al., 2013). Furthermore, analysis of MR and GR protein levels in the nuclear and cytosolic fractions of whole hippocampus of adolescent male mice exposed to alcohol during gestation indicates that levels of both MR and GR were not altered in the cytosolic fraction, whereas PAE reduced MR and elevated GR protein levels in the nuclear fraction (Caldwell et al., 2014). Our evaluation of stress-related receptor mRNA expression in specific subfields of dorsal and ventral hippocampus in adolescent and adult rats considerably extends these previous findings by demonstrating that there are indeed effects of PAE on these receptors but they are transient; PAE resulted in altered MR and GR mRNA expression in the dorsal and/or ventral hippocampus during adolescence but not in adulthood. Moreover, the effects of PAE were sex and region dependent as adolescent PAE male rats showed reduced MR mRNA expression in dorsal CA1, CA3 and DG while adolescent PAE female showed reduced GR mRNA expression in dorsal CA1 and DG. In addition, adolescent PAE female rats showed increased MR in ventral subiculum and GR in ventral CA1. In contrast to previous studies from our laboratory where adult PAE females showed reduced MR mRNA in the hippocampus (Sliwowska et al., 2008; Uban et al., 2013), we observed no effects of PAE in adult females in the current study. This discrepancy is probably due methodological differences: previous studies reported measures of basal receptor expression while the current study evaluated receptor expression following an acute stressor (e.g. forced swim test).
MR and GR are both nuclear receptors that bind to corticosterone, though GR binds corticosterone with a 10-fold lower affinity. Because of this difference in affinity, MR is already almost fully occupied at basal corticosterone levels, while GR becomes saturated at stress corticosterone levels (Reul and de Kloet, 1985; Spencer et al., 1993). Importantly, there is evidence that the nuclear type of MR can also be positioned in the membrane of hippocampal neurons (Karst et al., 2005). However, similar to GR, this membrane MR has a low affinity for corticosterone, responding only to high levels such as those present during stress-induced corticosterone release (Joëls et al., 2008). These findings suggest that both MR and GR in the hippocampus play a critical role in behavioral and hormonal stress responses, albeit in unique ways: whereas membrane MR is important for appraisal of the situation (i.e. how threatening is the stressor) and/or for choosing the appropriate response (i.e. determine the behavioral response to the stressor), GR is essential for returning brain activity to baseline after the stress response and for memory consolidation of the stressful event (de Kloet et al., 2005; Joëls et al., 2006; Oitzl and de Kloet, 1992; Schwabe et al., 2007, 2010, 2013). To this end, dysregulation of MR or GR expression in the hippocampus may affect different aspects of basal and/or stress response regulation. The current data show that PAE results in reduced MR in the dorsal hippocampus (CA1, CA3 and DG) of adolescent males and reduced GR in the dorsal hippocampus (CA1 and DG) of adolescent females. Taken together, these results suggest that the behavioral and hormonal alterations in response to acute and/or chronic stress observed in PAE animals (Hellemans et al., 2010; Weinberg et al., 2008) may be supported by different underlying mechanisms depending on the sex of the animal, with alterations in MR expression/regulation supporting the responses observed in PAE males, and alterations in GR expression/regulation supporting the responses observed in PAE females.
The current and previous data (Glavas et al., 2007; Kim et al., 1999; Uban et al., 2013) indicate that, in contrast to what is observed in adolescence, adult hippocampal MR and GR expression of PAE animals is not different from controls. It should be noted that the lack of PAE effects on the expression of stress-related receptor mRNA in adulthood does not necessarily preclude effects of PAE on protein levels, localization and/or function. Further investigation of underlying mechanisms, including epigenetic modifications will be important in future studies. Additionally, the PAE-related alterations in hippocampal MR and GR mRNA expression observed during adolescence in the current study may set the stage for the altered behavioral responses shown by PAE animals when faced with different concentrations of corticosterone in adulthood. Indeed, adrenalectomy in adulthood, which reduces corticosterone levels, resulted in an exacerbated increase in MR mRNA expression in dorsal CA3 of PAE females and in GR mRNA expression in dorsal CA3 of PAE males (Glavas et al., 2007). Additionally, corticosterone replacement in adrenalectomized animals was ineffective in normalizing MR mRNA expression in dorsal CA1 and DG of PAE males (Glavas et al., 2007). Together these data indicate that, even though the PAE effects of MR and GR mRNA expression in hippocampus appear to be transient, challenges to the system can uncover underlying alterations in the regulation of stress-related receptor expression in adulthood.
In addition to MR and GR, hippocampal CRHR1 also plays a role in emotional regulation. Indeed, abnormal activity of the central CRH system is associated with increased expression of many psychopathologies, including depression and anxiety (Binder and Nemeroff, 2010; Holsboer and Ising, 2008). Animal studies have confirmed this association by demonstrating that many of the behavioral abnormalities associated with depressive- and anxiety-like behaviors are mediated by dysregulated activity of CRHR1 in limbic areas, including the hippocampus. Specifically, increased activity of the CRH system is associated with increased anxiety-like behavior as indicated by the finding that intracerebroventricular administration of CRH increases expression of anxiety-like behaviors (Dunn and File, 1987; Britton et al., 1982). In contrast, decreased activity of the CRH system is associated with reductions in anxiety-like behavior, as shown in studies using CRHR1-deficient mice (Smith et al., 1998; Timpl et al., 1998), conditional inactivation of the CRHR1 in limbic areas (Müller et al., 2003), and CRHR1 antagonism in rats (Keck et al., 2001; Sandi et al., 2008; Veenit et al., 2014). Previous studies using animal models of PAE have demonstrated that rats exposed to alcohol during gestation show reduced CRHR1 mRNA expression in the amygdala, mPFC, and pituitary in adulthood (Glavas et al., 2007; Raineki et al., 2016). Here, we extend those findings by demonstrating that PAE also reduced CRHR1 mRNA expression in the hippocampus. Nevertheless, and in contrast with previous findings, the current data indicate that PAE effects on CRHR1 mRNA expression in the hippocampus are more evident during adolescence than adulthood. PAE reduced the expression of CRHR1 in the dorsal and ventral CA1 of adolescent males and in the ventral DG of adolescent females. In adulthood, however, PAE effects were only observed in females, with a reduction of CRHR1 mRNA expression in dorsal CA1 and DG. Consistent with previous findings on central CRHR1 expression following PAE (Caldwell et al., 2015; Glavas et al., 2007; Raineki et al., 2016), we also observed a reduction in CRHR1 mRNA expression in the hippocampus. This overall reduction in central CRHR1 expression could be a response to increased CRH production; however, extended investigation of CRH mRNA expression following PAE indicated that PAE increased CRH only in the central amygdala (Lan et al., 2015), with no changes were observed in the paraventricular nucleus of hypothalamus, mPFC, or nucleus accumbens (Glavas et al., 2007; Lan et al., 2015; Uban et al., 2013). More work is necessary to characterize fully the effects of PAE on CRH expression in areas that are known to produce and release CRH in the brain, especially in the hippocampus and locus coeruleus.
Besides CRHR1 and GR, MR has increasingly been recognized as playing a crucial role in the pathophysiology of depression (Chen et al., 2016; Hinkelmann et al., 2016; Mostalac-Preciado et al., 2011; Wingenfeld et al., 2016). In our previous study using the same animals as in the current study (Raineki et al., 2016), we demonstrated that PAE males showed depressive-like behaviors only during adolescence and not in adulthood. Similar to the behavioral effects of PAE on depressive-like behaviors, here, we observed a temporary reduction in MR mRNA expression in dorsal CA1, CA3, and DG and in CRHR1 in dorsal and ventral CA1 during adolescence in PAE males. However, those reductions in MR and CRHR1 were also transient and disappeared in adulthood. This temporal alignment between behavioral and receptor expression suggests that the depressive-like behavior in PAE animals may be mediated, at least in part, by deficits in CRHR1 and MR expression in the hippocampus.
Effects of adolescent CMS on stress-related receptors in the hippocampus
Exposure to chronic stress during adolescence has been shown to induce short- and long-term alterations to the structure and function of the hippocampus, including changes in the expression of stress-related receptors (Barha et al., 2011; Eiland and Romeo, 2013; Hueston et al., 2017; Iredale et al., 1996; Isgor et al., 2004; Li et al., 2015; McCormick et al., 2012; Sterlemann et al., 2008, 2010; Veenit et al., 2014). Nevertheless, the direction of these effects can diverge among studies; this is not necessarily surprising given that different studies vary in their use of specific stress paradigms, duration of stress exposure, animal ages, and methodologies to measure outcomes. Despite limiting our ability to generalize conclusions, the use of different adolescent stress protocols has underscored the importance of how each variable within a stress model can differentially impact the outcome. Notably, very little is known about how adolescent stress affects the expression of stress-related receptors in the hippocampus of females (Wulsin et al., 2016) because, the literature has almost exclusively focused on assessing males (Iredale et al., 1996; Isgor et al., 2004; Li et al., 2015; Sterlemann et al., 2008; Veenit et al., 2014). The current dataset provides valuable information on the differential short- and long-term responses to chronic stress in adolescent males and females. Furthermore, as MR, GR, and CRHR1 in the hippocampus are critical regulators of cognition, stress and emotional responses, the current data may help us better understand the underlying mechanisms by which adolescent stress can lead to sexually dimorphic effects in: 1) learning and memory (Toledo-Rodriguez et al., 2012; Toledo-Rodriguez and Sandi, 2007); 2) anxiety- and depressive-like behaviors (Bourke and Neigh, 2011; McCormick et al., 2008; Raineki et al., 2016; Toledo-Rodriguez and Sandi, 2011); and 3) HPA axis function (Barha et al., 2011; Bourke and Neigh, 2011; Raineki et al., 2016).
It has been shown that adolescent stress exposure leads to similar short- and long-term effects on hippocampal GR expression but in a sexually dimorphic manner, with males showing decreased GR mRNA expression in dorsal CA1 and DG in both the short- and long-term (Isgor et al., 2004), while females show no observable changes (Wulsin et al., 2016). The current study replicates these findings in males, showing that adolescent CMS reduced GR mRNA expression in dorsal CA1 during adolescence and in dorsal CA1, CA3, and DG in adulthood (Table 1). Nevertheless, we also saw increased GR mRNA expression in the ventral subiculum of adult males as well as increased GR in dorsal CA1, CA3, and DG in adolescent females and in ventral CA1 in adult females following adolescent CMS.
In contrast to GR, less in known about the effects of adolescent stress on MR expression in the hippocampus. It has been shown that, in male mice, adolescent stress reduces the MR mRNA expression in dorsal CA1, CA3 and DG in adulthood (Sterlemann et al., 2008). Though our current results do not corroborate this previous study, our data do expand knowledge about how adolescent stress exposure can have profound impacts on MR expression in the hippocampus. Indeed, adolescent CMS reduces MR mRNA expression in ventral subiculum of males and increases MR mRNA expression in the ventral CA1 of females in the short term. However, the long-term effects of adolescence CMS on MR were only observed in females, where adolescent CMS increased MR in the ventral CA3 and subiculum in adulthood.
Previous studies found that in the short-term, adolescent stress increases CRHR1 mRNA expression in whole hippocampus in males (Iredale et al., 1996). However, we observed that adolescent CMS did not alter the expression of CRHR1 in the hippocampus immediately after the end of stress exposure. The literature on the long-term effects of adolescent stress on CRHR1 expression in the hippocampus is somewhat mixed, with data indicating that adolescent stress can both increase CRHR1 mRNA expression (Veenit et al., 2014) and decrease CRHR1 protein expression (Li et al., 2015) in the hippocampus. This inconsistency is probably due to methodological differences between studies (as discussed above). Nevertheless, our data assessing the long-term effects of adolescent CMS indicate that both males and females show increased CRHR1 mRNA expression in the hippocampus in adulthood, although the effect was observed in different subfields of the hippocampus and was sex-dependent.
The effects of chronic stress during adolescence on brain and behavior can manifest differently depending on the age when observations are made. Certain effects are only present immediately after the end of the stress period, some are only observed long after the end of stress, and some are persistent and can be detected any time after stress exposure (Bourke and Neigh, 2011; McCormick and Green, 2013; Raineki et al., 2016; Toledo-Rodriguez and Sandi, 2007; Tsoory et al., 2008). The current data indicate that exposure to CMS during adolescence induces both short- and long-term effects on the expression of stress-related receptors in the hippocampus; however, the effects of CMS were more pronounced in adulthood, long after the termination of stress exposure (Table 1).
Lastly, the hippocampus of PAE animals appears to be less responsive to the effect of adolescent CMS, as CMS uniquely affected only CRHR1 in PAE animals compared to the altered MR, GR and CRHR1 mRNA expression observed in controls (Table 1). This blunted responsivity to adolescent CMS may indicate that the hippocampus of PAE animals shows reduced neuroplasticity, especially when facing chronic stress. Corroborating this suggestion are findings indicating that PAE is associated with long-term potentiation deficits in the hippocampus (Christie et al., 2005; Patten et al., 2013; Sutherland et al., 1997) and that chronic restraint stress in adulthood reduces hippocampal neurogenesis in control, but not PAE animals (Sliwowska et al., 2010). This PAE-induced blunted hippocampal responsivity to environmental stimuli (e.g. stress) and reduced neuroplasticity observed in animal models has important implications for individuals exposed to alcohol during gestation. Indeed, appropriate responding to environmental stimulation (e.g. stress) is a required property for the hippocampus to play its critical role in learning and memory as well as in emotional and stress regulation, all of which show some level of dysregulation following PAE. Clinical studies support this suggestion by demonstrating that PAE-induced disruptions in hippocampal development is directly associated with problems in memory and learning (Coles, et al., 2011; Gross et al., in press; Willoughby et al., 2008).
Conclusions
Together, the current results indicate that PAE and adolescent CMS induce dynamic alterations in the expression of stress-related receptors in the dorsal and ventral hippocampal formation, and that these alterations manifest differently depending on the age and sex of the animal. Due to the importance of hippocampal MR, GR, and CRHR1 for emotional and stress regulation as well as for learning and memory, the PAE- and adolescent stress-related alterations observed here may underlie some of the hippocampal-dependent behavioral deficits associated with those insults. Finally, the short-term impact of adolescent CMS on the expression of stress-related receptors in the hippocampus differs from the long-term effects, suggesting a possible incubation period for some of the effects of adolescent stress in altering the developmental trajectory of the hippocampus.
Supplementary Material
Acknowledgments
This research was supported by NIH/NIAAA grants R37 AA007789 and R01 AA022460, Kids Brain Health Network (Canadian Networks of Centers of Excellence) grant 20R64153 to JW. We thank all members of the Weinberg laboratory for their assistance. We also thank Dr. Wendy Comeau for her assistance with the animal work and for slicing a subset of brains and Parker J. Holman for help in editing the manuscript.
Grant sponsor: NIH/NIAAA; Grant number: R37 AA007789
Grant sponsor: NIH/NIAAA; Grant number: R01 AA022460
Grant sponsor: Kids Brain Health Network; Grant number: 20R64153
References
- Abel EL, Jacobson S, Sherwin BT. In utero alcohol exposure: Functional and structural brain damage. Neurobehavioral Toxicology and Teratology. 1983;5:363–366. [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LAM. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2011;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety – Insights from human genetic studies. Molecular Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sciences. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Burke MW, Ptito M, Ervin FR, Palmour RM. Hippocampal neuron populations are reduced in Vervet monkeys with fetal alcohol exposure. Developmental Psychobiology. 2015;57:470–485. doi: 10.1002/dev.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Tyler CR, Allan AM. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcoholism: Clinical and Experimental Research. 2014;38:392–400. doi: 10.1111/acer.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Goggin SL, Labrecque MT, Allan AM. The impact of prenatal alcohol exposure on hippocampal-dependent outcome measured is influenced by prenatal and early-life rearing conditions. Alcoholism: Clinical and Experimental Research. 2015;39:631–639. doi: 10.1111/acer.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang ZZ, Zhang S, Zuo W, Chen NH. Does mineralocorticoid receptor play a vital role in the development of depressive disorder? Life Sciences. 2016;152:76–81. doi: 10.1016/j.lfs.2016.03.022. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. European Journal of Neuroscience. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Coles CD, Goldstein FC, Lynch ME, Chen X, Kable JA, Johnson KC, Hu X. Memory and brain volume in adults prenatally exposed to alcohol. Brain and Cognition. 2011;75:67–77. doi: 10.1016/j.bandc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability to addiction. Pharmacology, Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Review Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ. Alcohol exposure in utero is associates with decreased gray matter volume in neonates. Metabolic Brain Disease. 2016;31:81–91. doi: 10.1007/s11011-015-9771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EL, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology and Behavior. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Kane CJ. Fetal alcohol spectrum disorders and neuroimmune changes. International Review of Neurobiology. 2014;118:41–80. doi: 10.1016/B978-0-12-801284-0.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has on anxiogenic action in the social interaction test. Hormones and Behavior. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine CJ, Patten AR, Sickmann HM, Helfer JL, Christie BR. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: Sex, age and methodological considerations. Neuroscience and Biobehavioral Reviews. 2016;64:12–34. doi: 10.1016/j.neubiorev.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: Interactive effects of dietary restriction and schedule feeding. Journal of Nutrition. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and adult neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Research Reviews. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu W, Weinberg J. Effects of prenatal alcohol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: Role of corticosterone. Alcoholism: Clinical and Experimental Research. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Green MR, Nottrodt RE, Simone JJ, McCormick CM. Glucocorticoid receptor translocation and expression of relevant genes in the hippocampus of adolescent and adult male rats. Psychoneuroendocrinology. 2016;73:32–41. doi: 10.1016/j.psyneuen.2016.07.210. [DOI] [PubMed] [Google Scholar]
- Gross LA, Moore EM, Wozniak JR, Coles CD, Kable JA, Sowell ER, Jones KL, Riley EP, Mattson SN, the CIFASD Neural correlates of verbal memory in youth with heavy prenatal alcohol exposure. Brain Imaging and Behavior. doi: 10.1007/s11682-017-9739-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience and Biobehavioral Reviews. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelmann K, Hellemann-Regen J, Wingenfeld K, Kuehl LK, Mews M, Fleischer J, Heuser I, Otte C. Mineralocorticoid receptor function in depressed patients and healthy individuals. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2016;71:183–188. doi: 10.1016/j.pnpbp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Hollis F, Isgor C, Kabbaj M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience. 2013;249:232–241. doi: 10.1016/j.neuroscience.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety – Evidence from clinical studies with CRH1 receptor antagonist. European Journal of Pharmacology. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Hueston CM, Cryan JF, Nolan YM. Stress and adolescent hippocampal neurogenesis: Diet and exercise as cognitive modulators. Translational Psychiatry. 2017;7:e1081. doi: 10.1038/tp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, Duman RS. Differential regulation of corticotropin-releasing factor1 receptor expression by stress and agonist treatments in brain and cultured cells. Molecular Pharmacology. 1996;50:1103–1110. [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihum JT, Chido LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and Psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, DeRijk R, de Kloet ER. The coming out of the brain mineralocorticoid receptor. Trends in Neuroscience. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Karst H, Benger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19204–10207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Welt T, Wigger A, Renner U, Engelmann M, Holsboer F, Lendgraf R. The anxiolytic effect of the CRH1 receptor antagonist R121919 depends on innate emotionality in rats. European Journal of Neuroscience. 2001;13:373–380. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- Kim CK, Yu W, Edin G, Ellis L, Osborn JA, Weinberg J. Chronic intermittent stress does not differentially alter brain corticosteroid receptor densities in rats prenatally exposed to ethanol. Psychoneuroendocrinology. 1999;24:585–611. doi: 10.1016/s0306-4530(99)00015-3. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity, and brain amine periodically. Endocrinology. 1974;95:1195–1202. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KGC, Ellis L, Weinberg J. Exposure to chronic mild stress differentially alters corticotropin-releasing hormone and arginine vasopressin mRNA expression in stress-responsive neurocircuitry of male and female rats prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2015;39:2414–2421. doi: 10.1111/acer.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. Journal of Neuroendocrinology. 2006;18:672–784. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Rivier C. Gender differences in the effects prenatal alcohol exposure on the hypothalamic-pituitary-adrenal axis responses to immune signals. Psychoneuroendocrinology. 1996;21:145–155. doi: 10.1016/0306-4530(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Li C, Liu Y, Yin S, Lu C, Liu D, Jiang H, Pan F. Long-term effect of early adolescent stress: Dysregulation of hypothalamic-pituitary-adrenal axis and central corticotropin releasing factor receptor 1 expression in adult male rats. Behavioural Brain Research. 2015;288:39–49. doi: 10.1016/j.bbr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Zeldin G, Diehl AM. Chronic ethanol consumption induces the production of tumor necrosis factor-α and related cytokines in liver and adipose tissue. Alcoholism: Clinical and Experimental Research. 1998;22:231S–237S. doi: 10.1097/00000374-199805001-00004. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Laing DG. Discrimination of odors from stressed rats by non-stressed rats. Physiology and Behavior. 1980;24:699–704. doi: 10.1016/0031-9384(80)90400-x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: Stress, spines, and CRH. Trends in Neuroscience. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural Brain Research. 2008;187:229–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2010;34:756–765. doi: 10.1016/j.pnpbp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Thomas CM, Sheridan CS, Nixon F, Flynn JA, Mathews IZ. Social instability stress in adolescent male rats alters hippocampal neurogenesis and produces deficits in spatial location memory in adulthood. Hippocampus. 2012;22:1300–1312. doi: 10.1002/hipo.20966. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: Investigation in rodent models. Neuroscience. 2013;249:242–257. doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: Cellular and molecular mechanisms for the effects of stressful experiences. Developmental Neurobiology. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nature Neuroscience. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostalac-Preciado CR, de Gortari P, López-Rubalcava C. Antidepressant-like effects of mineralocorticoid but not glucocorticoid anatagonists in the lateral septum: Interactions with the serotonergic system. Behavioural Brain Research. 2011;223:88–98. doi: 10.1016/j.bbr.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Müller MB, Zimmermann S, Sillaber I, Hagemeyer T, Deussing JM, Timpl P, Kromann MSD, Droste SK, Kühn R, Reul JMHM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediated anxiety-related behaviors and hormonal adaptations to stress. Nature Neuroscience. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcoholism: Clinical and Experimental Research. 2000;24:1084–1092. [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. Journal of Pediatric Psychology. 2006;31:50–64. doi: 10.1093/jpepsy/jsj021. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: The neuroendocrine regulation of puberty: Is the time ripe for a system biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Patten AR, Brocardo PS, Sakiyama C, Wortman RC, Noonan A, Gil-Mohapel J, Christie BR. Impairments in hippocampal synaptic plasticity following prenatal ethanol exposure are dependent on glutathione levels. Hippocampus. 2013;23:1463–1475. doi: 10.1002/hipo.22199. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning a functional expression of a rat brain corticotropin factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Raineki C, Hellemans KGC, Bodnar T, Lavigne KM, Ellis L, Woodward TS, Weinberg J. Neurocircuitry underlying stress and emotional regulation in animals prenatally exposed to alcohol and subjected to chronic mild stress in adulthood. Frontiers in Endocrinology. 2014;5:5. doi: 10.3389/fendo.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Chew L, Mok P, Ellis L, Weinberg J. Short- and long-term effects of stress during adolescence on emotionality and HPA function of animals exposed to alcohol prenatally. Psychoneuroendocrinology. 2016;74:13–23. doi: 10.1016/j.psyneuen.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Bodnar TS, Holman PH, Baglot SL, Lan N, Weinberg J. Effects of early-life adversity on immune function are mediated by prenatal environment: Role of prenatal alcohol exposure. Brain, Behavior, and Immunity. 2017;66:210–220. doi: 10.1016/j.bbi.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Developmental Medicine and Child Neurology. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: An overview. Neuropharmacology Review. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Science. 2004;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsores IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior – Modulation by trait anxiety and corticotropin-releasing factor system. European Journal of Neuroscience. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: Rodent and primate studies. Neuropharmacology Review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Philippsen C, Richter S, Bohringer A, Wippich W, Schachinger H. Stress modulates the use of special versus stimulus-response learning strategies in humans. Learning and Memory. 2007;14:109–116. doi: 10.1101/lm.435807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Schachinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. Journal of Cognitive Neuroscience. 2010;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Tegenthoff M, Höffken O, Wolf OT. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biological Psychiatry. 2013;74:801–808. doi: 10.1016/j.biopsych.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Lan N, Yamashita F, Halpert AG, Viau V, Weinberg J. Effects of prenatal ethanol exposure on regulation of basal hypothalamic-pituitary-adrenal activity and hippocampal 5-HT1A receptor mRNA levels in female rats across the estrous cycle. Psychoneuroendocrinology. 2008;33:1111–1123. doi: 10.1016/j.psyneuen.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwowska JH, Barker JM, Barha CK, Lan N, Weinberg J, Galea LAM. Stress-induced suppression of hippocampal neurogenesis in adult male rats is altered by prenatal ethanol exposure. Stress. 2010;13:302–314. doi: 10.3109/10253890903531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gould LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioural manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Miller AH, Moday H, Stein M, McEwen BS. Diurnal differences in basal and acute stress levels of type I and type II adrenal steroid receptor activation in neural and immune tissues. Endocrinology. 1993;133:1941–1950. doi: 10.1210/endo.133.5.8404640. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Müller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Hormones and Behavior. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Rammes G, Wolf M, Liebl C, Ganea K, Müller MB, Schmidt MV. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental & Behavioral Pediatrics. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Liu SH, Kokka N. Long-term effects of fetal ethanol exposure on pituitary-adrenal response to stress. Pharmacology, Biochemistry and Behavior. 1982;16:585–589. doi: 10.1016/0091-3057(82)90420-8. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JMHM, Stalla GK, Blanquer V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genetics. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plasticity. 2007;71203 doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Frontiers in Behavioral Neuroscience. 2011;5:7. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Pitiot A, Paus T, Sandi C. Stress during puberty boots metabolic activation associated with fear-extinction learning in hippocampus, basal amygdala and cingulate cortex. Neurobiology of Learning and Memory. 2012;98:93–101. doi: 10.1016/j.nlm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Guterman A, Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LAM. Prenatal ethanol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Hormone and Behavior. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Comeau WL, Ellis LA, Galea LAM, Weinberg J. Basal regulation of HPA and dopamine systems is altered differentially in males and females by prenatal alcohol exposure and chronic variable stress. Psychoneuroendocrinology. 2013;38:1953–1966. doi: 10.1016/j.psyneuen.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends in Neuroscience. 2012;35:284–292. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. Journal of Psychiatric Research. 2014;53:1–7. doi: 10.1016/j.jpsychires.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology. 2012;54:523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32:S16–S20. doi: 10.1016/j.psyneuen.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehavioral Toxicology and Teratology. 1984;6:261–269. [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. Journal of Neuroendocrinology. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Hodges CA, Black AC. Prenatal exposure to ethanol alters the organization of hippocampal mossy fibres in rats. Science. 1981;211:957–959. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuopsychological Society. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Kuehl LK, Dziobek I, Roepke S, Otte C, Hinkelmann K. Effects of mineralocorticoid receptor blockade on empathy in patients with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2016;16:902–910. doi: 10.3758/s13415-016-0441-4. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LAM. Alcohol and pregnancy: Effects in maternal care, HAP axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology. 2015;57:37–50. doi: 10.1016/j.psyneuen.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Wick-Carlson D, Packard BA, Morano R, Herman JP. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology. 2016;65:109–117. doi: 10.1016/j.psyneuen.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


