Abstract
Background
Diffuse axonal injury (DAI) on MRI has been associated with poor functional outcome after moderate-severe traumatic brain injury (msTBI). Yet, DAI assessment with highly-sensitive MRI-techniques is unfeasible in the acute trauma setting, and CT remains the key diagnostic modality despite its lower sensitivity. We sought to determine whether CT-defined-hemorrhagic-DAI (hDAI) is associated with discharge and favorable 3-and 12-month functional outcome (Glasgow Outcome Score ≥4) after msTBI.
Methods
We analyzed 361 msTBI patients from the single-center longitudinal OPTIMISM-study collected over six years (11/2009-11/2015) with prospective outcome assessments at 3- and 12-months. Patients with microhemorrhages on CT were designated “CT-hDAI-positive” and those without as “CT-hDAI-negative”. For secondary analyses “CT-hDAI-positive” was stratified into two phenotypes according to presence (“associated”) versus absence (“predominant”) of concomitant large acute traumatic lesions to determine whether presence vs. absence of additional focal mass lesions portends a different prognosis.
Results
Seventy patients (19%) were CT-hDAI-positive (n=36 predominant; n=34 associated hDAI). In univariate analyses, CT-hDAI-positive status was associated with discharge survival (p=0.004) and favorable outcome at 3-months (p=0.003) and 12-months (p=0.005). After multivariable adjustment, CT-hDAI-positivity was no longer associated with discharge survival and functional outcome (all p>0.05). Stratified by hDAI phenotype, predominant hDAI patients had worse trauma severity, longer ICU stays, and more systemic medical complications. Predominant hDAI, but not associated hDAI, was an independent predictor of discharge survival (adjusted OR 24.7; 95% CI 3.2–192.6; p=0.002) and favorable 12-month outcome (adjusted OR 4.7; 95% CI 1.5–15.2; p=0.01). Sensitivity analyses using Cox-regression confirmed this finding for 1-year survival (adjusted HR 5.6; 95% CI 1.3–23; p=0.048).
Conclusion
CT-defined-hDAI was not an independent predictor of unfavorable short- and long-term outcomes and should not be used for acute prognostication in msTBI patients. Predominant hDAI patients had good clinical outcomes when supported to ICU discharge and beyond.
Level of Evidence
Level III; prognostic study.
Keywords: Traumatic brain injury, TBI, diffuse axonal injury, traumatic axonal injury, computed tomography, CT, prognosis, patient outcomes, outcome prognostication, critical care
BACKGROUND
Moderate-to-severe traumatic brain injury (msTBI) is a leading cause of morbidity and mortality after trauma (1). Despite its significant public health burden, outcome prognostication after msTBI remains challenging in part owing to its complex and heterogeneous pathology. The presence of diffuse axonal injury (DAI), which is considered the pathological hallmark of many post-traumatic functional deficits, has been described to be a particularly poor prognostic sign in msTBI in several studies (2–5).
Brain imaging indices of DAI have been shown to correlate with the degree of axonal degeneration on post-mortem histology (2, 6–8). In particular, experimental brain magnetic resonance imaging (MRI) techniques are highly promising for the detailed evaluation of DAI (7, 9). However, although conventional MRI adds prognostic value with superior discrimination (10) compared to computed tomography (CT), (11, 12) it is frequently infeasible in the early acute phases of trauma (10, 13). This is due to patient instability for transport and the requirement to lie flat during the comparably slow image acquisition, increasing the risk for intracranial pressure elevation or hypoxemia with concurrent lung injury.
Therefore, given its widespread availability, rapid image acquisition, and absent contraindications, CT remains the standard diagnostic modality in the early phases of msTBI to evaluate for TBI-related hemorrhagic and non-hemorrhagic injuries (14). Yet, it is well recognized that CT only allows for indirect evaluation of DAI by identifying traumatic vascular injury associated with hemorrhagic DAI (hDAI), and typically underestimates the true extent of axonal injury (8). Considering the relatively low sensitivity of CT for the detection of DAI-related pathology, the presence of any hDAI on CT may be a particularly poor predictor for outcome based on the assumption that the actual, but invisible degree of DAI is much worse (so-called “stealth” pathology) (15). However, whether this supposition holds true in contemporaneous critical trauma care is uncertain. Accordingly, a better understanding of this issue is important because clinicians may provide premature and unduly grave prognoses for patients with CT-defined hDAI, particularly when they are comatose in the first few days after trauma (16–19).
The primary objectives of this study were to describe the prevalence of microhemorrhages on CT as markers of underlying hDAI and determine their association with the 3- and 12-month functional outcome in a contemporaneous and prospectively followed msTBI patient cohort. In secondary analyses we sought to determine whether the presence of traumatic microhemorrhages in isolation (i.e., in the absence of large focal mass lesions) portends a different prognosis than when present in conjunction with other traumatic lesions.
METHODS
Patient population
We conducted a prospective observational study at the University of Massachusetts Medical School (UMASS). It is the only level-1 trauma center in central Massachusetts with a 65-mile catchment area radius. All eligible adult (age ≥18 years without upper age limit) msTBI patients (Glasgow Coma Scale [GCS] ≤12) admitted between November 2009 and November 2015 were enrolled in the ongoing Outcome Prognostication in Traumatic Brain Injury (OPTIMISM) study with inclusion and exclusion criteria previously described in detail (20). Briefly, patients required a diagnosis of TBI based on the history and mechanism of injury, absence of a non-traumatic acute brain lesion on admission non-contrast head CT, and an enrollment GCS ≤12, recorded as the lowest post-resuscitation GCS in the first 24 hours of hospital admission and after sedation or intoxication subsided. Transfers from outside hospitals all occurred from emergency department (ED) to ED prior to admission and were included. There were no transfer requests after patients had already been admitted. This prospective observational study was approved by the local Institutional Review Board (IRB) with written informed consent obtained from the patient or surrogate decision-maker to perform follow-up. For non-survivors as well as those who declined consent for long-term follow-up after discharge, or in whom no surrogate decision-makers were available, the IRB granted a waiver for documentation of written consent for recording clinical data from the index hospitalization. Data were recorded in the Research Electronic Data Capture (REDCap) platform at UMASS (21).
Clinical management
The clinical patient management was described previously in detail (20). Briefly, after initial resuscitation in the ED trauma bay, subjects were admitted to a single closed neuro-trauma intensive care unit staffed by board-certified neuro-intensivists or a trauma-intensivist, and managed according to Brain Trauma Foundation guidelines (22). All patients with a GCS ≤8 and evidence of brain swelling or high-risk lesions received an intracranial pressure (ICP) monitoring device (intraparenchymal probe or extraventricular drain) as well as emergency neurosurgery based on clinical indications (22) and at the discretion of the consulting neurosurgeon. After 2012, additional brain tissue oxygen (PbtO2) monitoring with Licox© (Integra, Plainsboro, NJ) was performed. Both ICP and PbtO2 were treated according to an institutional protocol that mimicked the Brain Tissue Oxygen Monitoring in Traumatic Brain Injury (BOOST-2) protocol (23). Our standard institutional practice is to avoid early prognostication to the family in the first 5–7 days, unless the patient has irreversible brainstem herniation.
Measurements
Baseline demographics, trauma mechanism, pupillary reactivity, and vital signs including presence of hypoxia (oxygen saturation levels ≤89%) or hypotension (systolic blood pressure ≤89 mmHg) in the field or ED were collected. Out-of-hospital and UMASS ED data were obtained from written ED trauma attending and nursing reports. Enrollment GCS was recorded as the lowest post-resuscitation GCS in the first 24 hours of hospital admission and after sedation or intoxication subsided. The Injury Severity Score (ISS) was calculated from AIS scores assigned by an AIS-certified trauma registrar after complete medical record and diagnoses review by three trained AIS trauma registrars using the American College of Surgeons National Trauma Databank data dictionary criteria. Diagnoses, AIS and ISS scores were reviewed and validated weekly during regular group meetings of the trauma attendings at our institution. Patients were followed throughout the intensive care unit (ICU) stay. Consistent with the National Institutes of Health Common Data Elements for TBI and tbi-impact.org recommendations we collected information on race and ethnicity (24, 25). Occurrence of 28 pre-defined medical and neurological complications (20) were adjudicated weekly by three neurointensivists (R.C., W.H., S.M.). ICU complications are defined in the supplemental digital content.
Image analysis and DAI classification
In this study, hDAI was defined as the presence of one or more punctate hemorrhages on CT obtained on admission or the first follow-up CT performed within 24 hours of admission. All CTs were reviewed and adjudicated weekly by consensus between three neurointensivists for the presence of hDAI and other traumatic lesions and determination of the Marshall-CT-classification (11).
To gain a more granular understanding of the association of hDAI with the outcome in the presence versus absence of additional major pathology we also operationally classified hDAI according to three phenotypes (Supplemental Figure I): “predominant hDAI”, defined by the presence of hDAI on CT as the predominant pathology, without any other major large acute space-occupying pathologies exhibiting mass effect (i.e. subdural or epidural hematoma, contusions >10cc volume); “associated hDAI” with presence of other major acute pathologies as above; “CT-hDAI-negative”, with absence of any hDAI, irrespective of the presence of other major pathologies. To allow comparison with prior studies, we also classified hDAI as grade I (grey-white-matter), grade II (corpus callosum), and grade III (brainstem) (2, 26).
One trained physician (M.W.Q.) retrospectively measured the midline shift (MLS) at the septum pellucidum (in mm) and the hematoma volume (in ml) of the largest hematoma on the admission CT using the ABC/2 method (27, 28).
Outcome Assessments
Functional outcomes using the Glasgow Outcome Scale (GOS) were prospectively assessed at 3 and 12 months after trauma over the telephone by trained members of the study team using a standardized interview guide for the GOS (29). For quality control, the study PI conducted data review on randomly selected subjects and supervised phone interviews in person at least quarterly. Survival status was recorded for each patient at discharge, 3 and 12 months. If subjects or their families could not be contacted for follow-up at the respective time points, we searched the publicly available Social Security Death Index to retrospectively determine subjects’ survival status at the respective time follow-up time point.
Statistical analysis
We performed a complete case analysis using χ2 or Fisher’s Exact Test for categorical and Wilcoxon’s test for nonparametric continuous variables, as appropriate, comparing baseline differences between CT-positive-hDAI versus CT-hDAI -negative patients in our primary analysis. In our secondary analysis we compared CT-hDAI -negative patients to predominant and associated hDAI patients. The outcomes of interest were discharge and 1-year survival as well as favorable outcome (GOS 4 and 5) at 3 and 12 months, respectively.
For each outcome of interest we constructed a multivariable logistic regression model to determine whether CT-positive-hDAI status was independently associated with the outcome. For our predefined secondary analysis, we repeated all multivariable analyses stratified by hDAI phenotype. Multivariable logistic regression models were constructed incrementally by manually adding and removing variables due to the large number of univariate associations with the outcomes of interest. First, we included the validated IMPACT variables on admission (age, motor GCS, pupillary reactivity, hypoxia, hypotension, Marshall CT-classification, CT presence of traumatic subarachnoid hemorrhage and epidural mass) (30) and ISS in all multivariable logistic regression models. In order to prevent model overfitting, variables were only retained in the multivariable model if they remained significantly associated with the outcome (p<0.05 using Wald statistic). Next, we added variables to each model, which were significantly associated with the outcome of interest in the univariate analyses. Again, variables remained if they were independently associated with the outcome of interest (p<0.05 using Wald statistic) and resulted in the best model fit assessed by -2Log-likelihood. This resulted in parsimonious multivariable models with different retained variables without overfitting. The area under receiver-operator curves (c-statistics) and Hosmer-Lemeshow goodness of fit p-values were calculated as measures of discrimination and calibration, respectively. We used Kaplan-Meier and log-rank test for univariate comparisons of the 1 year survival between groups. Subjects that were lost to follow up were censored at either at the 90 days (no 3 month follow up) or 365 days follow up (3 month follow up completed but no 1-year follow up) time. To determine whether the CT hDAI status was independently associated with the 1 year survival we constructed Cox proportional hazards models adjusted for pertinent covariates. Variables were sequentially removed (likelihood ratio) from the models at a significance level of 0.1. The final models were adjusted for the ISS, age, sex, degree of midline shift (mm), Marshall CT score, hypotension, and pupillary reactivity. The proportional hazards assumption was assessed and satisfied in all models. Variables were examined for collinearity and interaction. Two-sided significance tests were used throughout and a two-sided p<0.05 was considered statistically significant unless stated otherwise. To calculate corrected levels of significance in cases of multiple comparisons in the univariate analyses we calculated an adjusted significance level after Benjamini and Hochberg (31). Analyses were conducted in SAS 9.4 (SAS Institute Inc., Cary, NC) and IBM SPSS Statistics Version 22 (IBM, Armonk, NY).
RESULTS
Over the 6-year study period, per the local trauma registry a total of 10,580 adult trauma patients were seen at our institution, of which 5917 patients had TBI. Of these, 4529 patients with TBI were admitted to the hospital. A total of 393 msTBI patients met inclusion criteria and were enrolled consecutively in the OPTIMISM-study. Five patients with hypoxic-ischemic brain injury after hanging were excluded as the mechanism of injury is considered different, leaving 388 patients. Twenty-seven patients were further excluded as they were enrolled very early in the OPTIMISM-study before IRB approval for long-term follow-up had been obtained. Finally, 361 patients were available for analysis (Figure 1). Overall, 70 patients (19.4%) were CT-hDAI -positive. Predominant hDAI was present in 10% of study patients (n=36) and associated hDAI in 9% (n=34). Baseline characteristics are summarized in Table 1.
Figure 1. Study flow chart.
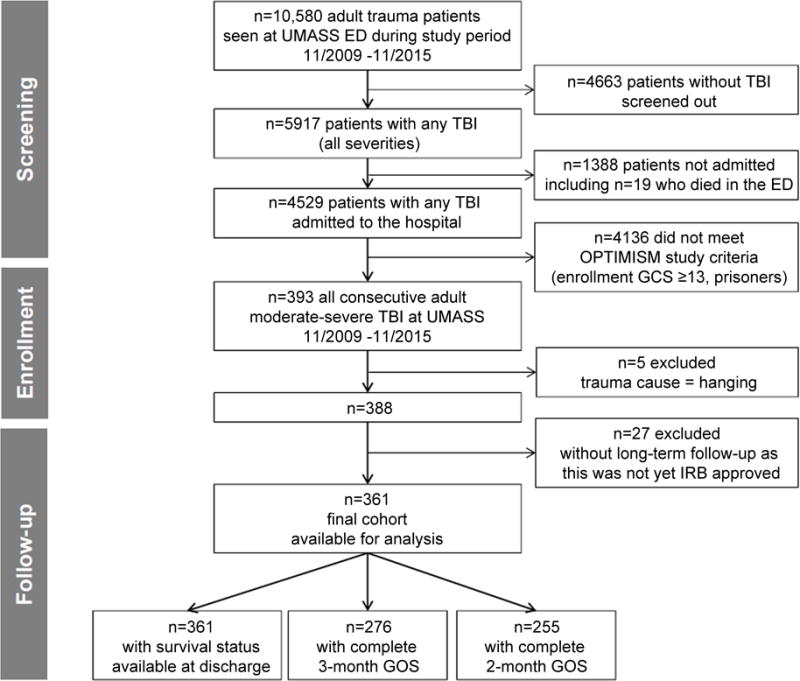
Five patients were excluded due to “hanging” resulting in hypoxic brain injury, which has a distinctly different pathophysiology from TBI. We further excluded 27 patients who had been enrolled in the early part of OPTIMISM prior to IRB approval for post-discharge assessments.
Table 1.
Baseline characteristics
| CT-hDAI-negative (n=291) |
CT-hDAI-positive (n=70) |
P | CT-hDAI-positive | ||||
|---|---|---|---|---|---|---|---|
| Predominant (n=36) |
P | Associated (n=34) |
P | ||||
| Demographics | |||||||
| Age (years) | 56 (36;72) | 32 (24; 52) | <.001 | 30 (24;47) | <.001 | 40 (25;55) | 0.004 |
| Male | 206 (71) | 54 (77) | 0.29 | 28 (78) | 0.44 | 26 (76) | 0.49 |
| Race | 0.22 | 0.92 | 0.07 | ||||
| White | 265 (91) | 64 (91) | 35 (97) | 29 (85) | |||
| Black | 10 (3) | 0 (0) | 0 (0) | 0 (0) | |||
| Asian | 5 (2) | 3 (4) | 0 (0) | 3 (9) | |||
| Other | 11 (4) | 3 (4) | 1 (3) | 2 (6) | |||
| Ethnicity | 1 | 0.49 | 0.51 | ||||
| Latino | 23 (8) | 5 (7) | 1 (3) | 4 (12) | |||
| Non-Latino | 268 (92) | 65 (93) | 35 (97) | 30 (88) | |||
| Trauma cause | <.001 | <.001 | 0.001 | ||||
| High velocity trauma | 94 (32) | 55 (79) | 32 (89) | 23 (68) | |||
| Fall | 156 (54) | 12 (17) | 3 (8) | 9 (26) | |||
| Assault/object hit head | 26 (9) | 3 (4) | 1 (3) | 2 (6) | |||
| Gunshot wound | 15 (5) | 0 (0) | 0 (0) | 0 (0) | |||
| Exam findings | |||||||
| Hypotension | 32 (11) | 15 (21) | 0.02 | 4 (11) | 1 | 11 (32) | <.001 |
| Hypoxia | 19 (7) | 8 (11) | 0.2 | 5 (14) | 0.16 | 3 (9) | 0.71 |
| Pupillary reactivity | 0.57 | 0.63 | 0.05 | ||||
| None | 70 (24) | 21 (30) | 6 (17) | 15 (44) | |||
| One | 26 (9) | 5 (7) | 3 (8) | 2 (6) | |||
| Both | 195 (67) | 44 (63) | 27 (75) | 17 (50) | |||
| mGCS | 0.16 | 0.69 | 0.1 | ||||
| 1. No motor response | 97 (33) | 32 (46) | 14 (39) | 18 (53) | |||
| 2. Extension to pain | 18 (6) | 3 (4) | 1 (3) | 2 (6) | |||
| 3. Flexion to pain | 8 (3) | 1 (1) | 0 (0) | 1 (3) | |||
| 4. Withdrawal from pain | 42 (14) | 9 (13) | 4 (11) | 5 (15) | |||
| 5. Localizing pain | 92 (32) | 23 (33) | 15 (42) | 8 (24) | |||
| 6. Obeys commands | 34 (12) | 2 (3) | 2 (6) | 0 (0) | |||
| ISS | 26 (24;35) | 36 (27; 45) | <.001 | 36 (28;45) | <.001 | 35 (27;43) | <.001 |
| Neuroimaging | |||||||
| Marshall CT score first CT | <.001 | <.001 | 0.19 | ||||
| I. No visible pathology | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| II. Cisterns intact | 160 (55) | 54 (77) | 35 (97) | 19 (56) | |||
| III. Cisterns compressed | 40 (14) | 10 (14) | 1 (3) | 9 (26) | |||
| IV. Midline shift >5 mm | 19 (7) | 1 (1) | 0 (0) | 1 (3) | |||
| V. Surgically evacuated lesion | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| VI. Non-evacuated lesion >25 ml | 72 (25) | 5 (7) | 0 (0) | 5 (15) | |||
| DAI grade I | – | 49 (70) | – | 29 (81) | – | 20 (59) | – |
| DAI grade II | – | 2 (3) | – | 2 (6) | – | 0 (0) | – |
| DAI grade III | – | 19 (27) | – | 5 (14) | – | 14 (41) | – |
| Subdural hematoma | 248 (85) | 44 (63) | <.001 | 15 (42) | <.001 | 29 (85) | 1 |
| Contusion | 160 (55) | 33 (47) | 0.24 | 10 (28) | 0.002 | 23 (68) | 0.16 |
| Epidural hematoma | 15 (5) | 1 (1) | 0.33 | 0 | 0.34 | 1 (3) | 1 |
| Traumatic subarachnoid hemorrhage | 230 (79) | 57 (81) | 0.66 | 28 (78) | 0.83 | 29 (85) | 0.5 |
| Midline shift (mm); median (IQR) | 3 (0;8) | 0 (0;3) | <.001 | 0 (0;0) | <.001 | 1.8 (0;6) | 0.44 |
| Hematoma volume (ml); median (IQR) | 20 (4;67) | 0 (0; 6) | <.001 | 0 (0;1) | <.001 | 4 (0;27) | 0.002 |
| Outcome | |||||||
| Discharge survival | 160 (55) | 52 (74) | 0.003 | 35 (97) | <.001 | 17 (50) | 0.58 |
| Favorable 3-month outcome | 46 (20) | 18 (35) | 0.02 | 12 (52) | <.001 | 6 (21) | 1 |
| Favorable 12-month outcome | 49 (23) | 20 (44) | 0.004 | 15 (75) | <.001 | 5 (20) | 0.81 |
All statistical comparisons were made to CT defined hemorrhagic DAI (hDAI)-negative patients. Midline shift and hematoma volume were assessed on the first available head CT. P-values ≤0.02 (corrected for multiple [n=60] testing) were considered significant and are listed in bold. mGCS indicates motor GCS; ISS= injury severity score; LOS= length of stay. Data are presented as n (%) or median (interquartile range). Data are complete for all included variables.
Compared to CT-hDAI -negative patients, CT-hDAI -positive patients were younger, more severely injured, and were more likely to be hypotensive in the field or emergency room. Expectedly, high-velocity trauma represented the most common trauma cause among patients with hDAI, whereas falls represented the most common trauma cause among CT-hDAI -negative patients. Rates of hypoxia, pupillary reactivity and motor GCS were not significantly different between CT-hDAI-positive and CT-hDAI-negative patients.
Imaging findings
In the CT-hDAI-positive group, DAI grade I was the predominant hDAI grade (70%), followed by DAI grade III (27%) and DAI grade II (3%). DAI grade I remained the prevailing type in both the predominant and associated hDAI groups. Subdural hematomas were significantly less common in the CT-hDAI-positive compared to the CT-hDAI-negative group.
After stratification, there was no difference in lesion prevalence between the associated hDAI and CT-hDAI-negative group, while (by definition) subdural hematomas and contusions were significantly less prevalent in the predominant hDAI vs. CT-hDAI-negative group. Epidural hematomas were rare in our cohort, and no difference in prevalence was found among the groups. MLS and hematoma volumes were greater in CT-hDAI-negative patients.
Outcome
Complete data for outcome analysis was available for all 361 patients at hospital discharge, 276 patients at 3-months, and 255 at 12-months. Overall, 212 (58.7%) patients survived the index hospitalization. 11 (3%) subjects died between hospital discharge and 3 month follow up and 8 (2%) subjects died between 3-months and 12-months follow up. Discharge survival rates were significantly higher among CT-hDAI-positive compared to CT-hDAI-negative patients (74% vs. 55%; p=0.003; Table 2), though this association only approached significance on multivariable logistic regression (Table 2). However, the 1-year risk of death was significantly lower among CT-hDAI-positive subjects as compared to CT-hDAI-negative patients (log rank p=0.002, Figure 2). This association remained significant after adjustment for potential confounders in Cox-regression analysis (HR 1.6; 95% CI 1.004-2.9; p=0.048; Supplemental Table I).
Table 2.
Short- and long-term outcomes stratified by CT-positive hDAI, predominant, and associated hDAI
| Primary analysis CT-positive hDAI | |||||||
|---|---|---|---|---|---|---|---|
| Crude | Adjusted | ||||||
| OR (95% CI) | p | C-statistic | OR (95% CI) | p | C-statistic | H-L p | |
| Discharge survival (n=361) | 2.4 (1.3–4.2) | 0.004 | 0.562 | 2.0 (0.99–3.9)a | 0.06 | 0.761 | 0.061 |
| Favorable 3-month outcome (n=276) | 2.1 (1.1–4.1) | 0.03 | 0.563 | 1.7 (0.8–3.9)a | 0.19 | 0.789 | 0.961 |
| Favorable 12-month outcome (n=255) | 2.6 (1.4–5.1) | 0.005 | 0.578 | 1.6 (0.7–3.4) b | 0.27 | 0.758 | 0.586 |
| Secondary analysis Predominant hDAI | |||||||
| Crude | Adjusted | ||||||
| OR (95% CI) | p | C-statistic | OR (95% CI) | p | C-statistic | H-L p | |
| Discharge survival (n=327) | 28.7 (3.9–211.9) | 0.001 | 0.586 | 24.7 (3.2–192.6)c | 0.002 | 0.773 | 0.71 |
| Favorable 3-month outcome (n=248) | 4.2 (1.8–10.2) | 0.001 | 0.575 | 2.3 (0.9–6.4)b | 0.1 | 0.761 | 0.313 |
| Favorable 12-month outcome (n=230) | 9.9 (3.4–28.5) | <.001 | 0.602 | 4.7 (1.5–15.2)c | 0.01 | 0.769 | 0.336 |
| Associated hDAI | |||||||
| Crude | Adjusted | ||||||
| OR (95% CI) | p | C-statistic | OR (95% CI) | p | C-statistic | H-L p | |
| Discharge survival (n=325) | 0.8 (0.4–1.7) | 0.58 | 0.509 | 1.12 (0.4–2.8)d | 0.96 | 0.772 | 0.072 |
| Favorable 3-month outcome (n=253) | 1.1 (0.4–2.8) | 0.90 | 0.503 | 1.3 (0.4–4.1)d | 0.69 | 0.801 | 0.857 |
| Favorable 12-month outcome (n=235) | 0.8 (0.3–2.3) | 0.71 | 0.509 | 0.5 (0.1–2.1)e | 0.25 | 0.851 | 0.836 |
Multivariable logistic regression models were adjusted for:
age, ISS, hypotension, Marshall CT classification;
age, ISS, and Marshall CT classification;
age, ISS, Marshall CT classification, and presence of contusion;
age, ISS, hypotension, and pupillary reactivity;
age, ISS, hypotension, pupillary reactivity and trauma cause. hDAI denotes hemorrhagic DAI; ISS, Injury Severity Score; H-L, Hosmer-Lemeshow; OR, odds ratio; CI, confidence interval. CT-hDAI negative patients served as reference group. A Hosmer-Lemeshow p-value of >0.05 indicates good model calibration.
Figure 2. Survival analysis for the one-year risk of death stratified by CT-hDAI status.
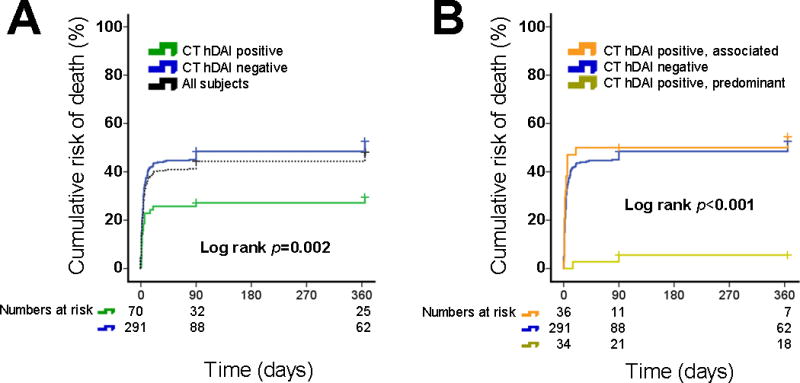
Shown are the Kaplan-Meier curves for the 1-year cumulative risk of death stratified by (A) absence vs. presence of CT-hDAI, and (B) CT-hDAI phenotype. Dotted line in panel A indicates the cumulative absolute risk of death in all included subjects (n=361) for reference only.
Similarly, a favorable functional outcome was significantly more common among CT-hDAI-positive patients compared CT-hDAI-negative patients at 3-months (35% vs. 20%; p=0.02) and 12-months (44% vs. 23%; p=0.004; Figure 3). However, hDAI was no longer significantly associated with a favorable 3- and 12-month outcome after multivariable adjustment (Table 2). Nevertheless, in these adjusted analyses, lower age, lower ISS, lower Marshall CT-score and lack of hypotension in the field or ED were all independently associated with a favorable outcome at 3 months (all p<0.01, except ISS p=0.01 and Marshall CT-score p=0.02; Supplemental Table II). At 12 months, hypotension in the field and ISS were no longer significantly associated with the outcome, but we retained ISS in the model to adjust for other injuries. Lower age and lower Marshall CT score remained significantly associated with 12-month favorable outcome (both p<0.01; Supplemental Table II).
Figure 3. Outcomes stratified by CT-defined DAI status.
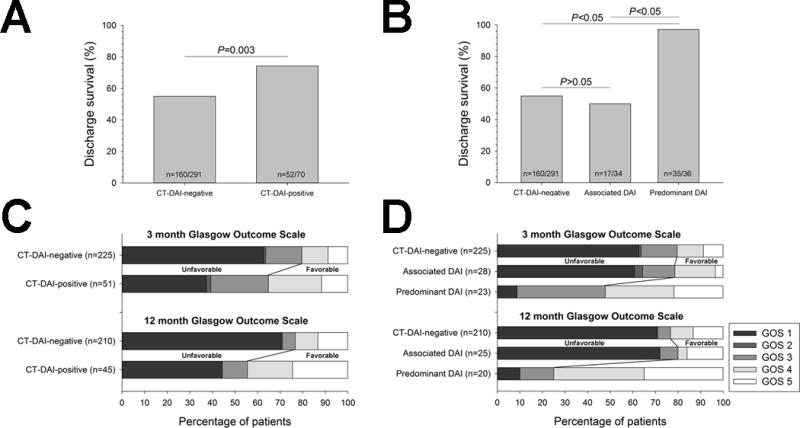
Proportion of patients with (A) discharge survival as well as (B) Glasgow Outcome Scale (GOS) distribution at respective 3- and 12-months stratified by absence versus presence of CT-defined hDAI in the primary analyses. Secondary analyses of the (C) discharge survival and (D) GOS distribution at respective 3- and 12-month outcomes further stratified by hDAI phenotype. Statistical comparisons of in-hospital survival were performed with χ2-test with post-hoc Bonferroni correction.
To better understand the clinical relevance of CT-defined hDAI in the absence or presence of any major acute space-occupying lesions, we compared CT-hDAI-negative with CT-hDAI-positive patients stratified by predominant versus associated hDAI. We found that only predominant CT-hDAI, but not associated CT-hDAI and CT-hDAI-negative status were associated with the 1 year survival in both unadjusted (log rank p<0.001, Figure 2) as well as adjusted analyses (Cox-regression; HR 5.6; 95% CI 1.3-23; p=0.018; Supplemental Table I). Likewise, favorable outcomes at discharge, 3 and 12 months were all significantly more common among patients with predominant hDAI compared to CT-hDAI-negative patients (all p<0.001), while there was no difference in any outcomes in associated hDAI vs. CT-hDAI-negative patients (Table 1). After multivariable adjustment, predominant hDAI remained independently associated with discharge survival and a favorable 12-month outcome (Table 2). Although the direction of association between predominant hDAI and outcome was similar for the 3-month GOS, the confidence intervals included one (Table 2). Associated hDAI was not associated with at the 3-month and 12-month outcomes (Table 2). We were unable to adjust for DAI grading in any of the models, due to the absence of any hDAI in CT-hDAI-negative patients by definition, resulting in quasi-complete separation of data points when including DAI grading in the logistic regression.
ICU course and complications
Next, we sought to determine whether our unexpected observation of a more favorable outcome among patients with predominant hDAI may have been related to differences in the incidence of key pre-defined ICU complications as collected in the OPTIMISM study (20). Table 3 summarizes the ICU length-of-stay and ICU complications stratified by CT-defined hDAI phenotypes.
Table 3.
ICU length-of-stay and ICU complications
| ICU complication | CT-hDAI-negative (n=291) |
CT-hDAI-positive (n=70) |
P | CT-hDAI-positive | |||
|---|---|---|---|---|---|---|---|
| Predominant (n=34) |
P | Associated (n=36) |
P | ||||
| ICU LOS (days) | 7 (2;15) | 16 (6; 24) | <.001 | 19 (11;24) | <.001 | 15 (2;24) | 0.09 |
| Herniation | 138 (47) | 20 (29) | 0.004 | 1 (3) | <.001 | 19 (56) | 0.35 |
| Brain edema requiring osmotherapy | 124 (43) | 33 (47) | 0.5 | 9 (25) | 0.04 | 24 (71) | 0.002 |
| Intracranial pressure crisis | 55 (57) | 25 (61) | 0.64 | 8 (47) | 0.46 | 17 (71) | 0.21 |
| Ischemic stroke | 29 (10) | 6 (9) | 0.83 | 0 (0) | 0.05 | 6 (18) | 0.17 |
| Seizure | 35 (12) | 3 (4) | 0.08 | 1 (3) | 0.15 | 2 (6) | 0.4 |
| Hyperglycemia | 235 (81) | 60 (86) | 0.34 | 28 (78) | 0.67 | 32 (94) | 0.06 |
| Pneumonia | 111 (38) | 38 (54) | 0.01 | 21 (58) | 0.02 | 17 (34) | 0.18 |
| Acute respiratory distress syndrome | 28 (6) | 11 (16) | 0.009 | 4 (11) | 0.28 | 7 (21) | 0.003 |
| Sepsis | 76 (28) | 29 (48) | 0.004 | 19 (58) | <0.001 | 10 (36) | 0.42 |
| Hypotension requiring vasopressors | 131 (45) | 38 (54) | 0.16 | 13 (36) | 0.31 | 25 (74) | 0.002 |
| Cardiac arrest in ICU | 24 (8) | 2 (3) | 0.19 | 0 (00 | 0.09 | 2 (6) | 1 |
| Fever | 180 (62) | 56 (80) | 0.004 | 33 (92) | <0.001 | 23 (68) | 0.51 |
| Acute renal failure | 27 (9) | 5 (7) | 0.81 | 1 (3) | 0.34 | 4 (12) | 0.55 |
P-values ≤0.02 (corrected for multiple [n=42] testing) were considered significant and are listed in bold. All comparisons were made against CT-hDAI-negative patients. Data are presented as median (IQR) or n (%). Data are complete for all included variables.
Overall, CT-hDAI-positive patients had significantly longer median ICU lengths-of-stay than CT-hDAI-negative patients (16 vs. 7 days; p<0.001), which was largely related to particularly long ICU lengths-of-stay of predominant hDAI patients (19 days). We found that CT-hDAI-positive patients suffered significantly more frequently systemic ICU complications, including pneumonia, acute respiratory distress syndrome, sepsis, and fever. Conversely, brain herniation occurred less frequently in CT-hDAI-positive compared to CT-hDAI-negative patients (29% vs. 47%; p=0.004). Additional stratification revealed that brain herniation was significantly less common in the predominant hDAI group (p<0.001) and similar among associated hDAI and CT-hDAI-negative patients (p=0.35). Likewise, compared to CT-hDAI-negative patients, brain edema requiring osmotherapy occurred more frequently in the associated hDAI group (71% vs. 43%; p=0.002), but less frequently in the predominant hDAI group (25% vs. 43%; p=0.04) as compared to CT-hDAI-negative patients.
DISCUSSION
The most important finding of our study was that in contrast to previous investigations (10, 32, 33) the presence of hDAI on head CT was not associated with a worse functional outcome. This observation was supported by additional analyses stratified by the hDAI phenotype. These analyses suggest that neither patients with predominant hDAI nor patients with associated hDAI had particularly worse outcomes than CT-hDAI-negative patients. Indeed, predominant hDAI was independently associated with the discharge and 1 year survival as well as a favorable outcome at 12-months. Our findings were surprising, and if validated, challenge the notion among clinicians, especially non-TBI specialists, as well as the portrayal in media content for non-specialists, that the presence of hDAI, at least when seen on CT in msTBI patients, is a universally poor prognostic sign (34).
The reasons for the lack of an association of hDAI with poor outcome in our study are probably multifactorial. However, the most likely explanation may be that the presence of other large intracranial mass lesions superseded the impact of hDAI on outcome as indicated by more frequent catastrophic neurological complications such as brain herniation in patients with mass lesions. In our cohort, associated hDAI was most often accompanied by SDH, a combination that has been correlated with a particularly grim outlook in prior studies (3, 35). Additional reasons may relate to the fact that the OPTIMISM-study does not include detailed neuropsychological follow-up assessments, which is necessary to ascertain possible long-term adverse cognitive effects of hDAI. Another likely explanation relates to differences in our definition of hDAI based on CT versus MRI and the inability of CT to provide sufficient discrimination of DAI lesion burden and location compared to MRI.
However, this last issue directly relates to the original motivation to conduct our study. We specifically chose CT to classify hDAI in order to mirror clinical practice at trauma centers. Although MRI is clearly superior to CT in identifying DAI, it remains unfeasible and impractical in the early phases of trauma for the commonly poly-traumatized msTBI patients. Yet, families desire a prognostic estimation from the treating physicians from the early phase onward. A large degree of variability in the prognostication and mortality of severe TBI patients between and within trauma centers has been described (16–18). Studies have shown that physicians form an opinion about the “Gestalt” of the patient’s injury severity during the early period of neurologically injured and comatose patients. This may result in forming an early fixed negative opinion and framing of a poor picture by the treating physician when discussing the presumed prognosis with patients’ families, which may lead to unwarranted premature decision-making, including withdrawal of care (16, 36).
While it is undisputed that MRI is much more sensitive to determining the presence and extent of DAI, our study closes an important knowledge gap for clinicians because it reflects the real life practice of using CT as the sole imaging modality to provide insights regarding expected outcomes in the early post-traumatic phase. In addition, it is important to remember that MRI-based DAI studies commonly suffer from ascertainment bias, as only survivors after initial decision-making are included and undergo an MRI; hence the results from MRI-based studies may not fully translate to all patients in the first days after trauma (10, 13, 33).
An additional important finding from our study was that patients with predominant hDAI had a prolonged ICU course and suffered systemic medical complications; yet they had lower rates of devastating neurologic complications and their outcome was not significantly worse than that of head-hCT-negative patients. In fact, despite the high injury severity and prolonged ICU course our data suggests that the presence of a hDAI predominant phenotype was associated with overall improved survival and more frequent favorable 12-month outcome among msTBI patients. This reinforces that clinicians should be particularly cautious in rendering early predictions using CT-based hDAI indices.
Study strengths and limitations
Important study strengths included the analysis of a large, contemporaneous, prospectively followed cohort of msTBI patients. In addition, we adhered to a rigorous, and pre-specified, CT- and ICU complications classification method to minimize bias and misclassification.
This study has limitations that deserve comment. First, as stated, it was our goal to determine the impact of hDAI as defined by CT, which is the clinically most relevant imaging modality for acute brain injury assessment in msTBI. However, the diagnosis of DAI can only be made with certainty by histologic examination of brain tissue at post-mortem(15). Therefore, by design, it is likely that we underestimated the true extent of DAI and, thus, patients in the CT-hDAI-negative group may have been misclassified. Conventional MRI, DTI MRI, and post-mortem pathology have previously identified non-hemorrhagic DAI (10, 37–39), which CT does not capture. Nevertheless, even when discovered on MRI, non-hemorrhagic DAI has not always been found to predict a poor outcome (32). Nonetheless, further research is warranted to determine whether CT-based hDAI lesion burden and pattern analysis may afford additional predictive insight. For example, the majority of the hDAI lesions in our predominant hDAI cohort were DAI grade 1 lesions limited to lobar white matter or cerebellum, rather than deep lesions as mostly seen in the associated hDAI group, which may explain the association of favorable outcome in the predominant hDAI group. Based on our simplified definition of the hDAI phenotypes it was not possible to include the DAI-grade as a marker of hDAI lesion burden and location into the logistic regression models because it would have resulted in quasi-complete separation of data points. Irrespective of these imaging based limitations, this study represents one of only few pragmatic DAI outcome studies based on acute CT. Therefore, until MRI (including advanced and highly sensitive techniques such as DTI) becomes a widespread standard modality for acute brain assessment in msTBI patients, our results provide critical insight given that CT remains the mainstay of imaging evaluation during the acute phases of trauma when clinicians are first asked to prognosticate. A second limitation includes that outcome data was incomplete. However, our follow-up rates align with other observational msTBI studies (40) and are reflective of poor compliance in trauma patients in general. Further, by employing Kaplan-Meier and Cox-regression analyses we accounted for missing follow-up data in the survival analyses. Nevertheless, due to the single-center design with limited racial diversity, our findings may not be generalizable to other msTBI cohorts. Accordingly, our findings require careful interpretation and validation in other TBI cohorts.
In summary, we found that the presence of hDAI on CT was not associated with worse short- and long-term outcome compared to CT-hDAI-negative patients. Further, predominant hDAI was independently associated with increased survival and improved 12-month outcomes, despite high injury severity and prolonged ICU lengths-of-stay. If validated, our results highlight the importance of avoiding acute unfavorable prognostication in msTBI patients based on CT-hDAI-indices. In particular, patients with hDAI and without major other intracranial pathology may have favorable outcomes that justify continued support through a prolonged ICU course until rehabilitation.
Supplementary Material
Supplemental Figure I. Representative head CTs demonstrating predominant and associated hemorrhagic diffuse axonal injury (hDAI). White arrows identify foci of hDAI in (A) predominant hDAI and (B) associated hDAI. Black arrows identify other main lesions in associated hDAI, such as contusions in this example.
Supplemental Table I. Cox regression models for prediction of 1-year survival by the CT hDAI status. *Adjusted for ISS, age, sex, degree of midline shift (mL), Marshall CT score, hypotension, and pupillary reactivity. ‡CT hemorrhagic DAI (hDAI) negative status served a reference group.
Supplemental Table II. Point estimates of the multivariable logistic regression models comparing CT-positive hemorrhagic DAI (hDAI) to CT-negative hDAI for the discharge survival as well as 3 and 12 month favorable outcome (defined as Glasgow Outcome Score ≥4). P<0.05 was considered statistically significant. CT-negative DAI is the reference group. *Hosmer-Lemeshow goodness of fit p=0.061; C-Statistic 0.761. †Hosmer-Lemeshow goodness of fit p=0.961; C-Statistic 0.789. ‡Hosmer-Lemeshow goodness of fit p=0.586; C-Statistic 0.758.
Acknowledgments
We thank Iryna Nieto (study coordinator) for her help with recruiting patients, collecting data and performing follow-up phone calls.
STUDY FUNDING:
Dr. Henninger is supported by 5K08NS091499 from the NIH/NINDS. Dr. Muehlschlegel is supported by NIH/NICHD grant 5K23HD080971. This project was supported by the University of Massachusetts Medical School Center for Clinical and Translational Science which is funded by the NIH Clinical and Translational Science Award to the University of Massachusetts Medical School (UL1TR000161).
Footnotes
DISCLOSURES:
Dr. Henninger serves on the advisory board of Omniox, Inc.
PRIOR PRESENTATION:
This study has been presented in part as a poster abstract at the 13th Annual Meeting of the Neurocritical Care Society, 10-09-2015 in Scottsdale, AZ.
AUTHOR CONTRIBUTIONS
NH was involved in data collection, data analysis, data interpretation, drafting of the manuscript and critical revision of the manuscript for important intellectual content. RAC was involved in the study design, data analysis, data interpretation, drafting of the manuscript. MWK, RC and WH were involved in data collection and critical revision of the manuscript for important intellectual content. SM was involved in the study design, data collection, data analysis, data interpretation, drafting of the manuscript and critical revision of the manuscript for important intellectual content.
Supplemental methods
ICU complications
References
- 1.Center for Disease Control. Traumatic Brain Injury Statistics: Center for Disease Control. 2016 [updated January 22, 2016 April 7, 2017]. Available from: http://www.cdc.gov/traumaticbraininjury/statistics.html.
- 2.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15(1):49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 3.Chelly H, Chaari A, Daoud E, Dammak H, Medhioub F, Mnif J, Hamida CB, Bahloul M, Bouaziz M. Diffuse axonal injury in patients with head injuries: an epidemiologic and prognosis study of 124 cases. J Trauma. 2011;71(4):838–46. doi: 10.1097/TA.0b013e3182127baa. [DOI] [PubMed] [Google Scholar]
- 4.Gentleman SM, Roberts GW, Gennarelli TA, Maxwell WL, Adams JH, Kerr S, Graham DI. Axonal injury: a universal consequence of fatal closed head injury? Acta Neuropathol. 1995;89(6):537–43. doi: 10.1007/BF00571509. [DOI] [PubMed] [Google Scholar]
- 5.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113(3):556–63. doi: 10.3171/2009.9.JNS09626. [DOI] [PubMed] [Google Scholar]
- 6.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18(4):307–16. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Tu TW, Williams RA, Lescher JD, Jikaria N, Turtzo LC, Frank JA. Radiological-pathological correlation of diffusion tensor and magnetization transfer imaging in a closed head traumatic brain injury model. Ann Neurol. 2016;79(6):907–20. doi: 10.1002/ana.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones NR, Blumbergs PC, Brown CJ, McLean AJ, Manavis J, Perrett LV, Sandhu A, Scott G, Simpson DA. Correlation of postmortem MRI and CT appearances with neuropathology in brain trauma: a comparison of two methods. J Clin Neurosci. 1998;5(1):73–9. doi: 10.1016/s0967-5868(98)90207-7. [DOI] [PubMed] [Google Scholar]
- 9.Haberg AK, Olsen A, Moen KG, Schirmer-Mikalsen K, Visser E, Finnanger TG, Evensen KA, Skandsen T, Vik A, Eikenes L. White matter microstructure in chronic moderate-to-severe traumatic brain injury: Impact of acute-phase injury-related variables and associations with outcome measures. J Neurosci Res. 2015;93(7):1109–26. doi: 10.1002/jnr.23534. [DOI] [PubMed] [Google Scholar]
- 10.Moen KG, Brezova V, Skandsen T, Haberg AK, Folvik M, Vik A. Traumatic axonal injury: the prognostic value of lesion load in corpus callosum, brain stem, and thalamus in different magnetic resonance imaging sequences. J Neurotrauma. 2014;31(17):1486–96. doi: 10.1089/neu.2013.3258. [DOI] [PubMed] [Google Scholar]
- 11.Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–92. [PubMed] [Google Scholar]
- 12.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. discussion –82. [DOI] [PubMed] [Google Scholar]
- 13.Moen KG, Skandsen T, Folvik M, Brezova V, Kvistad KA, Rydland J, Manley GT, Vik A. A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(12):1193–200. doi: 10.1136/jnnp-2012-302644. [DOI] [PubMed] [Google Scholar]
- 14.Provenzale JM. Imaging of traumatic brain injury: a review of the recent medical literature. AJR Am J Roentgenol. 2010;194(1):16–9. doi: 10.2214/AJR.09.3687. [DOI] [PubMed] [Google Scholar]
- 15.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit Care. 2013;19(3):347–63. doi: 10.1007/s12028-013-9925-z. [DOI] [PubMed] [Google Scholar]
- 17.Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L, Zygun DA, Bernard F, Meade MO, Dung TC, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581–8. doi: 10.1503/cmaj.101786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turgeon AF, Lauzier F, Burns KE, Meade MO, Scales DC, Zarychanski R, Moore L, Zygun DA, McIntyre LA, Kanji S, et al. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Crit Care Med. 2013;41(4):1086–93. doi: 10.1097/CCM.0b013e318275d046. [DOI] [PubMed] [Google Scholar]
- 19.Lobato RD, Cordobes F, Rivas JJ, de la Fuente M, Montero A, Barcena A, Perez C, Cabrera A, Lamas E. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg. 1983;59(5):762–74. doi: 10.3171/jns.1983.59.5.0762. [DOI] [PubMed] [Google Scholar]
- 20.Muehlschlegel S, Carandang R, Ouillette C, Hall W, Anderson F, Goldberg R. Frequency and impact of intensive care unit complications on moderate-severe traumatic brain injury: early results of the Outcome Prognostication in Traumatic Brain Injury (OPTIMISM) Study. Neurocrit Care. 2013;18(3):318–31. doi: 10.1007/s12028-013-9817-2. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 23.Lin CM, Lin MC, Huang SJ, Chang CK, Chao DP, Lui TN, Ma HI, Liu MY, Chung WY, Shih YH, et al. A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. Biomed Res Int. 2015;2015:529580. doi: 10.1155/2015/529580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath R, Raser KJ, Hajimohammadreza I, Wang KK. Thapsigargin induces apoptosis in SH-SY5Y neuroblastoma cells and cerebrocortical cultures. Biochem Mol Biol Int. 1997;43(1):197–205. doi: 10.1080/15216549700203971. [DOI] [PubMed] [Google Scholar]
- 25.IMPACT-collaborators. IMPACT TBI. [updated 20168/9/2017]. Available from: http://www.tbi-impact.org/.
- 26.Gentry LR. Imaging of closed head injury. Radiology. 1994;191(1):1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- 27.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–5. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 28.Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, Topol EJ, Mahaffey KW. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29(9):1799–801. doi: 10.1161/01.str.29.9.1799. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 30.Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165. discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 32.Paterakis K, Karantanas AH, Komnos A, Volikas Z. Outcome of patients with diffuse axonal injury: the significance and prognostic value of MRI in the acute phase. J Trauma. 2000;49(6):1071–5. doi: 10.1097/00005373-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Hilario A, Ramos A, Millan JM, Salvador E, Gomez PA, Cicuendez M, Diez-Lobato R, Lagares A. Severe traumatic head injury: prognostic value of brain stem injuries detected at MRI. AJNR Am J Neuroradiol. 2012;33(10):1925–31. doi: 10.3174/ajnr.A3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasserman JR. Diffuse Axonal Injury Imaging. 2016 [updated September 23, 2016April 7, 2017]. Available from: http://emedicine.medscape.com/article/339912-overview.
- 35.Cordobes F, Lobato RD, Rivas JJ, Cabrera A, Sarabia M, Castro S, Cisneros C, Torres ID, Lamas E. Post-traumatic diffuse axonal brain injury. Analysis of 78 patients studied with computed tomography. Acta Neurochir (Wien) 1986;81(1–2):27–35. doi: 10.1007/BF01456261. [DOI] [PubMed] [Google Scholar]
- 36.Ong CJ, Dhand A, Diringer MN. Early Withdrawal Decision-Making in Patients with Coma After Cardiac Arrest: A Qualitative Study of Intensive Care Clinicians. Neurocrit Care. 2016;25(2):258–65. doi: 10.1007/s12028-016-0275-5. [DOI] [PubMed] [Google Scholar]
- 37.Newcombe VF, Williams GB, Nortje J, Bradley PG, Harding SG, Smielewski P, Coles JP, Maiya B, Gillard JH, Hutchinson PJ, et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg. 2007;21(4):340–8. doi: 10.1080/02688690701400882. [DOI] [PubMed] [Google Scholar]
- 38.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil. 2001;82(10):1461–71. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 39.Ezaki Y, Tsutsumi K, Morikawa M, Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol. 2006;47(7):733–40. doi: 10.1080/02841850600771486. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Adam O, Rivet DJ, Flaherty S, et al. Outcome Trends Following US Military Concussive Traumatic Brain Injury. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I. Representative head CTs demonstrating predominant and associated hemorrhagic diffuse axonal injury (hDAI). White arrows identify foci of hDAI in (A) predominant hDAI and (B) associated hDAI. Black arrows identify other main lesions in associated hDAI, such as contusions in this example.
Supplemental Table I. Cox regression models for prediction of 1-year survival by the CT hDAI status. *Adjusted for ISS, age, sex, degree of midline shift (mL), Marshall CT score, hypotension, and pupillary reactivity. ‡CT hemorrhagic DAI (hDAI) negative status served a reference group.
Supplemental Table II. Point estimates of the multivariable logistic regression models comparing CT-positive hemorrhagic DAI (hDAI) to CT-negative hDAI for the discharge survival as well as 3 and 12 month favorable outcome (defined as Glasgow Outcome Score ≥4). P<0.05 was considered statistically significant. CT-negative DAI is the reference group. *Hosmer-Lemeshow goodness of fit p=0.061; C-Statistic 0.761. †Hosmer-Lemeshow goodness of fit p=0.961; C-Statistic 0.789. ‡Hosmer-Lemeshow goodness of fit p=0.586; C-Statistic 0.758.


