Abstract
Despite long-standing recognition of the importance of T cells in systemic sclerosis (SSc; scleroderma), the role of CD8+ T cells in disease pathogenesis has not been well studied. Our work has shown that over-production of the pro-fibrotic cytokine IL-13 by peripheral blood effector/memory CD8+ T cells is critical for predisposing patients to more severe forms of cutaneous fibrosis. Moreover, IL-13-producing CD8+ T cells induce a pro-fibrotic phenotype in normal and SSc dermal fibroblasts, and exhibit a strong cytotoxic activity ex vivo. We also found that CD8+ T cells are predominantly abundant in the skin lesions of patients in the early stages of diffuse cutaneous (dc)SSc compare to late-stage disease patients. Isolation of CD8+ T cells from the lesional skin of early active dcSSc patients, established that they are skin-resident, express cytolytic molecules and co-express extremely high levels of IL-13 and IFNγ. Other recent studies corroborate these findings and together strongly suggest that CD8+ T cells contribute to SSc pathogenesis through the production of high levels of cytokines with proinflammatory and pro-fibrotic function as well as by exhibiting a cytotoxic activity.
Keywords: CD8+ T cells, systemic sclerosis, cytokines, human, cytotoxicity, fibrosis
1-Introduction
Systemic sclerosis (SSc; scleroderma) is a highly debilitating connective tissue disease, characterized by inflammation, vasculopathy, and cutaneous and visceral fibrosis [1]. SSc is very heterogeneous in its clinical presentation, extent and severity of skin and internal organ involvement [2,3]. Patients are characterized into two main clinical subsets on the basis of the degree of cutaneous fibrosis and patterns of organ involvement [2–4]: diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc). Rapidly progressive fibrosis of the skin, lungs, and other internal organs characterizes dcSSc, while vascular manifestations, with generally mild skin and internal organ fibrosis are the most characteristic features of lcSSc. The complexity of SSc and its variable clinical course prevent a full understanding of disease pathogenesis. Tissue fibrosis is the most prominent clinical manifestation of SSc and results from the interaction of immune mediators and other growth factors with responsive tissue resident fibroblasts, leading in increased deposition of extracellular matrix proteins in affected tissues [5,6]. Macrophages and T lymphocytes represent the predominant cell types of the inflammatory infiltrate in the fibrotic tissues of patients [7–9] and produce cytokines that contribute to the inflammatory and fibrotic processes of SSc [10–12].
2-Detection of CD8+ T cells in affected SSc tissue
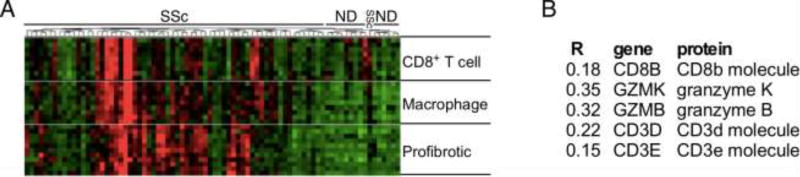
Despite the importance of T cells in SSc pathogenesis being well established, the phenotype and function of specific T-cell subsets involved in the different phases of the disease are not well defined. Multiple studies implicate CD4+ T-cell subsets such as Th1, Th17, Th22 in the inflammatory phase of SSc, while Th2 cells were shown to contribute to tissue fibrosis [13–20], dysregulation of T regulatory cells has also been observed [17]. However, few studies have focused on CD8+ T cells in SSc. Genome-wide expression profiling of skin biopsies from distinct scleroderma subsets previously identified expression of genes associated with CD8+ T cells (e.g: CD8A, GraK, GraH, and GraB) in lesional skin from the “inflammatory” subset of scleroderma patients [21,22]. This subgroup contained biopsies from patients with dcSSc, lcSSc, and localized scleroderma, which were characterized by a unified gene expression signature indicative of an early inflammatory response [22]. A CD8+ T-cell signature in SSc skin was confirmed by more recent genome-wide gene expression profiling [23,24]. Microarray data showing specifically the relationship between pro-fibrotic, macrophage, and CD8+ T-cell gene expression in SSc skin is depicted in Figure 1A. The correlation between CD8+ T-cell gene expression in SSc skin samples and skin thickness, measured by modified Rodnan Skin Score (MRSS) [25], is reported in Figure 1B. By immunohistochemical analyses of T-cell infiltrates in the lesional skin of dcSSc patients at different disease-stages, we showed that CD8+ T cells are predominant in the early stage (disease duration less than 3 years) [2] of SSc compared to CD4+ T cells [26], while more CD4+ lymphocytes are found in late-stage disease (disease duration more than 6 years) [2], suggesting that CD8+ T cells contribute to the initial phase of SSc while CD4+ T cells to its maintenance. High numbers of CD8+ T cells were also found in the skin of patients with active localized scleroderma [26,27]. In contrast, only scant numbers of CD8+ T cells were found in normal skin, matched for anatomical site [26,28]. Other studies report on an increased frequency of CD8+ compared to CD4+ T cells in bronchoalveolar lavage fluid [29] and lungs [30] of SSc patients with pulmonary fibrosis. Moreover, CD8+ T cells in lung and peripheral blood of SSc patients, present an activated phenotype [31,32] and antigen-driven oligoclonal expansion [33–35]. Although antigen-specificity is not yet known, previous studies reported the presence of Topoisomerase-1-specific CD8+ T-cell responses in patients with SSc [36]. Thus, oligoclonal CD8+ T cells are numerous in the affected tissues of patients with active, inflammatory SSc and likely contribute to the pathogenesis of the disease.
Figure 1. CD8+ T-cell gene expression signature in SSc skin.
Unsupervised clustering of microarray data [23,71]. A hierarchical relationship is suggested between CD8+ T cells driving macrophage driving pro-fibrotic gene expression. (G) CD8+ T cell correlations between gene expression and MRSS (R). (Data courtesy of Dr. R. Lafyatis).
3-Phenotype of SSc CD8+ T-cell subsets
Determination of cell surface expression of CD45RA and CD27 by multi-color flow cytometry allows identification of human CD8+ T-cell subsets [37]. We found that peripheral blood SSc CD8+ T cells are characterized by an increased proportion of effector (CD8+CD45RA+CD27−) and effector/memory (CD8+CD45RA−CD27−) cells compared to healthy controls [38]. Similar results were obtained by other groups [39,40]. In these studies, CD8+ T-cell subsets were identified by cell surface expression of CD62L and CCR7 [41,42]. Strikingly, we recently showed that CD8+ T cells in the lesional skin of patients with early active dcSSc uniformely present an effector/memory phenotype [28]. While we found an increased frequency of circulating SSc CD8+ T cells that express skin-homing receptors such as CLA and CCR10 [26], we demonstrated that CD8+ T cells in the lesional skin of dcSSc patients with active disease are skin-resident, as demonstrated by expression of the early acute activation marker CD69 and the αE integrin CD103 [28]. However, only few cells expressed CD103, such as skin-resident T cells confined to normal dermis [43]. This phenotype was further confirmed by immunohistochemical analysis of affected skin of dcSSc patients, showing that CD8+ T cells were restricted to the lower dermis and sub-dermal fat. In contrast, CD8+ T cells in peripheral blood were uniformly negative for CD69 and CD103 expression. While skin-resident memory T cells provide rapid in situ protection against most common pathogens [44,45], their dysregulation can contribute to autoimmune and inflammatory skin diseases [46], such as SSc.
4-Role of CD8+CD28− T cells in SSc
We [28] and others [19] found an increased frequency of CD8+CD28− T cells in the peripheral blood of patients with SSc. Loss of CD28 expression by human CD8+ T cells occurs with age and during chronic inflammatory conditions [47,48]. We demonstrated that CD8+CD28− T-cell expansion in blood and lesional skin of SSc patients is independent of patient age and correlates with the extent of skin fibrosis, an early and specific manifestation of SSc. Human CD8+CD28− T cells are defined as antigen-specific, oligoclonally expanded, terminally differentiated senescent T cells [49]. They exhibit functional heterogeneity, ranging from immunosuppressive to effector. Our studies indicate that circulating and skin-resident SSc CD8+CD28− T cells present an effector/memory phenotype and are cytotoxic [28]. Conversely, it has also been reported that peripheral blood SSc CD8+CD28− T cells may exert an in vitro suppressor activity [19]. While we could not detect any production of immunosuppressive cytokines such as IL-10 and TGFβ by ex vivo SSc CD8+CD28− lymphocytes [28], recent in vitro studies by Negrini et al. suggest that abnormal expression of CD39 and CD127 molecules, may impair maturation of SSc CD8+ Treg from their CD8+CD28− precursors [50]. Although the existence and relevance of such an inhibitory subset has yet to be examined in vivo, the cytotoxic and the suppressor subsets can both coexist as functional heterogeneity in CD8+CD28− T cells has been previously shown [47].
Persistent common viral infections, such as human cytomegalovirus (hCMV), are implicated in the expansion of the CD8+CD28− T-cell population with age [47,51]. Previous studies have shown that latent hCMV infection may contribute to progression of SSc through its ability to infect endothelial cells [52]. Additional evidence for the association between hCMV and SSc comes from the prevalence of anti-hCMV antibodies in patients affected by the disease [53]. In the absence of any medical indication of hCMV infection, we did not test for it. However, we performed preliminary experiment in several SSc patients and found no production of effector cytokines such as IFNγ by SSc CD8+CD28− lymphocytes when they were stimulated in vitro with hCMV pp65–recombinant protein, an immunodominant target of CD8+ T-cell responses to hCMV [54] (data not shown). While these studies suggest that CD8+CD28− T-cell accumulation is a response to a yet unidentified antigen, more in-depth studies are necessary do establish the exact role of hCMV or other chronic infections in SSc CD8+CD28− expansions.
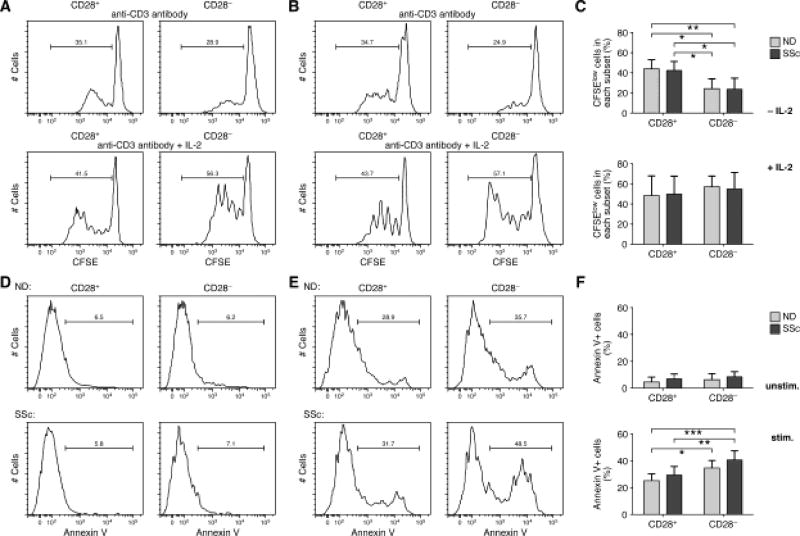
Multiple reports claim that CD8+CD28− T cells present features of cellular senescence such as shortened telomeres, reduced proliferation, and resistance to apoptosis [55–57]. Increasing evidence, however, indicates that this subpopulation is highly heterogeneous regarding its proliferative and apoptotic potential [47,58]. In order to establish whether SSc CD8+CD28− T cells presented features of cellular senescence, we analyzed their proliferative and apoptotic capacities. CFSE-labeled freshly isolated CD8+ T cells from SSc patients and age-matched controls were stimulated by anti-CD3 antibody with or without exogenous IL-2 and the dilution of the dye was studied in subsets of T cells by flow cytometry. As previously observed [47], we found that CD8+CD28− lymphocytes have a limited proliferative ability when stimulated by anti-CD3 alone over 5 days of culture. In contrast, CD8+CD28− cells proliferate at a comparable rate to their CD8+CD28+ counterparts in response to IL-2 (Figure 2A–C). Interestingly, we found that the proliferation rate of SSc CD8+CD28− T cells is comparable to that of age-matched healthy controls. Similar results were obtained when cells were stimulated with anti-CD3 and IL-15 [59] (data not shown). This is in agreement with recent studies showing that CD8+CD28− T cells are able to proliferate under certain conditions, such as in the presence of IL-2 [47] or IL-15 [59] and/or in response to specific co-stimulatory signals, such as OX40, 4-1BB, ICOS [58–60]. Significantly, high levels of IL-15 have been found in the serum of SSc patients [61], and we observed an increased expression of IL-15Rα by SSc CD8+CD28− T cells (data not shown). Additionally, we also found that SSc CD8+CD28− cells up-regulate OX-40 and 4-1BB expression on their surface (data not shown), suggesting that multiple factors in patients may contribute to their proliferation.
Figure 2. SSc CD8+CD28− T cells can proliferate and are susceptible to apoptosis.
Proliferation of CD8+CD28+/− T-cell subsets was determined by carboxyfluorescein diacetate succinimidyl ester assay (CFSE; Molecular Probes) (A–C). Freshly isolated CD8+CD28+/− T-cell subsets from patients and age-matched normal controls were analyzed for CSFE dilution profiles after 5 days stimulation with anti-CD3 mAb with or without IL-2. The cellular division capacity was tested by flow cytometry and analyzed by FlowJo software (Tree Star). The values of each histogram show the percentage of divided cells (CFSElow) and undivided cells (CFSEhigh). A representative experiment from a control (A) or a patient (B) is shown. (C) Mean percentage of CFSElow cells±SD in each CD8+ T-cell subset from 8 SSc patients and 8 NDs is shown. Statistics by ANOVA followed by post hoc Tukey's test. (D–E) Susceptibility to apoptosis of CD8+CD28+/− T-cell subsets was evaluated by Annexin V staining of unstimulated (D) and stimulated (E) with anti-CD3 mAb and IL-2 for 48 hours cells. Cells were stained with Annexin V-FITC and propidium iodide (PI, eBioscience) and analyzed by flow cytometry. Doublet cells were excluded from the CD3+CD8+ population and then the percentage of Annexin V+ cells was analyzed. Annexin V-positive, PI-negative cells were considered apoptotic. Representative examples are shown. (F) Mean percentage of apoptotic cells in each subset from 8 SSc patients and 8 age-matched healthy controls. Statistics by ANOVA followed by post hoc Tukey's test.
Another important feature of senescent cells is their resistance to apoptosis, leading to their progressive accumulation throughout a lifetime [47,62] and during autoimmune conditions [62]. Susceptibility to apoptosis of SSc CD8+CD28− T cells was analyzed by Annexin V uptake by apoptotic cells in freshly isolated cells and after 48 hours stimulation with immobilized anti-CD3 and IL-2. Results are depicted in Figure 2D–F and show that unstimulated CD8+CD28−/+ subsets from patients and controls exhibit a limited spontaneous apoptosis. In contrast, we detected discrete peaks of cells that showed Annexin V binding at high level after stimulation. The percentages of apoptotic cells in the CD28− subsets of patients and controls were significantly higher compared to their CD28+ counterparts after stimulation. Although we observed that SSc CD28− cells had a higher proportion of apoptotic cells compared to ND CD28− cells, this did not reach statistical significance.
Thus, our findings indicate that SSc CD8+CD28− lymphocytes can proliferate under certain stimulatory conditions and are susceptible to apoptosis, a key feature of cytotoxic T lymphocytes that die after elaborating their effector functions. In addition, while a study performed in patients with SSc and their family members reported shortened telomeres [63], controversy remains as to whether telomere shortening alone contributes to immune senescence [64]. Therefore, it appears that SSc CD8+CD28− lymphocytes are not truly senescent cells since they are able to proliferate and undergo apoptosis, but rather they represent a mixed population of highly antigen-experienced effector CD8+ T cells at different levels of differentiation [47]. Only those CD8+ CD28− T cells that become late-differentiated begin to gain properties of true cellular senescence, such as altered proliferative capacity and acquisition of resistance to apoptosis, and eventually become truly senescent.
In conclusion, SSc CD8+ T cells from the blood and skin of patients with active disease exhibit an effector/memory phenotype and an increased ability to migrate to site of inflammation. Importantly, we identified a specific subset of skin-resident CD8+ T cells in the lesional skin of dcSSc patients that likely play a critical role in the early stage of SSc skin disease.
5-Effector function of SSc CD8+ T cells
It was shown by us [28] and others [19] that peripheral blood SSc CD8+ T cells produce a variety of pro-inflammatory cytokines and expressed chemokines receptors not found in CD8+ T cells from healthy controls, which likely facilitate their migration to inflamed tissues. We established that dysregulated production of the pro-fibrotic cytokine IL-13 by peripheral blood effector SSc CD8+ T cells correlates with the extent of cutaneous fibrosis, while it is not observed in healthy controls or patients with other inflammatory conditions [38]. We found that also SSc CD4+ T cells produce high levels of IL-13 but to a lesser extent and more variable compared to SSc CD8+ T cells, indicating that IL-13 over-production is more a feature of CD8+ rather than CD4+ T cells in SSc [38]. Furthermore, we demonstrated that high numbers of CD8+IL-13+ cells are present in the sclerotic skin of patients, particularly in the early inflammatory stages of SSc [26,28]. We recently showed that skin-resident CD8+CD45RA−CD27-CD28− T cells isolated from the affected skin of dcSSc patients with active disease, co-express extremely high levels of IL-13 and IFNγ compared to circulating CD8+ T cells from the same patients [28]. Moreover, we demonstrated that IL-13 produced by SSc CD8+ T cells induce a pro-fibrotic phenotype in normal and SSc dermal fibroblasts [26,28]. IL-13 exerts its function by signaling through the receptor IL-13Rα1, which is highly expressed by fibroblasts and mononuclear cells in the skin of patients with early dcSSc [26], followed by activation of the Signal Transducer and Activator of Transcription (STAT-6) [26,65]. In parallel, IL-13 can also activate tissue-resident macrophages that in turn will produce TGFβ with pro-fibrotic activity [66]. IFNγ plays an essential role in the development and severity of autoimmune diseases [67], including SSc [13,17]. While the mechanism by which IFNγ contributes to SSc remains unclear, its critical role in Th1 cell differentiation that results in macrophage activation, inflammation and tissue damage [68,69] likely impacts disease severity. The effects of IFNγ on fibrosis are complex and although it is a well-documented anti-fibrotic agent in a variety of murine models of fibrosis, it has not been shown to be clinically beneficial in IPF [70]. Additionally, it was previously shown that IFN-regulated genes (both type I and type II) are elevated in both the skin and lungs of SSc patients [71,72]. Besides IL-13 and IFNγ, Klein et al. reported that peripheral blood SSc CD8+ T cells also produce other type-2 pro-fibrotic cytokines such as IL-4 and IL-5 [39,73] and IL-9 [39] as well as high levels of pro-inflammatory cytokines, such as TNFα [39] and IL-17 [39], which contribute to the inflammatory condition of SSc. Interestingly, CD8+ T cells from the bronchealveolar lavage fluid of SSc patients with lung disease mainly present a type-2 cytokine gene signature or a mixed type-1 and type-2 cytokine production phenotype, while in BAL fluids from healthy individuals CD8+ T cells preferentially expressed IFNγ mRNA with no or little type-2 cytokine mRNA [74], and this finding was correlated with the development of lung fibrosis [74].
Chemokine receptor expression mediate mononuclear blood cell migration to secondary lymphoid organs and into inflamed tissues [75]. Indeed, chemokine receptor ligands are highly expressed in SSc tissues [76,77]. We found that CCR4 and CCR10 expression confers increased skin-homing abilities to SSc CD8+ T cells. Strikingly, the functional ligand for CCR10, chemokine CCL27, was found highly expressed in the serum and mRNA from sclerotic skin of SSc patients [78], likely facilitating migration of CD8+ T cells to SSc skin lesions. Moreover, Klein et al. showed that specific patterns of chemokine receptor expression by SSc CD8+ T cells correlate with distinct cytokine profiles [39], with type-2 cytokines production negatively correlating with expression of chemokine receptors typical of type-1 responses such as CXCR3, CCR5 and CCR6, and IL-17 and IL-9 production correlating with expression of CD161 and CCR6 [39]. A recent DNA microarray study of effector/memory SSc CD8+T cells and healthy controls isolated from blood, showed that genes related to immune response and cell adhesion are differently expressed in patient samples compared to healthy controls [40]. In particular, expression of CD226, which is associated with both functions, was higher on SSc CD8+ T cells and correlated with the severity of skin and lung fibrosis [40]. Moreover, SSc CD8+CD226+ cells exhibited an increased IL-13 production and a cytotoxic activity against HUVECs [40]. Likewise, we demonstrated that blood and skin effector/memory SSc CD8+ T cells not only express cytolytic molecules such as perforin and Granzyme B [28], but also up-regulate cell-surface expression of CD107a upon activation [28,79], a marker of cytotoxic T lymphocyte degranulation, and exhibit a strong ex vivo cytolytic activity against allogeneic SSc fibroblasts [28]. Earlier studies showed that cytotoxic T lymphocyte-mediated killing using GraB represents a source of autoantigens in systemic autoimmune diseases [80] and, interestingly, self-protein fragments [81] generated by GraB are recognized by autoantibodies in a subset of SSc patients [80]. Furthermore, inhibition of endothelial cell growth in SSc by vascular injury appeared to be mediated by granzyme B and perforin [81]. Finally, CD8+ T cell cytotoxicity induces apoptosis of target cells [40], which may also contribute to fibrosis [82,83]. Thus, CD8+ T cells appear to participate directly in tissue-specific SSc pathogenesis by exerting multiple effector functions, including pro-inflammatory, pro-fibrotic and cytotoxic.
6-Conclusions
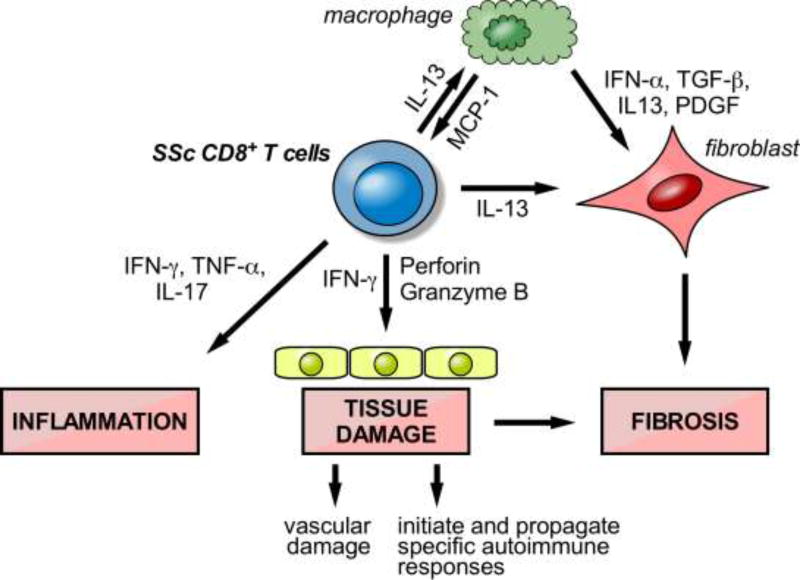
While better known for their involvement in type-1 immune responses and their ability to kill bacteria- or viral-infected cells, several studies reveal that CD8+ T cells have a much wider range of functions, including important roles in type-2-driven immune responses. Effector CD8+ T cells were shown to be the source of IL-13 in a mouse model of airway hyper-responsiveness and airway inflammation [84]. Moreover, IL-4- and IL-13-producing CD8+ T cells have been found in a number of human diseases including COPD and asthma [84–86]. Likewise, we and others demonstrated that CD8+ T cells producing type-2 cytokines are numerous in the affected tissues of patients with active SSc. In particular, we identified a specific subset of skin-resident, effector/memory CD8+ T cells in the lesional skin of patients with early active dcSSc that produce high levels of pro-fibrotic IL-13 and exhibit a potent cytotoxic activity ex vivo. These compelling results in the context of a robust framework of prior studies strongly suggest that CD8+ T lymphocytes are implicated in SSc pathogenesis by exerting a pro-inflammatory and pro-fibrotic activity as well as by inducing cytotoxic tissue damage. A model is proposed in Figure 3. This basic mechanistic knowledge is essential for approaching the treatment of SSc given the paucity of therapeutic options to reverse or even slow progression of this disease. Further, the existing therapies, such as immunoablation and stem cell transplant, and cyclophosphamide, lead to broad killing of immune cells and consequent toxicities, including death. Identifying the precise cell-type(s) and immune mechanism(s) driving SSc pathogenesis provides a framework for developing innovative therapies that selectively target the aberrant immune response, resulting in better efficacy and lower toxicity.
Figure 3. Model for the role of CD8+ T cells in SSc pathogenesis.
The schematic illustrates a mechanism through which tissue-resident CD8+ T cells, activated in response to antigen-driven stimulation, produce cytokines that contribute to inflammation and promote overproduction of collagen by fibroblasts which results in excessive fibrosis. The activation of cytotoxic, cell-mediated pathways may be involved in early vascular damage, and potentially in initiating and propagating a specific autoimmune response. CD8-induced apoptosis of target cells may also contribute to fibrosis.
Highlights.
CD8+ T cells are found numerous in the affected tissues of patients with active SSc;
SSc CD8+ T cells produce cytokines with pro-inflammatory and pro-fibrotic function;
SSc CD8+ T cells exhibit a strong ex vivo cytotoxic activity.
Acknowledgments
Funding This work was supported by National Institutes of Health /National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R03 AR065755 and University of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author declares that no competing financial interests or conflicts exist.
Bibliography
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. The New England journal of medicine. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheumatic diseases clinics of North America. 2003;29:255–273. vi. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 3.Medsger TA., Jr . Classification, Prognosis. In: Clements PJ, Furst DE, editors. Systemic Sclerosis. Philadelphia: Lippincott Williams & Williams; 2004. pp. 17–28. [Google Scholar]
- 4.Medsger TA, Jr, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42–46. [PubMed] [Google Scholar]
- 5.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37:11–25. doi: 10.1111/j.1346-8138.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 7.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–263. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmajer R, Perlish JS, Reeves JR. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977;20:975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- 9.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27:645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 10.Chizzolini C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Current opinion in rheumatology. 2008;20:707–712. doi: 10.1097/BOR.0b013e32830c45ae. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pendergrass SA, Lemaire R, Francis IP, Mahoney JM, Lafyatis R, Whitfield ML. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J Invest Dermatol. 2012;132:1363–1373. doi: 10.1038/jid.2011.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii H, Hasegawa M, Takehara K, Mukaida N, Sato S. Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clinical and experimental immunology. 2002;130:548–556. doi: 10.1046/j.1365-2249.2002.02017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truchetet ME, Brembilla NC, Montanari E, Lonati P, Raschi E, Zeni S, et al. Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheum. 2013;65:1347–1356. doi: 10.1002/art.37860. [DOI] [PubMed] [Google Scholar]
- 15.Truchetet ME, Brembilla NC, Montanari E, Allanore Y, Chizzolini C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13:R166. doi: 10.1186/ar3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brembilla NC, Montanari E, Truchetet ME, Raschi E, Meroni P, Chizzolini C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res Ther. 2013;15:R151. doi: 10.1186/ar4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radstake TR, van Bon L, Broen J, Hussiani A, Hesselstrand R, Wuttge DM, et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PLoS One. 2009;4:e5903. doi: 10.1371/journal.pone.0005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraut J, Michel L, Verrecchia F, Farge D. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmun Rev. 2010;10:65–73. doi: 10.1016/j.autrev.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Fenoglio D, Battaglia F, Parodi A, Stringara S, Negrini S, Panico N, et al. Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin Immunol. 2011;139:249–257. doi: 10.1016/j.clim.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology. 2012;51:1540–1549. doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt MB, Sargent JL, Farina G, Tsang K, Lafyatis R, Glimcher LH, et al. Interspecies Comparison of Human and Murine Scleroderma Reveals IL-13 and CCL2 as Disease Subset-Specific Targets. The American journal of pathology. 2012;180:1080–1094. doi: 10.1016/j.ajpath.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice LM, Stifano G, Ziemek J, Lafyatis R. Local skin gene expression reflects both local and systemic skin disease in patients with systemic sclerosis. Rheumatology. 2015 doi: 10.1093/rheumatology/kev335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan P, Silman A, Black C, Bernstein R, Coppock J, Maddison P, et al. Reliability of skin involvement measures in scleroderma. The UK Scleroderma Study Group. Br J Rheumatol. 1992;31:457–460. doi: 10.1093/rheumatology/31.7.457. [DOI] [PubMed] [Google Scholar]
- 26.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA., Jr Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum. 2013;65:236–246. doi: 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Zhang X, Wakasugi S, Makino T, Inoue Y, Ihn H. Immunohistochemical characterization of the cellular infiltrate in localized scleroderma. Int J Dermatol. 2008;47:438–442. doi: 10.1111/j.1365-4632.2008.03615.x. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Larregina AT, Domsic RT, Stolz DB, Medsger TA, Jr, Lafyatis R, et al. Skin-Resident Effector Memory CD8+CD28− T Cells Exhibit a Profibrotic Phenotype in Patients with Systemic Sclerosis. J Invest Dermatol. 2017;137:1042–1050. doi: 10.1016/j.jid.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yurovsky VV, Wigley FM, Wise RA, White B. Skewing of the CD8+ T-cell repertoire in the lungs of patients with systemic sclerosis. Hum Immunol. 1996;48:84–97. doi: 10.1016/0198-8859(96)00091-2. [DOI] [PubMed] [Google Scholar]
- 30.Luzina IG, Todd NW, Nacu N, Lockatell V, Choi J, Hummers LK, et al. Regulation of pulmonary inflammation and fibrosis through expression of integrins alphaVbeta3 and alphaVbeta5 on pulmonary T lymphocytes. Arthritis Rheum. 2009;60:1530–1539. doi: 10.1002/art.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luzina IG, Atamas SP, Wise R, Wigley FM, Choi J, Xiao HQ, et al. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 2003;48:2262–2274. doi: 10.1002/art.11080. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson R, Totterman TH, Klareskog L, Hallgren R. Increase in activated T cells and reduction in suppressor inducer T cells in systemic sclerosis. Ann Rheum Dis. 1990;49:40–45. doi: 10.1136/ard.49.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lympany PA, Southcott AM, Welsh KI, Black CM, Boylston AW, du Bois RM. T-cell receptor gene usage in patients with fibrosing alveolitis and control subjects. Eur J Clin Invest. 1999;29:173–181. doi: 10.1046/j.1365-2362.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 34.Tiev KP, Abriol J, Burland MC, Antonelli D, Klatzmann D, Cabane J, et al. T cell repertoire in patients with stable scleroderma. Clinical and experimental immunology. 2005;139:348–354. doi: 10.1111/j.1365-2249.2004.02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakkas LI, Platsoucas CD. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004;50:1721–1733. doi: 10.1002/art.20315. [DOI] [PubMed] [Google Scholar]
- 36.Boin F, Wigley FM, Schneck JP, Oelke M, Rosen A. Evaluation of topoisomerase-1-specific CD8+ T-cell response in systemic sclerosis. Ann N Y Acad Sci. 2005;1062:137–145. doi: 10.1196/annals.1358.016. [DOI] [PubMed] [Google Scholar]
- 37.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 38.Fuschiotti P, Medsger TA, Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60:1119–1128. doi: 10.1002/art.24432. [DOI] [PubMed] [Google Scholar]
- 39.Klein M, Schmalzing M, Almanzar G, Benoit S, Hamm H, Tony HP, et al. Contribution of CD8+ T cells to inflammatory cytokine production in systemic sclerosis (SSc) Autoimmunity. 2016;49:532–546. doi: 10.1080/08916934.2016.1217997. [DOI] [PubMed] [Google Scholar]
- 40.Ayano M, Tsukamoto H, Kohno K, Ueda N, Tanaka A, Mitoma H, et al. Increased CD226 Expression on CD8+ T Cells Is Associated with Upregulated Cytokine Production and Endothelial Cell Injury in Patients with Systemic Sclerosis. J Immunol. 2015;195:892–900. doi: 10.4049/jimmunol.1403046. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. 2015;7:279ra239. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 45.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7:269rv261. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 50.Negrini S, Fenoglio D, Parodi A, Kalli F, Battaglia F, Nasi G, et al. Phenotypic Alterations Involved in CD8+ Treg Impairment in Systemic Sclerosis. Frontiers in immunology. 2017;8:18. doi: 10.3389/fimmu.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallace DL, Masters JE, De Lara CM, Henson SM, Worth A, Zhang Y, et al. Human cytomegalovirus-specific CD8(+) T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects. Immunology. 2011;132:27–38. doi: 10.1111/j.1365-2567.2010.03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lunardi C, Bason C, Navone R, Millo E, Damonte G, Corrocher R, et al. Systemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cells. Nat Med. 2000;6:1183–1186. doi: 10.1038/80533. [DOI] [PubMed] [Google Scholar]
- 53.Neidhart M, Kuchen S, Distler O, Bruhlmann P, Michel BA, Gay RE, et al. Increased serum levels of antibodies against human cytomegalovirus and prevalence of autoantibodies in systemic sclerosis. Arthritis Rheum. 1999;42:389–392. doi: 10.1002/1529-0131(199902)42:2<389::AID-ANR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 54.Kern F, Bunde T, Faulhaber N, Kiecker F, Khatamzas E, Rudawski IM, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis. 2002;185:1709–1716. doi: 10.1086/340637. [DOI] [PubMed] [Google Scholar]
- 55.Dvergsten JA, Mueller RG, Griffin P, Abedin S, Pishko A, Michel JJ, et al. Premature cell senescence and T cell receptor-independent activation of CD8+ T cells in juvenile idiopathic arthritis. Arthritis Rheum. 2013;65:2201–2210. doi: 10.1002/art.38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Posnett DN, Edinger JW, Manavalan JS, Irwin C, Marodon G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28− cytotoxic effector clones. Int Immunol. 1999;11:229–241. doi: 10.1093/intimm/11.2.229. [DOI] [PubMed] [Google Scholar]
- 57.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–644. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 58.Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, et al. The loss of telomerase activity in highly differentiated CD8+CD28−CD27− T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178:7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 59.Chiu WK, Fann M, Weng NP. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J Immunol. 2006;177:7802–7810. doi: 10.4049/jimmunol.177.11.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stockl J, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wuttge DM, Wildt M, Geborek P, Wollheim FA, Scheja A, Akesson A. Serum IL-15 in patients with early systemic sclerosis: a potential novel marker of lung disease. Arthritis Res Ther. 2007;9:R85. doi: 10.1186/ar2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Artlett CM, Black CM, Briggs DC, Stevens CO, Welsh KI. Telomere reduction in scleroderma patients: a possible cause for chromosomal instability. Br J Rheumatol. 1996;35:732–737. doi: 10.1093/rheumatology/35.8.732. [DOI] [PubMed] [Google Scholar]
- 64.Weng NP. Telomeres and immune competency. Curr Opin Immunol. 2012;24:470–475. doi: 10.1016/j.coi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Reilly S, Ciechomska M, Fullard N, Przyborski S, van Laar JM. IL-13 mediates collagen deposition via STAT6 and microRNA-135b: a role for epigenetics. Sci Rep. 2016;6:25066. doi: 10.1038/srep25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-gamma and systemic autoimmunity. Discovery medicine. 2013;16:123–131. [PMC free article] [PubMed] [Google Scholar]
- 68.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 70.King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 71.Rice LM, Ziemek J, Stratton EA, McLaughlin SR, Padilla CM, Mathes AL, et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis & rheumatology. 2015;67:3004–3015. doi: 10.1002/art.39287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis & rheumatology. 2014;66:714–725. doi: 10.1002/art.38288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuschiotti P. CD8(+) T cells in systemic sclerosis. Immunol Res. 2011;50:188–194. doi: 10.1007/s12026-011-8222-1. [DOI] [PubMed] [Google Scholar]
- 74.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–1178. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 75.Bryant VL, Slade CA. Chemokines, their receptors and human disease: the good, the bad and the itchy. Immunol Cell Biol. 2015;93:364–371. doi: 10.1038/icb.2015.23. [DOI] [PubMed] [Google Scholar]
- 76.Tao J, Li L, Tan Z, Li Y, Yang J, Tian F, et al. Up-regulation of CC chemokine ligand 20 and its receptor CCR6 in the lesional skin of early systemic sclerosis. Eur J Dermatol. 2011;21:731–736. doi: 10.1684/ejd.2011.1469. [DOI] [PubMed] [Google Scholar]
- 77.Distler JH, Akhmetshina A, Schett G, Distler O. Monocyte chemoattractant proteins in the pathogenesis of systemic sclerosis. Rheumatology. 2009;48:98–103. doi: 10.1093/rheumatology/ken401. [DOI] [PubMed] [Google Scholar]
- 78.Hayakawa I, Hasegawa M, Matsushita T, Yanaba K, Kodera M, Komura K, et al. Increased cutaneous T-cell-attracting chemokine levels in sera from patients with systemic sclerosis. Rheumatology. 2005;44:873–878. doi: 10.1093/rheumatology/keh625. [DOI] [PubMed] [Google Scholar]
- 79.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 80.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kahaleh MB, Fan PS. Mechanism of serum-mediated endothelial injury in scleroderma: identification of a granular enzyme in scleroderma skin and sera. Clin Immunol Immunopathol. 1997;83:32–40. doi: 10.1006/clin.1996.4322. [DOI] [PubMed] [Google Scholar]
- 82.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. The European respiratory journal. 2008;32:1631–1638. doi: 10.1183/09031936.00176807. [DOI] [PubMed] [Google Scholar]
- 83.Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 2013;27:3893–3901. doi: 10.1096/fj.12-214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 85.Makris D, Lazarou S, Alexandrakis M, Kourelis TV, Tzanakis N, Kyriakou D, et al. Tc2 response at the onset of COPD exacerbations. Chest. 2008;134:483–488. doi: 10.1378/chest.07-2626. [DOI] [PubMed] [Google Scholar]
- 86.Betts RJ, Kemeny DM. CD8+ T cells in asthma: friend or foe? Pharmacol Ther. 2009;121:123–131. doi: 10.1016/j.pharmthera.2008.09.001. [DOI] [PubMed] [Google Scholar]