Abstract
Background
Transforming growth factor -β (TGF-β) acts as a tumor suppressor in normal epithelial cells but as a tumor promoter in advanced prostate cancer cells. PI3-kinase pathway mediates TGF-β effects on prostate cancer cell migration and invasion. PTEN inhibits PI3-kinase pathway and is frequently mutated in prostate cancers. We investigated possible role(s) of PTEN in TGF-β effects on proliferation and migration in prostate cancer cells.
Methods
Expression of PTEN mRNA and proteins were determined using RT-PCR and western blotting in RWPE-1 and DU145 cells. We also studied the role of PTEN in TGFβ effects on cell proliferation and migration in DU145 cells after transient silencing of endogenous PTEN. Conversely, we determined the role of PTEN in cell proliferation and migration after over-expression of PTEN in PC3 cells which lack endogenous PTEN.
Results
TGF-β1 and TGF-β3 had no effect on PTEN mRNA levels but both isoforms increased PTEN protein levels in DU145 and RWPE1 cells indicating that PTEN may mediate TGF-β effects on cell proliferation. Knockdown of PTEN in DU145 cells resulted in significant increase in cell proliferation which was not affected by TGF-β isoforms. PTEN overexpression in PC3 cells inhibited cell proliferation. Knockdown of endogenous PTEN enhanced cell migration in DU145 cells, whereas PTEN overexpression reduced migration in PC3 cells and reduced phosphorylation of AKT in response to TGF-β.
Conclusion
We conclude that PTEN plays a role in inhibitory effects of TGFβ on cell proliferation whereas its absence may enhance TGF-β effects on activation of PI3-kinase pathway and cell migration.
Keywords: PTEN, TGF-β, Cell Proliferation, Migration, PI3-Kinase, Prostate Cancer
Introduction
Prostate Cancer is the second leading cause of cancer deaths among men especially in the United States and in all racial groups 1, and the fourth most common tumor type worldwide 2. Major processes and molecular events are believed to represent important contributing factors in prostate carcinogenesis and have been associated with possible roles in cancer initiation and progression. Of these molecular mechanisms, tumor suppressor genes, which normally inhibit cell growth and prevent tumor formation, become functionally inactive in cancer cells through various mechanisms in the process of tumorigenesis. Insights into the molecular function of tumor suppressors could aid in understanding pathways and barriers that prevent the process of tumor progression 3. Phosphatase and tensin homolog (PTEN), a tumor suppressor gene, is frequently mutated in a variety of human cancers including prostate cancer4,5. PTEN negatively regulates the PI3-kinase pathway, a critical oncogenic signaling pathway, which regulates diverse cellular processes such as survival, proliferation, growth and cell migration. Several ligands acting through different receptors activate PI3-kinase which then converts PIP2 to PIP3. 6 AKT (also known as protein kinase B), an important downstream effector of PIP3 is then recruited to the membrane via PIP3 and is phosphorylated by phosphoinositide dependent protein kinase-1 (PDK1) at threonine 308. Mammalian target of rapamycin complex 2 (mTORC2) has been shown to be involved in phosphorylating AKT at serine 473 which, along with phosphorylation at threonine 308 by PDK1, is required for full activation of AKT which in turn activates mTORC1 complex6,7. PTEN negatively regulates the PI3-kinase/AKT/mTOR pathway by dephosphorylating PIP3 to PIP2 and directly opposes the action of PI3-kinase. PTEN has a crucial role in controlling cell death, cell proliferation, cell migration, and considerable evidences indicate that PTEN loss of function results in up-regulation of the PI3-kinase/AKT/mTOR signaling pathway in prostate cancer, primarily through the activation of AKT 8–11. Activating mutations of the PI3-kinase pathway and loss of PTEN are extremely common in advanced cancer tumor progression 12, and has been previously shown that PTEN mutations in prostate cancers have led to higher basal levels of phosphorylated AKT (pAKTSer473) and increased survival of cells 12–14.
Transforming growth factor-β (TGF-β) is a secreted cytokine and a major regulator of many cellular processes implicated as factors in cancer formation and progression such as proliferation, survival, migration/invasion and metastasis 15. TGF-β acts as a tumor suppressor inhibiting proliferation in normal epithelial cells and early stages of many cancers, while in later stages, it exerts pro-oncogenic roles by promoting epithelial to mesenchymal transformation (EMT) which converts static epithelial cells into highly invasive mesenchymal cells, a necessary pre-requisite for tumor cell metastasis 16–18. The three TGF-β isoforms, TGF-β1, TGF-β2, and TGF-β3 have been identified in mammalian cells and share a 70%–80% sequence homology in most organisms and play redundant roles in cancer cells 19–21. All three TGF-β isoforms signal by binding to TGF-β Receptor II (TGF-βRII) which activates TGF-β Receptor I (TGF-βRI) which in turn, recruit and phosphorylates receptor associated- Smads (R-Smads), Smad2 and Smad3,which then form heterodimeric complexes with Smad4 that are then translocated into the nucleus where it functions to regulate transcriptional target genes 20,22. It has been suggested that TGF-β1 and TGF-β3 share a similar receptor binding affinity to TGF-βRII and can exert similar biological effects on target cells. TGF-β2, on the other hand binds to TGF-βRII with an affinity that is 100–1,000 fold lower, and requires TGF-β Receptor III (TGF-βRIII) (β-glycan) to promote receptor assembly 19,23,24.
Previous studies also demonstrated that TGF-β3 increased the invasiveness of endometrial carcinoma cells via a Phosphatidylinositol-3 Kinase (PI3-kinase) –dependent pathway, which were distinct from TGF-β1 25,26. Our laboratory has shown TGF-β effects on migration and invasion of prostate cancer cells, and these effects are dependent on both TGF-βRI and Smad3, and are mediated via the PI3-kinase pathway 19. In our studies, TGF-β3 was found to be more potent than TGF-β1 in these effects. On the other hand, TGF-β1 and TGF-β3 isoforms have similar effects on proliferation of different prostate cancer cell lines 19,27–30. Both isoforms inhibit proliferation in DU145 cells, however PC3 cells do not respond to the inhibitory effects of either of the two isoforms 19,27–30, which indicates TGF-β differential effects on cell proliferation depending on the context of the cell. The specific roles of individual TGFβ isoforms in development and progression of prostate cancer in vivo have not been investigated.
Previous evidence has clearly suggested the involvement of the PI3-kinase signaling pathway mediating TGF-β effects on cancer cell invasion and metastasis 19,31,32. A recent report confirmed an isoform-specific role of TGF-β in the migration and invasion of the metastatic PC3 prostate cancer cells, which are dependent on the activation of the PI3-kinase pathway 19. However in DU145 prostate cancer cells, the effects of TGF-β isoforms on the activation of PI3-kinase and the phosphorylation of AKT were not observed 19. This suggests that one possible contributor of differential effects of TGF-β on the activation of PI3-kinase may be PTEN. PTEN is expressed in the DU145 prostate cancer cell line, but is deleted in PC3 cells 33–36. Another study indicated that the loss of PTEN expression in human cancers may contribute to a role for TGF-β as a tumor enhancer with specific effects on cellular motility and invasion 37.
Although previous studies confirmed a role of TGF-β in migratory and invasive behavior in metastatic prostate cancer cells, which are dependent on the activation of the PI3-kinase pathway, the role of PTEN in TGF-β effects on the activation of PI3-kinase in prostate cancer cells remains to be elucidated. In this study, we investigated the effects of TGF-β on PTEN expression in prostate cancer cells, and whether PTEN plays a role in TGF-β-induced effects on proliferation, migration, and the activation of PI3-kinase/AKT pathway.
Materials and Methods
Chemicals and Reagents
Recombinant human TGF-β1 and TGF-β3 were purchased from PeproTech (Rocky Hill, NJ). Mammalian expression vectors pcDNA3 GFP PTEN (plasmid # 10759), and pcDNA3 GFP (plasmid #20738) were obtained from Addgene (Cambridge, MA). Human PTEN siRNA (sc-29459) and control non-silencing siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX). Matrigel, rat tail collagen and transwell inserts were purchased from BD Biosciences (Bedford, MA). DAPI (4′, 6-Diamidine-2-phenylindole dihdochloride) was purchased from Roche Diagnostics, (Indiana, IN). The antibodies against PTEN, pAKTSer473, AKT (pan), pSmad2, pSmad3, and Smad2/3 were purchased from Cell Signaling Technology (Danvers, MA). Antibody against β-Actin (clone AC-15) was purchased from Sigma-Aldrich (St. Louis, MO). Goat anti-rabbit IgG HRP was obtained from Life Technologies (Grand Island, NY). Anti-mouse IgG HRP was obtained from GE Healthcare (Piscataway, NJ).
Cell Lines and Cell Culture
Prostate epithelial and prostate cancer cell lines were obtained from American Type Culture Collection (ATCC) (Rockville, MD), which include RWPE1 (immortalized human prostate epithelial cell line) 38, and prostate cancer cell lines isolated from distinct metastatic sites from prostate cancer patients such as androgen-dependent cell line LNCaP (derived from a lymph node lesion), and androgen-independent cell lines DU145 (derived from brain) and PC3 (derived from bone) 39. RWPE1 cells were maintained in keratinocyte serum free medium containing 50μg/ml gentamycin, 0.05mg/ml bovine pituitary extract (BPE), and 5ng/ml epidermal growth factor (EGF) (Invitrogen, Carlsbad, CA). LNCaP cells were maintained in RPMI-1640 medium containing 4mM L-glutamine, and 50μg/ml gentamycin. DU145 and PC3 cells were cultured in Eagles minimum essential medium with Earle’s salts with 0.1 mM of the following amino acid supplements: L-alanine, L-asparagine, L-aspartic acid, L-glutamic acid, L-proline, L-serine and L-glycine. The medium contained 4 mM L-glutamine, 2.5 g/l NaHCO3, 1.5 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B and 50 μg/ml gentamycin. MEM and RPMI media (Mediatech, Herndon, VA) were supplemented with 5% fetal bovine serum (HyClone, South Logan, Utah).
RNA isolation, cDNA synthesis, and RT-PCR
Total RNA was isolated from normal prostate and prostate cancer cells using TRIzol (Invitrogen, Carlsbad, CA). The RNA samples were quantified by optical density reading at 260nm as described previously 40; OD260/OD280 ratio for RNA samples were between 1.8 and 2.0. Total RNA (2 μg) was reverse transcribed in a 50 μl reaction mixture containing 0.5 mM dNTP (Fisher Scientific, Pittsburgh, PA), 0.5 mM dithiotreitol (Bio-Rad, Hercules, CA), 0.5 μg of oligo dT, and 400 U of M-MLV Reverse Transcriptase (Promega, Madison, WI) at 37°C for 1.5 hours. The reaction was terminated by heating the samples at 60°C for 5 min and subsequently cooled to 4°C. Polymerase chain reaction (PCR) was performed to detect mRNA levels of PTEN and L-19. The sequences of primer pairs that were used are as follows: human PTEN (forward 5′AGCTTCTGCCATCTCTCTCC-3′ and reverse 5′AATATTGTTCCTGTATACGCCTTC-3′) and L-19 (forward 5′GAAATCGCCAATGCCAACTC-3′ and reverse 5′TCTTAGACCTGCGAGCCTCA-3′). RT-PCR reactions were performed according to procedures described previously 41. L-19 (a ribosomal protein) was used as an internal control. The PCR products were visualized on 1.5% agarose gels (Amresco, Solon, OH) stained with ethidium bromide.
Cell Treatments
To determine the effects of TGF-β isoforms on PTEN, pAKTSer473, pSmad2, and pSmad3, cells were cultured in 6 well plates at a density of 2 × 105 cells/well in culture media with 5%FBS and allowed to attach overnight. Before each experiment, the cells were incubated in a serum free or supplement free media for 2 hours, followed by treatment with TGF-β1 or TGF-β3 (5ng/ml) over various time points. Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer (Cell Signaling Technology, Beverly, MA) containing 20mM Tris-HCL (pH 7.5), 150mM NaCl, 1mM Na2 EDTA, 1mM EGTA, 1% Triton, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 1μg/ml leptin and 1X protease inhibitor cocktail (Calbiochem, La Jolla, CA). Protein concentrations were determined by the Lowry HS assay using the Bio-Rad DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA).
Western Blot Analysis
Cell lysates (35–50μg protein) were subjected to SDS-PAGE in 8% or 10% gels and transferred to PDVF membranes (Millipore, Billerica MA). The membranes were blocked in 5% fat free milk in TBST (50mM Tris, pH 7.5, containing 0.15M NaCl, 0.05% Tween 20) for 1 hour at room temperature. The blots were then incubated overnight at 4°C in TBST containing 5% bovine serum albumin (BSA) with appropriate dilutions of specific primary antibodies (1:1000 dilution for anti-PTEN; anti-pAKTSer473, anti-Total AKT (pan), anti-pSmad2, anti-pSmad3, anti-Total Smad2/3; 1:5000 dilution for anti-β-Actin). After washing, blots were then incubated with anti-rabbit or anti-mouse IgG-HRP (dilution 1:20,000) for 1 hour. The blots were developed in Millipore Luminata Forte (EMD Millipore, Billeria, MA) for 5 minutes, exposed by using Syngene PXI 6 imaging system (Syngene, Feerick, MD). The density of specific protein bands was determined by ImageJ Software (NIH ver1.48) and values were normalized using β-actin.
Transwell Cell Migration Assay
In vitro cell migration assay was performed using a 24-well plate transwell inserts (8 μm) as previously described 42. Cells were washed with MEM and harvested from cell culture dishes by EDTA-trypsin into 50 ml conical tubes. The cells were centrifuged at 1000 RPM for 3 min at room temperature; the pellets were resuspended in MEM supplemented with 0.2% bovine serum albumin (BSA) at a cell density of 3 × 105 cells/ml. The outside of the transwell insert membrane was coated with 50 μl total volume. Chemoattractant solutions were made by diluting TGF-β1 and/or TGF-β3 (5ng/ml) or combination of both (TGF-β1 and TGF-β3), and EGF (10 ng/ml) in MEM for DU145 and PC3 cells, and RPMI for LNCaP cells supplemented with 0.2% BSA. MEM containing 0.2% BSA served as a negative control. EGF was used as a positive control 43. Migrated cells were counted from ten random fields. The results were expressed as migration index defined as: the sum of ten random fields for test substance/the sum of ten random fields for the medium control 41.
Invasion Assay
The invasive properties of DU145 cells were measured using the BD BioCoat Matrigel Invasion inserts. Inserts were coated with 50μl of a 1:4 Matrigel/medium dilution and allowed to solidify at 37 °C for 48 hours. Cells were resuspended (3 × 104 cells/ml) in MEM with 0.1% FBS and 500μl of cell suspension were added to each insert. Cells were treated with TGF-β1 and TGF-β3 (5ng/ml), or EGF (10ng/ml) and were allowed to invade through the porous membrane coated with Matrigel for 48 hours. Matrigel and non-invading cells were removed via cotton swabs. Invading cells on the membrane were fixed in 3.7% paraformaldehyde and stained using DAPI (Roche Diagnostics, Indiana, IN). Images were taken in ten different fields for sum of invading cells. The results were expressed as invasion index defined as: the sum of ten random fields for test substance/the sum of ten random fields for the medium control.
Cell Proliferation Assay
The cell growth assay was performed by counting the number of cells. Cells were seeded at a density of 1 × 105 cells overnight in 6 well plates and treated the next day with TGF-β1 or TGF-β3 (5ng/ml) or combination of both (TGF-β1 and TGF-β3), in culture media containing 1% FBS for specific time points. Cells were then trypsinized and counted using the Cellometer Vision System (Nexcelom Bioscience LLC, Lawrence, MA).
Transfection with specific plasmids and small interfering (si) RNAs
Cells were seeded at a density of 1 x105 cells in 6 well plates in 2ml of antibiotic-free normal growth medium supplemented with 5% FBS, and incubated overnight at 37°C. Plasmids (pcDNA3 GFP or pcDNA3 GFP PTEN) were transfected in PC3 cells using FuGene® HD transfection reagent (Promega, Madison, WI) following manufacturer’s instructions. Small interfering RNAs (60nM) for the PTEN or Control siRNA were transfected into DU145 cells using transfection reagent (Santa Cruz Biotechnology, Dallas, TX) following manufacturer’s recommendations. Forty-eight to seventy-two hours after transfection, cells were either treated with TGF-β1 or TGF-β3, or subjected to different functional analyses.
Statistical Analysis
All experiments were performed at least three times using different cell preparations. Data from representative experiments are presented in the figures. One-way analysis of variance and Duncan’s all pair-wise multiple comparison tests were employed to assess the significance of differences among treatment groups. SigmaPlot version 11.0 for Windows (Systat Software, Inc., San Jose, CA) was used for statistical analyses.
Results
Expression of PTEN in prostate cell lines
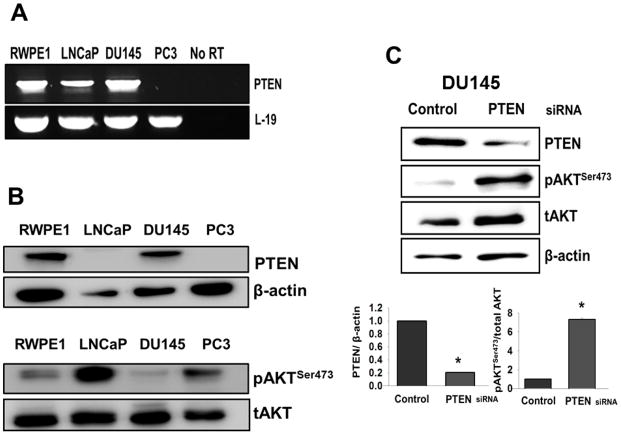
Gene expression of PTEN in prostate cells was determined using semiquantitative RT-PCR across four established prostate cell lines (RWPE1, LNCaP, DU145, and PC3). PTEN mRNA was detectable in three of the four cell lines with no detectable expression in PC3 cells (Fig. 1A). To examine the presence of PTEN protein and pAKTSer473 in these cell lines, the total cell lysate proteins were analyzed using western blotting (Fig. 1B). PTEN protein was detected in RWPE1 and DU145 cells, however, it was absent in LNCaP and PC3 cells. PAKTSer473 bands were detected in all cell lines with high levels of pAKTSer473 in PC3 and LNCaP cells compared with RWPE1 and DU145 cells.
Figure 1. Basal expression of PTEN and the levels of pAKTSer473 in prostate cell lines.
(A) RT-PCR was performed using total RNA from RWPE1, LNCaP, DU145, and PC3 cells to determine mRNA levels of PTEN. L-19 served as a loading control and was used to normalize mRNA levels in all cell line samples. No reverse transcriptase (RT) samples derived from the same RNAs were also included. (B) Levels of PTEN protein and pAKTSer473 as determined by western blotting analysis. β-actin and total AKT (tAKT) were used as loading controls. (C) Western blot analysis was performed to determine relative protein levels of PTEN and pAKTSer473 in DU145 cells after transfection with control siRNA or PTEN siRNA. Total AKT (tAKT) and β-actin were used as loading controls.
To determine whether the knockdown of PTEN in DU145 cells increased pAKTSer473 protein levels, we used a specific siRNA to transiently knockdown PTEN in DU145 cells (Fig. 1C). Control siRNA was also transfected to serve as a negative control. Protein levels of PTEN and pAKTSer473 were determined by western blot analysis, which confirmed that pAKTSer473 protein levels increased while PTEN protein levels decreased in comparison with cells transfected with control siRNA (Fig. 1C).
TGF-β isoforms increase intracellular PTEN protein levels in DU145 and RWPE1 cells
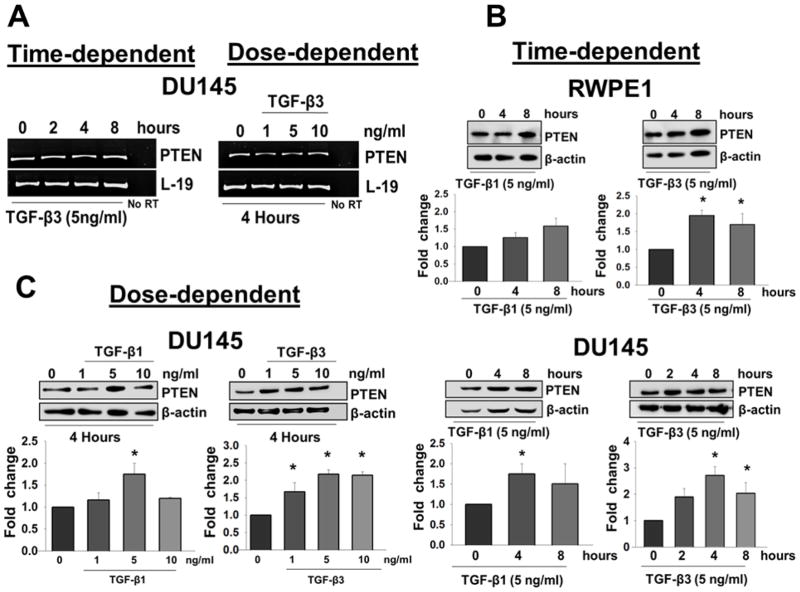
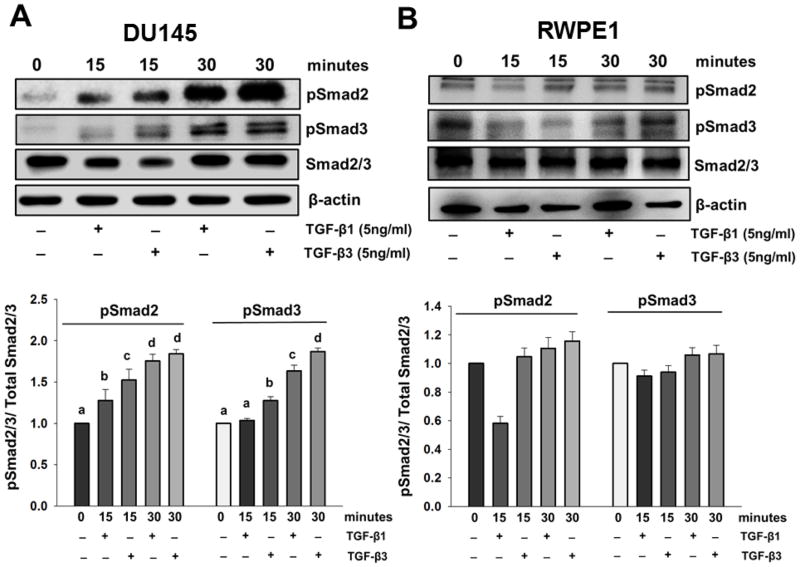
Next, we investigated the effects of TGF-β isoforms on the expression of PTEN in DU145 and RWPE1 cells. DU145 and RWPE1 cells were treated with different concentrations of TGF-β1 and/or TGF-β3 for specific times. As shown in Fig. 2A, TGF-β3 had no effect on the mRNA levels of PTEN in DU145 cells, as determined by RT-PCR analysis. We also confirmed the lack of TGF-β effects on PTEN mRNA levels by real time PCR (data not shown). However, as shown in Fig. 2B and 2C, both isoforms caused a dose dependent increase in PTEN protein levels at specific time points. Treatment with TGF-β3 (5ng/ml) significantly increased PTEN protein levels in DU145 cells at 4 hours (2.7 ± 0.34 fold; P < 0.05) and 8 hours (2.0 ± 0.40 fold; P < 0.05); and also in RWPE1 cells at 4 hours (2.0 ± 0.14 fold; P < 0.05) and 8 hours (1.7 ± 0.31 fold; P < 0.05) (Fig. 2B). As shown in Fig 2C, TGF-β3 treatments significantly increased PTEN protein levels in a dose dependent manner in DU145 cells after 4 hours at 1ng/ml (1.7 ± 0.26 fold; P < 0.05), 5ng/ml (2.2 ± 0.12 fold; P < 0.05), and 10ng/ml (2.15 ± 0.103 fold; P < 0.05). As shown in Fig 2B and 2C, TGF-β1 (5ng/ml) treatment also caused an increase in PTEN protein levels in both DU145 and RWPE1 cells. In DU145 cells, TGF-β1 treatment significantly increased PTEN protein levels at 4 hours (Fig. 2B) (1.9 ± 0.10 fold; P < 0.05), and in a dose dependent manner after 4 hours at 5ng/ml (1.38 ± 0.149 fold; P < 0.05) (Fig. 2C), however the increase in PTEN protein levels in TGF-β1 treated RWPE1 cells was not statistically significant (Fig.2C). These results suggest that both TGF-β1 and TGF-β3 increase PTEN protein levels; however TGF-β3 effects on both cell lines are more pronounced. TGF-β signaling is initiated by binding of the ligand to TGF-βRII that form heterodimers with TGF-βRI leading to Smad2 and Smad3 phosphorylation. Therefore, we examined whether TGF-β effects on PTEN are mediated by this canonical signaling pathway. We determined the effects of TGF-β on the phosphorylation of Smad2 and Smad3 in both DU145 and RWPE1 cells. Western blot analysis showed that TGF-β isoforms induced both Smad2 and Smad3 phosphorylation in a time-dependent manner (Fig. 3A and B).
Figure 2. TGF-β isoforms increase PTEN protein levels in DU145 and RWPE1 cells.
(A) RT-PCR analysis of PTEN gene expression in DU145 cells after treatment with TGF-β3 (5ng/ml) at specific time points and different doses of exogenous TGF-β3 for 4 hours. L-19 was used as a loading control. No reverse transcriptase (RT) samples derived from the same RNAs were also included. (B) Western blot analysis of PTEN protein levels in RWPE1 (upper panel) and DU145 (lower panel) cells after treatment for specific time periods with exogenous TGF-β1 and TGF-β3 (5ng/ml). Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) compared with untreated controls. (C) DU145 cells were treated with different doses of TGF-β1 or TGF-β3 for 4 hours. β-actin was used as a loading control. Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) compared with untreated controls.
Figure 3. TGF-β induces phosphorylation of Smad proteins in DU145 and RWPE1 cells.
(Western blot analyses of phosphorylated Smad2 (pSmad2) and Smad3 (pSmad3) in (A) DU145 cells and (B) RWPE1 cells after treatment with TGF-β1 or TGF-β3 (5ng/ml) for 15 and 30 minutes. Total Smad (Smad2/3) and β-actin were used as loading controls. Each bar represents mean ± SEM (n=3). Different letters denote significant differences among various groups (P < 0.05).
TGF-β isoforms exert differential effects on cell proliferation and migration in prostate cancer cells
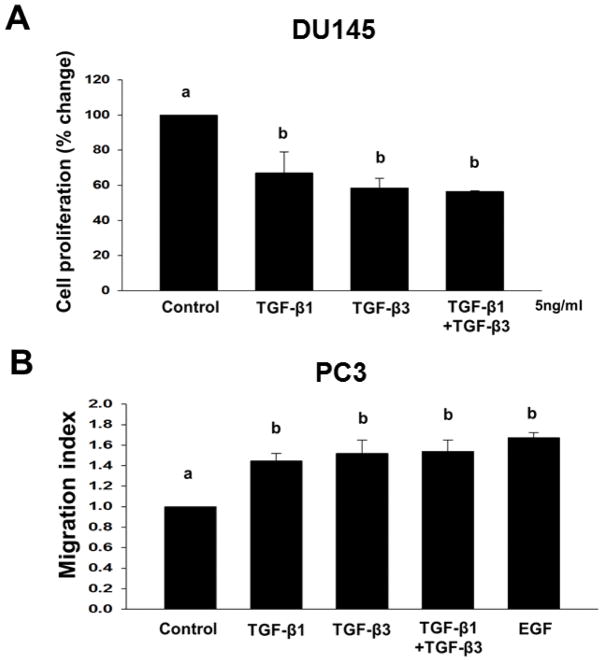
In previous studies, TGF-β has been shown to exert differential biological effects in different prostate cancer-derived cell lines 27,30. To confirm these studies, we first determined the effects of TGF-β1 and TGF-β3 (5ng/ml) and the combination of both (TGF-β1 and TGF-β3) on cell proliferation in DU145 prostate cancer cells (Fig. 4A). Both TGF-β isoforms caused a significant inhibition of cell proliferation in DU145 cells with no differences in the potencies of the two isoforms. Treatment with TGF-β1 resulted in 33% (P < 0.05) inhibition, TGF-β3 resulted in 42% (P < 0.05) inhibition, and both TGF-β1 and TGF-β3 combined resulted in 44% (P < 0.05) inhibition of proliferation in DU145 cells. There was no synergistic or additive effect of two isoforms on cell proliferation.
Figure 4. TGF-β isoforms have no synergistic or additive effect on cell proliferation and cell migration in different cell types.
(A) DU145 cells were treated with TGF-β1 or TGF-β3 (5ng/ml) or combined (TGF-β1 and TGF-β3) to determine cell proliferation. Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) when compared with appropriate controls. (B) Cell migration of PC3 cells across transwell membranes were assayed in response to TGF-β1 (5ng/ml), TGF-β3 (5ng/ml), TGF-β1 and TGF-β3 combined, or EGF (10ng/ml) treatments. EGF was used as a positive control. Each bar represents mean ± SEM (n=3). Different letters denote significant differences among various groups (P < 0.05).
We also determined the effects of TGF-β1 and TGF-β3 (5ng/ml) on cell migration in PC3 prostate cancer cells (Fig. 4B). Both TGF-β isoforms induced migration in PC3 cells (TGF-β1; 1.4 ± 0.75 migration index; P < 0.05) and TGF-β3; 1.52 ± 0.13 migration index; P < 0.05), TGF-β1 and TGF-β3; 1.54 ± 0.11 migration index; P < 0.05). The two isoforms did not exhibit synergistic or additive effects. Epidermal growth factor (EGF) used as a positive control, induced cell migration in PC3 (1.7 ± 0.05 migration index; P < 0.05) cells (Fig. 4B).
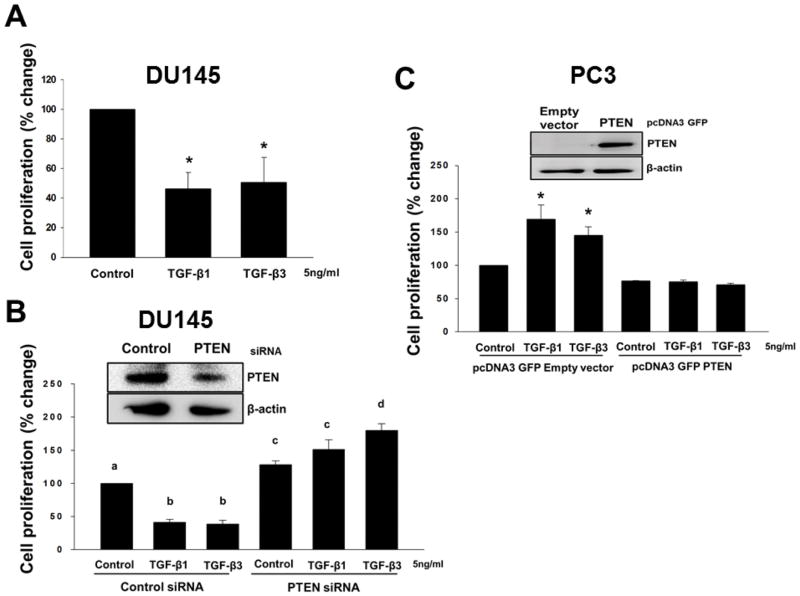
Reduction in levels of PTEN protein leads to attenuation of inhibitory effects of TGF-β on proliferation in prostate cancer cells
We have previously shown that TGF-β exerts differential effects on cell proliferation of different prostate cancer cell lines 28,30,44. TGF-β isoforms caused a significant inhibition of cell proliferation in DU145 cells (Fig. 5A)..
Figure 5. PTEN is required for inhibitory effects of TGF-β on cell proliferation in DU145 cells.
(A) DU145 cells were treated with TGF-β1 or TGF-β3 (5ng/ml) to determine cell proliferation. Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) when compared with appropriate controls. (B) Cell proliferation in DU145 cells after transfection with control siRNA or PTEN siRNA, and (C) PC3 cells after transfection with pcDNA3 GFP empty vector or pcDNA3 GFP PTEN vector, treated with TGF-β1 or TGF-β3 (5ng/ml) for 3 days. Each bar represents mean ± SEM (n=3). Different letters denote significant differences among various groups (P < 0.05). Levels of PTEN proteins after transfection with control siRNA or PTEN siRNA in DU145 cells, and empty vector or PTEN vector in PC3 cells were determined by western blotting analysis (inset).
The possible role of PTEN in proliferation of prostate cancer cells and its role in the effects of TGF-β on cell proliferation were determined using siRNAs to transiently knockdown PTEN proteins in DU145 cells (Fig. 5B). Expression of PTEN protein was determined by western blotting analysis, which confirmed reduced levels (~ 68 %) of PTEN protein in comparison with the cells transfected with control siRNA (Fig. 5B). DU145 cells were treated with TGF-β1 and TGF-β3 (5ng/ml) for 48 hours after transfection with PTEN siRNA or Control siRNA (Fig. 5B). As expected, after transfection with Control siRNA, we observed a significant decrease (P < 0.001) in proliferation of DU145 cells after treatment with both TGF-β isoforms. On the other hand, knockdown of endogenous PTEN resulted in a significant increase in cell proliferation which was further increased in TGF-β3 (P < 0.001) treated DU145 cells (Fig. 5B).
To further confirm the possible role of PTEN in inhibitory effects of TGF-β, we overexpressed PTEN in PC3 cells. PC3 were transiently transfected using the plasmid GFP-PTEN or empty vector and treated with TGF-β1 or TGF-β3 (5ng/ml) to determine the effects on cell proliferation. As shown in Fig. 5C, Expression of PTEN protein was determined by western blotting which confirmed PTEN overexpression in comparison with the cells transfected with the empty vector. TGF-β1 (70%; P < 0.05) and TGF-β3 (45%; P < 0.05) induced a significant increase in cell proliferation in PC3 cells transfected with the empty vector (Fig. 5C). However, proliferation was significantly reduced in PC3 cells overexpressing PTEN protein and TGF-β isoforms had no effects on proliferation in these cells.
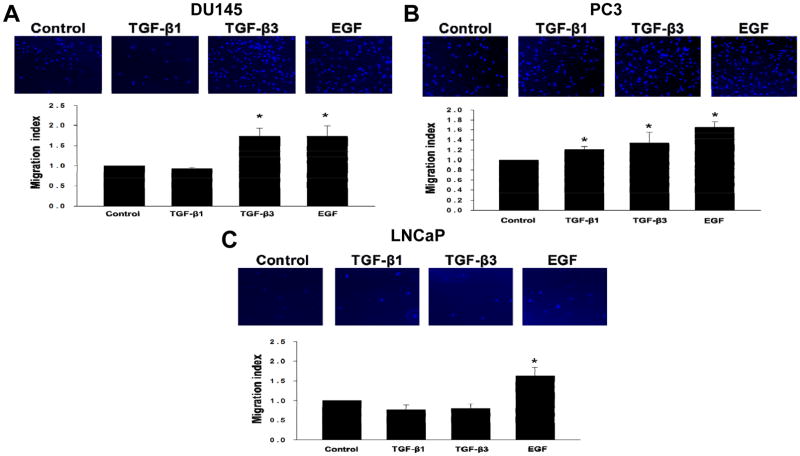
TGF-β isoforms exert differential effects on migratory and invasive properties in prostate cancer cell lines
TGF-β exerts effects on migration and invasion of specific prostate cancer cells. The effects of TGF-β1 and TGF-β3 on migration in selected prostate cell lines (DU145, PC3, and LNCaP) under identical experimental conditions were determined by using transwell cell migration assay. DU145, PC3, LNCaP cells were treated with either TGF-β isoforms and allowed to migrate according to established procedures. As shown in Fig. 6A and Fig. 6B, treatment with both isoforms induced cell migration in PC3 cells (TGF-β1; 1.2 ± 0.03 migration index; P < 0.05) and TGF-β3; 1.3 ± 0.13 migration index; P < 0.05), however TGF-β1 did not have any effect on migration in DU145 cells. Interestingly, TGF-β3 treatment significantly induced migration (1.7 ± 0.20 migration index; P < 0.05) in DU145 cells (Fig. 6A). In addition, we determined TGF-β effects on migration in LNCaP cells. It has been reported that LNCaP cells lack the TGF-βRII, a necessary receptor for TGF-β signaling 19. To confirm this notion, LNCaP cells were treated under the same experimental conditions and allowed to migrate. As expected, both TGF-β isoforms had no effect on migration in LNCaP cells (Fig. 6C). On the other hand, epidermal growth factor (EGF), used as a positive control, induced cell migration in DU145 (1.7 ± 0.26 migration index; P < 0.05), PC3 (1.6 ± 0.07 migration index; P < 0.05), and LNCaP (1.6 ± 0.20 migration index; P < 0.05) cells (Fig. 6A, B and C).
Figure 6. TGF-β isoforms exert differential effects on migratory properties in different prostate cancer cells.
(A–C) Cell migration of DU145, PC3, and LNCaP cells across transwell membranes were assayed in response to TGF-β1 (5ng/ml), TGF-β3 (5ng/ml), or EGF (10ng/ml) treatments. EGF was used as a positive control. Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) compared with untreated controls. Representative images of immunofluorescent cells using DAPI to stain the nucleus of the cells. Cells were visualized under 10X objective.
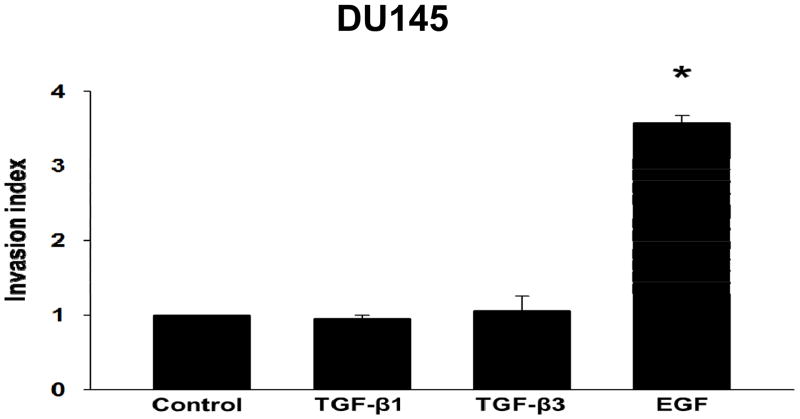
Previous studies in our laboratory have shown that TGF-β isoforms induced invasive behavior in PC3 cells, but not in DU145 cells 19,31. To confirm these studies, we determined the effects of TGF-β isoforms on cell invasion in DU145 cells using the BD BioCoat Matrigel Invasion inserts. As shown in Fig. 7, both TGF-β1 and TGF-β3 had no effect on invasiveness in DU145 cells, however, EGF induced invasion (2.9 ± 0.66 invasion index; P < 0.05) in DU145 cells. These results suggest that TGF-β isoforms may have differential effects on migration and invasion in prostate cancer cells.
Figure 7. TGF-β isoforms do not induce invasive behavior in DU145 cells.
Invasive properties of DU145 cells treated with TGF-β1 (5ng/ml), TGF-β3 (5ng/ml), or EGF (10ng/ml) were determined by an invasion assay. Cells were allowed to invade through a Matrigel coated porous membrane for 48 hours. Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) compared with untreated controls.
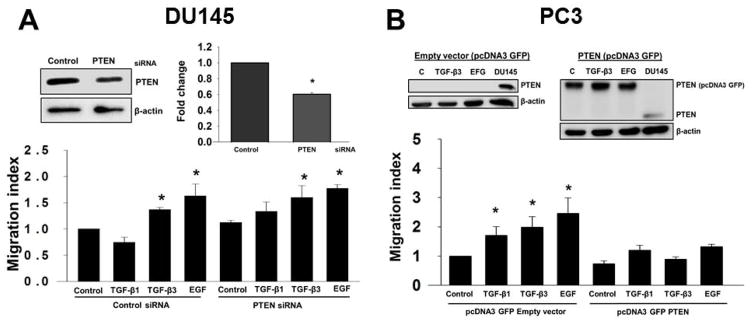
Re-expression of PTEN inhibits TGF-β induced cell migration in prostate cancer cells
To determine whether PTEN plays a role in TGF-β-mediated effects on migration in prostate cancer cells, we performed transient knockdown of PTEN in DU145 cells using siRNA specific for PTEN (Fig. 8A). PTEN protein levels were determined by Western blotting analysis, which confirmed a 54% reduction (P < 0.05) in DU145 cells transfected with PTEN siRNA compared to control siRNA transfected cells (Fig. 8A). We analyzed migration of DU145 cells after knockdown of PTEN (Fig. 8A). DU145 cells were treated with TGF-β1 and TGF-β3 (5ng/ml) for 48 hours after transfection with PTEN siRNA. We observed that both TGF-β isoforms induced migration with a significant increase with TGF-β3 (1.6 ± 0.23 fold; P < 0.008) and EGF (1.8 ± 0.07 fold; P < 0.008) (Fig. 8A).
Figure 8. Endogenous PTEN inhibits effects ofTGF-β on cell migration in prostate cancer cells.
(A) DU145 cells transfected with control siRNA or PTEN siRNA were treated with TGF-β1 or TGF-β3 (5ng/ml), or EGF (10ng/ml) to determine migratory properties in a transwell cell migration assay. Levels of PTEN proteins after transfection with control siRNA or PTEN siRNA in DU145 cells were determined by western blotting analysis (inset). Each bar represents mean ± SEM (n=3). *Significantly different (P < 0.05) compared with untreated controls. (B) PC3 cells after transfected with pcDNA3 GFP empty vector or pcDNA3 GFP PTEN vector were treated with TGF-β1 or TGF-β3 (5ng/ml), or EGF (10ng/ml) to determine migration using a transwell migration assay. Each bar represents mean ± SEM (n=4). *Significantly different (P < 0.05) when compared with untreated controls. Levels of PTEN proteins and β-actin (used as a loading control) after transfection with empty vector or PTEN vector, in the presence or absence of TGF-β3 or EGF in PC3 cells were determined by western blotting analysis (inset). DU145 was used as a positive control.
Next, we investigated the effects of PTEN expression on TGF-β induced migration in PC3 cells. We transiently overexpressed PTEN in PC3 cells (Fig. 8B). Expression of PTEN proteins were determined by western blotting analysis which confirmed overexpression of PTEN in PC3 cells. DU145 served as a positive control for PTEN protein expression (Fig. 8B). Effects of TGF-β isoforms on cell migration were significantly reduced in these cells compared with PC3 cells transfected with empty vector (Fig. 8B). These results suggest that PTEN overexpression in PC3 cells inhibited TGF-β-mediated effects on migration.
PTEN overexpression inhibit TGF-β effects on the phosphorylation of AKT in prostate cancer cells
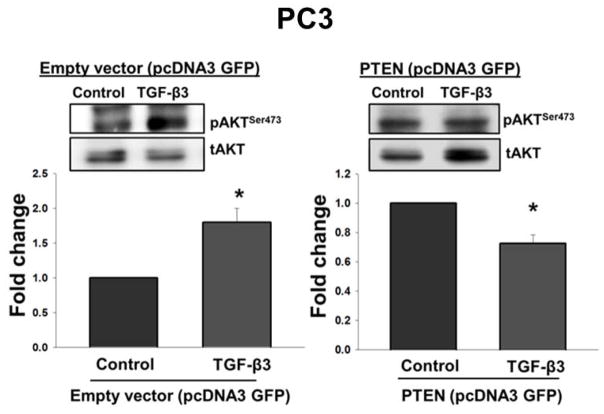
To determine whether or not overexpression of PTEN will affect TGF-β effects on the activation of PI3-kinase in PC3 cells treated with TGF-β3 (5ng/ml) (Fig. 9). Western blot analysis revealed that in response to TGF-β3, PTEN overexpressing PC3 cells had significantly lower levels (0.73 ± 0.06 fold: P < 0.05) of pAKTSer473 as compared to those transfected with empty vector (Fig. 9). These results suggest that overexpression of PTEN in PC3 cells attenuates TGF-β effects on the activation of PI3-kinase.
Figure 9. Overexpression of PTEN leads to reduced phosphorylation of AKT in response to TGF-β3 in PC3 cells.
Western blot analysis showing levels of phosphorylation of AKT (pAKTSer473) with or without TGF-β3 (5ng/ml) treatment in PC3 cells after transfection with pcDNA3 GFP empty vector or pcDNA3 GFP PTEN vector. Total AKT (tAKT) was used as a loading control. Each bar represents mean ± SEM (n=4). *Significantly different (P < 0.05) when compared with untreated controls.
Discussion
In this study we investigated the role of PTEN in TGF-β effects on proliferation and migration of prostate epithelial cancer cells. We report that TGF-β has differential effects on PTEN protein and RNA levels in prostate cancer cells. We also show that PTEN may mediate anti-proliferative effects of TGF-β in prostate epithelial cells. Our results also revealed that lack of PTEN may enhance TGF-β effects on cell migration in prostate cancer cells.
Prostate cancer transpires and progresses as a result of accumulated genomic mutations that lead to unchecked cellular growth and survival of the mutated and dividing cells 45. The principle problem arising from prostate cancer and its high rate of mortality, is due to metastasis of the primary tumor to secondary sites 46. Cellular migration and invasion play fundamental roles in cancer metastasis. Among multiple growth factors, TGF-β has been implicated in the regulation of prostate cancer cell proliferation, progression and/or metastasis 28–30,44,47–50 TGF-β is a multiple functional protein that acts as a tumor suppressor in normal epithelial cells and in early stages of epithelial cancers by inhibiting proliferation and inducing apoptosis 51. However in later stages of cancer, cells become resistant to growth inhibitory effects of TGF-β, in which TGF-β acts as a tumor promoter due to its role in epithelial to mesenchymal transformation (EMT) or promoting the degradation of ECM; all of which aid invasion and metastasis 19,52–54 In addition, previous studies also show that TGF-β effects on migration and invasion of metastatic prostate cancer cells are dependent on activation of the PI3-kinase pathway 19,55 by TGF-β, as shown by increased phosphorylation of AKT 55–59. However, the intracellular mechanisms involved in TGF-β activation of PI3-kinase pathway are largely unknown. The PI3-kinase pathway mediates cellular processes such as proliferation, cell survival, and migration, and is negatively regulated by PTEN which inhibit those cellular functions. Therefore, PTEN may play a role in differential effects of TGF-β on proliferation, and migratory behavior by antagonizing PI3-kinase activity in prostate cancer cells.
PTEN mutations and deletions in prostate cancer cells 60–63 lead to the loss of PTEN and constitutive activation of the PI3-kinase/AKT pathway. Several prostate cancer derived cell lines have also been shown to lack expression of PTEN such as a homozygous deletion in PC3 cells and frame shift mutation in LNCaP cells 63. Other cell lines derived from normal or cancer cells such as RWPE-1 and DU145 cells maintain normal expression of PTEN. These cell lines provide convenient model systems to study the role of PTEN and PI3-kinase pathway in prostate cancer cell proliferation, migration and invasion. Our results showed higher constitutive levels of pAKTSer473 in LNCaP and PC3 cells as a consequence of PTEN loss, but not in RWPE1 and DU145 cells. On the other hand, knockdown of endogenous PTEN by specific siRNA in DU145 and RWPE1 cells resulted in increased levels of pAKTSer473, indicating enhanced activation of PI3-kinase pathway.
Earlier studies have addressed the interaction of the PI3-kinase/PTEN pathway and TGF-β signaling pathways in various cell types. Previous reports have shown that TGF-β1 regulates PTEN expression in keratinocytes 4 and causes a reduction of PTEN mRNA levels in pancreatic cancer cells 64. Moreover, inactivation of TGF-β signaling and loss of PTEN cooperate to induce colorectal cancer formation and progression by suppressing cell cycle inhibitors 65. Although it is known that loss of PTEN expression seems to be more frequent in many human tumors, the expression of PTEN has been associated with inhibitory effects on proliferation, migration, and induction of apoptosis in vitro and in vivo 64,66,67. In the current study, we studied the effects of TGF-β1 and TGF-β3 on PTEN expression in DU145 and RWPE1 cells. Our results show that while TGF-β isoforms had no effect on PTEN mRNA levels; they induced an increase in PTEN protein in both DU145 and RWPE1 cells. TGF-β induced increase in PTEN protein levels may suggest that the level of PTEN may be regulated by TGF-β via other mechanisms such as protein stability and protein degradation. It has been reported previously that PTEN activity may be highly regulated on many levels by posttranscriptional and posttranslational mechanisms 68. It has also been demonstrated that TGF-β can regulate protein stability via posttranslational modifications 69–72. One study revealed that TGF-β increases nuclear p27 levels by preventing proteasomal degradation, specifically by downregulating ubiquitin E3 ligase subunits in endometrial carcinoma cells 72. Therefore, it is possible that TGF-β may stabilize PTEN protein levels by inhibiting the proteasomal degradation pathway in prostate cancer cells.
Our results suggest that increased PTEN protein levels in DU145 cells and RWPE1 cells, in response to TGF-β, may lead to a reduction in cell proliferation in these cells. Our laboratory has previously shown that TGF-β inhibits cell proliferation in RWPE1 and DU145 cells, whereas PC3 (PTEN null) prostate cancer cells are resistant to these growth inhibitory effects 28. Our results show that PTEN knockdown by specific siRNA resulted in a significant increase in cell proliferation in the presence of TGF-β1 and TGF-β3 isoforms. These results indicate that reduced PTEN protein levels in DU145 cells enhanced TGF-β effects and that PTEN may be required for inhibitory effects of TGF-β on cell proliferation in these cells. The possible role of PTEN in cell proliferation was also confirmed by our experiments where we transiently overexpressed PTEN in PC3 cells, which led to inhibition of cell proliferation. Our results are very similar to recently reported findings indicating that the overexpression of PTEN in PC3 cells inhibited cell proliferation, and decreased IGF-I-induced phosphorylation of IRS-1, the downstream substrate of IGF-IR 73.
Previous studies have shown that TGF-β1 and TGF-β3 exert differential effects on migration and invasion in prostate cancer cells 19,27,50,74–81. It has been indicated that TGF-β3 exerts more potent effects on migratory and invasive behavior. Although both TGF-β isoforms induced migratory and invasive behavior in PC3 cells 19,27, both isoforms had no effects on migratory behavior in DU145 cells 19. In another study, TGF-β1 induced cell migration and invasion in PC3 cells but not in in DU145 cells using the transwell insert cell migration assay 27,31; TGF-β3 was not investigated in these studies. Interestingly, our results show that while TGF-β1 had no effects on migration in DU145 cells, TGF-β3 significantly induced migration in both DU145 and PC3 cells. These results support previous findings that TGF-β3 and TGF-β1 exert differential effects on invasive behavior in prostate and other cancer cells 19,27,31. This could either be because of differential potencies of individual isoforms or due to inherent specific signaling pathways utilized by TGF-β1 and TGF-β382. However, the exact mechanisms involved in these differential effects remain unknown. Interestingly, TGF-β3 had no effects on invasive behavior in DU145 cells although it induced cell migration in these cells. These data indicate that TGF-β3 may exert differential effects on intracellular mechanisms which are involved in induction of cell migration and/or invasion which may play significant role in the progression of invasive and metastatic disease 19,20,32. These observed effects in both DU145 and PC3 cells are specific to TGF-β, since LNCaP cells (used as negative controls due to lack of TGF-βRII) were unresponsive to both isoforms of TGF-β 19,83. Similar differential role of TGF-β isoforms is also supported in breast carcinomas, where high expression of TGF-β3 correlates with decreased overall survival rate 84,85. It has been confirmed that the isoform specific roles of TGF-β on cell migration and invasion are mediated by TGF-βRI and Smad3 dependent activation of PI3-kinase pathway in metastatic prostate cancer cells 19. Previous studies have implicated a role for PTEN in repressing the pro-tumorigenic effects of TGF-β; reconstitution of PTEN expression in PTEN-null cells blocked TGF-β-induced invasion, but did not modulate TGF-β-mediated growth regulation 37. Similarly, in keeping with these studies, our results show that TGF-β induced migration in PTEN knockdown DU145 cells, whereas TGF-β reduced migratory behavior in PTEN overexpression PC3 cells. These results indicate PTEN may play a role in TGF-β mediated effects in regulating tumor cell metastasis. In PC3 cells, overexpression of PTEN has been shown to be associated with a decrease in the levels of pAKTSer473 73. Previously, it has been shown that TGF-β can activate PI3-kinase pathway in prostate cancer cells as shown by increased phosphorylation of AKT 55–59, and activation of this pathway is required for its effects on increased migration and invasion of these cells 32. Here, we show that overexpression of PTEN in PC3 cells reduced the phosphorylation of AKT and invasive behavior in response to TGF-β3. These results suggest that PTEN inhibits PI3-kinase dependent AKT phosphorylation, and that the ability of TGF-β to activate PI3-kinase signaling may be inhibited in the presence of PTEN. Our results indicate that PTEN may play a role in differential effects of TGF-β on proliferation and invasion of prostate cancer cells during different stages of disease progression. Hence a loss of PTEN which is extremely common in prostate cancers may lead to enhanced effects of TGF-β on cell migration and invasion in advanced stages of cancer and loss of TGF-β effects on cell proliferation. PTEN mutations in prostate cancer, and TGF-β’s pro-oncogenic functions in advanced stages of the disease, are positively correlated with tumor aggressiveness and poor prognosis, which may suggest that PTEN may modulate the “switch” of TGF-β from anti- to pro-tumorigenic in prostate cancer.
Conclusion
In conclusion, our results demonstrate that TGF-β increases protein levels of PTEN in prostate cancer cells; however, TGF-β has no effect on mRNA levels of PTEN in these cells. We also show that lack of PTEN led to increased TGF-β effects on migration in prostate cancer cells, whereas overexpression of PTEN, in PTEN null prostate cancer cells, inhibited TGF-β effects on cell migration. In addition, overexpression of PTEN in these cells decreased TGF-β-induced phosphorylation of AKT, as a result of inhibition of the PI3-kinase/AKT signaling pathway. Increased proliferation and enhanced TGF-β effects on cell migration and invasion after knockdown of endogenous PTEN could explain the upregulation of TGF-β signaling and increased tumor growth and metastatic behavior in prostate cancer cells that have deletions or inactivating mutations of PTEN. Therefore advancing the knowledge of molecular mechanisms involved in enhanced TGF-β effects on proliferation and migration and loss of PTEN may lead to the identification of therapeutic strategies for prostate cancer patients.
Acknowledgments
This study was supported by grants from NIH/NIMHD/RCMI grant #G12MD007590 and NIH/NIMHD grant #5P20MD002285.
Footnotes
Disclosure Statement
The authors have nothing to disclose.
References
- 1.American Cancer Society. [Accessed April 20, 2017];Information and Resources about for Cancer: Breast, Colon, Lung, Prostate, Skin. https://www.cancer.org/
- 2.Cancer Genome Atlas Research Network TCGAR. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Mendonca J, Ying J, et al. The prostate metastasis suppressor gene NDRG1 differentially regulates cell motility and invasion. Mol Oncol. 2017;11:655–669. doi: 10.1002/1878-0261.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 5.Dong JT, Li CL, Sipe TW, Frierson HF. Mutations of PTEN/MMAC1 in primary prostate cancers from Chinese patients. Clin Cancer Res. 2001;7:304–308. [PubMed] [Google Scholar]
- 6.Inoki K, Ouyang H, Li Y, Guan K-L. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GV, Horvath S, Smith BL, et al. Antibody-based profiling of the phosphoinositide 3-kinase pathway in clinical prostate cancer. Clin Cancer Res. 2004;10:8351–8356. doi: 10.1158/1078-0432.CCR-04-0130. [DOI] [PubMed] [Google Scholar]
- 9.Chen M-L, Xu P-Z, Peng X, et al. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulholland DJ, Dedhar S, Wu H, Nelson CC. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25:329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 11.Shen MM, Abate-Shen C. Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res. 2007;67:6535–6538. doi: 10.1158/0008-5472.CAN-07-1271. [DOI] [PubMed] [Google Scholar]
- 12.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Blanco-Aparicio C, Renner O, Leal JFM, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 14.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 15.Massagué J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chantry A. WWP2 ubiquitin ligase and its isoforms: new biological insight and promising disease targets. Cell Cycle. 2011;10:2437–2439. doi: 10.4161/cc.10.15.16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006;27:2148–2156. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 19.Walker L, Millena AC, Strong N, Khan SA. Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis. 2013;30:13–23. doi: 10.1007/s10585-012-9494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Themsche C, Chaudhry P, Leblanc V, Parent S, Asselin E. XIAP gene expression and function is regulated by autocrine and paracrine TGF-beta signaling. Mol Cancer. 2010;9:216. doi: 10.1186/1476-4598-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 22.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 23.Baardsnes J, Hinck CS, Hinck AP, O’Connor-McCourt MD. TbetaR-II discriminates the high- and low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs. Biochemistry. 2009;48:2146–2155. doi: 10.1021/bi8019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheifetz S, Hernandez H, Laiho M, ten Dijke P, Iwata KK, Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990;265:20533–20538. [PubMed] [Google Scholar]
- 25.Felgueiras J, Silva JV, Fardilha M. Prostate cancer: the need for biomarkers and new therapeutic targets. J Zhejiang Univ Sci B. 2014;15:16–42. doi: 10.1631/jzus.B1300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17(Suppl 2):S72–9. doi: 10.3747/co.v17i0.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo BT, Cody B, Cao Y, Khan SA. Differential role of Sloan-Kettering Institute (Ski) protein in Nodal and transforming growth factor-beta (TGF-β)-induced Smad signaling in prostate cancer cells. Carcinogenesis. 2012;33:2054–2064. doi: 10.1093/carcin/bgs252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong N, Millena AC, Walker L, Chaudhary J, Khan SA. Inhibitor of differentiation 1 (Id1) and Id3 proteins play different roles in TGFβ effects on cell proliferation and migration in prostate cancer cells. Prostate. 2013;73:624–633. doi: 10.1002/pros.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokeshwar BL, Block NL. Isolation of a prostate carcinoma cell proliferation-inhibiting factor from human seminal plasma and its similarity to transforming growth factor beta. Cancer Res. 1992;52:5821–5825. [PubMed] [Google Scholar]
- 30.Millena AC, Vo BT, Khan SA. JunD Is Required for Proliferation of Prostate Cancer Cells and Plays a Role in Transforming Growth Factor-β (TGF-β)-induced Inhibition of Cell Proliferation. J Biol Chem. 2016;291:17964–17976. doi: 10.1074/jbc.M116.714899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vo BT, Morton D, Komaragiri S, Millena AC, Leath C, Khan SA. TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology. 2013;154:1768–1779. doi: 10.1210/en.2012-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Themsche C, Mathieu I, Parent S, Asselin E. Transforming growth factor-beta3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase c-dependent induction of matrix metalloproteinas. J Biol Chem. 2007;282:4794–4802. doi: 10.1074/jbc.M608497200. [DOI] [PubMed] [Google Scholar]
- 33.Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao C, Subhawong T, Albert JM, et al. Inhibition of Mammalian Target of Rapamycin or Apoptotic Pathway Induces Autophagy and Radiosensitizes PTEN Null Prostate Cancer Cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 35.Festuccia C. Molecular aspects of gefitinib antiproliferative and pro-apoptotic effects in PTEN-positive and PTEN-negative prostate cancer cell lines. Endocr Relat Cancer. 2005;12:983–998. doi: 10.1677/erc.1.00986. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Cheville JC, Pan Y, Roche PC, Schmidt LJ, Tindall DJ. PTEN induces chemosensitivity in PTEN-mutated prostate cancer cells by suppression of Bcl-2 expression. J Biol Chem. 2001;276:38830–38836. doi: 10.1074/jbc.M103632200. [DOI] [PubMed] [Google Scholar]
- 37.Hjelmeland AB, Hjelmeland MD, Shi Q, et al. Loss of phosphatase and tensin homologue increases transforming growth factor beta-mediated invasion with enhanced SMAD3 transcriptional activity. Cancer Res. 2005;65:11276–11281. doi: 10.1158/0008-5472.CAN-05-3016. [DOI] [PubMed] [Google Scholar]
- 38.Kovalenko PL, Zhang Z, Cui M, Clinton SK, Fleet JC. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genomics. 2010;11:26. doi: 10.1186/1471-2164-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinmetz NF, Maurer J, Sheng H, et al. Two Domains of Vimentin Are Expressed on the Surface of Lymph Node, Bone and Brain Metastatic Prostate Cancer Lines along with the Putative Stem Cell Marker Proteins CD44 and CD133. Cancers (Basel) 2011;3:2870–2885. doi: 10.3390/cancers3032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millena AC, Reddy SC, Bowling GH, Khan SA. Autocrine regulation of steroidogenic function of Leydig cells by transforming growth factor-alpha. Mol Cell Endocrinol. 2004;224:29–39. doi: 10.1016/j.mce.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhong M, Boseman ML, Millena AC, Khan SA. Oxytocin induces the migration of prostate cancer cells: involvement of the Gi-coupled signaling pathway. Mol Cancer Res. 2010;8:1164–1172. doi: 10.1158/1541-7786.MCR-09-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zigmond SH, Foxman EF, Segall JE. Chemotaxis assays for eukaryotic cells. Curr Protoc Cell Biol. 2001;Chapter 12(Unit 12.1) doi: 10.1002/0471143030.cb1201s00. [DOI] [PubMed] [Google Scholar]
- 43.Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343:848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 44.Vo BT, Khan SA. Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: autocrine effects on cell proliferation and migration. Prostate. 2011;71:1084–1096. doi: 10.1002/pros.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11:57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dos Reis ST, Pontes-Júnior J, Antunes AA, et al. Tgf-β1 expression as a biomarker of poor prognosis in prostate cancer. Clinics (Sao Paulo) 2011;66:1143–1147. doi: 10.1590/S1807-59322011000700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones E, Pu H, Kyprianou N. Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–234. doi: 10.1517/14728220802705696. [DOI] [PubMed] [Google Scholar]
- 49.MARTIKAINEN P, KYPRIANOU N, ISAACS JT. Effect of Transforming Growth Factor-β 1 on Proliferation and Death of Rat Prostatic Cells*. Endocrinology. 1990;127:2963–2968. doi: 10.1210/endo-127-6-2963. [DOI] [PubMed] [Google Scholar]
- 50.Collazo J, Zhu B, Larkin S, et al. Cofilin drives cell-invasive and metastatic responses to TGF-β in prostate cancer. Cancer Res. 2014;74:2362–2373. doi: 10.1158/0008-5472.CAN-13-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 52.Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 53.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi A, Cao D. TGF-beta signaling, tumor microenvironment and tumor progression: the butterfly effect. Front Biosci (Landmark Ed) 2010;15:180–194. doi: 10.2741/3614. [DOI] [PubMed] [Google Scholar]
- 55.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 57.Viñals F, Pouysségur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol. 2001;21:7218–7230. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin I, Bakin AV, Rodeck U, Brunet A, Arteaga CL. Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol Biol Cell. 2001;12:3328–3339. doi: 10.1091/mbc.12.11.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkes MC, Mitchell H, Penheiter SG, et al. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–10440. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 60.Reiss K, Porcu P, Sell C, Pietrzkowski Z, Baserga R. The insulin-like growth factor 1 receptor is required for the proliferation of hemopoietic cells. Oncogene. 1992;7:2243–2248. [PubMed] [Google Scholar]
- 61.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–825. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stambolic V. PTEN: a new twist on beta-catenin? Trends Pharmacol Sci. 2002;23:104–106. doi: 10.1016/s0165-6147(00)01984-2. [DOI] [PubMed] [Google Scholar]
- 63.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 64.Ebert MPA, Fei G, Schandl L, et al. Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer. 2002;86:257–262. doi: 10.1038/sj.bjc.6600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu M, Trobridge P, Wang Y, et al. Inactivation of TGF-β signaling and loss of PTEN cooperate to induce colon cancer in vivo. Oncogene. 2014;33:1538–1547. doi: 10.1038/onc.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 67.Davies MA, Koul D, Dhesi H, et al. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res. 1999;59:2551–2556. [PubMed] [Google Scholar]
- 68.Worby Ca, Dixon JE. Pten Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]
- 69.Coricor G, Serra R. TGF-β regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 71.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor β1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006:281. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 72.Lecanda J, Ganapathy V, D’Aquino-Ardalan C, et al. TGFbeta prevents proteasomal degradation of the cyclin-dependent kinase inhibitor p27kip1 for cell cycle arrest. Cell Cycle. 2009;8:742–756. doi: 10.4161/cc.8.5.7871. [DOI] [PubMed] [Google Scholar]
- 73.Zhao H, Dupont J, Yakar S, Karas M, LeRoith D. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene. 2004;23:786–794. doi: 10.1038/sj.onc.1207162. [DOI] [PubMed] [Google Scholar]
- 74.Barrett CSX, Millena AC, Khan SA. TGF-β Effects on Prostate Cancer Cell Migration and Invasion Require FosB. Prostate. 2017;77:72–81. doi: 10.1002/pros.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mu Y, Zang G, Engström U, Busch C, Landström M. TGFβ-induced phosphorylation of Par6 promotes migration and invasion in prostate cancer cells. Br J Cancer. 2015;112:1223–1231. doi: 10.1038/bjc.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thakur N, Gudey SK, Marcusson A, et al. TGFβ-induced invasion of prostate cancer cells is promoted by c-Jun-dependent transcriptional activation of Snail1. Cell Cycle. 2014;13:2400–2414. doi: 10.4161/cc.29339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuura I, Chiang K-N, Lai C-Y, et al. Pin1 promotes transforming growth factor-beta-induced migration and invasion. J Biol Chem. 2010;285:1754–1764. doi: 10.1074/jbc.M109.063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buczek ME, Miles AK, Green W, et al. Cytoplasmic PML promotes TGF-β-associated epithelial–mesenchymal transition and invasion in prostate cancer. Oncogene. 2016;35:3465–3475. doi: 10.1038/onc.2015.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Festuccia C, Bologna M, Gravina GL, et al. Osteoblast conditioned media contain TGF-beta1 and modulate the migration of prostate tumor cells and their interactions with extracellular matrix components. Int J cancer. 1999;81:395–403. doi: 10.1002/(sici)1097-0215(19990505)81:3<395::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 80.Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming growth factor-beta promotes invasion in tumorigenic but not in nontumorigenic human prostatic epithelial cells. Cancer Res. 2006;66:8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Q, Tong S, Zhao X, et al. Periostin Mediates TGF-β-Induced Epithelial Mesenchymal Transition in Prostate Cancer Cells. Cell Physiol Biochem. 2015;36:799–809. doi: 10.1159/000430139. [DOI] [PubMed] [Google Scholar]
- 82.Laverty HG, Wakefield LM, Occleston NL, O’Kane S, Ferguson MWJ. TGF-beta3 and cancer: a review. Cytokine Growth Factor Rev. 2009;20:305–317. doi: 10.1016/j.cytogfr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Y, Kyprianou N. Overexpression of transforming growth factor (TGF) beta1 type II receptor restores TGF-beta1 sensitivity and signaling in human prostate cancer cells. Cell Growth Differ. 1998;9:185–193. [PubMed] [Google Scholar]
- 84.Amatschek S, Koenig U, Auer H, et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004;64:844–856. doi: 10.1158/0008-5472.can-03-2361. [DOI] [PubMed] [Google Scholar]
- 85.Li C, Wang J, Wilson PB, et al. Role of transforming growth factor beta3 in lymphatic metastasis in breast cancer. Int J cancer. 1998;79:455–459. doi: 10.1002/(sici)1097-0215(19981023)79:5<455::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]