Abstract
BACKGROUND
The goal of this study was to determine a set of timing, shape, and statistical features available through non-invasive monitoring of maternal electrocardiogram and photoplethysmography that identifies preeclamptic patients.
METHODS
Pregnant women admitted to Labor and Delivery were monitored with pulse oximetry and electrocardiogram for 30 min. Photoplethysmogram features and heart rate variability were extracted from each data set and applied to a sequential feature selection algorithm to discriminate women with preeclampsia with severe features, from normotensive and hypertensive controls. The classification boundary was chosen to minimize the expected misclassification cost. The prior probabilities of the misclassification costs were assumed to be equal.
RESULTS
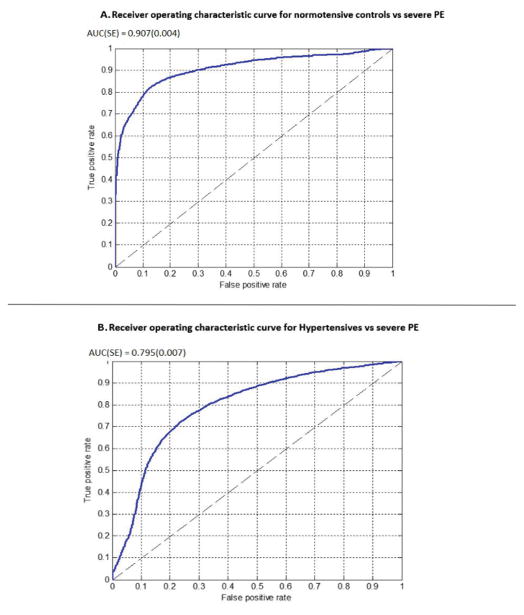
Thirty-seven patients with clinically diagnosed preeclampsia with severe features were compared with 43 normotensive controls; all were in early labor or beginning induction. Six variables were used in the final model. The area under the receiver operating characteristic curve was 0.907 (SE = 0.004) (sensitivity 78.2% (SE = 0.3%), specificity 89.9% (SE = 0.1%)) with a positive predictive value of 0.883 (SE = 0.001). Twenty-eight subjects with chronic or gestational hypertension were compared with the same preeclampsia group, generating a model with five features with an area under the curve of 0.795 (SE = 0.007) (sensitivity 79.0% (SE = 0.2%), specificity 68.7% (SE = 0.4%)) and a positive predictive value of 0.799 (SE = 0.002).
CONCLUSIONS
Vascular parameters, as assessed non-invasively by photoplethysmography and heart rate variability, may have a role in screening women suspected of having preeclampsia, particularly in areas with limited resources.
Preeclampsia (PE) affects 3% of pregnancies in the United States and remains a leading cause of maternal and fetal morbidity and mortality.1 The pathophysiology of PE remains an area of intense research, the outcome of which should lead to novel prevention and treatment strategies. Of current interest is the endothelial dysfunction that is presumed related to placental ischemia and the release of various soluble vasoreactive factors. Although these proteins can be assayed, at significant expense, the resulting alteration in vascular performance (e.g., arterial stiffness and distensibility) may be more readily measured.
Analysis of the peripheral pulse wave has long been recognized to indicate arterial resistance characteristics. Radial artery pulse waveforms obtained via applanation tonometry have shown some promise in the prediction and diagnosis of PE.2–5 The photoplethysmographic (PPG) pulse, obtained via a standard finger pulse oximeter, may replace the more labor-intensive and expensive tonometry. The former technology has been applied to vasomotor responsiveness and endothelial function analysis in non-pregnant patients.6
Heart rate variability (HRV) analysis is gaining acceptance in the evaluation of cardiovascular disease, particularly for assessing the state of the autonomic nervous system.7 Its use in PE has been limited8 and coupled with the more difficult-to-obtain blood pressure variability.9
Based on the above evidence, our hypothesis was that parameters of the PPG pulse and electrocardiogram (ECG) alone would distinguish women with preeclampsia with severe features from non-preeclamptic patients.
METHODS
The study protocol was approved by the University of Florida Institutional Review Board (UF IRB #189-2011), and all subjects provided written informed consent. Exclusion criteria included proteinuria prior to pregnancy, labor beyond 5 cm cervical dilation, and presence of neuraxial anesthesia. Three groups of women were included in this analysis: those with PE with severe features, chronic hypertension (presenting prior to 20 weeks gestation) or gestational hypertension (combined as HTN), and normotensive pregnant women. Women with chronic hypertension and those with gestational hypertension were analyzed together because both cause high blood pressure in a patient presenting after 20 weeks gestation. If limited data were available prior to this point, the exact diagnosis would not be known. This poses a diagnostic dilemma common in patient care environments with limited frequency of contact. Excluded from this analysis were subjects with PE without severe features or an incomplete evaluation (e.g., hypertension but no analysis for urine protein), those who developed a confounding disease state during admission (e.g., vasculitis or renal or liver disease unrelated to PE), and those with less than 15 min of reliable ECG/PPG data available.
Severe PE was initially defined based on the 2002 American College of Gynecologists criteria12. This earlier definition was used because the study was initiated prior to the 2014 update, and to facilitate comparison with other studies presented in the Discussion. Subsequent analysis found that all patients designated severe would be similarly classified based on the new criteria. The severe PE group was compared first with normotensive women and then with subjects with HTN without superimposed PE.
Continuous, standard three-lead ECG from the maternal chest, and pulse oximetry waveforms (PPG) from the right middle finger were obtained for 30 min with the patient at rest in bed with head elevated. All signals and data were collected through a custom amplifier and data collection system designed to acquire and synchronize the unprocessed signals. The PPG signal was pre-processed to remove artifacts such as baseline wander and powerline noise. The resulting signal was low-pass filtered using a linear phase FIR (finite impulse response) filter (cutoff 15 Hz). A pulse identification algorithm was used to identify the start and end points of individual pulses along with the systolic and diastolic peaks. To stabilize the baseline and facilitate extraction of individual features, the second order derivative of the PPG signal was taken, yielding an acceleration plethysmogram, so called because it relates to acceleration of blood in the finger10 (Fig. 1).
Figure 1.
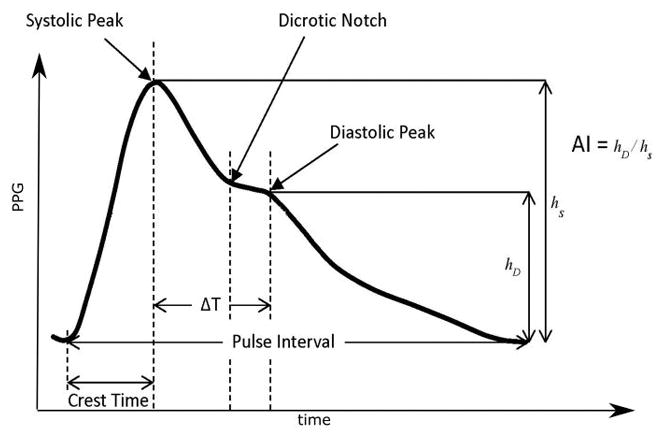
The plethysmogram waveform, examples of the extracted features, and its second derivative, the acceleration plethysmogram. Crest time, delta T, pulse width and AI: augmentation index.
The ECG signal was pre-processed to remove artifacts and processed using the Pan-Tompkins algorithm to identify the R peaks. Ectopic beats were removed and the interval between successive normal R peaks (beat-to-beat interval termed “NN”) was calculated. These inter-beat intervals are represented by a tachogram and they capture HRV.
Statistical Methods
Differences in continuous measures (age, BMI, blood pressure, gestational age) across the three groups were analyzed with ANOVA, with post hoc tests directly comparing PE versus control and PE versus HTN; ANOVA on Ranks was used for non-normally distributed measures (cervical dilation, pain). Logistic regression was used to compare PE versus control and PE versus HTN for categorical measures (race/ethnicity, nulliparity, intrauterine growth restriction, active labor, antihypertensive use, oxytocin, and magnesium administration).
Following a literature search of parameters used in similar studies, more than 60 timing, shape, and HRV features were analyzed. The most informative features were selected using the Least Absolute Shrinkage and Selection Operator (LASSO) procedure.11 LASSO is a popular and attractive technique for variable selection for high-dimensional data. It uses a regularization technique to select the parameters most likely to create a good model of the data and has been shown to work very well for problems with many variables and limited sample sizes.
After selecting the model parameters using the LASSO procedure, linear discriminant analysis was used as the classifier, using normalized features. The features were normalized to have zero mean and unit variance. The normalization parameters are considered part of the model, they were calculated in the training set and applied in the test set. The classification simulations included the following steps:
Dataset partitioned into training /test sets using five-fold cross-validation.
Feature extraction of training /test sets for each fold.
Classifier model trained for each fold.
Model tested on training /test sets for each fold.
Classification performance computed and aggregated over all the folds.
Steps 1 through 5 repeated 1000 times and classification results aggregated.
We used the expected misclassification cost as our criterion for choosing the classification boundary between the two classes. We assume that the prior probabilities and misclassification costs are equal for the two classes, but the methodology could be weighted based on most clinically relevant prediction – rule-out preeclampsia, identify high risk patients, etc.
To explore the significance of each selected variable and provide insight on the information that each classifier is exploiting, we tested the distributions of the groups of interest using the non-parametric Mann-Whitney U test. Since each classifier was trained independently from the other, we test the variables of each classifier separately. While the above analysis of the involved variables was not used in the variable selection procedure, it provides useful information that helps interpret the results and supports the effectiveness of the classifier. P < 0.05 was considered statistically significant after adjustment using a false discovery rate approach.
Assuming a prevalence of PE of 30%, based on our selection criteria, a total n = 67 – 70 would be required to detect a minimum sensitivity and specificity of 80% and 70%, respectively. This estimation assumes 80% power and alpha = 0.05.
RESULTS
One hundred eight parturients were included in the analysis: severe PE, 37; normotensive control, 43; and HTN, 28 (chronic, 8; gestational, 20). Subject characteristics are listed in Table 1. Maternal demographic characteristics did not differ between groups. PE deliveries were at a younger gestational age compared to controls and HTN. Systolic blood pressure was higher in the PE group than in either comparative group, and diastolic blood pressure was higher in the PE group than in controls. Antihypertensive use within six hours of data collection, and magnesium administration during data collection were more frequent in PE group compared to controls, but did not significantly differ between PE and HTN groups.
Table 1.
Subject Characteristics
| Characteristics | Control (n = 43) | Preeclampsia (n = 37) | Hypertension (n = 28) | Pa | Pairwise Comparisonsb | |
|---|---|---|---|---|---|---|
| Preeclampsia vs control | Preeclampsia vs hypertension | |||||
| Maternal age, mean years ± SD | 26.4 ± 5.5 | 26.6 ± 5.4 | 27.8 ± 6.1 | 0.650 | ||
| Body mass index, mean ± SD | 34.5 ± 8.9 | 33.7 ± 7.0 | 37.4 ± 9.2 | 0.254 | ||
| Diastolic blood pressure, mean mmHg ± SD | 68.3 ± 9.0 | 86.0 ± 10.4 | 81.5 ± 8.7 | <0.001 | <0.001 | 0.11 |
| Systolic blood pressure, mean mmHg ± SD | 117.7 ± 1.9 | 146.2 ± 15.0 | 134.8 ± 11.4 | <0.001 | <0.001 | <0.001 |
| Gestational age, mean weeks ± SD | 36.8 ± 4.3 | 32.2 ± 3.6 | 36.3 ± 3.1 | <0.001 | <0.001 | <0.001 |
| Race/Ethnicity, n (%) | 0.732 | |||||
| Caucasian | 24 (55.8%) | 18 (48.7%) | 17 (60.7%) | |||
| African-American | 15 (34.9%) | 14 (37.8%) | 10 (35.7%) | |||
| Hispanic | 2 (4.7%) | 3 (8.1%) | 1 (3.6%) | |||
| Asian | 2 (4.7%) | 0 (0%) | 2 (5.4%) | |||
| Nulliparity, n (%) | 27 (62.8%) | 18 (48.7%) | 12 (42.9%) | 0.267 | ||
| Intrauterine growth restriction, n (%) | 6 (14.0%) | 7 (18.9%) | 3 (10.7%) | 0.676 | ||
| Cervical dilation, median cm (range) | 1.0 (0–4.0) | 1.0 (0–5.0) | 0 (0–3.0) | 0.139 | ||
| Pain Score, median (range) | 0 (0–8.0) | 0 (0–6.5) | 0 (0–9.0) | 0.252 | ||
| In active labor, n (%) | 20 (46.5%) | 10 (27.0%) | 9 (32.1%) | 0.173 | ||
| Antihypertensive use, n (%) | 0 (0%) | 27 (73.0%) | 10 (43.5%) | <0.001 | <0.001 | 0.065 |
| Oxytocin administered, n (%) | 19 (44.2%) | 8 (21.6%) | 9 (32.1%) | 0.097 | ||
| Magnesium administered, n (%) | 3 (7.0%) | 32 (86.5%) | 5 (17.9%) | <0.001 | <0.001 | 0.064 |
P values from ANOVA or ANOVA on Ranks for continuous measures or chi-square test (calculated from logistic regression output) for categorical measures
For Pairwise comparisons, Dunnett’s test was used for continuous measures and logistic regression was used for categorical measures.
Analysis of PE versus controls resulted in a six-dimensional feature vector, including three PPG-based features and three HRV metrics per patient (Supplemental Table 1). The area under the ROC curve was 0.907 (SE =0.004) (Fig. 2A). Table 2 presents the mean value and standard error of the mean for the variables used in the model. All of the variables significantly differed (P < 0.001) between the two groups. The classifier used the combined contributions of all the variables to construct a classification score used for the decision.
Figure 2.
Receiver operating characteristic curve for (A) normotensive controls (negative class) versus severe preeclampsia (positive class). AUC (SE) 0.907 (0.004) and (B) hypertensives (negative class) versus severe preeclampsia (positive class). AUC (SE) 0.795 (0.007).
Table 2.
Comparison of the Features Distinguishing Preeclampsia from Controls
| Features | Preeclampsia (mean ± SD) | Control (mean ± SD) |
P Mann-Whitney U |
|---|---|---|---|
| Low Frequency Power (amplitude2) | 799.5 ± 67.2 | 1219.2 ± 71.5 | <0.001 |
| Poincare SD2 (seconds2) | 0.078 ± 0.005 | 0.099 ± 0.006 | 0.021 |
| Multiscale Entropy Scales 1–5 slope ( ) | 0.272 ± 0.014 | 0.349 ± 0.011 | <0.001 |
| Delta T (seconds) | 0.235 ± 0.007 | 0.283 ± 0.004 | <0.001 |
| Crest Time (seconds) | 0.199 ± 0.008 | 0.154 ± 0.003 | <0.001 |
| Spring Constant (PPG amplitude/seconds2) | 126.0 ± 10.1 | 202.8 ± 10.8 | <0.001 |
Comparing PE with HTN resulted in a five-dimensional feature vector, including three PPG-based features and two HRV metrics per patient (Supplemental Table 2). The area under the ROC curve was 0.795 (SE =0.007) (Fig. 2B). Table 3 presents the variables used in the model discriminating PE from HTN. In this case, the pRR50, peak-to-peak interval of the PPG pulse, and variance of crest time did not individually present significant differences between the two groups, although the combination proved predictive during the feature selection procedure.
Table 3.
Comparison of the Features Distinguishing Preeclampsia from HTN
| Features | Preeclampsia (mean ± SD) | Control (mean ± SD) |
P Mann-Whitney U |
|---|---|---|---|
| pRR50 (percent) | 6.39 ± 1.85 | 12.75 ± 2.88 | 0.081 |
| Low Frequency / (Low Frequency + High Frequency) (percent) | 0.193 ± 0.016 | 0.262 ± 0.024 | 0.019 |
| Peak to Peak Interval (seconds) | 0.751 ± 0.019 | 0.721 ± 0.015 | 0.395 |
| Variance of Crest Time (seconds2) | 4.4*10−4 ± 0.69*10−4 | 9.3*10−4 ± 2*10−4 | 0.063 |
| Variance of Spring Constant (PPG amplitude/seconds2)2 | 2164 ± 351) | 5296 ± 1219 | 0.011 |
Table 4 lists the classification performance on the test set aggregated over 1000 simulations.
Table 4.
Mean of the Test Set Performance over 1000 Simulations at the Optimal Receiver Operating Characteristic Point for the two classifiers
| Metric* | Severe preeclampsia vs controls (mean ± SE) (n = 37 vs 43) | Severe preeclampsia vs Hypertension (mean ± SE) (n = 37 vs 28) |
|---|---|---|
| Accuracy | 0.843 (0.001) | 0.748 (0.002) |
| Sensitivity | 0.782 (0.003) | 0.790 (0.002) |
| Specificity | 0.899 (0.001) | 0.687 (0.004) |
| Positive predictive value | 0.883 (0.001) | 0.799 (0.002) |
| Negative predictive value | 0.823 (0.001) | 0.689 (0.002) |
| False positive rate | 0.101 (0.001) | 0.313 (0.004) |
Accuracy measures the percent of correct decisions of the classifier for both classes. Sensitivity measures the percent of the preeclampsia patients identified as such, while specificity measures the percent of control or HTN patients correctly identified, respectively. The False positive rate measures the percent of control/HTN subjects identified as preeclamptic. Positive Predictive Value measures the chance that a positive preeclampsia prediction is correct, while the Negative Predictive Value measures the chance that a negative preeclampsia prediction is correct. The average value of the 1000 iterations is reported for all measures. The Standard error of the mean is calculated by taking the standard deviation of the estimated bootstrapped means.
To test the effectiveness of the model used in new, unseen data, and evaluate the external validity of the classifier, we split each study’s data based on collection date. We used the first 70% percent for training a classifier, using the selected features in Supplemental Table 1 (for controls) and Supplemental Table 2 (for HTN). The remaining 30% of the data were used for testing.
For PE vs control, we trained the classifier on a dataset consisting of 31 control and 25 PE subjects. The test set consisted of 12 control and 12 PE subjects. The classifier accuracy on the test set was 87.5%, with one false positive and two false negative predictions. The sensitivity was 0.833 and the specificity 0.910.
The classifier for discriminating between HTN and PE subjects was trained using 20 HTN and 25 PE subjects. The test set consisted of 8 HTN and 12 PE subjects. The classifier accuracy on the test set was 80%, with two false positive and two false negative predictions. The sensitivity was 0.833 and the specificity 0.75.
The results are comparable to those of the cross-validation procedure and indicate that the classifiers should be able to generalize to new data without significant deterioration of the classification accuracy.
DISCUSSION
Worldwide, PE causes 76,000 maternal and 500,000 fetal or neonatal deaths and is the second most common cause of maternal mortality.13 In some cases, the diagnosis is simple: a gravid woman presents with newly elevated blood pressure and significant proteinuria after 20 weeks gestation. Management decisions are then based on disease severity and gestational age. However, nearly one-third of preeclamptics do not present as clearly,14 and there is no predictive test with adequate discrimination power. Even in women with eclampsia, nearly one-half (43%) were not previously diagnosed with both hypertension and proteinuria.15 In recognition of the high prevalence of atypical presentation,16,17 and due to a sense that failure to treat a false-negative poses greater risk than treating a false-positive,18 a large number of women without disease are treated. They are hospitalized, maintain prolonged bedrest, experience increased monitoring, incur additional costs, and possibly suffer complications from aggressive treatment of presumed preeclampsia (e.g., preterm delivery or magnesium toxicity). At particular risk is the unfamiliar patient who presents with hypertension of unknown duration.
Recent work on prediction models has focused on vasoreactive markers identified in maternal blood. Low placental growth factor (PlGF) and/or an elevated ratio of soluble Fms-like tyrosine kinase-1 (sFlt-1)-to-PlGF improves the prediction of complications in early-onset PE (≤34 weeks).19 Commercial immunoassays are available, although not yet in the United States.20 As for so many screening tools, the greatest utility of these predictive tests may be their negative predictive value (NPV).
Chappell et al.21 studied plasma PlGF (<5th percentile for gestational age) in women between 20 and 35 weeks gestation presenting with suspected PE using a primary outcome of delivery within 14 days for PE. The area under the curve for the ROC was 0.87, but the NPV of normal PlGF was 98%.
Zeisler et al.22 investigated sFlt-1-to-PlGF ratios in women 24 to 36 weeks gestation with singleton gestations in whom PE was suspected. After identifying a cutoff value of 38 in a development cohort of 50 women, they prospectively validated the test on 550 women. The ratio of ≤38 had an NPV for development of PE in 1 week of 99.3% with 80.0% sensitivity and 78.3% specificity. The positive predictive value was much lower: 36.7% for a diagnosis of PE when the time frame was extended to 4 weeks (sensitivity 66.2%, specificity 83.1%).
In an economic modeling analysis comparing the routine use of vascular factor testing (PlGF-1 and sFlt-1) with current practice, Hadker et al.23 identified a 42% cost saving, despite the cost of laboratory analysis, due to reduction in treatment of false-positive (115/1000) and false-negative (15/1000) results. Although promising, the cost and availability of this testing will remain a hurdle, particularly in the developing world. A reliable, inexpensive diagnostic device that requires minimal training and no maintenance would improve the provision of care.
Identification of the cardiovascular changes unique to PE may provide an additional tool for diagnosis. Current understanding of the disorder identifies endothelial dysfunction caused by the release of mediators from the abnormal placentation and resulting in increased vascular reactivity and a high resistance state.16 Using pulse wave analysis, Avni et al.5 demonstrated that aortic stiffness in preeclamptics exceeds that of both normotensive and HTN parturients. Pulse wave analysis employs a tonometer applied to the radial artery that converts the signal into an aortic pressure waveform and then measures various features of the resulting tracing. This technology has been found to distinguish preeclamptic, hypertensive, and normotensive pregnancies in the third trimester2,3,24 and can also predict eventual PE (79% sensitivity with 11% false positives) at 11 to 14 weeks gestation.4 Tonometry, however, requires training and remains an expensive technology. PPG, using a pulse oximeter probe, may be a simpler alternative to obtain similar data.
The digital volume pulse, the waveform from a pulse oximeter, is generated by forward pressure due to blood ejection from the left ventricle (systolic peak), as well as reflected waves from vessel branching (diastolic peak or inflection point). Numerous features can be extracted by taking first and second derivatives of the waveform. These have been correlated with large and small arterial stiffness and distensibility. Indices from the PPG have been correlated with age, blood pressure, risk for coronary artery disease, and the presence of atherosclerosis.10 Specific to PE, Arioz et al.25 studied the PPG signal and identified a 50% increase in arterial stiffness (5.9 ± 0.8 m/s vs. 8.8 ± 1.2, P < 0.0001) in the presence of the disease.
HRV reflects, among other things, the balance between the sympathetic and parasympathetic systems. Since at least 1996, when Schobel et al.26 recorded sympathetic nerve activity at skeletal muscle blood vessels, PE has been associated with sympathetic overactivity, yet a review of studies looking at autonomic function testing found no consistent difference between preeclamptic and normotensive pregnancies.27 However that review did not look at HRV parameters. Tejera et al.8 investigated several HRV parameters using a neural network and separated normotensive, hypertensive, and preeclamptic patients with area under the curve >0.95. Our work differs in that their model depended largely on additional information, most importantly, blood pressure. Even the sex of the fetus factored in. For use in the developing world, these parameters are less accessible. An accurate model that requires no additional inputs, or limits those to readily accessible information requiring no equipment or training, is preferable.
The current study successfully separated severe preeclamptic from normotensive pregnant subjects with high sensitivity and specificity (0.782 (SE=0.003) and 0.899 (SE=0.001), respectively), rivaling that of the circulating vascular factor studies. The performance separating hypertensive from severe preeclamptic subjects was only slightly less accurate (sensitivity 0.790 (SE=0.002); specificity 0.687 (SE=0.004). We also demonstrate that features of PPG and ECG are altered in PE, and that those features are not related solely to elevated blood pressure. With further prospective studies, we hope to reduce the need for evaluation on Labor and Delivery for “rule-out preeclampsia,” reducing laboratory, nursing and administrative expenses, as well as patient inconvenience.
Severe PE, chosen as the subject of study here, is likely one point on a spectrum of disease with artificial subdivisions of the phenotype. Some researchers promote an inclusive definition, considering the disease a multi-systemic disorder in which not all systems are involved in every patient (thus, even hypertension and proteinuria are not required for diagnosis).17 Others argue that only a renal biopsy showing glomerular endotheliosis can prove PE and that 76% of multiparas are misdiagnosed (25% of primiparas); therefore, only the latter should be included in related research.28 Recently, Myatt et al.29 advocated consideration of PE as a syndrome rather than a specific disease. By restricting our comparison group to those patients diagnosed with severe features, we hoped to identify parameters present in those most in need of higher level care. Recognizing those patients prior to the onset of severe symptoms is the subject of an ongoing study. Furthermore, identifying those who will NOT develop complications, and therefore do not require continued hospitalization or iatrogenic preterm delivery, would further improve healthcare and reduce costs.
Some limitations should be considered when reviewing the results of this study. First, while our predictive model was able to discriminate among our groups well, case-control studies can inflate diagnostic accuracy estimates, thus prospective studies are needed to confirm our findings. These estimates may also be inflated because only those with severe PE were included; however, as discussed above, we intended to focus our prediction on identifying patients who would need a higher level of care. Furthermore, our study may have been underpowered to detect differences in patient characteristics, including BMI, nulliparity, and intrauterine growth restriction. However, despite these limitations, our study provides support that various vascular features may be useful in screening women at risk for PE.
An inexpensive, non-invasive, reliable test for PE would reduce healthcare costs and improve safety for mothers and babies. While this is of interest for all obstetric care providers, it is particularly true in low and middle income countries where even blood pressure screening is complicated by training of healthcare workers and maintenance of calibrated equipment.30 Furthermore, the ability to identify these patients earlier in gestation would allow for the implementation of prevention strategies such as aspirin,31 which is most effective when administered during the first trimester.
Based on the predictive value of first trimester pulse wave analysis by Khalil et al.,4 and on the current data that appear to validate PPG and HRV as a replacement for this technology at later gestation, future efforts by the authors will investigate the value of PPG and HRV analysis in predicting PE early in pregnancy.
Supplementary Material
Acknowledgments
This material is based upon work supported by the National Institutes of Health (grant no. 1R41HD075550-1).
The authors thank the data collectors at UF Health, including Teresa Lyles, PhD, as well as our editor, Corey Astrom. Finally, we would like to thank the nursing and physician staff at UF Health.
Footnotes
Tammy Y. Euliano, MD: This author helped design the study and wrote the manuscript, Kostas Michalopoulos, PhD: This author helped design the algorithms to interpret the signals and wrote the methods sections of the manuscript, Savyasachi Singh, PhD: This author helped design the algorithms to interpret the signals, Anthony R. Gregg, MD: This author helped with study design and implementation, Mariem Del Rio, MPH: This author helped oversee data collection and contributed to study design, Terrie Vasilopoulos, PhD: This author helped with statistical analysis and wrote the statistical section of the manuscript, Amber M. Johnson, BS: This author helped collect data and contributed to study design, Allison Onkala, MPAS: This author helped oversee data collection and contributed to study design, Shalom Darmanjian, PhD: This author helped with device design and implementation, Neil R. Euliano, PhD: This author helped oversee the engineering side of the project and contributed to the manuscript, Monique Ho, MD: This author helped with study design and implementation.
NRE (TYE’s husband) is the President, and Drs. Michalopoulos and Singh are employees, and Dr. Darmanjian was an employee of Convergent Engineering. TYE is listed on patents filed for some of the technology described in this paper. The remaining authors report no conflicts of interest.
Clinical trial identification number: UF IRB: 189-2011; URL of the registration site: http://irb.ufl.edu
References
- 1.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol. 2013;25:124–132. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- 2.Rönnback M, Lampinen K, Groop PH, Kaaja R. Pulse wave reflection in currently and previously preeclamptic women. Hypertens Pregnancy. 2005;24:171–180. doi: 10.1081/PRG-200059871. [DOI] [PubMed] [Google Scholar]
- 3.Coppadoro A, Berra L, Bittner EA, Ecker JL, Pian-Smith MCM. Altered arterial compliance in hypertensive pregnant women is associated with preeclampsia. Anesth Analg. 2013;116:1050–1056. doi: 10.1213/ANE.0b013e318282dc58. [DOI] [PubMed] [Google Scholar]
- 4.Khalil AA, Cooper DJ, Harrington KF. Pulse wave analysis: a preliminary study of a novel technique for the prediction of pre-eclampsia. BJOG. 2009;116:268–276. doi: 10.1111/j.1471-0528.2008.01906.x. discussion 276–277. [DOI] [PubMed] [Google Scholar]
- 5.Avni B, Frenkel G, Shahar L, Golik A, Sherman D, Dishy V. Aortic stiffness in normal and hypertensive pregnancy. Blood Press. 2010;19:11–15. doi: 10.3109/08037050903464535. [DOI] [PubMed] [Google Scholar]
- 6.Millasseau SC, Ritter JM, Takazawa K, Chowienczyk PJ. Contour analysis of the photoplethysmographic pulse measured at the finger. J Hypertens. 2006;24:1449–1456. doi: 10.1097/01.hjh.0000239277.05068.87. [DOI] [PubMed] [Google Scholar]
- 7.Acharya U, Joseph K, Kannathal N, Lim C, Suri J. Heart rate variability: a review. Med Biol Engineer Comput. 2006;44:1031–1051. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 8.Tejera E, Jose Areias M, Rodrigues A, Ramõa A, Manuel Nieto-Villar J, Rebelo I. Artificial neural network for normal, hypertensive, and preeclamptic pregnancy classification using maternal heart rate variability indexes. J Matern Fetal Neonatal Med. 2011;24:1147–1151. doi: 10.3109/14767058.2010.545916. [DOI] [PubMed] [Google Scholar]
- 9.Walther T, Wessel N, Malberg H, Voss A, Stepan H, Faber R. A combined technique for predicting pre-eclampsia: concurrent measurement of uterine perfusion and analysis of heart rate and blood pressure variability. J Hypertens. 2006;24:747–750. doi: 10.1097/01.hjh.0000217858.27864.50. [DOI] [PubMed] [Google Scholar]
- 10.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8:14–25. doi: 10.2174/157340312801215782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc B. 1996;58:267–288. [Google Scholar]
- 12.American College of Gynecologists. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 13.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 14.von Dadelszen P, Payne B, Li J, et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7. [DOI] [PubMed] [Google Scholar]
- 15.Douglas K, Redman C. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steegers E, von Dadelszen P, Duvekot J, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 17.Pettit F, Brown M. The management of pre-eclampsia: what we think we know. Eur J Obstet Gynecol Reprod Biol. 2012;160:6–12. doi: 10.1016/j.ejogrb.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 18.Meads C, Cnossen J, Meher S, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2008;12:1–270. doi: 10.3310/hta12060. [DOI] [PubMed] [Google Scholar]
- 19.Chaiworapongsa T, Romero R, Korzeniewski S, et al. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287.e1–287.e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benton S, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 2011;205:469.e1–e8. doi: 10.1016/j.ajog.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Chappell L, Duckworth S, Seed P, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia a prospective multicenter study. Circulation. 2013;128:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 22.Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 23.Hadker N, Garg S, Costanzo C, et al. Financial impact of a novel preeclampsia diagnostic test vs. standard care: a decision-analytic modeling analysis from a German health care payer perspective. Value Health. 2009;12:A321. [Google Scholar]
- 24.Elvan-Taspinar A, Franx A, Bots ML, Bruinse HW, Koomans HA. Central hemodynamics of hypertensive disorders in pregnancy. Am J Hypertens. 2004;17:941–946. doi: 10.1016/j.amjhyper.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Arioz DT, Saglam H, Demirel R, et al. Arterial stiffness and dipper/nondipper blood pressure status in women with preeclampsia. Adv Ther. 2008;25:925–934. doi: 10.1007/s12325-008-0090-2. [DOI] [PubMed] [Google Scholar]
- 26.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia - a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 27.Rang S, Wolf H, Von Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy-associated hypertensive disorders: a review. J Hypertens. 2002;20:2111–2119. doi: 10.1097/00004872-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 1981;60:267–276. [PubMed] [Google Scholar]
- 29.Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17:8. doi: 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- 30.Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract Res Clin Obstet Gynaecol. 2011;25:537–548. doi: 10.1016/j.bpobgyn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007:CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Wei CC. Developing an effective arterial stiffness monitoring system using the spring constant method and photoplethysmography. IEEE Trans Biomed Eng. 2013;60:151–154. doi: 10.1109/TBME.2012.2207384. [DOI] [PubMed] [Google Scholar]
- 33.Kamath MV, Fallen EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng. 1993;21:245–311. [PubMed] [Google Scholar]
- 34.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 35.Bravi A, Longtin A, Seely AJ. Review and classification of variability analysis techniques with clinical applications. Biomed Eng Online. 2011;10:90. doi: 10.1186/1475-925X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumert M, Javorka M, Seeck A, Faber R, Sanders P, Voss A. Multiscale entropy and detrended fluctuation analysis of QT interval and heart rate variability during normal pregnancy. Comput Biol Med. 2012;42:347–352. doi: 10.1016/j.compbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Usman S, Reaz M, Ali M. Determining the arterial stiffness through contour analysis of a PPG and its association with HbA1c among diabetic patients in Malaysia. Maringa. 2014;36:6. [Google Scholar]
- 38.Seitsonen ER, Korhonen IK, van Gils MJ, et al. EEG spectral entropy, heart rate, photoplethysmography and motor responses to skin incision during sevoflurane anaesthesia. Acta Anaesthesiol Scand. 2005;49:284–292. doi: 10.1111/j.1399-6576.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 39.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 40.DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19:78–81. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.