eTOC Blurb
In humans, there is a consistent relation that has been reported between self-control and general intelligence. Beran and Hopkins report the same relation in chimpanzees that were given a test of delay of gratification and a battery of social and cognitive tasks that measure general intelligence.
Keywords: Chimpanzees, Pan troglodytes, Intelligence, Delay of Gratification, Self-Control
Summary
For humans, there appears to be a clear link between general intelligence and self-control behavior, such as sustained delay of gratification [1–9]. Chimpanzees also delay gratification [10–12], and can be given tests of general intelligence (‘g’) [13–15], but these two constructs have never been compared within the same sample of nonhuman animals. We presented 40 chimpanzees with the Hybrid Delay Task (HDT) [16–17] which measures inter-temporal choices and the capacity for sustained delay of gratification and the Primate Cognitive Test Battery (PCTB) which measures general intelligence in chimpanzees [13–15]. Importantly, none of the sub-tasks in the PCTB directly assesses self-control or other forms of behavioral inhibition. Rather, they assess areas of physical cognition (e.g., quantity discrimination) or social cognition (e.g., gaze following). In three phases of testing, we consistently found that the strongest relation was between chimpanzee ‘g’ scores and efficiency in the HDT. Chimpanzee ‘g’ was not most closely related to the proportion of trials the chimpanzees chose to try to wait for delayed rewards, but rather was most closely related to how good they were at waiting for those rewards when they chose to do so. We also found the same strong relation between HDT efficiency and those factors in the PCTB that loaded most strongly on chimpanzee ‘g.” These results highlight that, as with humans, there is a strong relation between chimpanzees’ self-control and their overall intelligence, a relation that likely reflects the role of successful inhibitory control during cognitive processing of information and intelligent decision-making.
Results
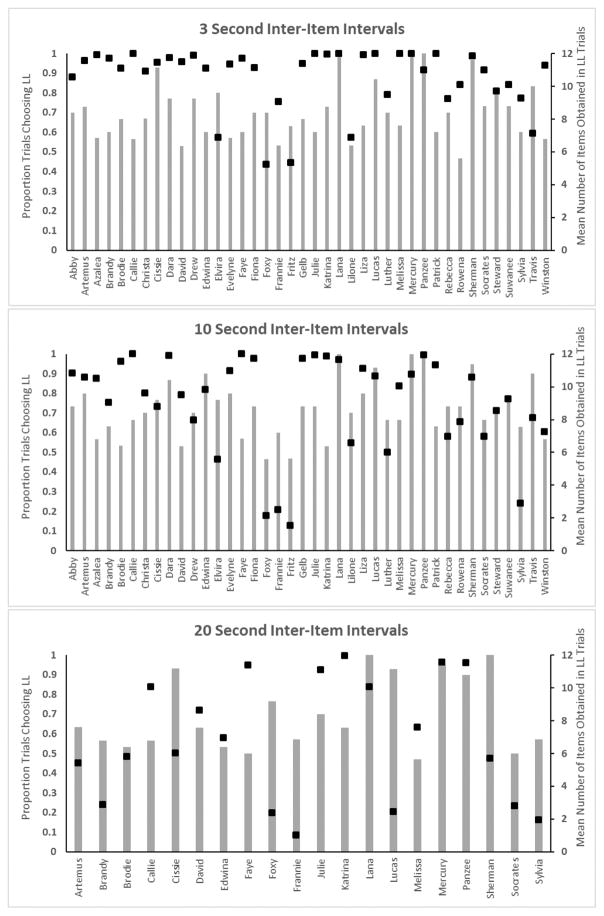
Figure 1 presents the performance of each chimpanzee in each condition of the HDT in terms of how often the delayed option was selected, and how many items were accumulated on those trials. Of specific interest was whether performance on the HDT task was associated with domain general intelligence. Domain general intelligence, or “g,” refers to a latent, general problem solving skill [18–20] that some have argued was selected for in primate evolution and accounts for the wide range of cognitive specializations observed in humans compared to more distantly related species [21–23]. There is debate as to whether the construct of a general intelligence factor more accurately reflects the nature of primate cognition versus a more modular conception of intelligence [24–27]. Our hypothesis, given past testing with chimpanzees that indicated evidence of a “g” factor for chimpanzee intelligence [14] was that efficient engagement of delay of gratification should be associated with better overall cognitive performance, and we predicted that significant positive associations would be found between HDT performance and overall “g” scores derived from the PCTB rather than specific subscale scores. Delay of gratification is a form of self-control, and in the HDT it is reflected in the accumulation of larger quantities over time, where immediate consumption of available reward ended any additional accumulation.
Figure 1. Performance of each chimpanzee in the HDT.
The bars shown the proportion of trials on which each chimpanzee choose the LL option. The points indicate the mean number of items obtained on those LL trials (maximum = 12).
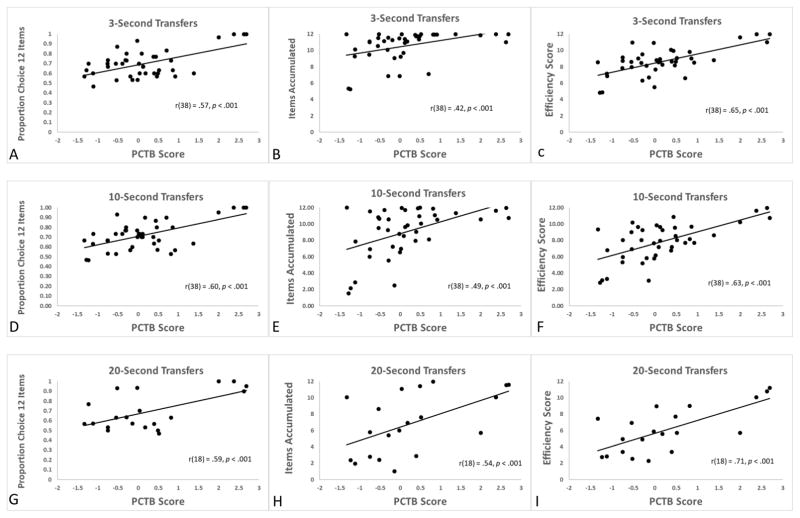
Figure 2 presents the relation of composite PCTB “g” scores to three measures from the HDT in each of the three conditions. First, PCTB scores are shown in relation to the proportion of choices of the LL option in each condition (panels A, D, G). As a reminder, this measure reflects how often the chimpanzees pointed to 12 grapes rather than 4 grapes. Second, PCTB scores are shown in relation to the average number of items accumulated in the delay phase of trials where the 13-item set was selected (panels B, E, H). As a reminder, these panels would reflect the relation of general intelligence to how long chimpanzees could wait for accumulating rewards when they had chosen to wait for those rewards. Third, PCTB scores are shown in relation to a measure of overall task efficiency (panels C, F, I). This measure reflects the average number of grapes eaten across all trials, whether the smaller-sooner (SS) or larger-later (LL) option was selected. It is designated as a measure of efficiency because it allows for ranking chimpanzees in terms of how much food they obtained but is agnostic as to whether a given chimpanzee should have chosen the SS or LL option on a given trial. Rather, it indicates how the overall performance pattern of the chimpanzee in its choices and in its delay of gratification during LL trials produced reward. Thus, it could be viewed as a measure of cognitive monitoring of self-control resources rather than just the proportion of self-control choices that are made.
Figure 2. Relationship between PCTB scores and HDT performance.
This is shown in terms of the percentage of choice of the LL set (left panels), HDT mean number of items accumulated when the LL set had been selected (center panels), and HDT efficiency score (right panels). For all inter-item transfer rates (3 seconds, 10 seconds, and 20 seconds), the efficiency score best predicted PCTB performance in this sample of chimpanzees.
For the 3-second delay condition, there was a significant correlation of PCTB score and proportion of LL choices made, r(38) = .57, p < .001. There also was a significant correlation of PCTB score and the average accumulation performance on LL trials, r(38) = .42, p < .001. And, there was a significant correlation of PCTB score and efficiency score, r(38) = .65, p < .001. Given that all of these HDT measures showed individual significant relations with PTCB performance, we conducted a multiple regression analysis with those three factors to determine which of them best predicted PCTB score. That analysis showed that the efficiency score best predicted PCTB score, t(38) = 5.30, p < .001, and it accounted for 42.4% of the variance. The other two factors, proportion choice LL and accumulation performance in LL trials, did not account for statistically significant additional levels of variance: proportion choice, t(38) = 1.61, p = .11; accumulation t(38) = 1.61, p = .11.
Crucially, there also was not a significant relation between proportion choice of the LL response and accumulation performance on LL trials, r(38) = .11, p = .51. This indicates that those chimpanzees that waited longer when accumulating were not necessarily those who also chose most often the LL option.
For the 10-second delay, there was a significant correlation of PCTB score and proportion of LL choices made, r(38) = .60, p < .001. There also was a significant correlation of PCTB score and the average accumulation performance on LL trials, r(38) = .49, p < .001. And, there was a significant correlation of PCTB score and efficiency score, r(38) = .63, p < .001. Given that all of these HDT measures showed individual significant relations with PTCB performance, we conducted a multiple regression analysis with those three factors to determine which of them best predicted PCTB score. That analysis showed that the efficiency score best predicted PCTB score, t(38) = 4.96, p < .001, and it accounted for 39.3% of the variance. The other two factors, proportion choice LL and accumulation performance in LL trials, did not account for statistically significant additional levels of variance: proportion choice, t(38) = 1.85, p = .06; accumulation t(38) = 1.88, p = .06.
For this condition, there was a significant relation between proportion choice of the LL response and accumulation performance on LL trials, r(38) = .42, p = .001.
For the 20-second delay, there was a significant correlation of PCTB score and proportion of LL choices made, r(18) = .59, p < .001. There also was a significant correlation of PCTB score and the average accumulation performance on LL trials, r(18) = .54, p < .001. And, there was a significant correlation of PCTB score and efficiency score, r(18) = .71, p < .001. Again, we conducted a multiple regression analysis with those three factors to determine which of them best predicted PCTB score. That analysis showed that the best model included efficiency score, t(18) = 2.43, p < .001, which accounted for 49.7% of the variance and accumulation performance, t(18) = 3.04, p < .001, which accounted for an additional 17.7% of the variance. Proportion choice of the LL set did not account for statistically significant additional levels of variance, t(18) = 0.82, p = .43.
As in condition 1, for condition 3 there was not a significant relation between proportion choice of the LL response and accumulation performance on LL trials, r(18) = .17, p = .49. This again highlights that those chimpanzees that waited longer when accumulating were not necessarily those who also chose most often the LL option.
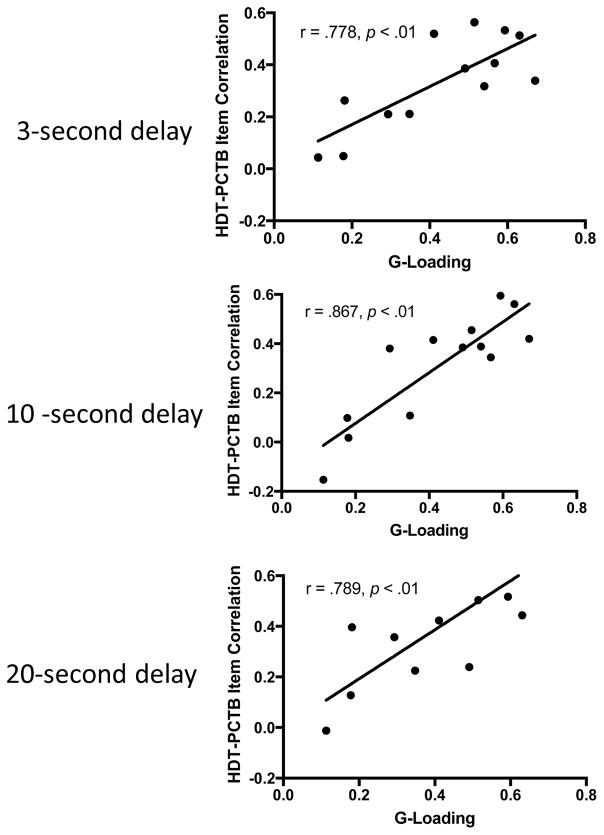
Significant positive correlations were found between HDT performance and several PCTB tasks including object permanence, rotation, transposition, numbers, tool use, gaze/point comprehension, gesture production, and attention getting behaviors (Table 1). Figure 3 shows that significant positive associations were found between PCTB task loading weights and the correlation coefficients between each measure and the HDT measures at 3 seconds, 10 seconds and 20 seconds. Thus, for PCTB tasks that more strongly loaded on the principal axis factor (PAF) analysis derived “g,” these same measures also showed stronger associations with HDT performance across the three delay intervals.
Table 1.
PCTB Tasks, Number of Trials Administered, PAF Score Weighting, and Pearson Product Moment Correlations Between PCTB Tasks and HDT Performance at Each Delay Interval
| Task | Trials | PAF Weighting | 3 Seconds | 10 Seconds | 20 Seconds |
|---|---|---|---|---|---|
| Physical Cognition Tasks | |||||
| Spatial Memory | 6 | .348 | .211 | .108 | .225 |
| Object Permanence | 18 | .671 | .339* | .420** | .788* |
| Rotation | 18 | .567 | .406** | .344* | .602** |
| Transposition | 18 | .540 | .318* | .388* | .733* |
| Quantity | 24 | .515 | .564** | .455* | .504* |
| Causality (Noise) | 12 | .181 | .363 | .018 | .396 |
| Causality (Visual) | 12 | .293 | .210 | .380* | .357 |
| Tool Use | 12 | .411 | .520** | .415** | .423 |
| Tool Properties | 12 | .178 | .047 | .098 | .128 |
| Social Cognition Tasks | |||||
| Gaze/Point Comprehension | 12 | .593 | .533** | .595** | .517** |
| Gesture Production | 8 | .631 | .513** | .561** | .444* |
| Attention State | 18 | .491 | .386* | .385* | .239 |
| Gaze Following | 6 | .113 | .044 | −.153 | −.012 |
p < .05,
p < .01.
Figure 3. The relation of weighted score of each PCTB task and HDT performance.
Weighted scores were computed from the PAF analysis and the correlation coefficient value found between each task and the HDT scores at 3 second, 10 second and 20 second delays (see Table 1).
As with the PAF scores, the correlations of unit-weighted factor (UWF) scores and HDT scores were positive and significant for the 3 second delays, r(38) = .610, p < .001, the 10 second delays, r(38) = .562, p < .001, and the 20 second delays, r(18) = .643, p < .01. Thus, using a UWF instead of a PAF score to reflect general intelligence does not change the results.
Discussion
As is true with humans, chimpanzee general intelligence is clearly and consistently related to self-control capacities, and particularly to delay of gratification. This result mirrors the impressive and long-lasting relation between performance on tests as simple as the marshmallow task, and many later outcome variables that reflect crucial aspects of human psychological well-being [1–9]. In addition, the same factors that most strongly loaded on chimpanzee “g” measures also best predicted performance on the HDT. This also makes the strong case that self-control and intelligence are linked, and the fact that such a relation exists in species other than humans likely reflects something foundational about the role of inhibitory, cognitive processes in general intelligence. From an evolutionary perspective, the present data suggest that this relation has, at minimum, a shared origin within humans and apes. Future research with other species will be needed to illustrate whether other primates, and perhaps non-primate species, also show this relation, in which case one could argue for a strong selective pressure for cognitive processes that relied on inhibitory control of action, and perhaps even precursor mechanisms that assessed present and future options during choice behavior.
It is important to note that the best relation within the HDT outcomes to chimpanzee “g” scores was not the percentage of trials choosing the LL option (or SS option). Nor was the best relation with “g” how well chimpanzees accumulated food items. Rather, it was the relation of “g” to efficiency in the HDT. The efficiency measure makes no claim as to what a chimpanzee should choose at the trial outset. Rather, it places a premium on being able to maintain delay, if one chooses the delayed reward option. In this way, it allows for chimpanzees to score more highly in terms of efficiency if they choose the LL option less often, but then are very good at accumulating rewards. This would seem to reflect a very important issue in human choice behavior – namely that it is not always in one’s best interests to choose later, better options. Sometimes, taking something less preferred, but more immediately, is important as well. Otherwise, there is the risk that one might show pathological levels of self-control [28–30]. The present results suggest that, among the generally more “intelligent” chimpanzees, HDT performance reflected this same occasional “failure” to delay, which might instead reflect the occasional need to disengage from self-control. This need to sometimes not show self-control is quite reasonable, and thus should be seen as a sign of intelligent behavior. The constant effort to wait for later things can be stressful [31–32], and this seems particularly true for animals in the wild such as chimpanzees that must consider immediate predation, competition from conspecifics, perishable resources, and the uncertainty of future outcomes that may not persist. In other words, sometimes taking a more immediate reward or benefit, such as a lower quality food that puts something in the stomach now, could boost future success in delaying gratification for other better, but delayed, rewards [31]. At the same time, flexible intelligence might lend itself to being able to recognize times when delayed gratification may not work, due either to internal factors (e.g., being too hungry, feeling too anxious) or external factors (e.g., uncertainty about the delayed reward still be available later). Thus, intelligence decision making would reflect the right balance of engaging in delay of gratification when it was best warranted but not when it was least warranted. This is certainly an interesting avenue for further investigation within comparative research.
Past research [16–17] reported different qualitative outcomes for capuchin monkeys and chimpanzees using the HDT. Those results suggested that there may be a strong concern about choice tasks assessing self-control that involve direct points to food rewards, where pointing to more food is considered to reflect the more self-controlled response. Instead, the concern is that such points may be largely driven by an impulsive drive to reach toward more of something that is valuable [31]. The present results support that concern. In two of three conditions, there was no relation between how often chimpanzees selected the LL set, and how well those chimpanzees were at delaying gratification through the maintenance part of the trial. This indicates that those two behavioral outcomes do not underlie to the same degree the presumed construct (self-control) that they are claimed to reflect when tested alone. Chimpanzee intelligence scores were not best matched by choices for the LL set, but rather by efficiency in obtaining rewards (which was a combination of choice behavior, and delay maintenance). Thus, the present results also highlight additional need for caution about using food sets as response options for intertemporal choice tasks when there is no way to confirm that such choices reflect true self-control [16–17]. The HDT avoids this concern, and its close relation with “g” in chimpanzees offers a strong parallel between human and chimpanzee self-control behavior (as reflected in both intertemporal choices and delay of gratification) and intelligence.
The significant phenotypic associations between HDT performance and “g” in chimpanzees raises the question of whether similar or different neural and genetic mechanisms underlie their expression. For instance, Latzman, Taglialatela, and Hopkins [33] recently reported in a sample of chimpanzees that the acquisition of delay of gratification performance, as measured by the accumulation task, was associated with increasing white matter connectivity between the striatum and prefrontal cortex. In light of the fact that HDT performance in the chimpanzees was associated with “g,” then it might be predicted that the volume of white matter tracts connecting these regions may similarly correlate with 1) HDT performance and 2) the PAF “g” scores, assuming they have a common neural substrate. Similarly, quantitative genetic analyses have shown that chimpanzee “g” intelligence is significantly heritable [14] but whether similar heritability is evident for delay of gratification or HDT performance remains unknown. Further, determining the genetic (as opposed to phenotypic) correlation between chimpanzee intelligence and HDT performance would provide important insight into whether common genes underlie their expression. From an evolutionary perspective, this would provide critical data on whether evolutionary factors that selected for increased self-control (or “g”) had the collateral consequence of influencing overall intelligence (or vice versa).
In summary, the findings reported here show for the first time that individual differences in self-control, as measured by the HDT task, are associated with general intelligence in chimpanzees. In short, more intelligent chimpanzees have better self-control abilities. Specifically, these individuals are better at waiting when they decide to do so, but they also seem to balance those choices with other choices for occasional immediate reward, perhaps when successful delay maintenance is unlikely. Another possibility for these results (perhaps in conjunction with the last comment) is that more intelligent chimpanzees learned the contingencies of the HDT better or more quickly, and thus showed more optimal performance. Related to both of these points, items from the PCTB that contribute more strongly to “g” are also more strongly associated with individual performance in the HDT task. Crucially, these items are not themselves tests of inhibition (as shown in Table 1). This strongly suggests that self-control and intelligence have a common, but as yet unidentified, neurobiological foundation within chimpanzees. Further, the findings reinforce previous results showing that the HDT task is a more sensitive measure of self-control by dissociating choice behavior, as reflected in the intertemporal choice tasks, from the requirement that subjects wait for the accumulation of chosen food rewards [16–17]. Together, these results demonstrate a clear and important similarity between humans and chimpanzees with regard to self-control behavior, and how the capacity for such self-control relates to general intelligence across these species. The behaviors that come from various delay of gratification tests used across species offer important insights into the nature of intelligence, and highlight that studying individual differences in self-control matter for multiple species in understanding other differences in broader aspects of cognition and general intelligence.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael Beran (mberan1@gsu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Subjects
We tested 40 adult chimpanzees at the Yerkes Primate Research Center of Emory University and the Language Research Center of Georgia State University. The chimpanzees ranged in age from 18 to 48 years of age (Mean = 27.3 years, SD = 7.19). There were 26 females and 14 males, and these specific apes were selected for use in the study because they were currently available for behavioral testing and not assigned to other protocols at the time of data collection. All chimpanzees lived in social groups in which they were housed with 2 to 3 other individuals. Al chimpanzees had access daily to indoor and outdoor areas, as well as to a variety of species-appropriate enrichment items. Chimpanzees were maintained on a veterinarian-approved diet and were not food or water restricted for the purposes of experimental testing. Chimpanzees voluntarily engaged in all experimental sessions, choosing to approach the apparatus and engage with the experimenter. Chimpanzees were never restrained, and no invasive procedures were conducted during this study. Table S1 provides additional details about each chimpanzee including their sex, age and rearing history. Under the factor rearing, we have designated the apes as either mother-reared, nursery-reared or wild caught. In captive settings, most females exhibit competent infant care (mother-reared) but sometimes females exhibited inadequate or poor maternal care of their offspring immediately after birth. To minimize the risk of injury or death to these apes, the infants were removed and raised in a human nursery setting (nursery-reared). A complete description of nursery-rearing practices at the Yerkes National Primate Research Center can be found in [34]. Lastly, a small number of chimpanzees were captured in the wild and brought to captivity before 1974 when CITES banned all importation of wild chimpanzees to the United States; thus, these apes were relatively older individuals who had been in captivity for an extended period of time.
This project was reviewed and approved by the IACUC committee of the Yerkes National Primate Research Center (IACUC #YER-2001074-041718GA) and the IACUC of Georgia State University (IACUC #A15018). All guidelines outlined in the Institute of Medicine report for the ethical use of chimpanzees in research were adhered to (and always have been) during all aspects of this research.
Apparatus
Trials were presented on a bench with a mounted sliding tray. The bench was placed outside the enclosure but flush with the mesh, so that chimpanzees could reach through to point at one of the two option that was presented. Those options were two sets of discrete food items (grape slices for Yerkes chimpanzees, cereal pieces for LRC chimpanzees) that were placed on small plates (approximately 10 cm in diameter). For Yerkes chimpanzees, a clear plastic tube with a stopper at the midpoint was placed halfway through the mesh of the caging. This allowed the grapes to accumulate in the tube without falling through while chimpanzees were able to pull in the tube at any time to eat the grapes. Once they pulled in the tube, they could eat the items in it, and then return it to the experimenter. For LRC chimpanzees, the food items accumulated in a bowl on the floor rather than a tube, given different logistical aspects of the two laboratories. In both cases, these setups allowed chimpanzees easily to retrieve their food items during trials when they chose to do so.
METHOD DETAILS
All chimpanzees had completed the PCTB testing prior to this experiment [14, 31]. Importantly, they all had done so as adult animals, and so the resulting data were not from ages when developmental trends would be expected.
All 40 chimpanzees completed the first two conditions of the HDT, and a subset of chimpanzees (N = 20, 14 females and 6 males) completed the third condition. That subset reflected that some chimpanzees at the Yerkes Primate Center were transferred to another facility before having the chance to complete the third condition. Some chimpanzees were newly tested for this specific project, and the data from other animals came from [17]. Table S1 provides more information.
There were two phases to each trial – the choice phase and the accumulation phase. In the choice phase, the experimenter presented two quantities of food items randomized for left-right placement on the bench tray - 4 items were placed on one plate, and 12 items on the other. The experimenter then slid the tray forward, allowing the chimpanzee to make a choice by pointing to one of the two food sets. The chimpanzees were highly familiar with this response routine. After they indicated a set of food items, the experimenter removed whichever set the chimpanzee did not choose, and those items were placed behind the bench and out of view. If a chimpanzee chose the set of four items (the SS option), then in the accumulation phase of the trial, the experimenter immediately transferred the four items, all at once, into the tube or bowl so the chimpanzees could obtain them. However, if a chimpanzee chose the set of 12 items (LL), then the accumulation phase of the trial was more extensive. The experimenter transferred the twelve items, one at a time, into the tube in the mesh (Yerkes) or the bowl (LRC) while all remaining items in that set remained visible. This transfer of items continued until all were delivered or until the chimpanzee began eating the items, at which point the experimenter stopped transferring items and the trial was finished. Each trial in a session was of a fixed duration, meaning that the same amount of time passed between the start of every trial and the start of the subsequent trial. This duration depended on the experimental phase (see below for more details).
We conducted the same three conditions of increasing delay duration for choices of the LL set that were used in past studies with chimpanzees [17] so that all newly tested animals experienced those conditions. In the first condition, the experimenter transferred food items from the larger set at a rate of one item every 3 seconds. Thus, the total amount of time required to accumulate all twelve items was 36 seconds. In this condition, the total time that passed between the beginning of one trial and the beginning of the next was 120 seconds. Each test session consisted of eight trials. The first two trials of the session were forced trials in which only one set of grapes was presented (one trial with four items and one trial with 12 items). These allowed the chimpanzees to experience the outcomes of choosing each set. The remaining six trials were test trials as described above. Chimpanzees completed five 8-trial sessions in this condition for a total 30 test trials and 10 forced trials.
Condition 2 changed only the transfer rate – now to 10 seconds per item – and the total trial duration - now to 180 seconds. Chimpanzees completed five sessions of eight trials each. Condition 3 changed the transfer rate to 20 seconds per item and the total trial duration to 300 seconds. Chimpanzees completed five sessions of eight trials each.
For all choice trials, with both options available, we recorded which set of items was selected, and when it was the LL set, we recorded how many items were accumulated before the chimpanzees ate those items. Given the perfect relation of delay length and accumulated number of items, we used the latter measure, although for all analyses and results, one can interpret those results in terms of delay time as well as a function of accumulated reward amount.
PCTB Testing and Calculation of “g”
The methods and procedures for administering the PCTB tasks has been described in detail elsewhere [14, 35]. Briefly, the PCTB consists of 13 tasks aimed at quantifying domain general and domain specific intelligence, notably physical and social cognition. Nine tasks are considered to measure physical cognition and four quantify social cognition, and they are listed in Table 1 along with the number of trials administered for each task. Trial counts are not large for most tasks because the battery was designed to assess more spontaneous performance rather than the trained performances of nonhuman primates [13–15]. Each chimpanzee received two test sessions on the complete 13-item PCTB test battery that were separated by at least two weeks. Administration of the PCTB tasks was distributed over several days of testing, depending on subject availability and motivation. A complete set of PCTB test data were obtained on all measures before starting on the second series of tests. Food rewards were provided for all operationally defined correct responses for each task. To calculate the domain general intelligence “g” score, the percentage of correct responses was computed for each task across trials. The PCTB performance measures were then subjected to a principal axis factor (PAF) analysis with no rotation. The outcome measure from this analysis was a weighted PAF score and these values were subsequently correlated with the HDT values.
QUANTIFICATION AND STATISTICAL ANALYSIS
To assess the relation between PAF “g” scores and HDT performance, we calculated Pearson Product Moment Correlation coefficients. We also conducted multiple regression analysis with the three factors of HDT performance with PCTB scores. T-tests were conducted to assess whether individual factors accounted for statistically significant variance in the regression models. These analyses were conducted for each delay length independently. To assess the relation of PCTB task weighting and HDT performance, a principal axis factor (PAF) analysis was performed on the 13 performance measures from the PCTB and one piece of output data from this analysis is the factor matrix, which indicates the relative weight that each item or measure within the PCTB contributes to the structure of the PAF “g” scores (see Table 1). Thus, items with higher values contributed more to the structure of the “g” PAF scores than items with lower values. In light of the fact that overall PAF “g” scores were positively associated with the delay of gratification scores derived from the HDT test, then it follows that PCTB tasks that load more strongly on the PAF derived “g” scores would be more strongly associated with HDT performance than tasks that contribute less to the derived measure of “g.” To test this hypotheses, we ran two additional sets of correlations. We calculated Pearson Product Moment Correlation coefficients between the PCTB performance scores and the efficiency measure derived from the HDT measures at each delay interval. We correlated the weighted score of each PCTB task computed from the PAF analysis and the correlation coefficient value found between each task and the HDT scores at each delay length (see Table 1).
The individual PAF scores for a given subject are their weighted contribution to the creation of a latent factor, and this is considered a parsimonious representation of observed correlations between variables. We also performed correlations between the HDT scores and unit-weighted factor (UWF) values which converts performance measures from the different tasks to z-scores, and then averages the z-scores across all tasks to derive a single measure of intelligence.
Alpha was set at .05 for all analyses. For all analyses, data were included from all chimpanzees that were tested, and analyses were conducted using SPSS (Version 23).
DATA AND SOFTWARE AVAILABILITY
All data acquired and used in this study will be made available to anyone who directly requests it from either M. J. Beran (mberan1@gsu.edu) or W. D. Hopkins (whopkins4@gsu.edu).
Additionally, the raw data will be placed in the publication repository for the National Chimpanzee Brain Resource (chimpanzeebrain.org).
KEY RESOURCES
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Critical Commercial Assays | ||
| Deposited Data | ||
| Raw data | This paper and National Chimpanzee Brain Resource | chimpanzeebrain.org |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Pan troglodytes | Yerkes National Primate Research Center; Language Research Center, Georgia State University | |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Other |
Supplementary Material
Highlights.
In humans, delay of gratification appears to be related to general intelligence. Chimpanzees completed an intelligence test and a test of delay of gratification. Intelligence scores were most closely related to delay of gratification efficiency. Factors that loaded most strongly on “g” scores were most related to delay scores.
Acknowledgments
This research was supported by grants HD-060563 and NS-42867 from the National Institutes of Health. The authors thank Joe McIntyre, Jamie Russell, and Jennifer Schaeffer for their assistance with data collection and other aspects of this project. Fabio Paglieri, Theodore Evans, and Elsa Addessi made crucial contributions to the original development of the Hybrid Delay Task, for which we are grateful. The authors declare that they have no conflicts of interest that attach to this article or this program of research.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
Author Contributions:
Conceptualization, M.J.B. and W.D.H.; Methodology, M.J.B. and W.D.H; Investigation, W.D.H.; Writing – Original Draft, M.J.B.; Writing – Review & Editing, W.D.H.; Funding Acquisition, W.D.H.; Resources, M.J.B., W.D.H.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mischel W. The Marshmallow Test: Mastering Self-Control. New York: Little, Brown and Company; 2014. [Google Scholar]
- 2.Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 3.Mischel W, Shoda Y, Peake PK. The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol. 1988;54:687–696. doi: 10.1037//0022-3514.54.4.687. [DOI] [PubMed] [Google Scholar]
- 4.Boisvert D, Stadler W, Vaske J, Wright JP, Nelson M. The interconnection between intellectual achievement and self-control. Crim Just and Behav. 2013;40:80–94. [Google Scholar]
- 5.Duckworth AL, Quinn PD, Tsukayama E. What No Child Left Behind leaves behind: The roles of IQ and self-control in predicting standardized achievement test scores and report card grades. J Ed Psychol. 2012;104:439–451. doi: 10.1037/a0026280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meldrum RC, Petkovsek MA, Boutwell BB, Young JT. Reassessing the relationship between general intelligence and self-control in childhood. Intelligence. 2017;60:1–9. [Google Scholar]
- 7.Mischel W, Metzner R. Preference for delayed reward as a function of age, intelligence, and length of delay interval. J Abnorm Soc Psychol. 1962;64:425–431. doi: 10.1037/h0045046. [DOI] [PubMed] [Google Scholar]
- 8.Petkovsek MA, Boutwell BB. Childhood intelligence and the emergence of self-control. Crim Justice Behav. 2014;41:1232–1249. [Google Scholar]
- 9.Shamosh NA, Gray JR. Delay discounting and intelligence: A meta-analysis. Intelligence. 2008;36:289–305. [Google Scholar]
- 10.Beran MJ, Savage-Rumbaugh ES, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees (Pan troglodytes) Dev Psychobiol. 1999;34:119–127. doi: 10.1002/(sici)1098-2302(199903)34:2<119::aid-dev5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) J Gen Psychol. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- 12.Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biol Lett. 2007;3:599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins WD, Russell JL, Schaeffer J. Chimpanzee intelligence is heritable. Curr Biol. 2014;24:1649–1652. doi: 10.1016/j.cub.2014.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt V, Pankau B, Fischer J. Old World monkeys compare to apes in the primate cognition test battery. PLoS One. 2011;74:e32024. doi: 10.1371/journal.pone.0032024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paglieri F, Focaroli V, Bramlett J, Tierno V, McIntyre J, Addessi E, … Beran MJ. The hybrid delay task: Can capuchin monkeys (Cebus apella) sustain a delay after an initial choice to do so. Behav Process. 2013;94:45–54. doi: 10.1016/j.beproc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beran MJ, Evans TA, Paglieri F, McIntyre JM, Addessi E, Hopkins WD. Chimpanzees (Pan troglodytes) can wait, when they choose to: A study with the hybrid delay task. Anim Cogn. 2014;17:197–205. doi: 10.1007/s10071-013-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deary IJ. Intelligence. Ann Rev Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- 19.Herrnstein RJ, Murray C. The Bell Curve. New York: Free Press; 1994. [Google Scholar]
- 20.Jensen AR. The g factor and the design of education. In: Sternberg RJ, Williams WM, editors. Intelligence, Instruction, and Assessment: Theory into Practice. Mahwah, NJ: Lawrence Erlbaum; 1998. pp. 111–131. [Google Scholar]
- 21.Burkart JM, Schubiger MN, van Schaik CP. The evolution of general intelligence. Behav Brain Sci. 2016:1–65. doi: 10.1017/S0140525X16000959. [DOI] [PubMed] [Google Scholar]
- 22.Reader SM, Laland KN. Social intelligence, innnovation, and enhanced brain size in primates. Proc Nat Acad Sci USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth G, Dicke U. Evolution of brain and intelligence. Trends Cogn Sci. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Amici F, Barney B, Johnson VE, Call J, Aurelli F. A modular mind? A test using individual data from seven primate species. PLoS ONE. 2012;7:e51918. doi: 10.1371/journal.pone.0051918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann E, Call J. Are there geniuses among the apes? Philos Trans R Soc B Biol Sci. 2012;367:2753–2761. doi: 10.1098/rstb.2012.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrmann E, Hernández-Lloreda MV, Call J, Hare B, Tomasello M. The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychol Sci. 2010;21:102–110. doi: 10.1177/0956797609356511. [DOI] [PubMed] [Google Scholar]
- 27.Shaw RC, Schmelz M. Cognitive test batteries in animal cognition research: Evaluating the past, present and future of comparative psychometrics. Anim Cogn. 2017;20:1003–1018. doi: 10.1007/s10071-017-1135-1. [DOI] [PubMed] [Google Scholar]
- 28.Kivetz R, Simonson I. Self-control for the righteous: Toward a theory of precommitment to indulge. J Consum Res. 2002;29:199–217. [Google Scholar]
- 29.Kivetz R, Keinan A. Repenting hyperopia: An analysis of self-control regrets. J Consum Res. 2006;33:273–282. [Google Scholar]
- 30.Keinan A, Kivetz R. Remedying hyperopia: the effects of self-control regret on consumer behavior. J Marketing Res. 2008;45:676–689. [Google Scholar]
- 31.Paglieri F, Addessi E, Sbaffi A, Tasselli MI, Delfino A. Is it patience or motivation? On motivational confounds in intertemporal choice tasks. J Exp Anal Behav. 2015;103:196–217. doi: 10.1002/jeab.118. [DOI] [PubMed] [Google Scholar]
- 32.Killeen P. An additive-utility model of delay discounting. Psychol Rev. 2009;116:602–619. doi: 10.1037/a0016414. [DOI] [PubMed] [Google Scholar]
- 33.Latzman RD, Taglialatela JP, Hopkins WD. Delay of gratification is associated with white matter connectivity in the dorsal prefrontal cortex: A diffusion tensor imaging (DTI) study in chimpanzees (Pan troglodytes) Proc R Soc B. 2015;22:20150764. doi: 10.1098/rspb.2015.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bard KA. Evolutionary roots of intuitive parenting: Maternal competence in chimpanzees. Infant Child Dev. 1994;3:19–28. [Google Scholar]
- 35.Russell JL, Lyn H, Schaeffer JA, Hopkins WD. The role of sociocommunicative rearing environments in the development of social and physical cognition in apes. Dev Sci. 2011;14:1459–1470. doi: 10.1111/j.1467-7687.2011.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.