Abstract
Consistent associations have been found between higher cardiorespiratory fitness (CRF) and indices of enhanced brain health and function, including behavioral measures of cognition as well as neuroimaging indicators such as regional brain volume. Several studies have reported that higher CRF levels are associated with a larger hippocampus, yet associations between volume and memory or functional connectivity metrics with the hippocampus remain poorly understood. Using a multi-modal framework, we hierarchically examine the association between CRF and hippocampal volume and resting state functional connectivity (rsFC) in younger adults (ages 22–38), as well as the relationship between these imaging markers and memory function. We conducted theoretically driven rsFC and volumetric analyses with seeds in the anterior and posterior portions of the hippocampus, as well as control seeds located in the caudate nucleus. We tested (1) whether hippocampal connectivity with prefrontal cortical regions was associated with CRF in an adult sample much younger than traditionally tested, (2) whether associations between CRF and rsFC remains significant after adjusting for hippocampal volume, and (3) whether volume and rsFC are related to memory performance. We found that higher CRF levels were associated with larger left anterior hippocampal volume, as well as stronger, more positive rsFC of the (bilateral) anterior hippocampus to several regions including the prefrontal cortex. In addition, rsFC accounted for significant variance in CRF, above and beyond hippocampal volume. Higher CRF can thus be independently linked to increased anterior hippocampal volume, as well as stronger hippocampal rsFC in a population much younger than those typically tested. This suggests that CRF may be a critical factor for maintaining multiple aspects of brain health in younger adults, as well as in older adults, the population most often studied in the context of CRF and brain health.
Keywords: cardiorespiratory fitness, functional connectivity, hippocampal subfield, resting state
Introduction
Consistent associations have been found between higher cardiorespiratory fitness (CRF) and indices of enhanced brain health and function that include behavioral measures of mood and cognition as well as neuroimaging indicators such as regional brain volume, evoked neuroelectric responses, and white matter microstructure (Erickson, Hillman, & Kramer, 2015; Erickson, Leckie, & Weinstein, 2014; Etnier et al., 1997; Sibley & Etnier, 2003). A great many of these studies have focused on the hippocampus, a brain region central for learning and episodic and relational memory, that is relatively easy to identify and segment, and that is sensitive both to wheel running in rodents and to numerous neurologic and psychiatric conditions in humans (e.g., depression, schizophrenia, Alzheimer’s disease). (S. J. Colcombe et al., 2006; S. Colcombe & Kramer, 2003; Erickson et al., 2009, 2011; Niemann, Godde, & Voelcker-Rehage, 2014; ten Brinke et al., 2015).
Despite recognition that the volume of the hippocampus is an important clinical marker for many conditions (e.g., pathological aging), it is often only weakly associated with behavioral outcomes (e.g., relational memory performance). In fact, while associations between CRF and hippocampal volume have been consistently observed (e.g., L. Chaddock-Heyman et al., 2014; Laura Chaddock-Heyman et al., 2015; Erickson et al., 2009, 2011), many studies do not examine, or report, the association between CRF-related variation in hippocampal volume with memory performance, and those that do assess these associations often report small effect sizes (e.g., Erickson et al., 2009; 2011; Chaddock et al., 2010; Honea et al., 2009). Such limited evidence for associations between behavior and hippocampal volume in the context of CRF, begs the scientific field to look beyond traditional neuroimaging measures of volume and to approach the hippocampus (and the rest of the brain) from a multi-modal neuroimaging perspective. By combining metrics from more than one neuroimaging technique into a single analytic model, we may improve our understanding of the association between CRF and hippocampal volume and function, and might also improve our understanding of the links between the hippocampus and the behaviors that it supports.
Functional connectivity is one such neuroimaging technique that could inform the associations between CRF and hippocampal function. In fact, several studies have now reported associations between higher CRF and increased functional connectivity (FC) between numerous cortical and subcortical nodes (Herting & Nagel, 2013; Voss, Erickson, et al., 2010). For example, in exploratory analyses of memory-related task-evoked activation patterns, Herting and colleagues (2013) found that high and low CRF groups differed in the connectivity between the hippocampus and several prefrontal and parietal regions comprising the default mode network (DMN) during memory tasks. Although these group differences in connectivity were not associated with differences in memory performance, these associations suggest that variation in CRF relates to connectivity measures of the hippocampus. However, Herting and colleagues (2013) did not control for variation in hippocampal volume, so it is unknown whether these associations were confounded by, or independent of, volume.
In addition, to assessing connectivity during a task, higher CRF has also been associated with the FC between brain regions during rest, referred to as intrinsic or resting state functional connectivity (rsFC) (Smith et al., 2009). Using a data-driven approach across the entire brain, Voss and colleagues found that higher levels of CRF were related to stronger connectivity amongst nodes making up the DMN, such that older adults with higher CRF had rsFC levels similar to that of younger adults within this network (Voss, Erickson, et al., 2010). Notably, these relationships held even after controlling for variance associated with gray matter volume, suggesting a unique contribution of CRF to brain FC. The data-driven approach taken in this paper, however, did not isolate the hippocampus as a theoretically-derived seed region, so it remained unknown as to how rsFC of the hippocampus varied as a function of CRF and whether variation in hippocampal volume accounts for any CRF-related differences in rsFC.
Data-driven approaches have also shown that the rsFC of largescale networks, including the DMN and Frontal Executive Network, increase following moderate-intensity exercise training in older adults (Burdette et al., 2010; Voss et al., 2016; Voss, Prakash, et al., 2010). Intervention-related changes in rsFC were specific to frontal and temporal connections of the DMN (Voss, Prakash, et al., 2010), and changes in the global efficiency of these largescale networks were related to an estimated doubling in hippocampal connectivity within the exercise group (Burdette et al., 2010). Thus, even using data-driven analytical approaches, the hippocampus emerges as a key region showing sensitivity to CRF.
As the evidence above suggests, prior rsFC work has relied predominately upon data-driven, whole-brain, exploratory approaches to examine relationships between rsFC and CRF, or have focused on large-scale networks rather than specific regions of interest (but see Prakash, Patterson, Janssen, Abduljalil, & Boster, 2011 for one exception). While such exploratory approaches provide valuable opportunities for identification of whole-brain connectivity patterns, no rsFC studies have focused on the hippocampus as a seed region, despite overwhelming evidence for its unique sensitivity to CRF in both volumetric and morphometric domains of brain health. Further, only one prior rsFC study to our knowledge (Voss, Erickson, et al., 2010) has examined both volume and functional connectivity data in the same participants in order to examine the hierarchical nature of these metrics in relation to CRF. In the present study, we conducted theoretically driven rsFC analyses with seeds in the hippocampus. Our goals were to determine (1) whether hippocampal connectivity with prefrontal cortical regions is associated with CRF in an adult sample much younger than those traditionally tested, (2) whether associations between CRF and rsFC remains significant after adjusting for volume, and (3) to examine whether volume and rsFC are related to memory performance in a cognitively normal and healthy young adult sample. We conducted this analysis using anterior and posterior hippocampal seeds separately since several studies have reported stronger associations between CRF and anterior regions of the hippocampus than posterior regions (Laura Chaddock-Heyman et al., 2015; Erickson et al., 2011; Killgore, Olson, & Weber, 2013). In addition to the hippocampal seeds of interest, we also included analyses of the left and right caudate as negative control seeds. We predicted that hippocampal (but not caudate) volume would be positively associated with CRF and memory and that this relationship would be specific to the anterior hippocampus. We also predicted that connectivity of the hippocampus, but not caudate, would vary as a function of CRF and that differences might be particularly salient for anterior hippocampal rsFC. Finally, we predicted that rsFC would explain unique variance in CRF and memory function, above and beyond that explained by the association between CRF and hippocampal volume.
Methods
Subjects
A cross sectional sample of 50 young adults ranging in age from 20–38 (M ± SD = 25.22 ± 5.11; 28 female) was recruited from the University of Pittsburgh and surrounding community. All participants reported no neurological or health conditions that could affect central nervous system functioning, such as history of psychiatric disease, epilepsy, or metabolic disorder. In addition, all participants were deemed MRI-safe via screening prior to the start of the study and indicated that they were physically healthy enough to engage in PA. All protocols and procedures were approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained in accordance with the principles set forth in the Declaration of Helsinki.
Fitness Testing
CRF was assessed by graded maximal exercise testing on a motor-driven treadmill (VO2max). All study participants were acclimated to the general environment and test procedures. All participants began the test by walking at 3.0 mph and 0% incline as a warm-up. All study participants completed a modified Bruce protocol in which Stage 1 started at 3.5 mph and 2.0% incline. The modified Bruce protocol was chosen for this sample because it gives larger metabolic equivalents (MET) increases per stage compared to other protocols. The speed increased .50 mph and grade increased in increments of 2% every 2min. This protocol was designed to increase exercise intensity in a linear format over time in order to achieve a workload that the participant would be unable to maintain within an 8 to 12-minute duration. A trained exercise physiologist continuously monitored measurements of oxygen uptake, heart rate and blood pressure. Gas exchange values were measured from expired air samples averaged at 15s intervals using a Parvo Medics metabolic cart. Expired air was collected via a mouth piece connected to a two-way valve. The mouthpiece was supported by a comfort fitted head gear. During the test, participants wore nose clips in order to ensure that all expired air was collected. All equipment was worn until a maximal VO2 was attained either due to symptom limitation and/or self-reported exhaustion. VO2max was defined as the highest recorded VO2 value. A test was defined as maximal for each participant when they met one of the following two criteria: (1) a plateau in VO2 peak between two or more workloads (.15 Liters/minute or 2.0 ml/kg/min), or (2) two of the following three criteria were met: a respiratory exchange ratio >1.10, a heart rate within 10 beats of their age predicted maximum (i.e., 220 - Age), or a Rating of Perceived Exertion (RPE) equal to or greater than 17. Forty-nine of the 50 participants met one of the two criteria listed above, and one participant did not meet either but stopped the test from volitional exhaustion.
Cognitive Testing
Participants completed a relational memory task related to hippocampal functioning. The task employed was a variant of the spatial reconstruction task developed by Watson and colleagues (Watson, Voss, Warren, Tranel, & Cohen, 2013) and described in detail by Monti and colleagues (2015). The task involves relational memory binding, and several different variants of the task, including the outcome measure “swaps” or “swap errors” (see below), have been shown to be highly sensitive to the structural integrity and/or volume of the hippocampus (Watson et al., 2013; Monti et al., 2015; Schwarb et al, 2016), as well as to be positively associated with CRF (Monti, Hillman, & Cohen, 2012). Briefly, on each trial of the task participants study the spatial arrangement of five novel line drawings and are told to remember the arrangement for a later test. Study time is self-paced, and participants are instructed to use the mouse to click on each stimulus. Following the study phase, there is a 4000ms delay in which participants see a blank screen; a self-paced test phase begins after this delay. In the test phase, stimuli appear aligned at the top of the screen, and participants use the mouse to click and drag them to where they were positioned during the study phase. Participants completed three practice trials and 15 experimental trials of the task (2000ms ITI).
Memory errors committed during the test phase were the primary outcome measures from the spatial reconstruction task. Errors were assessed using 4 metrics: (1) average item misplacement (in pixels), (2) edge resizing (in pixels), (3) edge displacement (in radians), and (4) swaps (proportion of all possible pairwise relationships). Detailed descriptions and examples of the various errors are provided in Watson et al., (2013). In all cases, higher values indicate worse memory performance. We created a single metric of relational memory performance by creating a composite score, computed as the sum of the normalized values of each type of possible error.
Resting State MRI
Acquisition
Each participant completed a functional magnetic imaging (MRI) scan, which included acquisition of resting state and structural images, within 2 weeks of VO2max testing. All images were collected on a 3T head-only Siemens Allegra MRI scanner. High-resolution T1-weighted brain images were acquired using a 3D Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) protocol with 176 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures (echo time (TE) = 2.48ms, repetition time (TR) = 1.4s, field of view (FOV) = 256mm, acquisition matrix 256mm × 256mm, flip angle = 8). The resting-state fMRI (rsfMRI) data consisted of a series of 180 scans acquired using a Gradient Echo Pulse Sequence with TR = 1.7s while participants rested with eyes open, fixating on a centrally located crosshair inside the MRI scanner for 5:11 minutes (33 slices; TE = 25ms; FOV = 205 x 205mm; acquisition matrix 64 x 64mm; 90 degree flip angle; voxel dimensions 3mm isotropic).
Preprocessing
After skull stripping, the structural image was spatially normalized to MNI space. All rsfMRI frames were aligned to correct for head motion during the scan, co-registered to each participant’s structural image, and spatially normalized to MNI space. The rsfMRI timecourses were then band-pass filtered (0.009–0.08Hz) to attenuate respiration and other physiological noise. In addition, six affine transformation parameters from the alignment process, as well as the mean time courses from the brain parenchyma including white matter tissue and ventricles were included as covariates in order to further account for motion and physiological noise. The data were of high quality in this healthy young adult sample, and no subjects were eliminated due to excessive motion (mean framewise displacement ranged from .04 to .26mm; M±SD= .09± .03) or physiological noise. The residualized parameter estimate maps were converted to z scores (via Fishers r to z transform) to achieve normality and were entered into higher level analyses.
Seed Creation
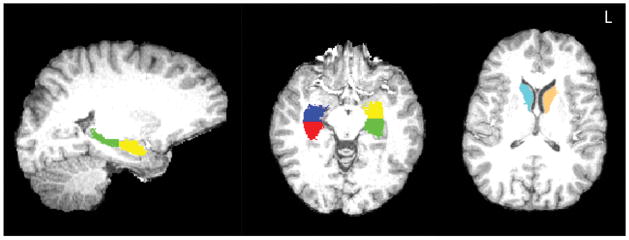
For the functional connectivity and volumetric analysis of the hippocampus and control (caudate nucleus) seeds, we employed FMRIB’s Integrated Registration and Segmentation Tool (FIRST) in FMRIB’s Software Library (FSL) version 5.0. FIRST is a semi-automated model-based subcortical segmentation tool which uses a Bayesian framework from shape and appearance models obtained from manually segmented images from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston, MA, USA (see Patenaude, Smith, Kennedy, & Jenkinson, 2011 for further description of this method). Briefly, FIRST runs a two-stage affine registration to a standard space template (MNI space) with 1mm resolution using 12 degrees of freedom and uses a subcortical mask to exclude voxels outside subcortical regions. Second, subcortical regions, including hippocampus, are segmented (both hemispheres separately). Manual volumetric region labels are parameterized as surface meshes and modeled as a point distribution model. The hippocampus segmentation from FIRST was then split based on the center of gravity of the region into anterior and posterior subregions (for each hemisphere separately). This resulted in separate anterior and posterior hippocampal seeds for each participant, for each hemisphere. This procedure for dividing the hippocampus shows differences as a function of CRF and exercise (Erickson et al., 2009, 2011). The final segmentations of both the hippocampus and caudate nucleus seeds were visually inspected for quality. The volume of each seed region was obtained from FIRST in mm3. Figure 1 shows the masks for all seeds on a representative participant’s MPRAGE.
Figure 1.
Location of the hippocampal and caudate seed regions in each hemisphere derived from FIRST segmentation. The seed masks are presented on a representative subject’s MPRAGE. Green: Left posterior hippocampus; Yellow: Left anterior hippocampus; Red = Right posterior hippocampus; Blue = Right anterior hippocampus; Cyan = Left caudate; Teal = Right caudate.
Statistical Analyses
First, we examined the relationship between (anterior and posterior) hippocampal volume, caudate volume, and CRF using 6 separate linear regressions. Results were corrected for multiple comparisons using the Benjamini Hochberg procedure implemented in SPSS with a false discovery rate (q) value of .10. This method and specific value of q was chosen because a) we had a very specific apriori hypothesis regarding which seed volumes should relate to CRF and 2) this method is less susceptible to false negatives when a small number of pre-planned comparisons are made compared to other methods of controlling for false discovery (Benjamini & Hochberg, 1995).
Next, voxelwise functional connectivity network maps were then constructed for each seed, for each participant using the pre-processed rsfMRI data. These first-level seed maps were then entered into (separate) group-level linear regressions to identify regions where connectivity with the seed covaried with V02max scores. Gender and mean framewise displacement (in mm) were included as nuisance regressors in the group-level analyses of functional connectivity, and gender was included in group analyses of volume. All variables were mean-centered prior to being entered into group-level models. Results were corrected for multiple comparisons at P < .05 using FSL’s automatic FEAT cluster-based thresholding, which is a method of Family-Wise Error correction based on Gaussian Random Field Theory.
Finally, we conducted a hierarchical regression to compare the extent to which volume explains the variability in any rsFC-CRF relationships. Sex and seed volume were entered as explanatory variables in the first-wave model. CRF was subsequently entered in wave 2, in order to determine the unique variance in rsFC accounted for by each of these variables (i.e., volume vs. CRF). Finally, we examined associations between CRF, volume, rsFC, and memory performance.
Results
Consistent with previous research in older adults and patient populations, there was a wide range of variability in V02max scores in our healthy young adult sample. Scores ranged from a minimum of 25.4 to a maximum of 60.4 ml/kg/min (M ± SD = 44.9 ± 7.9), corresponding to a CRF percentile range of 4.4 and 87.2, respectively. Such variability was well suited for examining individual differences in brain structure and function. Age was limited to 18–38 and was unrelated to any variable or outcome of interest and was, therefore, not included as a covariate in any of the analyses described below.
Volume-Fitness Correlations
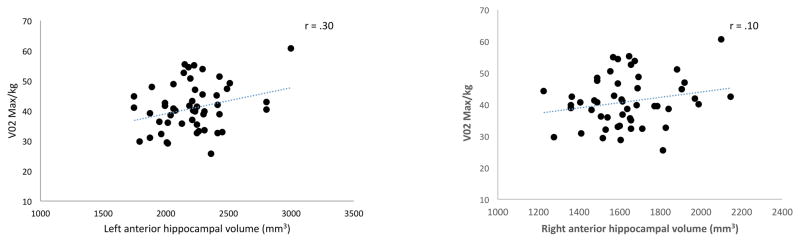
We observed a positive correlation between the volume of the left anterior hippocampus and CRF, β =.01, p =.04. This relationship was specific to the anterior seed (Figure 2). There were no other significant relationships between volume of the other hippocampal and caudate seeds and CRF (ps >.12, βs <.26). Because we did not detect a significant correlation in both hemispheres, we did a posthoc analysis to examine hemispheric differences in hippocampal volume and whether these differences varied as a function of CRF. While there was a main effect of hemisphere, such that hippocampal volume of the left anterior hippocampus was on average larger (M ± SD = 2216.34 ± 256.10) than that of the right anterior hippocampus (M ± SD = 2216.34 ± 197.58; F(1,47) = 518.32, p < .001), there was no evidence for a hemispheric interaction with CRF, F(1,47) = .09, p = .76.
Figure 2.
Positive correlation between left anterior hippocampal volume and cardiorespiratory fitness (V02max) in healthy younger adults.
Connectivity-Fitness Correlations
Left anterior hippocampus seed
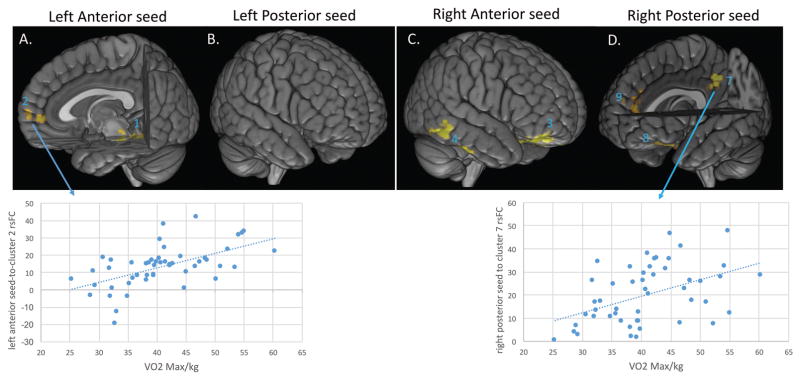
Greater CRF was associated with greater connectivity between the left anterior hippocampus to clusters located in the frontal pole/middle frontal gyrus, as well as posterior hippocampus/brain stem (Figure 3; Table 1). Because MRI signal in brain stem regions is highly susceptible to physiological noise/artifacts (e.g., see Beissner, Schumann, Brunn, Eisenträger, & Bär, 2014), we do not further consider the CRF-related cluster that mostly includes brain stem regions in the analyses below. There were no significant negative relationships for this seed.
Figure 3.
Positive correlations between resting state connectivity of the anterior and posterior hippocampus seeds and cardiorespiratory fitness (V02max score) in healthy younger adults. Panel (A) depicts results from the left anterior hippocampus seed. (B) depicts (NS) results from the left posterior hippocampal seed. Panel (C) depicts results from the right anterior seed, and (D) results from the right posterior hippocampal seed. Representative scatter plots are presented for clusters 2 and 7. Numbers in blue within each panel correspond to the cluster labels in Table 1. Clusters 5 and 6 are not visible in panel C. Results were corrected based on the voxel z-score and extent of activity given the correlated nature of the voxels. Specifically, the voxel-wise z-score was 2.3 (p<.01) and the clusters were significant at p<.05).
Table 1.
Significant clusters in which hippocampal connectivity positively covaries with cardiorespiratory fitness (CRF). There were no significant CRF-related rsFC connections for the left posterior hippocampus seed.
| Seed | Nature of Correlation | Cluster Label | Cluster Location | k | Peak Z | Peak MNI coordinates |
|---|---|---|---|---|---|---|
| Left Anterior Hippocampus | Positive | 1 | L brain stem/posterior parahippocampal gyrus | 415 | 3.70 | (−16, −30, −20) |
| Positive | 2 | L frontal pole/medial prefrontal/anterior cingulate | 242 | 3.41 | (−10, 68, −4) | |
| Right Anterior Hippocampus | Positive | 3 | R frontal pole | 2037 | 4.41 | (26, 46, −22) |
| Positive | 4 | R inferior/middle temporal gyrus | 865 | 3.59 | (66, −46, −16) | |
| Positive | 5 | L inferior/middle temporal gyrus | 239 | 4.31 | (−34, −26, −30) | |
| Negative | 6 | R precentral gyrus | 453 | 3.69 | (36, 10, 62) | |
| Right Posterior Hippocampus | Positive | 7 | R precuneus | 365 | 3.82 | (8, −60, 38) |
| Positive | 8 | L frontal pole/anterior cingulate | 244 | 3.17 | (−30 42, −20) | |
| Positive | 9 | L superior frontal/paracingulate gyrus | 229 | 2.26 | (−2, 60, 14) |
Left posterior hippocampus seed
No CRF-rsFC relationships survived correction for the left posterior hippocampus seed.
Right anterior hippocampus seed
Greater CRF was associated with greater connectivity of the right anterior hippocampus to three clusters located in the frontal pole extending to middle frontal gyrus, and posterior parahippocampal cortex (Figure 3; Table 1). In addition to these positive correlations, greater CRF was associated with less connectivity between the right anterior hippocampus seed to a cluster in the right superior frontal gyrus (Table 1).
Right posterior hippocampus seed
Greater CRF was associated with greater connectivity of the right posterior hippocampus to three clusters in the frontal pole extending into anterior cingulate, paracingulate/superior frontal gryus, and precuneus. There were no negative relationships for this seed (Figure 3; Table 1).
Left caudate (control) seed
No CRF-rsFC relationships survived correction for the left caudate seed.
Right caudate (control) seed
No CRF-rsFC relationships survived correction for the right caudate seed.
Hierarchical Regression Results
We next conducted a hierarchical regression to compare the extent to which CRF-related rsFC (detected only in the hippocampal seeds) could be explained by independent vs. overlapping variability in hippocampal volume. Given that the volume-CRF correlation was specific to the left anterior hippocampus seed, we chose to focus on this seed in the hierarchical regression analysis. Wave 1 of the model, in which sex and seed volume were entered as the explanatory variables, was not significant, F(2,49) = .70, p = .50. Together, these variables only accounted for 2.9% of the variance in rsFC, suggesting volume does not share much overlapping variance with rsFC. Adding CRF into the model greatly improved model fit and accounted for an additional (and significant), 35.6% of variance in rsFC. A total of 38.5% of variance in rsFC was explained by the addition of CRF into wave 2, F(3, 49) = 9.60, p <.0001. These results are summarized in Table 2.
Table 2.
Summary of hierarchical regression results. Results of wave 2 demonstrate that CRF accounts for significant portion of variance in rsFC, even after controlling for volume of the left anterior hippocampal seed. Wave 1 of the model (which controls for sex and volume only) was not significant. For each wave, all coefficients are reported, but the coefficient of interest (i.e., that for volume or CRF) is highlighted.
| Model Wave | Variables included | Standardized Beta | SE Beta | R squared | Model p-value |
|---|---|---|---|---|---|
| Wave 1 | Sex, volume | −.17, −.06 | 3.4, .01 | .03 | .50 |
| Wave 2 | Sex, volume, CRF | .17, −.21, .71 | 3.1, .01, .20 | .39 | <.0001 |
Correlations with Memory
Relational memory data was missing for 2 participants; we report data from the remaining 48 participants for this task. We first examined correlations between memory and hippocampal volume controlling for the confounding influence of gender. We focused on the left anterior hippocampus given the specificity of the volume-CRF correlation to this seed. There were no significant relationships between volume and performance on the relational memory task (β =−11.2, p = .26).
Next, we examined associations between memory and rsFC. As with volume, we focused specifically on CRF-related rsFC. However, no significant relationships between rsFC and relational memory performance were detected (β =.32, p = .49).
Finally, we examined correlations between CRF and memory performance. There was a marginal relationship between CRF and relational memory performance, such that those with higher CRF tended to commit fewer relational memory errors, β= −.51, p =.06.
Discussion
Consistent with our predictions and with previous literature in older adults and children (L. Chaddock-Heyman et al., 2014; Erickson et al., 2009, 2011; Niemann et al., 2014), higher CRF was associated with greater volume of the left anterior hippocampus in healthy young adults. There were no relationships between the volume of the other hippocampal seeds, nor caudate nucleus seeds, and CRF. In addition, higher CRF was associated with greater rsFC of the left anterior hippocampus seed to the frontal pole, middle frontal gyrus, and parahippocampus. These rsFC patterns appeared to be specific to the anterior hippocampal seeds in that a very similar pattern of CRF-related rsFC was observed for the right anterior seed, but not for the left or right posterior seeds. One major difference, however, between the left and right anterior seeds was the presence of a negative correlation between CRF and right anterior hippocampal connectivity to the right superior frontal gyrus. This association suggests CRF-related hemispheric differences of hippocampal connectivity and other brain areas. The negative correlation is difficult to interpret, but could be related to a shift in allocation of resources or attentional focus. As predicted, the caudate nucleus seeds showed no significant rsFC relationships in either direction to CRF. Finally, we demonstrated that CRF accounts for a unique portion of variance in the rsFC of the left anterior hippocampus to the middle frontal gyrus, above and beyond the variance explained by the left anterior hippocampal volume.
Results in the context of the broader field of exercise and brain
The specificity of the rsFC-CRF relationship to the anterior hippocampus extends findings of volumetric studies in the CRF/exercise literature by demonstrating that CRF-rsFC relationships are also largely confined to the anterior hippocampal subregion.
Individuals with a higher CRF exhibited stronger connectivity at rest between the anterior hippocampus and prefrontal and temporal cortical regions often implicated in supporting attention, declarative memory, and inhibition, namely the frontal pole, middle frontal gyrus, and parahippocampal gyrus. (Beaty, Benedek, Kaufman, & Silvia, 2015; Yang & Li, 2012; Zuo, Di Martino, et al., 2010; Zuo, Kelly, et al., 2010). Thus, the stronger rsFC of the anterior hippocampus to these regions suggests CRF may modulate the tonic intrinsic communication of specific networks supporting executive cognitive functions.
In addition to the positive rsFC-CRF relationships observed in the present study, one unexpected finding was that of a negative relationship between CRF and rsFC between the right hippocampal seed and right superior frontal gyrus. Such decreased connectivity in the context of increasing CRF is interesting and rarely, if ever discussed. Decreased connectivity in some cases is beneficial for behavior and long-term goals. In fact, this effect could be related to a difference in the brain circuits associated with attentional allocation or resources as a function of fitness, but in this context such an interpretation is highly speculative.
The results of our hierarchical regression demonstrate that the CRF-rsFC relationships we detected are not simply artifacts of the CRF-volume relationship. That is, once the variance in rsFC and volume is statistically accounted for, CRF was able to account for a significant portion of additional variance in rsFC. This suggests that CRF may have both overlapping and distinct influences on structural and functional brain health in this age group.
Finally, the CRF-anterior hippocampal volume correlation that we report here has been found numerous times in both cross-sectional and intervention studies (e.g., Erickson et al., 2009, 2011; Niemann et al., 2014). However, the present results are novel in that they are the first to replicate this relationship in a sample of healthy young adults not likely to be experiencing many developmental changes or age-related atrophy. Thus, our results suggest that CRF is a key component of brain health not only in youth and older adults, but also in younger adults. For example, our results suggest that higher CRF may play a protective role in maintaining neural connections key to memory and other cognitive functions--in this case both structural integrity and functional connectivity of the anterior hippocampus at rest--in younger adults, as has been previously documented in older adults and children (Chaddock et al., 2010; Erickson et al., 2011; Monti et al., 2012; Schwarb et al., 2017). However, in terms of how CRF-related brain integrity may translate to behavior in younger adults, we found only very weak evidence that higher CRF was related to better relational memory performance. One interpretation of our results is that CRF has a larger effect on behavior in other age groups compared to healthy younger adults. However, at least one other study has previously reported a significant association between CRF and relational memory in a young adult sample using a different relational memory task with a more implicit measure of relational memory (looking time as opposed to explicit choice) (Baym et al., 2014). Thus, there is some ambiguity as to whether CRF is as strongly related to relational memory performance in younger adults as it is in older adults and children. More research on possible age group moderation of CRF is needed in order to clarify whether age group may be a moderator of CRF.
Interestingly, the association between CRF and anterior hippocampal volume in our young adult sample was only significant for the left hippocampus and did not reach statistical significance for the right hemisphere. Despite the non-significant hemisphere interaction term, the apparent laterality of the results suggests that the left hemisphere may be more sensitive to modifications by CRF. In fact, other cross-sectional and intervention studies have reported similar asymmetrical associations with CRF (Niemann et al., 2014), although some studies have reported bilateral effects (Erickson et al., 2009, 2011).
Potential Mechanisms
As is the case with hippocampal volume (e.g., Erickson et al., 2011), the mechanisms underlying associations between CRF and hippocampal rsFC are unknown. However, the fact that both anterior hippocampal volume and rsFC show associations with CRF suggests there might be some common mechanisms across these endpoints. These mechanisms may include cellular and molecular changes, such as neurogenesis or angiogenesis or changes in vasculature, myelination, or dendritic complexity. CRF may also induce higher-level changes, including changes in brain or socioemotional functioning (Stillman, Cohen, Lehman, & Erickson, 2016), which may contribute to CRF associations with behavior in humans.
Findings from the animal literature helps provide clues regarding the potential mechanisms of CRF-brain relationships in humans. The majority of the physical activity-related animal findings center on the hippocampus, which was the impetus for choosing hippocampal seeds in the present study. Experimental studies employing animal models have established that animals with higher CRF (most typically manipulated through aerobic exercise training) show improved cognitive function compared to less fit animals, especially in cognitive domains dependent on the hippocampus, such as spatial or relational learning and memory, object recognition (e.g., Bechara & Kelly, 2013; Hopkins & Bucci, 2010), and avoidance learning (e.g., Baruch, Swain, & Helmstetter, 2004; Chen et al., 2008) (see van Praag, 2008 for review ). In addition, exercise increases long-term potentiation, a cellular analog of learning and memory, in a hippocampal sub-region known as the dentate gyrus (e.g., van Praag, Christie, Sejnowski, & Gage, 1999). Animal models have been critical in establishing that the changes initiated by exercise extend beyond behavior into cognition, prompting further research into the mechanisms underlying exercise-induced synaptic, and downstream cognitive, changes.
Behavioral associations, however, were not observed in the present sample. We found no significant relationships between memory and CRF. We also failed to detect significant associations between rsFC and memory, although we observed one relationship between CRF and relational memory in the hypothesized (positive) direction that did not meet statistical significance. The fact that there were few correlations with behavior is surprising. One possible explanation for the lack of associations between memory and CRF, rsFC, and volume in this sample could relate to the range of performance in this high-functioning young adult sample. For example, it is possible that given the typically high/ceiling performance of young adults on cognitive tasks and CRF is more strongly predictive of cognitive performance in older samples in which the range of variability is larger. Thus, the influence of individual-level (e.g., genetics) and environmental factors (e.g., PA) on behavior tends to be stronger in older age groups (McCormack, Shiell, Doyle-Baker, Friedenreich, & Sandalack, 2014; Woodard et al., 2012). Future CRF research should include a variety of cognitive tasks, including those with adaptive levels of difficulty, in studies of younger adults in order to examine whether the CRF-rsFC associations specifically support enhanced memory performance, or whether CRF-related rsFC may support enhanced performance in broader cognitive domains.
Limitations
The results of the current study should be interpreted in the context of some limitations. First, we chose a seed-based approach to analyze our resting state data. We chose this approach because we had a theoretically-driven hypothesis focusing on the hippocampus and its subregions. However, by doing so we did not examine whether connectivity between other brain regions was associated with rsFC. There are many approaches for analyzing resting state data, including data-driven approaches that are more suited for capturing larger, network-level, whole-brain associations with CRF. Of course, the tradeoff is that data-driven approaches may be more atheoretical or miss regionally-specific associations, which often makes the results of such approaches difficult to interpret in the context of the broader literature that focuses on particular brain areas. Second, we only included two brain metrics (volume and rsFC) in our test of shared vs. distinct variance in hippocampal integrity accounted for by CRF. Future studies employing multimodal techniques could expand upon these findings by including additional measures of hippocampal structure or functioning (e.g., cerebral blood flow, white matter integrity). Finally, the present results are limited by the cross-sectional nature of the study design. Ideally, in order to demonstrate that exercise itself modulates anterior hippocampal structure and/or function across age groups, it would be ideal to include a more continuous age range, such as youth or older adults, in a randomized clinical trial that manipulated and controlled the levels of exercise and the magnitude of change in CRF.
Conclusions
Despite these limitations, we can draw several broad and important conclusions from these data. The first is that higher CRF can be independently linked to increased anterior hippocampal volume, as well as stronger hippocampal rsFC. Second, these neurobiological markers of CRF can be observed in populations much younger than those typically tested. Finally, and more generally, CRF may be a critical factor for maintaining structural and functional brain health, even in young adults.
Supplementary Material
Conjunction maps showing overlap between each seed’s average connectivity and fitness-related connectivity. (A) left posterior (B) right anterior (C) and right posterior (D) hippocampal seeds. All seeds exhibited diffuse connectivity to the rest of the brain on average. (Z=3.1, P<.001). There were no significant clusters showing fitness-related correlations for the left posterior seed, and hence there was no overlap with this seeds average connectivity.
References
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behavioral Neuroscience. 2004;118(5):1123–1127. doi: 10.1037/0735-7044.118.5.1123. https://doi.org/10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Baym CL, Khan NA, Pence A, Raine LB, Hillman CH, Cohen NJ. Aerobic fitness predicts relational memory but not item memory performance in healthy young adults. Journal of Cognitive Neuroscience. 2014;26(11):2645–2652. doi: 10.1162/jocn_a_00667. https://doi.org/10.1162/jocn_a_00667. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Kaufman SB, Silvia PJ. Default and Executive Network Coupling Supports Creative Idea Production. Scientific Reports. 2015;5:10964. doi: 10.1038/srep10964. https://doi.org/10.1038/srep10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara RG, Kelly ÁM. Exercise improves object recognition memory and induces BDNF expression and cell proliferation in cognitively enriched rats. Behavioural Brain Research. 2013;245:96–100. doi: 10.1016/j.bbr.2013.02.018. https://doi.org/10.1016/j.bbr.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Beissner F, Schumann A, Brunn F, Eisenträger D, Bär KJ. Advances in functional magnetic resonance imaging of the human brainstem. NeuroImage. 2014;86:91–98. doi: 10.1016/j.neuroimage.2013.07.081. https://doi.org/10.1016/j.neuroimage.2013.07.081. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. https://doi.org/10.2307/2346101. [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, … Rejeski WJ. Using Network Science to Evaluate Exercise-Associated Brain Changes in Older Adults. Frontiers in Aging Neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00023. https://doi.org/10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M, … Kramer AF. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. https://doi.org/10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Mackenzie MJ, Zuniga K, Cooke GE, Awick E, Roberts S, … Kramer AF. Higher cardiorespiratory fitness levels are associated with greater hippocampal volume in breast cancer survivors. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00465. https://doi.org/10.3389/fnhum.2015.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock-Heyman L, Watson PD, Paul EJ, Zamroziewicz MK, Cooke GE, Allen C, … Barbey AK. Hippocampal volume mediates the association between aerobic fitness and relational memory in younger and middle-aged adults 2014 [Google Scholar]
- Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, … Jen CJ. Treadmill exercise enhances passive avoidance learning in rats: The role of down-regulated serotonin system in the limbic system. Neurobiology of Learning and Memory. 2008;89(4):489–496. doi: 10.1016/j.nlm.2007.08.004. https://doi.org/10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, … Kramer AF. Aerobic Exercise Training Increases Brain Volume in Aging Humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Hillman CH, Kramer AF. Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences. 2015;4:27–32. https://doi.org/10.1016/j.cobeha.2015.01.005. [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiology of Aging. 2014;35(Supplement 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. https://doi.org/10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, … Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. https://doi.org/10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, … Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. https://doi.org/10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell P. The Influence of Physical Fitness and Exercise upon Cognitive Functioning: A Meta-Analysis. Journal of Sport and Exercise Psychology. 1997;19(3):249–277. https://doi.org/10.1123/jsep.19.3.249. [Google Scholar]
- Herting MM, Nagel BJ. Differences in Brain Activity during a Verbal Associative Memory Encoding Task in High- and Low-fit Adolescents. Journal of Cognitive Neuroscience. 2013;25(4):595–612. doi: 10.1162/jocn_a_00344. https://doi.org/10.1162/jocn_a_00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF Expression in Perirhinal Cortex is Associated with Exercise-Induced Improvement in Object Recognition Memory. Neurobiology of Learning and Memory. 2010;94(2):278–284. doi: 10.1016/j.nlm.2010.06.006. https://doi.org/10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Li CR. The Right Superior Frontal Gyrus and Individual Variation in Proactive Control of Impulsive Response. Journal of Neuroscience. 2016;36(50):12688–12696. doi: 10.1523/JNEUROSCI.1175-16.2016. https://doi.org/10.1523/JNEUROSCI.1175-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Olson EA, Weber M. Physical Exercise Habits Correlate with Gray Matter Volume of the Hippocampus in Healthy Adult Humans. Scientific Reports. 2013;3:3457. doi: 10.1038/srep03457. https://doi.org/10.1038/srep03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack GR, Shiell A, Doyle-Baker PK, Friedenreich CM, Sandalack BA. Subpopulation differences in the association between neighborhood urban form and neighborhood-based physical activity. Health & Place. 2014;28:109–115. doi: 10.1016/j.healthplace.2014.04.001. https://doi.org/10.1016/j.healthplace.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Monti JM, Cooke GE, Watson PD, Voss MW, Kramer AF, Cohen NJ. Relating Hippocampus to Relational Memory Processing across Domains and Delays. Journal of Cognitive Neuroscience. 2015;27(2):234–245. doi: 10.1162/jocn_a_00717. https://doi.org/10.1162/jocn_a_00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Hillman CH, Cohen NJ. Aerobic fitness enhances relational memory in preadolescent children: the FITKids randomized control trial. Hippocampus. 2012;22(9):1876–1882. doi: 10.1002/hipo.22023. https://doi.org/10.1002/hipo.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Godde B, Voelcker-Rehage C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00170. https://doi.org/10.3389/fnagi.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. https://doi.org/10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Patterson B, Janssen A, Abduljalil A, Boster A. Physical activity associated with increased resting-state functional connectivity in multiple sclerosis. Journal of the International Neuropsychological Society: JINS. 2011;17(6):986–997. doi: 10.1017/S1355617711001093. https://doi.org/10.1017/S1355617711001093. [DOI] [PubMed] [Google Scholar]
- Schwarb H, Johnson CL, Daugherty AM, Hillman CH, Kramer AF, Cohen NJ, Barbey AK. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. NeuroImage. 2017;153:179–188. doi: 10.1016/j.neuroimage.2017.03.061. https://doi.org/10.1016/j.neuroimage.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The Relationship between Physical Activity and Cognition in Children: A Meta-Analysis. Pediatric Exercise Science. 2003;15(3):243–256. https://doi.org/10.1123/pes.15.3.243. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox MP, Mackay CE, … Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. https://doi.org/10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman CM, Cohen J, Lehman ME, Erickson KI. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Frontiers in Human Neuroscience. 2016;10 doi: 10.3389/fnhum.2016.00626. https://doi.org/10.3389/fnhum.2016.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. British Journal of Sports Medicine. 2015;49(4):248–254. doi: 10.1136/bjsports-2013-093184. https://doi.org/10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and Exercise: Past and Future Directions. NeuroMolecular Medicine. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. https://doi.org/10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, … Kramer AF. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48(5):1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. https://doi.org/10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, … Kramer AF. Plasticity of Brain Networks in a Randomized Intervention Trial of Exercise Training in Older Adults. Frontiers in Aging Neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00032. https://doi.org/10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Burzynska AZ, Wong CN, Cooke GE, Clark R, … Kramer AF. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage. 2016;131:113–125. doi: 10.1016/j.neuroimage.2015.10.044. https://doi.org/10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23(7):570–580. doi: 10.1002/hipo.22115. https://doi.org/10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Nielson KA, Sugarman MA, Smith JC, Seidenberg M, Durgerian S, … Rao SM. Lifestyle and Genetic Contributions to Cognitive Decline and Hippocampal Integrity in Healthy Aging. Current Alzheimer Research. 2012;9(4):436–446. doi: 10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li P. Brain Networks of Explicit and Implicit Learning. PLoS ONE. 2012;7(8):e42993. doi: 10.1371/journal.pone.0042993. https://doi.org/10.1371/journal.pone.0042993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, … Milham MP. The Oscillating Brain: Complex and Reliable. NeuroImage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. https://doi.org/10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. https://doi.org/10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conjunction maps showing overlap between each seed’s average connectivity and fitness-related connectivity. (A) left posterior (B) right anterior (C) and right posterior (D) hippocampal seeds. All seeds exhibited diffuse connectivity to the rest of the brain on average. (Z=3.1, P<.001). There were no significant clusters showing fitness-related correlations for the left posterior seed, and hence there was no overlap with this seeds average connectivity.