Abstract
The effects of light on cognitive function have been well-documented in human studies, with brighter illumination improving cognitive performance in school children, healthy adults and patients in early stages of dementia. However, the underlying neural mechanisms are not well understood. The present study examined how ambient light affects hippocampal function using the diurnal Nile grass rats (Arvicanthis niloticus) as the animal model. Grass rats were housed in either a 12:12hr bright light-dark (brLD, 1000 lux) or dim light-dark (dimLD, 50 lux) cycle. After 4 weeks, the dimLD group showed impaired spatial memory in the Morris Water Maze (MWM) task. The impairment in their MWM performance were reversed when the dimLD group were transferred to the brLD condition for another 4 weeks. The results suggest that lighting conditions influence cognitive function of grass rats in a way similar to that observed in humans, such that bright light is beneficial over dim light for cognitive performance. In addition to the behavioral changes, grass rats in the dimLD condition exhibited reduced expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, most notably in the CA1 subregion. There was also a reduction in dendritic spine density in CA1 apical dendrites in dimLD compared to the brLD group, and the reduction was mostly in the number of mushroom and stubby spines. When dimLD animals were transferred to the brLD condition for 4 weeks, the hippocampal BDNF and dendritic spine density significantly increased. The results illustrate that not only does light intensity affect cognitive performance, but that it also impacts hippocampal structural plasticity. These studies serve as a starting point to further understand how ambient light modulates neuronal and cognitive functions in diurnal species. A mechanistic understanding of the effects of light on cognition can help to identify risk factors for cognitive decline and contribute to the development of more effective prevention and treatment of cognitive impairment in clinical populations.
Keywords: light, hippocampus, diurnal, synaptic plasticity, BDNF
Introduction
Environmental lighting conditions influence a vast array of physiological and behavioral processes in humans, i.e. circadian rhythms, alertness/arousal, as well as mood and cognition (Chellappa et al., 2011; LeGates et al., 2014; Vandewalle et al., 2009). The effects of light in regulating cognitive processes have been documented across diverse populations, with brighter illumination yielding improved cognitive performance. For example, brighter illumination in the classroom enhances the performance of elementary school students in math and reading (Barkmann et al., 2012; Heschong, 2002; Heschong et al., 2002; Mott et al., 2012); bright office lighting improves the performance of adults in the work environment (Baron et al., 1992; Mills et al., 2007; Viola et al., 2008) and bright light therapy has been shown to attenuate cognitive deterioration in mild/early-stage dementia (Forbes et al., 2009; Riemersmavan der Lek et al., 2008; Yamadera et al., 2000). However, the neural mechanisms through which light modulates cognitive functions are not well understood.
For diurnal species, including humans, light promotes alertness, which is essential for optimal cognitive function (Brainard, 2005). Humans receiving bright light exposure during the day have lower sleepiness and fatigue scores compared to those in a dim light condition (Ruger et al., 2006). Neuroimaging studies have shown that daytime bright light exposure instantly increases activity in the subcortical regions that support alertness/arousal even before affecting cortical areas involved in cognitive processes and performance (Vandewalle et al., 2006). Similar results are obtained when using blue-enriched light at a ~ 460nm wavelength (Vandewalle et al., 2007a; Vandewalle et al., 2007b), which is the preferred wavelength for the retinal ganglion cells that are responsible for non-image-forming photoreception (Do and Yau, 2010; Lucas et al., 2014).
Light also modulates human attention and executive functions involved in cognitive processing. Measuring brain activities using electroencephalogram (EEG), shows that daytime exposure to blue light increases the amount of attentional resource allocated to cognitive tasks (An et al., 2009; Okamoto and Nakagawa, 2015). Furthermore, bright light therapy has been used in patients with attention-deficit/hyperactivity disorder, who show improvement in measures of both attention and executive function (Rybak et al., 2006).
In addition to the acute effects of bright-light exposure on arousal and attention, chronic changes in ambient lighting conditions can also produce long-lasting effects on brain and behavior. For example, laboratory rats housed in constant light during early development are resistant to the disruptive effects of constant light on circadian rhythms throughout their adulthood, suggesting that alterations in ambient illumination can lead to long-term changes in the brain (Cambras et al., 1998). Mice housed under different photoperiods or day-length over early development also show enduring difference in their dorsal raphe serotonin neurons, including their electrical properties and neurotransmitter content (Green et al., 2015). In postmortem human brain tissue, the number of midbrain dopaminergic neurons is higher in those who died in summer compared to those in winter (Aumann et al., 2016). Our own work using Nile grass rats (Arvicanthis niloticus), a diurnal rodent species, shows an increased number of dopaminergic and serotoninergic neurons in animals that had been housed over 4 weeks under daytime bright light (~ 1000 lux) compared to those kept under daytime dim light (~ 50 lux) (Deats et al., 2015; Leach et al., 2013a). Based on these findings, we hypothesized that long-lasting changes in the brain, beyond temporary enhancement of arousal or attention, are likely to contribute to the superior cognitive performance associated with brighter illumination.
To test this hypothesis, the present study utilized the diurnal Nile grass rat and hippocampal-dependent spatial learning/memory as model systems to explore the neural mechanisms through which ambient lighting conditions impact cognitive functions. Spatial learning and memory was assessed using the Morris Water Maze (MWM) task, which has been has been widely used in rodent species (Morris, 1981; Vorhees and Williams, 2006). Successful performance in MWM task has been shown to rely upon an intact hippocampus (Aggleton et al., 1986; Morris et al., 1982; Scoville and Milner, 2000) and is strongly correlated with hippocampal expression of brain-derived neurotrophic factor (BDNF) and dendritic plasticity (Conrad et al., 2012; Kesslak et al., 1998). Therefore, we focused our investigation on the hippocampus by examining its expression of BDNF and its dendritic spine morphology. The present study provides novel insights into the mechanisms responsible for the effects of ambient light on cognitive function, and has identified the grass rat as a useful diurnal animal model to further elucidate the underlying neural substrates for the behavioral effects of differential light exposure.
Experimental Procedures
Subjects
Male unstriped Nile grass rats (Arvicanthis niloticus) from our breeding colony at Michigan State University were used for all experiments. All animals were entrained to a 12:12 hr light-dark (LD, ~300 lux during the day) cycle and were given food (PMI Nutrition Prolab RMH 2000, Brentwood, MO, USA) and water ad libitum. All grass rats were group-housed prior to the start of the behavioral testing and then single-housed for the duration of the study in Plexiglas cages (34×28×17 cm), under either a 12:12hr bright light-dark (brLD, ~1000 lux during the day) or dim light-dark (dimLD, ~ 50 Lux) cycle as described in our previous studies (Deats et al., 2014; Leach et al., 2013a). A PVC tube was provided in the cage as a form of enrichment and as a hut for the animals. All experiments were performed in compliance with guidelines established by the Michigan State University Institutional Animal Care and Use Committee (IACUC), and the National Institutes of Health (NIH) guide for the Care and Use of Laboratory Animals.
Morris Water Maze
Three cohorts of animals (n=8/lighting condition) were used in this experiment. In the first cohorts, animals were housed in either brLD or dimLD for 1 week prior to being trained on the Morris Water Maze (MWM); while in the 2nd cohort, animals were housed in each condition for 4 weeks prior to MWM training. For the 3rd cohort, animals either remained in the colony condition (~300 lux) and then transferred to dimLD for 4 weeks prior to training on the MWM, or housed in dimLD for 4 weeks before being transferred to brLD for an additional 4 weeks prior to training. For all cohorts and conditions, during the last week before training, the animals were handled daily for 10 minutes to reduce novelty-induced stress that may stem from the experimenter’s handling of the animals (Leger et al., 2013). Handling was performed in the animals’ home cage in the behavioral testing room. Animals were trained and tested during zeitgeber time (ZT) 5–7, lights on was defined as ZT0; the light intensity in the testing room was ~ 300 lux. Training on the MWM was performed as previously described using a circular pool (60 cm depth × 122 cm diameter) with a platform (15-cm diameter) located 2cm under the water level and approximately 30cm away from the perimeter of the pool (Martin-Fairey and Nunez, 2014). The water was made opaque with non-toxic white tempera paint and kept at 26±2°C; different geometrical cues were posted up on each wall of the room for spatial orientation. Prior to the hidden-platform training, animals underwent a one-day cued-platform training, during which the water was clear and the platform was kept above water. This was done to ensure that any deficits seen during the hidden platform training were not due to impaired motor functions (Vorhees and Williams, 2006). As a prerequisite, all animals included in the following experiments located the platform in less than two minutes when it was visible. For the hidden platform procedure, each animal completed two training trials per day over 5 days with each trial being a maximum of two minutes in length with an inter-trial interval of 30 seconds. If the animal failed to locate the platform at the end of the two-minute period, it was guided towards the platform, and given a latency score of 120 seconds. On the sixth day, reference memory was tested 24 hr after the last training session by removing the platform from the MWM and allowing each grass rat to swim for one minute to measure the following parameters: time spent in the goal quadrant where the platform had been located, swim speed, and thigmotaxis, i.e. time spent swimming next to the wall (Morris, 1984). All behavior videos were loaded into Noldus Ethovision (XT 8.5, Noldus Information Technology, Netherlands) and scored by a experimenter who was blind to the experimental conditions.
Immunohistochemistry (IHC)
Animals tested in the MWM were left undisturbed for two days before being used for the IHC analysis. Another group of animals that was housed under the same lighting conditions, i.e. 4 weeks of either brLD or dim LD, but without behavioral training/testing, was also used for the IHC analysis. All animals were transcardially perfused at ZT 5–7 with saline followed by 4% paraformaldehyde (PFA). Brains were post-fixed and cryo-protected, then 3 alternate sets of 40μm sections were collected using a cryostat. 10 sequential sections containing the dorsal HPC from one alternate set were processed for IHC using anti-BDNF primary antibody (1:5000, raised in rabbit, ab101747, Abcam, Cambridge, UK). The specificity of the antibody in grass rats has been verified in a previous study (Martin-Fairey and Nunez, 2014). The IHC procedures were carried out as described in our previous studies using 3,3′-Diaminobenzidine (DAB) and 4% Nickel Sulfate for colorimetric reaction (Adidharma et al., 2012; Deats et al., 2014; Leach et al., 2013a; Leach et al., 2013b). After the IHC reaction, sections were mounted, dehydrated/clarified and then coverslipped using Permount (Fisher Scientific, NH, USA). Photomicrographs of the dorsal hippocampus were taken using a CCD camera attached to a Nikon light microscope and analyzed using Image J (NIH) as described in previous studies (Adidharma et al., 2012; Deats et al., 2014; Leach et al., 2013a; Leach et al., 2013b). The number of BDNF-ir cells was determined for the CA1, CA3 and Dentate Gyrus (DG) subregions of the hippocampus with a 200×400μm counting box (Suppl. Fig. 1).
Golgi staining
Behaviorally naïve animals were used in this study. Grass rats were housed in either brLD or dimLD (n=7/condition) for 4 weeks prior to transcardial perfusion (at ZT 5–7) with a phosphate buffer followed with a Rapid-Golgi fixative solution (modified from (Patro et al., 2013)). Brains were post-fixed in the same solution for 24 hours, then transferred to 3% potassium dichromate for three days before immersion in 1% AgNO3 for eight days. Brains were placed in 20% sucrose for 48 hours prior to sectioning at 100 μm using a cryostat. Sections were processed through an ethanol dehydration series and were clarified with xylene. Sections were mounted onto gelatin-coated slides and coverslipped with Permount (Fisher Scientific, NJ, USA). For quantification, images of dendritic spines were captured using a CCD video camera (CX9000, MBF bioscience, VM, USA) attached to a light microscope using an oil immersion lens (Nikon Instruments Inc., NY, USA) and spines were quantified using ImageJ with the AnalyzeSkeleton plug-in (Ignacio Arganda-Carreras, http://fiji.sc/wiki/index.php/). CA1 apical dendritic spines were analyzed from 20μm segments of four distinct dendritic branches per neuron, a total of six neurons were analyzed per brain (Pyter et al., 2005).
Spine morphology
Animals were housed in either brLD or dimLD for 4 weeks (n = 6/condition) without behavioral training or testing (behaviorally naïve). They then received bilateral injection into dorsal hippocampus of herpes simplex virus expressing green fluorescent protein (HSV-GFP, Massachusetts Institute of Technology Viral Core Facility) (Rodriguez et al., 2008). 26-gauge needles (Hamilton Company, Reno, NV) were placed bilaterally at the following coordinates from bregma: −0.1mm A–P (anteroposterior); ± 2.0 mm L–M (mediolateral); −2.7mm D–V (dorsoventral) from brain surface. Purified high-titer HSV-GFP (0.5μl) was infused at a rate of 0.1μl/minute, after infusions the needle rested at the site for five minutes prior to extraction.
After 48h post-surgery to allow for maximal GFP expression, animals were perfused transcardially with PBS followed by 4% paraformaldehyde. Sections were obtained at 100 μm thickness and mounted onto subbed glass slides with ProLong® Diamond Antifade Mountant (ThermoFisher Scientific, Waltham, MA). For detailed morphological analyses of dendritic spines, samples were imaged on a Nikon A1Rsi laser scanning confocal microscope utilizing a 100× Plan Apo TIRF DIC-oil immersion objective (total magnification of 1,000x). To visualize GFP, the samples were excited with a 488nm laser and the fluorophore emission was captured by a 525/50 band-pass (BP) filter. A z-stack was obtained for each sample for dendritic spine analysis.
For each animal, five neurons (two dendritic segments/neuron) were analyzed. Z-stacks were were used to achieve three-dimensional reconstruction utilizing the NeuronStudio freeware morphometric program, which allows for accurate visualization and aids in reducing experimenter bias (Rodriguez et al., 2008). Dendritic spine density for the dendritic segments was quantified and grouped by subtypes (e.g. thin, stubby, and mushroom) based on neck length and head diameter. Thin and mushroom spine subtypes are classified as having visible necks with the major difference being that the head diameter of thin spines is not notably different from the neck diameter, while mushroom spines’ head diameters are clearly larger than their neck diameter. Stubby spines are characterized as having a large head diameter along with no neck presence (Bourne and Harris, 2008; Hering and Sheng, 2001).
Data analysis
Statistical analysis was performed using SPSS (version 24, IBM, Armonk, North Castle, NY). For MWM behavioral data, the latency to reach the platform was analyzed using 2 × 5 Mixed ANOVAs with lighting condition as the between–subjects factor and training days as the repeated measures factor for trials 1 and 2 separately. In the case that there was a significant interaction, Holm-method comparisons were used to evaluate group differences across all five training days; when there was no interaction, only main effects were interpreted. Two-tailed independent samples student’s t-tests were used to assess group differences on the amount of time spent in the goal quadrant, swim speed, and thigmotaxis (i.e. time spent swimming in the periphery not representative of a search pattern) during the probe tests. The number of BDNF-ir cells in each subregion (Suppl. Fig. 1) and dendritic spine density in CA1 were compared between lighting conditions using two-tailed independent samples student’s t-tests. The threshold for statistical significance for all analyses was established at p<0.05.
Results
Chronic daylight deficiency impairs MWM performance
For the first trial of each day, both groups kept under either dim- or br-LD for 4 weeks showed significant improvement in their performance (i.e., latency to find the platform) over the 5 training days, but the performance of the brLD group was superior to that of the dimLD animals (Fig. 1A; main effect of training days: F(4,56)= 16.493, p<0.001; main effect of lighting condition: F(1,14)=4.652, p< 0.05). There was no significant interaction between training days and lighting condition (F(4,56)= 0.953, p>0.05). That group difference was absent for trial 2 (F(1,14)= 1.377, p > 0.05), which was conducted 30 seconds after trial 1 (Figure 1B). By the last two training days, the majority of the animals successfully located the platform during training trails (Suppl. Fig. 2). During the probe trial when the platform was removed, the brLD animals concentrated the search within the goal quadrant, in contrast to the dimLD animals (Fig. 1C). Comparison of the time spent on the goal quadrant by each group showed a significant difference with the brLD spending more time on the quadrant than the dimLD group (Fig. 1D; t(14)= 2.98, p= 0.01). The performance of the dimLD group was not significantly different from chance (15 sec; t(7)= −.057, p= .956). The groups did not differ significantly with respect to swim speed (t(14)= 0.002, p>0.05) and thigmotaxic behavior (t(14)= −0.76, p>0.05). In contrast to the animals housed in each condition for 4 weeks, identical testing of animals kept in brLD or dimLD for just one week did not result in group differences on any of the dependent variables (Suppl. Fig. 3).
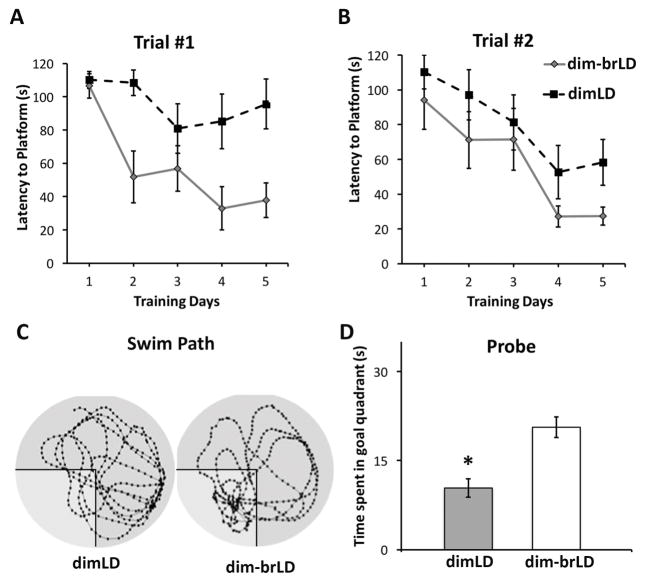
Figure 1.
Impaired MWM performance of grass rats housed in dimLD compared to those in brLD condition over 4 weeks. (A) Latency of animals to locate the platform during trial 1 (24-hour delay) over the 5 training days. Grass rats housed in brLD were able to locate the platform significantly faster in the than those housed in dimLD (main effect of training days: F(4,56)= 16.493, p<0.001; main effect of lighting condition: F(1,14)=4.652, p< 0.05); interaction between training days and lighting condition (F(4,56)= 0.953, p>0.05). (B) Latency of animals to locate the platform during trial 2 (30-second delay), there were no significant differences between the two groups. (C) Representative track plots of a grass rat in each lighting condition during the probe trial (with goal quadrant highlighted). (D). Grass rats housed in brLD nearly spent twice as much amount of time searching for the platform in the goal quadrant in the probe test when compared to grass rats in the dimLD group. *, p < 0.05.
Impaired MWM performance resulting from daytime light deficiency can be restored by transferring to brLD condition
To determine if the impairments in spatial learning/memory due to light restriction are reversible, the animals initially housed for 4 weeks in dimLD were transferred to brLD and kept there for 4 weeks before testing. The transferred animals showed superior performance compared to those kept in dimLD. For trial 1 there was a significant main effect of training days (F(4,56)= 15.05, p<0.001) and housing condition on the latency to reach the platform (F(1,14)= 12.942, p= 0.003), with no significant interaction (Fig. 2A; F(4,56)= 2.38, p= 0.062). Individual group comparisons showed superior performance for the animals in the reversal condition (i.e., dimLD-brLD) over those in the dimLD group for days 2, 4 and 5 of training. No group differences were detected for the latency data for trial 2 (Fig. 2B). During the probe trial, animals transferred from dimLD to brLD concentrated the search for the platform within the goal quadrant in contrast to the dimLD animals (Fig. 2C). The transferred animals also spent more time in the goal quadrant than the dimLD group (Fig. 2D, t(14)= 4.387, p= 0.001). There were no significant differences in swim speed (t(14)= 0.488, p>0.05) or thigmotaxis (t(14)=0.116, p>0.05) between groups.
Figure 2.
Subsequent brLD housing (dim-brLD) restored the impaired MWM performance of animals housed in dimLD conditions for 4 weeks. (A) Latency of animals to locate the platform during the first trial (main effect of training days: (F(4,56)= 15.05, p<0.001); main effect of housing condition : (F(1,14)= 12.942, p= 0.003); interaction between training days and housing condition: F(4,56)= 2.38, p= 0.062). (B) Latency of animals to locate the platform during the 2nd trial, there were no significant differences between the two groups. (C) Representative track plots of a grass rat in each lighting condition during the probe trial (with goal quadrant highlighted). (D). Time spent searching for the platform in the goal quadrant in the probe test. *, p < 0.05.
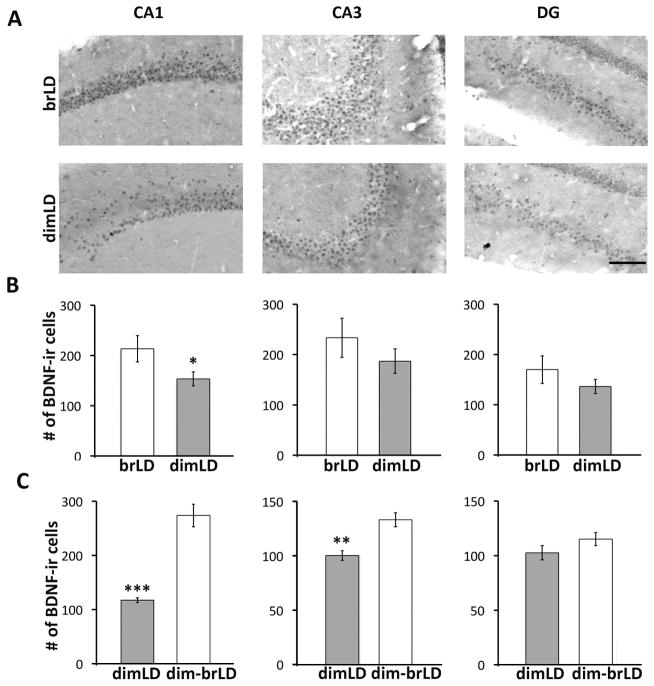
Ambient lighting condition modulates hippocampal BDNF expression
BDNF-ir in the hippocampus was reduced in the dimLD group compared to the brLD group (Fig. 3A). The average number of BDNF-ir cells was analyzed in CA1, CA3 and DG. The number of BDNF-ir cells were consistently lower across the three areas for the dimLD group, although statistical significance was reached only for CA1 (t(10)= 3.05, p=0.012, Fig. 3B). The data reported here are from animals that had been through MWM training. A separate cohort of animals without any behavioral testing was also compared for BDNF-ir (Suppl. Fig. 4). Similar results were obtained, with lower number of BDNF-ir cells in the CA1 of dimLD compared to brLD condition in these naïve animals (t(10)= 6.798, p<0.001). Similar to the behavioral reversal in MWM performance seen when the dimLD animals were transferred to brLD for 4 weeks, there was an increase in hippocampal BDNF-ir in the transferred animals compared to those kept in dimLD (Fig. 3C). A significant increase was observed in both CA1 (t(10)= 7.307, p<0.001) and CA3(t(10)= 4.183, p= 0.002).
Figure 3.
Ambient light condition modulates hippocampal BDNF expression. (A) Representative photomicrographs of BDNF immunochemical staining within the CA1, CA3, and DG of the hippocampus of grass rats housed in brLD or dimLD condition. (B) Number of BDNF-labeled cell bodies in each subregion of the hippocampus in animals housed in brLD or dimLD condition. (C). Number of BDNF-labeled cell bodies in each subregion of the hippocampus in animals housed in dimLD and those initially housed in dimLD then switched to brLD. Scale bar, 100 μm. *, p<0.05; **, p < 0.01; ***, p < 0.001.
Ambient light modulates CA1 apical dendritic morphology
In addition to BDNF expression, ambient light also modulates structural plasticity in the hippocampus. The morphology of Golgi-stained apical dendrites was analyzed in CA1 of animals from different lighting condition (Fig. 4). When the brLD and dimLD groups were compared (Fig. 4A, B), there was a significant reduction of apical dendritic spine density in the dimLD (t(8)= 5.103, p=0.001). Following transferring to the brLD condition (Fig. 4C, D), the dimLD-brLD group showed a significant increase in apical dendritic spine density compared to those kept in dimLD (t(10)= 10.062, p<0.001).
Figure 4.

Golgi staining of CA1 apical dendrites. (A) Representative photomicrograph and (B) quantification of dendritic spines of grass rats housed in either brLD or dimLD condition for 4 weeks. (C) Representative photomicrograph and (D) quantification of dendritic spines of grass rats housed in dimLD or initially in dimLD then transferred to brLD. Scale bar, 5μm. *, p < 0.01; **, p < 0.001.
The morphology of apical dendritic spines was further analyzed using HSV-GFP expression in hippocampal neurons (Fig. 5). Examples of labelled apical dendrites from animals kept four weeks in brLD or dimLD are shown in Fig. 5A. Group comparisons of the abundance of different types of spines in CA1 found significant higher density of mushroom (t(9)= 2.680, p< 0.05) and stubby spines (t(9)= 4.605, p=0.001) in the BLD group compared to the DLD animals, with no significant group differences for density of thin spines (Fig. 5B).
Figure 5.
Hippocampal apical dendrites visualized by HSV-GFP expression. (A) The injection sites of the HSV-GFP. (B) Representative photomicrophs of HSV-GFP expression. (C) Quantification of the density of dendritic spine sub-types. Scale bar, 5μm. *, p < 0.01.
Discussion
We show here that in diurnal Nile grass rats, chronic conditions of ambient lighting can influence cognition in a way similar to that observed in humans, such that bright light is beneficial over dim light for cognitive performance. In addition, we found that lighting condition can modulate the level of hippocampal BDNF expression as well as structural plasticity within the hippocampus.
The Nile grass rat is a well-established diurnal rodent model that has been used in various research areas including circadian rhythms and sleep/arousal systems to show how the control of those functions differs from that of nocturnal rodents (Castillo-Ruiz et al., 2010; Gall et al., 2013; Gall et al., 2014; Nixon and Smale, 2007; Novak et al., 2000; Ramanathan et al., 2008; Schwartz and Smale, 2005). Because of the different, and often opposite, effects of light on diurnal and nocturnal species (e.g. light promotes wakefulness/arousal in diurnal animals including humans, but induces sleep in nocturnal ones), a diurnal model is of crucial importance for a mechanistic understanding how light modulates cognition in humans (Challet, 2007; Smale et al., 2003). Diurnal rodent models i.e. Mongolian gerbils, fat sand rats and Nile grass rats have been used for investigating the impact of lighting condition on brain and behavior in various lighting paradigms including total darkness, short-photoperiod or dim light exposure at night (Ashkenazy-Frolinger et al., 2010; Einat et al., 2006; Lau et al., 2011) (Fonken et al., 2012). The lighting paradigm used in the present study was designed to manipulate daytime light intensity while keeping the day-length or photoperiods constant. By having photoperiods remain constant, our findings aim to be more ecologically relevant to humans because, unlike animals in nature under sunlight, much of our living environment is comprised of artificial light. Therefore, we do not experience drastic changes in day-length, but rather variations in the quality of light i.e. spectrum or intensity (Hubert, 1998).
After being kept in dim light during the day (dimLD) for 4 weeks, grass rats showed a deficit in the MWM task compared to the performance of animals kept under brLD for the same duration. The deficit was evident for both the first trial of each training day and for the probe test, in which the amount of time the dimLD animals spent in the goal quadrant was at chance level (Fig. 1). There were no group differences for animals kept in the two lighting conditions for just one week, thus suggesting that the detrimental effects of dim light develop over time (Suppl. Fig. 3). Further, since both groups were tested under identical intermediate lighting conditions, the superior performance of the animals in brLD for 4 weeks is not due to the acute effects of bright light on performance, as has been reported in human studies (Knez, 1995; Riemersma-van der Lek et al., 2008; Royer et al., 2012; Yamadera et al., 2000). Swimming speed did not differ across groups; therefore, group differences are not likely to reflect deficits in motivation (Lubbers et al., 2007) or sensory-motor functions (Vorhees and Williams, 2006). Even though dimLD housing has been shown to be anxiogenic for grass rats in the open field test (Ikeno et al., 2016), the lack of group differences in the display of thigmotaxis in the MWM suggests that the memory deficit of the dimLD animals is unlikely the result of enhanced anxiety during training/testing. When the dimLD grass rats were rehoused under the brLD condition for another 4 weeks, the animals performed significantly better than those in dimLD, suggesting that subsequent exposure to bright light can restore impaired spatial memory due to previous light restriction (Fig. 2).
Interestingly, the effect of long-term light restriction on latency to find the platform during training, was only significant for trial 1 of each day (Fig. 1A, 2A), suggesting that the retention of the memory for the platform location was impaired in the dimLD animals after a 24 hr interval but not for the 30 second between-trial interval; a similar conclusion is supported by the results of the probe trial, which occurs a day after the last training trial. However, the effect of lighting condition on latency to find the platform was not significant for trial 2 of each day (Fig. 1B, 2B), indicating that exposure to the water maze on trial 1 of each day was sufficient to bring the performance of the dimLD animals to the level of the brLD group in the second trial 30 seconds later. In the MWM, working memory, which involves the prefrontal cortex (Jones, 2002), is engaged as the animal searches for the escape platform on subsequent trials of the same training day, whereas hippocampal-dependent reference memory is necessary for remembering the location of the platform from one training day to the next. Thus, the normal performance of dimLD animals on trial 2 may reflect an intact working memory and lack of dysfunction in the prefrontal cortex. Alternatively, the experience of trial 1 each day may reactivate a relatively weak reference memory (de Hoz et al., 2004), which then supports the normal performance of the dimLD animals on trial 2. Regardless of the possible explanations for the improved performance of the dimLD animals on trial 2, our results point to an inability to consolidate a robust hippocampal-dependent memory over a 24-hr interval. The results collectively suggest that long-term (4 weeks) light deficiency impairs consolidation of spatial memories, which is indicated by the rapid forgetting over a 24-hour interval displayed by the dimLD animals. This rapid forgetting of reference memory in the MWM has been seen in studies with other animal models of hippocampal deficits e.g., epilepsy (Barkas et al., 2012), and hippocampal insulin resistance (Grillo et al., 2015).
MWM performance has been linked to hippocampal expression of BDNF (Kesslak et al., 1998), a member of the neurotrophin family of growth factors, which has been shown to be involved in learning and is crucial for long-term memory (Bekinschtein et al., 2007; Bekinschtein et al., 2008; Lu et al., 2008). Analyses of hippocampal BDNF expression and apical dendritic spines revealed a significant effect of ambient light on the structural plasticity of the hippocampus. Following 4 weeks of dimLD housing, there was a significant reduction in the number of BDNF-ir cells in the CA1 sub-region, compared to the animals in brLD and to those initially housed in dimLD then switched to brLD for another 4 weeks (Fig. 3). It is noteworthy that the brain samples from the two cohorts of animals i.e. 4 weeks in brLD vs dimLD (Fig. 3B) and 4 weeks in colony lighting (~300 lux) followed by 4 weeks dimLD vs. 4 weeks dimLD followed by 4 weeks brLD (dim-brLD, Fig. 3C) were processed for ICC separately, therefore, the results cannot be directly compared. Nonetheless, the differences between the dimLD and dim-brLD in the last cohort (Fig. 3C) appear to be greater than that in Fig. 3B when dimLD and brLD were compared. The greater differences in the last cohort (Fig. 3C) may due to the interaction between the lighting condition and prolonged (8 weeks) singly housing of the animals. Since these animals had been through MWM training/testing, the differences observed in BDNF-ir could have resulted from their housing condition, or alternatively, from their experience with the MWM, or the interaction of the two factors. Thus, we repeated the analysis in naïve animals. The results revealed a similar pattern with higher BDNF-ir in brLD as seen in the animals exposed to MWM training, thus suggesting that the difference in BDNF-ir was indeed due to the effects of the lighting condition and not the result of differential performance of the two groups on the MWM (Suppl. Fig. 4).
BDNF signaling modulates dendritic spine growth in the CA1 (Tyler and Pozzo-Miller, 2003). The growth of dendritic spines in the hippocampus, particularly within the CA1 region, has been linked to the formation of new synapses and improved learning and memory (Matsuzaki et al., 2004; Moser et al., 1994; Tsien et al., 1996). We found reduced CA1 apical dendritic spine density in dimLD animals compared to brLD and dimLD-to-brLD groups (Fig. 4), suggesting a possible change in CA3-CA1 connectivity, a crucial circuit for spatial memory (Kodama et al., 2005; Marston et al., 2008). Following 4 weeks of rehousing the dimLD group in the brLD condition, we found a significant increase in both BDNF-ir and dendritic spine density in the hippocampus. These findings indicate restored hippocampal function underlies the improvement of MWM performance of animals under the same lighting regimen (Fig. 2). It should be noted that the animals in the present study were all young adults (4–6 months old). Whether this level of plasticity is retained in older animals and how aging may impact the modulatory effects of light on hippocampal function requires further investigation.
It has been proposed that most excitatory synapses are located at dendritic spines (Harris, 1999; Matus, 2000), and their retraction or generation may underlie the neural mechanisms for learning and memory (Segal, 2017). A more detailed morphometric analysis on CA1 apical dendritic segments revealed a significant reduction of stubby and mushroom spines in dimLD compared to the brLD group (Fig. 5). Various studies demonstrate that after tetanic stimulation or behavioral training engaging the hippocampus, the spine apparatus prevalent in mushroom spines (Spacek and Harris, 1997) recruits a wide array of molecules that enhance synaptic plasticity (Engert and Bonhoeffer, 1999; Matsuo et al., 2008; Ostroff et al., 2002). Therefore, the observed lower number of CA1 mushroom spines may reflect a degradation of synaptic plasticity that is correlated with impaired performance in the MWM. The changes in BDNF expression, dendritic spine density, as well as spine morphology within the CA1, collectively suggest that ambient light modulates structural plasticity in the hippocampus.
The functional and structural changes in the hippocampus support the hypothesis that long-lasting changes in the brain, beyond temporary enhancement of arousal or attention, contribute to the superior cognitive performance associated with brighter illumination. Consistent with this hypothesis, seasonal variation has been reported in human cognitive brain responses, measured by P300 event-related brain potentials (Kosmidis et al., 1998; Polich and Geisler, 1991) and fMRI (Meyer et al., 2016). And the P300 amplitude has been shown to be influenced by seasonal variation in the available amount of sunshine (Polich and Geisler, 1991). Enhanced cognitive function by light has traditionally been explained in reference to sleep and circadian rhythms (Chellappa et al., 2011), and sleep and circadian regulation certainly play a role in memory and hippocampal functions (Smarr et al., 2014; Walker and Stickgold, 2006). However, lighting conditions can also influence learning/memory through circadian-independent mechanisms likely to involve melanopsin-based photoreception (LeGates et al., 2014). Our results provide evidence that lighting condition modulates the functional connectivity of the neural circuit within the hippocampus. More work is required to further elucidate the neural pathways mediating the effects of ambient light on the hippocampus. A possible candidate would be the hypothalamic orexin/hypocretin neurons, which have been implicated in many important functions including wakefulness, energy homeostasis, emotion and cognition (Gerashchenko and Shiromani, 2004; Tsujino and Sakurai, 2009). Our previous work in grass rats has shown that the number of orexin-ir neurons and the density of orexin-ir fibers are affected by lighting conditions, with higher levels of orexin-ir in brLD than in dimLD groups (Deats et al., 2014); and orexin pathways mediate the effects of light on other brain regions, i.e. the dorsal raphe (Adidharma et al., 2012) and hypothalamic dopaminergic neurons (Deats et al., 2015). Orexinergic cells project directly to the hippocampus in both nocturnal laboratory rats and diurnal grass rats (Nixon and Smale, 2007; Peyron et al., 1998). Thus, the orexin system is well positioned to mediate the effects of light on hippocampal-dependent learning and memory, an idea that will be further explored in future studies.
The present study is a first step towards a better understanding of how ambient light modulates cognitive functions in diurnal species. Such knowledge is significant for the design of lighting environments that promote optimal cognitive function. In the United States, a majority of the population spends ~90% of their time indoors, where the lighting is less bright than outdoors (Klepeis et al., 2001). Even in optimal environments, light deficiency can occur as a result of reduced ocular transmission related to retinal disease or aging (Chen et al., 2013; Coleman et al., 2008; de Zavalia et al., 2011; Fernandez et al., 2013; Higuchi et al., 2013). Although light pollution or light exposure at night has recently been recognized as a negative factor for ecology and human health (Chepesiuk, 2009; Falchi et al., 2011), the consequence of insufficient light during the day has received less attention. Our finding that 4 weeks of daytime light deficiency leads to a reduction in the functional connectivity within the hippocampus and to impairments in spatial learning and memory underscore the salient effects of light on our brain and behavior. A mechanistic understanding of the effects of light on cognition can help to identify risk factors for cognitive decline and contribute to the development of more effective prevention and treatment of cognitive impairment in clinical populations.
Supplementary Material
The sampling boxes used to count the number of BDNF-ir cells in the CA1, CA3 and DG in the hippocampus. Scale bar, 500 μm
The number of the animals successfully locating the platform during trial 1 (A) and trial 2 (B) over the 5 training days.
After 1 week in brLD and dimLD, there were no significant effect of lighting condition in the latency to locate the platform between the two groups in either the first (A) or the second trial (B). (C) Representative track plots of a grass rat in each lighting condition during the probe trial (with goal quadrant highlighted). (D). Time spent searching for the platform in the goal quadrant in the probe test.
Number of BDNF-labeled cell bodies in the CA1, CA3 and DG of behaviorally naïve animals animals housed in brLD or dimLD condition. *, p<0.05; **, p < 0.01.
Acknowledgments
We would like to thank Sheneé Martin and Alexandra Schulte for technical assistance. This work was supported by NIH grants MH111276 to LY and NS098173 to AN and LY. JS is supported by an NINDS Research Supplements to Promote Diversity in Health-Related Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding agencies.
References
- Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neuroscience. 2012;220:201–7. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19(2):133–46. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- An M, Huang J, Shimomura Y, Katsuura T. Time-of-day-dependent effects of monochromatic light exposure on human cognitive function. J Physiol Anthropol. 2009;28(5):217–23. doi: 10.2114/jpa2.28.217. [DOI] [PubMed] [Google Scholar]
- Ashkenazy-Frolinger T, Kronfeld-Schor N, Juetten J, Einat H. It is darkness and not light: Depression-like behaviors of diurnal unstriped Nile grass rats maintained under a short photoperiod schedule. J Neurosci Methods. 2010;186(2):165–70. doi: 10.1016/j.jneumeth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Raabus M, Tomas D, Prijanto A, Churilov L, Spitzer NC, Horne MK. Differences in Number of Midbrain Dopamine Neurons Associated with Summer and Winter Photoperiods in Humans. PLoS One. 2016;11(7):e0158847. doi: 10.1371/journal.pone.0158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas L, Redhead E, Taylor M, Shtaya A, Hamilton DA, Gray WP. Fluoxetine restores spatial learning but not accelerated forgetting in mesial temporal lobe epilepsy. Brain. 2012;135(Pt 8):2358–74. doi: 10.1093/brain/aws176. [DOI] [PubMed] [Google Scholar]
- Barkmann C, Wessolowski N, Schulte-Markwort M. Applicability and efficacy of variable light in schools. Physiol Behav. 2012;105(3):621–7. doi: 10.1016/j.physbeh.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Baron R, Rea M, Daniels S. Effects of indoor lighting (illuminance and spectral distribution) on the performance of cognitive tasks and interpersonal behaviors: the potential mediating role of positive affect. Motivation and Emotion. 1992;16(1):1–33. [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53(2):261–77. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105(7):2711–6. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP. Photons, Clocks, and Consciousness. Journal of Biological Rhythms. 2005;20(4):314–325. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- Cambras T, Vilaplana J, Torres A, Canal MM, Casamitjana N, Campuzano A, Diez-Noguera A. Constant bright light (LL) during lactation in rats prevents arrhythmicity due to LL. Physiol Behav. 1998;63(5):875–82. doi: 10.1016/s0031-9384(98)00006-7. [DOI] [PubMed] [Google Scholar]
- Castillo-Ruiz A, Nixon JP, Smale L, Nunez AA. Neural activation in arousal and reward areas of the brain in day-active and night-active grass rats. Neuroscience. 2010;165(2):337–49. doi: 10.1016/j.neuroscience.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148(12):5648–55. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Gordijn MC, Cajochen C. Can light make us bright? Effects of light on cognition and sleep. Prog Brain Res. 2011;190:119–33. doi: 10.1016/B978-0-444-53817-8.00007-4. [DOI] [PubMed] [Google Scholar]
- Chen M, Hombrebueno JR, Luo C, Penalva R, Zhao J, Colhoun L, Pandi SP, Forrester JV, Xu H. Age- and light-dependent development of localised retinal atrophy in CCL2(−/−)CX3CR1(GFP/GFP) mice. PLoS One. 2013;8(4):e61381. doi: 10.1371/journal.pone.0061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect. 2009;117(1):A20–7. doi: 10.1289/ehp.117-a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372(9652):1835–45. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2012;126(1):142–56. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoz L, Martin SJ, Morris RG. Forgetting, reminding, and remembering: the retrieval of lost spatial memory. PLoS Biol. 2004;2(8):E225. doi: 10.1371/journal.pbio.0020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zavalia N, Plano SA, Fernandez DC, Lanzani MF, Salido E, Belforte N, Sarmiento MI, Golombek DA, Rosenstein RE. Effect of experimental glaucoma on the non-image forming visual system. J Neurochem. 2011;117(5):904–14. doi: 10.1111/j.1471-4159.2011.07260.x. [DOI] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Lonstein JS, Yan L. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience. 2014;272:252–60. doi: 10.1016/j.neuroscience.2014.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deats SP, Adidharma W, Yan L. Hypothalamic dopaminergic neurons in an animal model of seasonal affective disorder. Neurosci Lett. 2015;602:17–21. doi: 10.1016/j.neulet.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90(4):1547–81. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Kronfeld-Schor N, Eilam D. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav Brain Res. 2006;173(1):153–7. doi: 10.1016/j.bbr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage. 2011;92(10):2714–22. doi: 10.1016/j.jenvman.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Fernandez DC, Sande PH, de Zavalia N, Belforte N, Dorfman D, Casiraghi LP, Golombek D, Rosenstein RE. Effect of experimental diabetic retinopathy on the non-image-forming visual system. Chronobiol Int. 2013;30(4):583–97. doi: 10.3109/07420528.2012.754453. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Kitsmiller E, Smale L, Nelson RJ. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms. 2012;27(4):319–27. doi: 10.1177/0748730412448324. [DOI] [PubMed] [Google Scholar]
- Forbes D, Culum I, Lischka AR, Morgan DG, Peacock S, Forbes J, Forbes S. Light therapy for managing cognitive, sleep, functional, behavioural, or psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2009;(4):CD003946. doi: 10.1002/14651858.CD003946.pub3. [DOI] [PubMed] [Google Scholar]
- Gall AJ, Smale L, Yan L, Nunez AA. Lesions of the Intergeniculate Leaflet Lead to a Reorganization in Circadian Regulation and a Reversal in Masking Responses to Photic Stimuli in the Nile Grass Rat. PLoS One. 2013;8(6):e67387. doi: 10.1371/journal.pone.0067387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Yan L, Smale L, Nunez AA. Intergeniculate leaflet lesions result in differential activation of brain regions following the presentation of photic stimuli in Nile grass rats. Neurosci Lett. 2014;579:101–5. doi: 10.1016/j.neulet.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29(1):41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Green NH, Jackson CR, Iwamoto H, Tackenberg MC, McMahon DG. Photoperiod programs dorsal raphe serotonergic neurons and affective behaviors. Curr Biol. 2015;25(10):1389–94. doi: 10.1016/j.cub.2015.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes. 2015;64(11):3927–36. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9(3):343–8. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Heschong L. Daylighting makes a difference. Educational Facility Planner. 2002;37:5–14. [Google Scholar]
- Heschong L, Wright R, Okura S, Klein P, Simner M, Berman S, Clear R. Daylighting impacts on human performance in school. Journal of Illuminating Engineering Society. 2002;31:101–114. [Google Scholar]
- Higuchi S, Hida A, Tsujimura S, Mishima K, Yasukouchi A, Lee SI, Kinjyo Y, Miyahira M. Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose-dependent manner. PLoS One. 2013;8(3):e60310. doi: 10.1371/journal.pone.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert M, Dumont M, Paquet J. Seasonal and Diurnal Patterns of Human Illumination Under Natural Conditions. Chronobiology International. 1998;15(1):59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- Ikeno T, Deats SP, Soler J, Lonstein JS, Yan L. Decreased daytime illumination leads to anxiety-like behaviors and HPA axis dysregulation in the diurnal grass rat (Arvicanthis niloticus) Behav Brain Res. 2016;300:77–84. doi: 10.1016/j.bbr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2002;2(7):639–47. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112(4):1012–9. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–52. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Knez I. Effects of indoor lighting on mood and cognition. Journal of Environmental Psychology. 1995;15(1):39–51. [Google Scholar]
- Kodama T, Usui S, Honda Y, Kimura M. High Fos expression during the active phase in orexin neurons of a diurnal rodent, Tamias sibiricus barberi. Peptides. 2005;26(4):631–8. doi: 10.1016/j.peptides.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Kosmidis MH, Duncan CC, Mirsky AF. Sex differences in seasonal variations in P300. Biol Psychol. 1998;49(3):249–68. doi: 10.1016/s0301-0511(98)00043-x. [DOI] [PubMed] [Google Scholar]
- Lau BW, Ren C, Yang J, Yan SW, Chang RC, Pu M, So KF. Light deprivation induces depression-like behavior and suppresses neurogenesis in diurnal mongolian gerbil (Meriones unguiculatus) Cell Transplant. 2011;20(6):871–81. doi: 10.3727/096368910X539065. [DOI] [PubMed] [Google Scholar]
- Leach G, Adidharma W, Yan L. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus) PLoS One. 2013a;8(2):e57115. doi: 10.1371/journal.pone.0057115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach G, Ramanathan C, Langel J, Yan L. Responses of brain and behavior to changing day-length in the diurnal grass rat (Arvicanthis niloticus) Neuroscience. 2013b;234:31–9. doi: 10.1016/j.neuroscience.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–54. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T. Object recognition test in mice. Nat Protoc. 2013;8(12):2531–7. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89(3):312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers ME, van den Bos R, Spruijt BM. Mu opioid receptor knockout mice in the Morris Water Maze: a learning or motivation deficit? Behav Brain Res. 2007;180(1):107–11. doi: 10.1016/j.bbr.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37(1):1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N, Piggins HD. Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol Brain. 2008;1:19. doi: 10.1186/1756-6606-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fairey CA, Nunez AA. Circadian modulation of memory and plasticity gene products in a diurnal species. Brain Res. 2014;1581:30–9. doi: 10.1016/j.brainres.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319(5866):1104–7. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290(5492):754–8. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- Meyer C, Muto V, Jaspar M, Kusse C, Lambot E, Chellappa SL, Degueldre C, Balteau E, Luxen A, Middleton B, et al. Seasonality in human cognitive brain responses. Proc Natl Acad Sci U S A. 2016;113(11):3066–71. doi: 10.1073/pnas.1518129113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills P, Tomkins S, Schlangen L. The effect of high correlated colour temperature office lighting on employee wellbeing and work performance. J Circadian Rhythms. 2007;5(2):2–10. doi: 10.1186/1740-3391-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learning and motivation. 1981;12(2):239–260. [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91(26):12673–5. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott M, Robinson D, Walden A, Bernette J, Rutherford A. Illuminating the Effects of Dynamic Lighting on Student Learning. Sage Open. 2012 Apr-Jun;:1–9. [Google Scholar]
- Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Harris JA, Smale L, Nunez AA. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus) Brain Res. 2000;874(2):147–57. doi: 10.1016/s0006-8993(00)02572-5. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Nakagawa S. Effects of daytime light exposure on cognitive brain activity as measured by the ERP P300. Physiol Behav. 2015;138:313–8. doi: 10.1016/j.physbeh.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35(3):535–45. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Patro N, Kumar K, Patro I. Quick Golgi method: modified for high clarity and better neuronal anatomy. Indian J Exp Biol. 2013;51(9):685–93. [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Geisler MW. P300 seasonal variation. Biol Psychol. 1991;32(2–3):173–9. doi: 10.1016/0301-0511(91)90008-5. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005;25(18):4521–6. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Nunez AA, Smale L. Daily rhythms in PER1 within and beyond the suprachiasmatic nucleus of female grass rats (Arvicanthis niloticus) Neuroscience. 2008;156(1):48–58. doi: 10.1016/j.neuroscience.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–55. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3(4):e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer M, Ballentine NH, Eslinger PJ, Houser K, Mistrick R, Behr R, Rakos K. Light therapy for seniors in long term care. J Am Med Dir Assoc. 2012;13(2):100–2. doi: 10.1016/j.jamda.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Ruger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1413–20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(10):1527–35. doi: 10.4088/jcp.v67n1006. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Smale L. Individual differences in rhythms of behavioral sleep and its neural substrates in Nile grass rats. J Biol Rhythms. 2005;20(6):526–37. doi: 10.1177/0748730405280924. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12(1):103–13. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines: Morphological building blocks of memory. Neurobiol Learn Mem. 2017;138:3–9. doi: 10.1016/j.nlm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18(5):356–66. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ. A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci. 2014;128(3):283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17(1):190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–38. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61(2):162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553(Pt 2):497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16(16):1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, Maquet P. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007a;17(12):2788–95. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–38. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, Berken PY, Balteau E, Degueldre C, Luxen A, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007b;2(11):e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34(4):297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54(3):352–3. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The sampling boxes used to count the number of BDNF-ir cells in the CA1, CA3 and DG in the hippocampus. Scale bar, 500 μm
The number of the animals successfully locating the platform during trial 1 (A) and trial 2 (B) over the 5 training days.
After 1 week in brLD and dimLD, there were no significant effect of lighting condition in the latency to locate the platform between the two groups in either the first (A) or the second trial (B). (C) Representative track plots of a grass rat in each lighting condition during the probe trial (with goal quadrant highlighted). (D). Time spent searching for the platform in the goal quadrant in the probe test.
Number of BDNF-labeled cell bodies in the CA1, CA3 and DG of behaviorally naïve animals animals housed in brLD or dimLD condition. *, p<0.05; **, p < 0.01.