Abstract
Background
Focal segmental glomerulosclerosis (FSGS) is a common cause of end stage renal disease (ESRD) with a high rate of recurrence after kidney transplantation. Several factors such as white race, rapid progression, and previous allograft failure due to recurrence were found to be risks of recurrence. Data are limited on the benefits of rituximab and/or therapeutic plasma exchange (TPE) in preventing recurrence. In this study, we sought to assess the efficacy of rituximab and TPE for the prevention and treatment of recurrent FSGS post kidney transplantation.
Methods
We enrolled 66 patients with FSGS in this prospective observational study and followed their outcomes. Patients with high risk for recurrence received preventative therapy with TPE and/or rituximab.
Results
Twenty three of the thirty seven (62%) who received preventative therapy developed recurrence compared to fourteen recurrences out of the twenty seven (51%) who did not receive any therapy (p=0.21). There was a trend for less relapse when rituximab was used as a therapy for recurrent FSGS, (6/22 versus 9/18, p=0.066). We utilized a clinical score of 5 values to assess the prediction of FSGS recurrence. A score of 3 or more had a predictive Receiver Operating Characteristic (ROC) curve of 0.72. Treatment with TPE and/or rituximab resulted in better allograft survival than historical studies. Allograft failure due to recurrent FSGS occurred in only 6 patients (9%).
Conclusion
Preventative therapies do not decrease the recurrence rate of recurrent FSGS. However, prompt treatment of recurrence with these therapies may result in improved outcomes.
Introduction
Focal segmental glomerulosclerosis (FSGS) is the leading cause of nephrotic syndrome in adults and commonly progresses to end stage renal disease (ESRD). FSGS is known to recur after kidney transplantation. FSGS is either idiopathic (ie, primary) or secondary to a variety of etiologies, such as genetic, infections, drugs, and adaptive. In general, only idiopathic FSGS recurs following kidney transplantation. The etiology of idiopathic FSGS remains unknown. However, circulating permeability factors have been suggested in the pathogenesis. The rate of posttransplant idiopathic FSGS recurrence after transplantation is not well defined but it is reported to average around 30% 1–4.
Several studies have attempted to identify risk factors for FSGS recurrence. White race, young age at presentation, low serum albumin, mesangial hypercellularity on initial biopsies, rapid progression to ESRD, and pervious failed transplant due to FSGS recurrence, all have been described as risk factors for posttransplant FSGS recurrence 2,5–11.
FSGS recurrence is associated with poor outcomes and a graft loss rate as high as 60% 12,13. Management of FSGS recurrence remains a great clinical challenge. Therapeutic plasma exchange (TPE) is the current standard of care with success rate of around 50% 13–15. Rituximab, which is a monoclonal antibody directed against CD-20 expressed in B-lymphocytes, has been used in posttransplant FSGS recurrence. Its beneficial effect on posttransplant FSGS recurrence was reported initially in 2006 16. Subsequently, only few studies 14,17–20 have assessed the efficacy of rituximab in the prevention and treatment of FSGS recurrence.
In this manuscript, we report the results of an observational prospective cohort study to evaluate risk factors for posttransplant FSGS recurrence, describes its course, and to determine the efficacy of rituximab and TPE in its prevention and treatment.
Materials and Methods
Study design
This a prospective observational cohort study that included patients with end stage renal disease secondary to FSGS who underwent kidney transplantation at our institution. Inclusion criteria for the study were: 1) diagnosis of idiopathic FSGS as original disease or patients who developed “de novo” FSGS after transplant but their pretransplant course is very suggestive of idiopathic FSGS, 2) age ≥ 18 years, 3) agree to participate in the study. Double organ transplantation recipients were excluded from the study. The study was approved by the Institutional Review Board at our center. Table 4 shows the type of FSGS in the native kidney.
Table 4.
Biopsy findings before and after transplant
| N=64 | Nonrecurrence, n=27 | Recurrence, n=37 | De novo, n=2 (No biopsy before transplant) | |
|---|---|---|---|---|
| Biopsy findings | Before transplant | Before transplant | Post transplant | Post transplant |
| Podocyte effacement, no changes on light microscopy | 0 | 7 | ||
| Not-Otherwise Specified (NOS) | 10 | 9 | 17 | |
| Collapsing variant | 4 | 5 | 5 | |
| Perihilar variant | 1 | 1 | ||
| Tip variant | 1 | 5 | 2 | |
| Cellular variant | ||||
| Tip+Collapsing | 1 | |||
| Unknown type | 12 | 20 | ||
| No biopsy | 2 | 1 | ||
Subjects who are deemed high risk for recurrence post kidney transplantation received preventative therapy that includes TPE and/or rituximab. We also, evaluated subjects’ response to the preventive therapies and the treatment methods in those who developed recurrence. Response to therapy is defined as reduction of proteinuria to less than 1 g/g. At the end of the study, we assessed risk factors for recurrence and created a 5-point clinical score to predict FSGS recurrence post kidney transplantation and assessed its ability to predict FSGS recurrence.
Study Population
The study included total of 66 patients; 64 patients with the diagnosis of idiopathic FSGS in their native kidneys and 2 patients who developed “de novo” FSGS after transplant but had a pretransplant course very suggestive of idiopathic FSGS. The transplants were performed between September 2008 and December 2015. Table 1 shows the patients’ characteristics. Donor and baseline recipient clinical variables were collected prospectively and from medical records. Patients were followed as per routine posttransplant care. Clinical and laboratory variables are collected periodically throughout the follow up period.
Table 1.
Patients characteristics
| Characteristic | N=66 (%) |
|---|---|
|
| |
| Ethnicity, n (%) | |
| White | 37 (56%) |
| African American | 21 (32%) |
| Asian | 5 (7%) |
| Hispanic | 3 (4%) |
|
| |
| Gender, n (%) | |
| Male | 34 (51%) |
|
| |
| Mean Age at Diagnosis (Yr) ± SD* | 29.9 ± 17.2 |
|
| |
| Mean Age at RRT† initiation (Yr) ± SD* | 36.7 ± 17 |
|
| |
| Median Time from diagnosis to RRT†, Yr | 4 (0–9) |
|
| |
| Median Duration of Dialysis (Yr) | 2.5 (0–9.5) |
|
| |
| Mean Age at Current Transplant (Yr) ± SD* | 38 ± 16.5 |
|
| |
| First transplant, n (%) | 42 (63%) |
|
| |
| Donor Source, n (%) | |
| Deceased | 25 (37%) |
| Living Unrelated | 25 (37%) |
| Living Related | 16 (25%) |
|
| |
| Induction with depleting agent, n (%) | 61 (92%) |
|
| |
| Maintenance Immunosuppression, n (%) | |
| Tacrolimus, MMF, Prednisone | 61 (92%) |
| Cyclosporine, MMF, Prednisone | 5 (8%) |
SD: standard deviation;
RRT: renal replacement therapy
Statistical analysis
We performed our statistical analyses using STATA 13 statistical software (StataCorp LP, College Station, Texas, USA). Descriptive statistics were used to estimate the frequencies, means, medians, and proportions of the study variables. We checked normality of distribution for continuous variables using box plots, normal probability plots, and Shapiro/Wilk normality test. Continuous data were expressed as median and range or mean and standard deviation. We used survival analysis/Kaplan-Meier curve to present all allografts’ survivals. We used Cox regression models to compare between the recurrence and nonrecurrence groups. Area under the receiver operator curves that predicted recurrence for different values of the scoring system was used. P-value of <0.05 is considered statistically significant.
Results
The study included total of 66 patients. Mean age at diagnosis of FSGS was 29.9 years (range: 18–59 years). Thirty four (51%) patients were male. Thirty seven patients (56%) of our cohort were Caucasian, twenty-one (32%) were African American, five (7%) were Asian, and three (4%) were Hispanic. The median time from diagnosis of FSGS to initiation of the first renal replacement therapy was 4 years (range 0–9 years). Data on the treatment of FSGS in the native kidneys was available in 55 patients; 28 (55%) of them received immunosuppressive therapy in the form of corticosteroids, calcineurin inhibitors, cyclophosphamide, or combination of these therapies. In forty-two of the sixty-six patients (64%) this was their first kidney transplantation. In fifteen (22%) this was the second, in seven (10%) this was their third, and in the remaining 2 this was their fourth transplantation. Twenty-five (37%) patients received their allografts from living unrelated donors, sixteen (25%) received their allografts from living related donors, and twenty-five (37%) received their allograft from a deceased donors. Forty four (65%) recipients were cytomegalovirus immunoglobulin G (CMV IgG) positive at the time of transplant. Median duration of follow up from time of transplantation was 29.5 months.
Preventative Strategies
In regards to the therapies used to prevent FSGS recurrence, thirty seven (57%) received perioperative rituximab and twenty eight (44%) received both perioperative rituximab and therapeutic plasma exchange therapies. Rituximab was given in 1 or 2 doses (375 mg/m2 per dose). The perioperative TPE sessions were started anytime between day 7 before transplant to postoperative day 2, depending on the source of donor and the availability of vascular access; and continued for 3–10 sessions. Patients were elected to receive a preventative therapy based on their pre transplant risk of recurrence. Risk factors taken into account were white race, young age at presentation defined by disease onset at less than thirty years old, rapid disease progression defined by development of ESRD within 5 years of disease onset in the native kidney, albumin level of less than 3 g/dL during the disease course, and history of failed kidney transplantation due to FSGS recurrence. Patients who had 2 risk factors were deemed high risk and recommended to receive preventative therapy with TPE and Rituximab. Nine patients did not receive TPE due to lack of vascular access or due to patients’ or providers’ preference. Out of the patients who were deemed low risk, 6 received a form of preventative therapy because of presence of donor specific antibodies (DSA) at the time of transplant (Table 2).
Table 2.
Prevention sub-groups
| N=64 | No prevention group n=27 | Prevention group n=37 | |
|---|---|---|---|
| Negative DSA n=31 | Positive DSA n=6 | ||
| Rituximab | 0 | 31 | 6 |
| TPE | 0 | 22 | 6 |
DSA: donor specific antibody, TPE: therapeutic plasma exchange
Recurrence
Excluding patients with de novo FSGS after transplant, thirty eight (59%) experienced recurrence of FSGS after transplant. Median time to recurrence was 1.25 months (range: 1 day–30 months). Mean urine protein-creatinine ratio at time of recurrence was 5.81 gram/gram (g/g) of creatinine, with range of 2.1–17 g/g. Twenty three of the thirty-seven patients (62%) who received a preventative therapy developed recurrence. On the other hand, fourteen of the twenty seven (51%) who did not receive a preventative therapy developed recurrence. The difference between the 2 groups was not statistically significant (HR 1.714, CI 0.62–4.71, p = 0.21).
The 2 patients who had de novo FSGS after transplant developed proteinuria at 7 and 64 months after transplant. Their allograft biopsies showed severe podocyte effacement (more than 50%). Table 3 shows these 2 patients’ characteristics.
Table 3.
Patients with de novo FSGS post transplant
| Patient 1 | Patient 2 | |
|---|---|---|
| Age at current transplant | 64 | 52 |
| Gender | Female | Male |
| Number of current transplant | 1 | 1 |
| Source of transplant | Living related | Living related |
| Pretransplant diagnosis | Diabetic nephropathy | Diabetic nephropathy |
| Pretransplant treatment with TPE or Rituximab | TPE and Rituximab | None |
| Time to FSGS | 7 | 64 |
| Peak proteinuria (g/g) | 9.01 | 6.3 |
| Treatment | TPE and Rituximab | TPE and Rituximab |
| Response to treatment | No | Yes |
| Most recent proteinuria (g/g) | 6 | 0.25 |
All patients with recurrent FSGS received kidney biopsy to confirm the diagnosis except 1 patient. Table 4 shows all biopsy data before and after transplant.
We assessed the association between several clinical variables and FSGS recurrence and we created a clinical scoring system to predict FSGS recurrence. The system included 5 clinical parameters with 1 point for each one of them. The clinical parameters are: white race, young age at presentation defined by disease onset at less than thirty years old, rapid disease progression defined by development of ESRD within 5 years of disease onset in the native kidney, albumin level of less than 3 g/dL during the disease course, and history of failed kidney transplantation due to FSGS recurrence. When analyzed as a single factor, we did not detect an association between race, gender, age at diagnosis, time to ESRD, and source of transplant, and risk of recurrence (Table 5). However, when analyzed the performance of the clinical scoring system in predicting FSGS recurrence, the calculated area under the curve (AUC) was 0.7227 when using a score of 3. Using a score of 2, the AUC is 0.614 and using a score of 4, the AUC is 0.581. Figure 1 shows area under the receiver operator curves for the scoring system performance to predict FSGS recurrence
Table 5.
Predictive values of clinical variables
| Variable | Hazard Ratio | CI* | P value |
|---|---|---|---|
| White race | 1.15 | (0.4–3.26) | 0.787 |
| Female gender | 0.76 | (0.27–2.08) | 0.579 |
| Age at diagnosis | 0.99 | (0.96–1.02) | 0.793 |
| Time to ESRD† | 1.04 | (0.97–1.12) | 0.201 |
| Dialysis duration | 1.01 | (0.85–1.19) | 0.883 |
| Previous recurrence | 0.92 | (0.19–2.05) | 0.107 |
| Source of transplant (Living related) | 0.88 | (0.28–2.77) | 0.83 |
| Use of Rituximab | 0.733 | (0.29–1.26) | 0.163 |
| Use of Rituximab and TPE§ | 0.632 | (0.18–1.15) | 0.225 |
| DGF¶ (In deceased donor) | 1.42 | (0.27–7.51) | 0.67 |
| Clinical score 3 and above | 1.445 | (1.16–1.72) | 0.009 |
CI: Confidence Interval;
ESRD: end stage renal disease;
TPE: therapeutic plasma exchange;
DGF: delayed graft function
Figure 1.
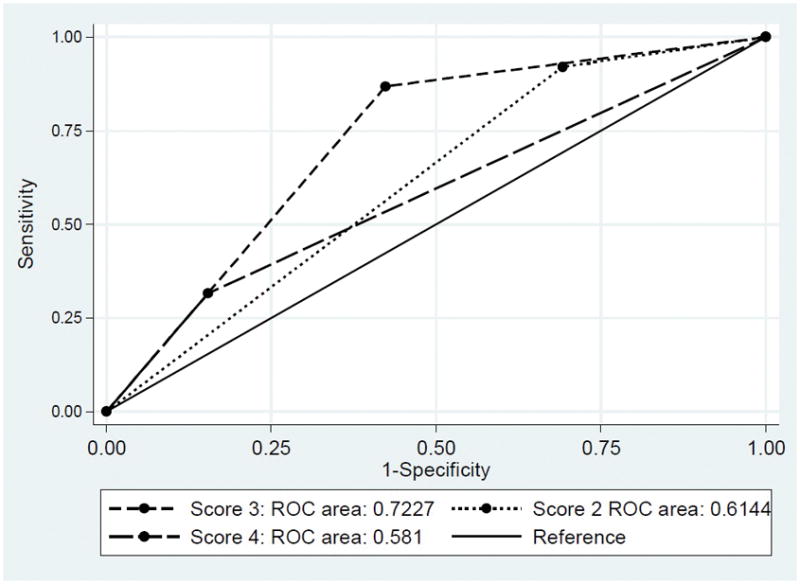
ROC curves for detecting patients with highest risk of recurrence stratified by their pretransplant risk scoring system
Treatment and Outcome of Recurrence
All patients who experienced FSGS recurrence and the 2 patients with de novo FSGS received standardized treatment consisted of TPE and low dose intravenous immunoglobulins (IVIG) 200 mg/kg. Additionally, twenty patients (50%) received rituximab. TPE course consisted of at least 10 sessions. The first 3–5 sessions were daily and followed by IVIG. The remaining sessions were scheduled 3 times a week, 2 of which were followed by IVIG. We used replacement fluids of 100% Albumin. TPE sessions are continued for at least 10 sessions. We stop the treatment after that if there is response to treatment (defined by decrease in proteinuria by >50%). Eight patients needed a longer course of TPE (range of 16–35 sessions) at a weaning schedule. Four additional patients required much longer term TPE that has been going on for 1–2 years to keep FSGS in remission. These 4 patients received TPE on 1 session every 2–3 weeks schedule. Thirty-five patients (87%) were placed on angiotensin converting enzyme inhibitor (ACEi) (after the completion of TPE) or angiotensin receptor blocker (ARB). All but 5 (87%) responded to this initial therapy. Four (10%) patients lost their allografts due to FSGS recurrence. These 4 did not receive rituximab.
Fourteen of the thirty-five (40%) responders developed at least 1 relapse after the initial treatment and 2 of them lost their allograft. There were more relapses in the patients who did not receive rituximab at the time of recurrence [9/18 versus 6/22, HR 0.285, CI 0.069–1.167, p = 0.066]. Nine of the relapses were observed in patients who did not receive rituximab at the initial treatment.
There were 8 total allografts losses; 4 due to the initial recurrence, 1 due to cardiovascular death, 2 due to subsequent relapse, and 1 due to rejection.
Table 6 shows the differences in serum Creatinine, eGFR (calculated by CKD-EPI equation), serum albumin, and urine protein creatinine ratio between the groups of recurrence and without recurrence at the end of the study. Serum Creatinine and urine protein-creatinine ratio were higher and eGFR was lower in the recurrence group. Survival analysis showed that there was a trend toward better renal allograft survival in nonrecurrent group compared to the recurrent group (P log rank of 0.0662) (Figure 2).
Table 6.
Clinical outcome of patients with and without recurrent FSGS
| Laboratory value | Nonrecurrence Value (CI*) | Recurrence Value (CI*) | P value |
|---|---|---|---|
| Serum Creatinine (mg/dL) | 1.33 (1.21–1.45) | 1.72 (1.43–2.02) | 0.02 |
| eGFR† (mL/min/1.73 m2) | 61.8 (53.9–69.6) | 49.3 (39.9–58.6) | 0.0451 |
| Albumin (g/dL) | 4.3 (4.0–4.5) | 4.1 (3.9–4.2) | 0.162 |
| UPC§ (g/g) | 0.12 (0.09–0.16) | 1.40 (0.80–1.99) | 0.0002 |
CI: confidence interval;
eGFR: estimated glomerular filtration rate;
UPC: urine protein-creatinine ratio
Figure 2.
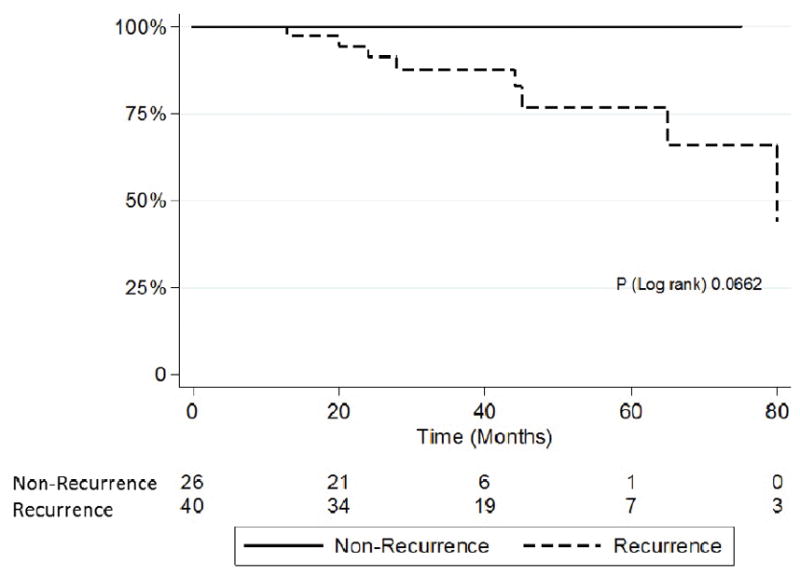
Kaplan Meier curve of allografts’ survival in patients with FSGS recurrence
Discussion
Our study is one of the largest prospective studies that describes the natural course of FSGS after transplant and gives insight on the efficacy of using interventions such as TPE and rituximab in the prevention and treatment of posttransplant FSGS recurrence. Idiopathic FSGS remains a challenge to transplant centers because of its high risk of recurrence and associated poor outcomes. Although several studies have assessed different therapies to prevent and treat FSGS recurrence, most of them were small and more importantly, they included a mixture of FSGS type. Therefore, larger studies that include only patients with idiopathic FSGS are needed so transplant centers can develop strategies to manage such patients. Our study included only patients with high degree of certainty of having idiopathic FSGS.
The etiology of idiopathic FSGS is still unknown. Circulating permeability factors have been suggested in the pathogenesis 21. This is based on the observation of development of proteinuria within hours after transplantation and response to TPE 22–24. In one case, kidney transplant recipient developed early recurrence of FSGS with evidence of foot process effacement on biopsy that resolved after re-transplanting the same kidney into a second recipient 24. The identity of the circulating permeability factor is yet to be determined, although the serum soluble urokinase receptor (suPAR) has been suggested in several studies 21. Regardless of the type of this factor, TPE seems to be effective in removing it, and therefore, it has been used in its treatment and suggested as a preventative therapy.
The rate of idiopathic FSGS recurrence after transplantation has been difficult to ascertain. In most of the published studies, it is challenging to determine whether FSGS is idiopathic or secondary. The reported FSGS recurrence rate in most of these studies averages approximately 30% 1–4. However, this rate is probably an underestimation given the reasons mentioned above. In our study, which included only patients with idiopathic FSGS, the observed recurrence rate was 59%. This is much higher than what has been previously published. For example, the reported recurrence rate in the European Renal Association-European Dialysis and Transplant Association registry was 24% 25. In a cohort of thirty highly selected idiopathic FSGS patients, the reported recurrence rate was 47% 14.
A number of factors have been reported to be associated with an increased risk of recurrence. Patients who develop ESRD rapidly after the onset of the disease are at higher risk compared to those who had their disease for a longer period of time 2,5,6. White race has also been described as a risk factor 3,9. Mesangial hypercellularity on initial renal biopsy has also been described as a risk factor for recurrence 6,7. Patients with recurrence tended to be younger at the onset of the disease when compared with those without recurrence 6,8,9. Also, recurrence of FSGS in previous transplants has been identified as a risk factor 2. While earlier reports suggested that collapsing FSGS tend to recur more, later studies did not support this finding 10. Lower albumin level is associated with increased risk of recurrence 11 and worse outcome 3. We have developed a predictive clinical scoring system that incorporates the most studied risk factors in addition to our clinical observation, to help identify patients with the highest risk of recurrence. Our study is the first one to develop a prediction clinical scoring system. We assessed its prediction for development of FSGS recurrence and we found that using a score of 3 and above, the calculated AUC was 0.7227. This suggests that this system is a fair test to predict risk for posttransplant FSGS recurrence. However, because of the relatively small number of patients, we acknowledge that the scoring system needs to be validated on larger cohorts once such cohorts are available.
There is no proven therapy that has been effective in preventing posttransplant FSGS recurrence. Several studies used TPE and/or rituximab as preventative strategies. TPE has been used for this purpose with variable success. In one study, TPE did not reduce the incidence of FSGS recurrence 26. Rituximab is a chimeric mouse/human monoclonal antibody directed against CD-20 expressed on B-lymphocytes. Rituximab has several applications in treating glomerular conditions, such as acute allograft rejection and nephrotic syndrome. The mechanism by which rituximab affects the FSGS course does not seem to be related to its anti CD-20 activity 19. In vitro studies showed possible cross-reactivity of rituximab with sphingomyelin-phosphodiesterase-acid-like-3b (SMPDL-3b) 27 and in vitro exposure to rituximab in lymphoma cells regulates the activity of acid-sphyngomyelinase (ASMase) in raft microdomains. These are essential for the organization of receptors and signaling molecules in highly specialized cells such as the podocytes 18. Its beneficial effect on posttransplant FSGS recurrence was reported initially in 2006 16. Subsequently, only few studies assessed the efficacy of rituximab in prevention of FSGS recurrence 17,19,20. However, these studies included only a small number of patients. In one study, rituximab treatment was associated with a lower incidence of posttransplant proteinuria and stabilization of glomerular filtration rate 19. In another study, rituximab was effective in preventing FSGS recurrence in 4 patients who had failed previous kidney transplants due to FSGS recurrence 17.
In our study, we did not observe a difference in the rate of posttransplant FSGS recurrence between patients who received a preventative therapy and those who did not (62% versus 51%, p 0.21). However this should be interpreted with caution since patients who received preventative therapy were deemed to have a higher risk for recurrence.
The observed average response rate of TPE is 50% to 70% in published literature 13,14,23. We observed a higher rate of response to TPE 87% (35/40). From those thirty-five, fourteen patients needed a long course or became TPE dependent. Half of the patients received rituximab at the time of recurrence. The high response rate to TPE, although it is difficult to prove, could be explained by the additive use of rituximab.
Among patients who responded initially to treatment, 40% developed recurrence. There was a trend toward more relapses in patients who did not receive rituximab at initial recurrence. This is consistent with the findings of Hickseon et al in which the 4 patients with FSGS recurrence who received TPE at recurrence and rituximab therapy at some time posttransplant sustained remission of proteinuria as well as stable graft function 14. Graft survival was inferior in patients who developed recurrence compared to those who did not. There were a total of 8 graft losses in the recurrence group, 6 of which were due to FSGS recurrence.
Our study has limitations. Although our data constitutes one of the largest prospective analyses of recurrent FSGS post kidney transplant, however it remained small sized study, and a larger multi centers prospective is required to validate the clinical score and the outcome of prevention and therapies that we used in our cohort. Additionally, there are possibly other clinical factors that may predict FSGS recurrence, however, the parameters we utilized in our clinical score were the most relevant and significant in previously published data and our clinical observation.
For different logistic reasons, we were unable to enroll in our study all FSSG patients who were transplanted in our center; in particular patients without recurrence. Therefore, it is possibly that the rate of recurrence is less than the reported rate. Furthermore, complete data of the native biopsies were unavailable to us in spite of all the effort to obtain these data. A large number of these biopsies were performed in other centers and many years in the past. We have relied on the patients’ nephrology record to confirm the diagnosis of FSGS in native kidney. Other limitation of our findings is the fact that the therapies are heterogeneous and not randomized; the reason is that the therapies depended on patient’s response. Some patients responded to short course of TPE and 1 dose of rituximab; hence the recurrent FSGS therapies were stopped completely and patients were maintained on ACEi or ARB. However, some patients required longer duration of TPE and more than 1 dose of rituximab.
Acknowledgments
Sources of support:
NIH R01DK101350-04, Nada Alachkar
ABBREVIATIONS
- FSGS
Focal segmental glomerulosclerosis
- ESRD
End stage renal disease
- TPE
therapeutic plasma exchange
- ROC
Receiver Operating Characteristic
- CMV
Cytomegalovirus
- DSA
donor specific antibodies
- HR
Hazard Ratio
- CI
Confidence interval
- AUC
area under the curve
- ACEi
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- eGFR
estimated glomerular filtration rate
- suPAR
serum soluble urokinase receptor
- SMPDL-3b
sphingomyelin-phosphodiesterase-acid-like-3b
Footnotes
Authorship Page:
Sami Alasfar: data collection, data analysis and interpretation, drafting the article
Dany Matar: data collection
Robert A Montgomery: critical revision of the article
Niraj Desai: critical revision of the article
Bonnie Lonze: critical revision of the article
Vikas Vujjini: data collection
Michelle M. Estrella: critical revision of the article
John Manllo Dieck: data collection, critical revision of the article
Gebran Khneizer: data collection
Sanja Sever: critical revision of the article
Jochen Reiser: critical revision of the article
Nada Alachkar: design of the work, data collection, data analysis and interpretation, drafting the article, final approval of the version to be published
Disclosures:
JR and SS are cofounders and stock holders of Trisaq, a biotechnological company developing novel therapeutics for chronic kidney diseases and FSGS, and have pending and issued patents in the therapeutic and diagnostic space regarding kidney diseases.
References
- 1.Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(11):2535–2542. doi: 10.1111/j.1600-6143.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- 2.Pinto J, Lacerda G, Cameron JS, Turner DR, Bewick M, Ogg CS. Recurrence of focal segmental glomerulosclerosis in renal allografts. Transplantation. 1981;32(2):83–89. doi: 10.1097/00007890-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Abbott KC, Sawyers ES, Oliver JD, 3rd, et al. Graft loss due to recurrent focal segmental glomerulosclerosis in renal transplant recipients in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;37(2):366–373. doi: 10.1053/ajkd.2001.21311. [DOI] [PubMed] [Google Scholar]
- 4.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. The New England journal of medicine. 2002;347(2):103–109. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 5.Dantal J, Baatard R, Hourmant M, Cantarovich D, Buzelin F, Soulillou JP. Recurrent nephrotic syndrome following renal transplantation in patients with focal glomerulosclerosis. A one-center study of plasma exchange effects. Transplantation. 1991;52(5):827–831. doi: 10.1097/00007890-199111000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Senggutuvan P, Cameron JS, Hartley RB, et al. Recurrence of focal segmental glomerulosclerosis in transplanted kidneys: analysis of incidence and risk factors in 59 allografts. Pediatric nephrology (Berlin, Germany) 1990;4(1):21–28. doi: 10.1007/BF00858431. [DOI] [PubMed] [Google Scholar]
- 7.Pardon A, Audard V, Caillard S, et al. Risk factors and outcome of focal and segmental glomerulosclerosis recurrence in adult renal transplant recipients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(4):1053–1059. doi: 10.1093/ndt/gfk005. [DOI] [PubMed] [Google Scholar]
- 8.Banfi G, Colturi C, Montagnino G, Ponticelli C. The recurrence of focal segmental glomerulosclerosis in kidney transplant patients treated with cyclosporine. Transplantation. 1990;50(4):594–596. doi: 10.1097/00007890-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Nehus EJ, Goebel JW, Succop PS, Abraham EC. Focal segmental glomerulosclerosis in children: multivariate analysis indicates that donor type does not alter recurrence risk. Transplantation. 2013;96(6):550–554. doi: 10.1097/TP.0b013e31829c2431. [DOI] [PubMed] [Google Scholar]
- 10.Canaud G, Dion D, Zuber J, et al. Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS) Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(4):1321–1328. doi: 10.1093/ndt/gfp500. [DOI] [PubMed] [Google Scholar]
- 11.Tejani A, Stablein DH. Recurrence of focal segmental glomerulosclerosis posttransplantation: a special report of the North American Pediatric Renal Transplant Cooperative Study. Journal of the American Society of Nephrology : JASN. 1992;2(12 Suppl):S258–263. doi: 10.1681/ASN.V212s258. [DOI] [PubMed] [Google Scholar]
- 12.Hariharan S, Adams MB, Brennan DC, et al. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR) Transplantation. 1999;68(5):635–641. doi: 10.1097/00007890-199909150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F. Recurrent focal glomerulosclerosis: natural history and response to therapy. The American journal of medicine. 1992;92(4):375–383. doi: 10.1016/0002-9343(92)90267-f. [DOI] [PubMed] [Google Scholar]
- 14.Hickson LJ, Gera M, Amer H, et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87(8):1232–1239. doi: 10.1097/TP.0b013e31819f12be. [DOI] [PubMed] [Google Scholar]
- 15.Artero ML, Sharma R, Savin VJ, Vincenti F. Plasmapheresis reduces proteinuria and serum capacity to injure glomeruli in patients with recurrent focal glomerulosclerosis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1994;23(4):574–581. doi: 10.1016/s0272-6386(12)80381-7. [DOI] [PubMed] [Google Scholar]
- 16.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. The New England journal of medicine. 2006;354(18):1961–1963. doi: 10.1056/NEJMc055495. [DOI] [PubMed] [Google Scholar]
- 17.Audard V, Kamar N, Sahali D, et al. Rituximab therapy prevents focal and segmental glomerulosclerosis recurrence after a second renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2012;25(5):e62–66. doi: 10.1111/j.1432-2277.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 18.Bezombes C, Grazide S, Garret C, et al. Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood. 2004;104(4):1166–1173. doi: 10.1182/blood-2004-01-0277. [DOI] [PubMed] [Google Scholar]
- 19.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Science translational medicine. 2011;3(85):85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HS, Hong Y, Sun IO, et al. Effects of pretransplant plasmapheresis and rituximab on recurrence of focal segmental glomerulosclerosis in adult renal transplant recipients. The Korean Journal of Internal Medicine. 2014;29(4):482–488. doi: 10.3904/kjim.2014.29.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alachkar N, Wei C, Arend LJ, et al. Podocyte effacement closely links to suPAR levels at time of posttransplantation focal segmental glomerulosclerosis occurrence and improves with therapy. Transplantation. 2013;96(7):649–656. doi: 10.1097/TP.0b013e31829eda4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport RD. Apheresis treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation: re-analysis of published case-reports and case-series. Journal of clinical apheresis. 2001;16(4):175–178. doi: 10.1002/jca.10007. [DOI] [PubMed] [Google Scholar]
- 23.Matalon A, Markowitz GS, Joseph RE, et al. Plasmapheresis treatment of recurrent FSGS in adult renal transplant recipients. Clinical nephrology. 2001;56(4):271–278. [PubMed] [Google Scholar]
- 24.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. The New England journal of medicine. 2012;366(17):1648–1649. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 25.Briggs JD, Jones E. Recurrence of glomerulonephritis following renal transplantation. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14(3):564–565. doi: 10.1093/ndt/14.3.564. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez E, Ettenger R, Rianthavorn P, Tsai E, Malekzadeh M. Preemptive plasmapheresis and recurrence of focal segmental glomerulosclerosis in pediatric renal transplantation. Pediatric transplantation. 2011;15(5):495–501. doi: 10.1111/j.1399-3046.2011.01478.x. [DOI] [PubMed] [Google Scholar]
- 27.Perosa F, Favoino E, Caragnano MA, Dammacco F. Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood. 2006;107(3):1070–1077. doi: 10.1182/blood-2005-04-1769. [DOI] [PubMed] [Google Scholar]


