Abstract
Background
We hypothesized C1q binding de novo donor specific antibody (DSA) after heart transplant (HT) is a higher risk for development of coronary artery vasculopathy (CAV) in children.
Methods
A retrospective analysis of 127 pediatric heart transplant (HT) recipients transplanted between January 2005 and December 2014 was used to determine complement (C1q)-binding de novo DSA on the outcomes of HT and the ability of the C1q assay to predict CAV development.
Results
Out of 127 patients, 59 (46.4%) developed de novo DSA, of those 37 had C1q+ DSA. There was no difference in baseline characteristics except patients who developed C1q+ DSA more often received a donor heart from a female compared to C1q− DSA group (p=0.034). The DSA median fluorescent intensity (MFI) value of ≥7000 had 80% sensitivity and 80% specificity (C statistics 0.89, p <0.05) for predicting positive C1q binding. Multivariate analyses identified C1q binding DSA as an independent risk for CAV with a hazard ratio (HR) of 3.25 (95% confidence interval of 1.33–7.93, p= 0.0095). In multivariable Cox proportional hazard models, the covariates associated with graft loss included: C1q+ DSA (HR 3.2, 95% CI, 1.34–7.86; p<0.009), pre-HT renal insufficiency (HR 11.3, 95% CI, 3.71–34.29, p<0.0001) and pre-HT ventilator support (HR 3.3, 95% CI 1.39–7.81, p=0.007).
Conclusions
The DSA strength in MFI correlates with positive C1q binding activity and hence functional capabilities of DSA. Close monitoring of DSA strength in MFI and function (C1q assay) may be useful for identifying pediatric HT recipient at risk for development of CAV.
Introduction
There is equivocal evidence in the literature regarding the role of de novo donor-specific anti-HLA antibodies (DSA) and its association with increased incidence of coronary artery vasculopathy (CAV), acute rejection and decreased survival in pediatric heart transplant (HT) recipients. Currently, detection of HLA antibodies is routinely performed using single antigen beads (SAB) with solid-phase assays (SPA). In the last decade, SPA has replaced for the most part less sensitive cell based assays. SPA technologies have the advantage of not only determining the presence of HLA antibodies but also the class and specificity. However, SPA may be oversensitive by not distinguishing those DSA with clinical significance. Ho et al, have shown that antibody-mediated rejections (AMR) and poor long-term graft survival was associated with DSA identified by the complement dependent cytotoxicity assay but not by SPA.1 In our previous study, we showed that, DSA-positive patients had significantly higher rate of CAV compared with DSA-negative patients (36% vs. 13%), but we did not evaluate the impact of complement binding and noncomplement binding DSAs on acute rejections, graft loss and development of CAV.2
The capacity of DSA to bind complement fraction C1q, which is the first step in the activation of the classic complement cascade, determines the cytotoxic potential of these antibodies, and an assessment of their complement binding capacity may be useful for risk stratification. Numerous studies have clearly demonstrated that C1q binding DSAs are strongly associated with AMR and graft loss in solid organ transplants including heart,3–4 kidney,5–6 and lung7 transplants. In the most comprehensive analysis to date, Loupy et al, found that patients who develop C1q positive DSA in the first year after renal transplantation, showed worse graft survival than those with C1q negative (C1q−) DSA (54% vs. 93% 5-year graft survival).6 Zeevi and colleagues showed similar results in HT recipients.4 However, studies by Yell et al,7 and Tambur et al,8 showed that in fact, C1q binding represents higher levels of DSA and is not necessarily a reflection of a difference in DSA function. We hypothesized that the C1q-binding de novo DSA after pediatric HT is associated with a higher risk for development of CAV. This study aimed, (1) to determine the impact of C1q+ de novo DSA on adverse outcomes in pediatric HT; (2) to find a cut-off value for DSA in median fluorescent intensity (MFI) as predictor of C1q binding activity (functional ability of DSA); and (3) to evaluate the ability of C1q assay to predict the development of CAV in pediatric HT recipients.
Materials and Methods
Patients
This retrospective analysis comprised pediatric HT recipients transplanted at our center between January 2005 and December 2014 and was approved by our Institutional Review Board. All patients included in this study had negative T and B cell retrospective flow cytometric crossmatch on post-HT day 0, and no DSA were identified by Luminex SAB assay prior to transplant. Our SAB tests detected the presence of IgG antibodies but not IgM. In the case of patients who underwent retransplantation, only their first HT outcomes were included in this study. We used the term DSA to describe de novo DSA in the remainder of the manuscript.
All patients received a quadruple sequential immunosuppression. Induction therapy consisted of methyl prednisolone and basiliximab (Simulect, Novartis) in 99% of cases (only 2 patients received antithymocyte globulin). Standard maintenance immunosuppression included triple therapy of tacrolimus/cyclosporine, mycophenolate mofetil, and steroids. Steroids were routinely withdrawn after 1 year unless there was more than 1 rejection event within the first year after transplant. Patients who developed renal insufficiency or CAV received sirolimus instead of a calcineurin inhibitor. The medical records, pathologic reports, coronary angiograms were reviewed to obtain the baseline characteristics and clinical data.
Donor-specific antibodies and C1q testing
Prior to 2006, donors and recipients were typed for HLA-A, B, DRB1/3/4/5 and DQB1 using a serological method and/or sequence-specific primer (SSP), SSP Unitray (Invitrogen Inc.). After 2006, all patients and donors where typed for HLA-A, B, C, DRB1/3/4/5 and DQB1 using sequence-specific oligonucleotide (SSO), LabType SSO (One Lambda, Canoga Park, USA). Some patients and donors typed prior to 2006 were retyped using SSO or high resolution sequence based typing (in house) when needed to confirm presence of DSA. As needed, patients were also typed for HLA-DQA1 and DPB1.
All patients were tested for the presence of circulating DSA in serum samples collected with each cardiac catheterization and endomyocardial biopsy (EMB) as described in our previous study.2 Luminex LABScreen Single Antigen beads (One Lambda, Canoga Park, CA) was used to detect class I and class II HLA-antibodies and the intensity of the HLA antigen-antibody interaction was expressed in MFI. We defined patients as having positive DSA only when the DSA was persistent for 2 consecutive samples and DSA MFI was ≥ 1000 for this study as previously reported cut-off for “true” antibodies by Chin et al.3 The negative control bead MFI value was subtracted from each bead MFI value prior to applying the cutoff to adjust for background. We identified the peak serum samples for the circulating immunodominant DSAs (iDSAs) for each patient with positive DSA. For this study, we analyzed those samples for the presence of C1q binding DSA per manufacturer’s protocol (C1qScreen and LAB Screen Single Antigen, One Lambda).9 DSAs identified by single antigen assay with the capacity to bind human C1q were considered to be C1q+ DSA, and those without this capacity were considered C1q− DSA. The positive cutoff for the C1q assay was set at 500 MFI after comparisons were made to the background MFI and there was a 50% increasing over the background at cutoff values of 500 MFI. Positive and negative control sera were used in this assay as controls.
Endomyocardial Biopsy
Routine surveillance EMBs were performed per our institutional protocol: every 2 weeks for the first 3 months, every month for the next 3 months, at 9 months, and then at 12 months after HT. The frequency of EMB 1 year post-HT was annual unless clinical rejection was suspected. With each annual EMB, hemodynamics from right and left heart were obtained, coronary angiograms performed and 5 pieces of the right ventricular myocardium were obtained and sent for histopathology.
Acute Rejections
Acute rejection events included all clinical rejections requiring antirejection therapy, acute cellular rejection (ACR) 2R or higher ISHLT grade10 or AMR. Since this was a retrospective study, we did not use the 2013 ISHLT guidelines for diagnosis of AMR11 rather we defined AMR per our institutional practice if the patient had 3 of the following 4 criteria: 1) evidence of graft dysfunction on echocardiogram, 2) positive C4d and CD68 by immunohistochemical stains, 3) biopsy with interstitial edema and endothelial swelling (usually without evidence of ACR), and 4) presence of DSA. Patients, who had acute clinical rejection but had not undergone prior-EMB due to hemodynamic instability or those who had EMB proved pathological ACR ≥ 2R, were first treated with intravenous methylprednisolone at 20 mg/kg (maximum 1000mg) daily for 3 days. We repeated EMB 2 weeks after completion of corticosteroid treatment to confirm resolution of rejection. If there was persistent ACR grade 2R or higher after the first treatment, pulsed intravenous methylprednisolone was repeated. A repeat biopsy was performed at the completion of the second course of steroids. If there was persistence of ACR or the patient had hemodynamically compromising clinical rejection, the patient was treated with antithymocyte globulin (1–1.5 mg/kg/dose for 10–14 doses). For patients who had AMR, the treatment course was rituximab (375mg/m2 IV weekly × 4 doses) and intravenous immunoglobulin G (1 gram/kg/dose every 2 weeks × 2 doses), followed by a repeat EMB after 4 weeks of completion of rituximab to confirm recovery.
Cardiac Allograft Vasculopathy Screening
The diagnosis of CAV was based on coronary angiography in all patients and in addition 2 patients who had pathologic evidence of CAV on autopsy. For all patients, first coronary angiogram screening for CAV was performed 1-year after HT and annually thereafter. Additional angiograms were performed if there was unexplained graft dysfunction. All angiograms were reviewed retrospectively for evidence of CAV by 1 of the authors (BD). For this study, CAV was defined as any new-onset coronary artery stenosis by coronary angiogram irrespective of whether a branch or main vessel was involved.
Graft loss
Graft loss was defined as patient death or relisting for HT. Relisting for transplantation was considered when a patient developed heart failure due to ventricular dysfunction or life threatening arrhythmia. The date of relisting was considered as the endpoint of graft loss for those patients who had significant irreversible graft dysfunction (n=8). All 8 patients who were relisted underwent subsequent retransplantation. Five of the patients who satisfied the above criteria for graft loss were not relisted for transplantation at our center due to social issues or noncompliance, all of whom died and their time of death was included as time of graft loss. Some patients (n=2) who were considered for relisting but subsequently improved and did not require transplant were not included under graft loss.
Statistical Analysis
Independent t test, ANOVA, Fisher’s exact test and chi square test were employed to compare variables between groups. The Wilcoxon–Mann–Whitney test was performed when the normality assumption did not hold. Kaplan-Meier curves were constructed to present time to event outcomes. Univariate and Multivariate Cox regression models were employed to assess risk factors associated with the time to event for graft loss and CAV. Univariate analysis was performed for each covariates: recipient age at transplant, donor age, donor and recipient gender, pretransplant diagnosis (congenital heart disease versus cardiomyopathy), pretransplant Panel Reactive Antibodies (PRA), number of HLA mismatches, previous surgical procedure, pretransplant use of mechanical circulatory support (MCS), inotropic support, ventilator support, serum albumin, bilirubin, renal insufficiency (defined as estimated glomerular filtration rate <50 ml/min/1.73m2), withdrawal of steroid at 1 year posttransplant, presence of DSA, strength of DSA in MFI, C1q positive status of DSA, and era of HT (2005–2010 versus 2011–2014). C1q status and MFI were included as time-dependent covariates such that all subjects were DSA negative at baseline but some patient became DSA positive during follow-up, at which point C1q+/− and MFI were determined. Then, covariates with p values less than 0.2 in univariate analysis were retained as candidate predictors for multivariate analysis and stepwise variable selection procedure was performed on the candidate predictors to obtain the final multivariate model. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
A total of 127 patients who underwent primary HT at Children’s Medical Center Dallas between 2005 and 2014 were included in the analysis. DSA was detected in 59/127 patients after HT. Patients were classified in 3 groups, per the presence or absence of DSA and C1q binding capacity: DSA negative group (N=68), C1q+ DSA group (N=37) and C1q− DSA group (N=22). Table 1 shows the clinical, laboratory and demographic characteristics of all patients included in this study. No difference in baseline characteristics was noted between those with or without DSA. Patients who developed C1q+ DSA received a donor heart from a female gender more often than a male compared to C1q− DSA group (p=0.034).
Table 1.
Summary of patients per presence or absence of C1q binding DSA.
| Characteristics | All patients (N=127) |
Patients with no DSA (N= 68) |
Patients with DSA (N=59) | p value | |
|---|---|---|---|---|---|
| C1q+ (N=37) |
C1q− (N=22) |
||||
| Donor age-yr. | 9.1±8.6 | 8.3±8.4 | 9.5±8.9 | 10.5±9 | 0.673 |
| Donor gender (male), n (%) | 71 (55.9) | 43 (63.2) | 14 (37.8) | 14 (66.6) | 0.034 |
| Ischemic time, min | 192.7±57.7 | 199.5±59.8 | 191.7±49.9 | 173.3±62.5 | 0.223 |
| Age at HT-yr. | 6.6±6.2 | 5.6±5.9 | 7.0±6.4 | 8.1±6.6 | 0.574 |
| Recipient gender (male), n (%) | 66 (51.9) | 33 (48.5) | 23 (62.1) | 10 (47.6) | 0.282 |
| Pre-HT diagnosis-CHD, n (%) | 54 (42.5) | 26 (38.4) | 17 (45.9) | 10 (47.6) | 0.902 |
| Pre-HT cardiac surgery, n (%) | 52 (40.9) | 26 (38.2) | 17 (45.95) | 8 (38.1) | 0.561 |
| Pre-HT PRA ≥10%, n (%) | 32 (25.2) | 16 (23.5) | 10 (27) | 5 (23.8) | 0.788 |
| Pre-HT MCS ECMO, n (%) VAD, n (%) |
33 11 (33.3) 21(63.6) |
19 7 (35) 12 (65) |
9 4 (44.4) 5 (55.5) |
4 0 (0.0) 4 (100) |
0.228 |
| Inotropes at HT, n (%) | 124 (97.6) | 66 (97) | 36 (97.3) | 22 (100) | 1.000 |
| Ventilator at HT, n (%) | 23 (18.1) | 15 (22) | 7 (18.9) | 1 (4.7) | 0.236 |
| Renal insufficiency, n (%) | 17(13.3) | 10 (14.7) | 4 (10.8) | 3 (14.2) | 0.695 |
| Albumin (mg/dL) | 3.52±0.5 | 3.59±0.5 | 3.39±0.6 | 3.6±0.6 | 0.21 |
| Bilirubin (mg/dL) | 1.89±1.1 | 1.84±1.3 | 1.9±0.8 | 1.9±0.8 | 0.70 |
| HLA mismatch, n | 7.8±1.9 | 7.8±1.9 | 7.2±1.6 | 7.7±1.2 | 0.239 |
| Steroid off 1-yr post-HT, n (%) | 111(87%) | 63 (92%) | 31(83%) | 17 (77%) | 0.534 |
| Class I DSA, n (% of total DSA positive patients) | 4/59 (6.7%) |
1 | 3 | 0.043 | |
| Class II DSA, n (% of total DSA positive patients) | 27/59 (45.7%) |
18 | 9 | 0.67 | |
| Class I and II DSA, n (% of total DSA positive patients) | 28/59 (47.5%) |
18 | 10 | 0.53 | |
Among the 59 patients who were positive for DSA, DSAs were first identified at a median of 689 days post-HT. Of patients with positive DSA, 4(6.7%) patients had class I DSA (mean MFI 2653 with 25th to 75th percentile: 1223–4296), 27(45.7%) patients had class II DSA (mean MFI 10 784 with 25th to 75th percentile: 4291–15 869) and 28(47.6%) patients had both Class I and Class II DSA (mean MFI 10 521 with 25th to 75th percentile: 4279–16 200). Among class II HLA antibodies, DQ was prevalent in our cohort. Antibodies against DQB1, DQA1 or combo DQA1/DQB1 were included under the DQ specificity. The receiver-operating characteristic (ROC) curve (Figure 1) showed that a DSA at ≥ 7000 MFI identified most C1q positive cases with an 80% sensitivity and 80% specificity (C statistics 0.89 and p <0.05).
Figure 1.
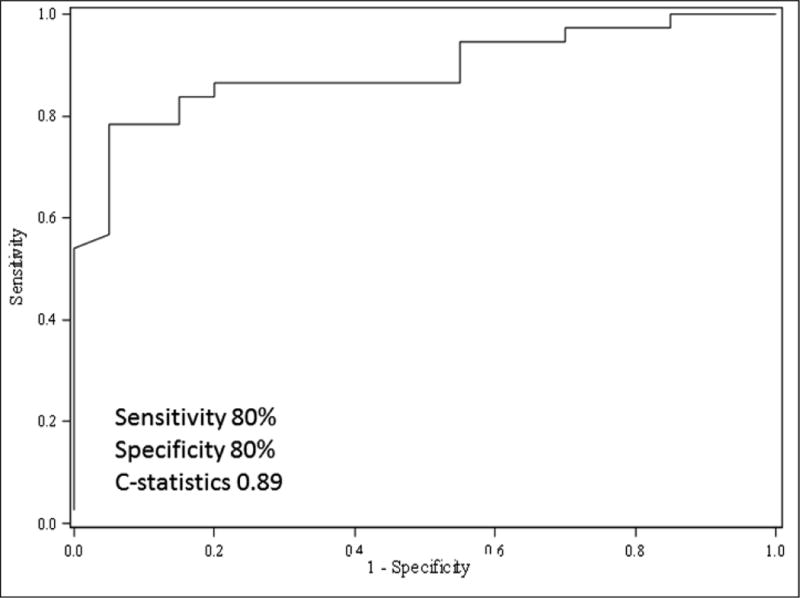
Receiver-operating characteristic (ROC) curve showing DSA MFI ≥7000 identifies C1q positivity with 80% sensitivity and 80% specificity. (C statistics 0.89)
Acute Rejections
Table 2A showed no significant differences in rejection episodes per patient-year between DSA negative and C1q− DSA positive patients, 0.5±0.9 vs. 0.8±1 (P =0.2119). The number of acute rejection events per patient-year between patients who had C1q+ DSA and C1q− DSA were 1.9±1.4 vs. 0.8±1 respectively (p=0.0018). Similarly, patients with DSA ≥7000 MFI had 2.0±1.4 rejections per patient year compared to 1±1.1 in patient with DSA MFI <7000 MFI (p=0.0059). The diagnostic accuracy of C1q binding activity and elevated DSA MFI value for detecting acute rejections were comparable (Table 2B).
Table 2A.
Comparisons of adverse outcome of heart transplantation by C1q assay.
| p valuea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Outcome Variable | No DSA (N=68) |
DSA with Positive C1q (N=37) |
DSA with Negative C1q (N=22) |
All patients (N=127) |
All patients | No DSA vs DSA with Positive C1q | No DSA vs DSA with Negative C1q | Positive C1q vs Negative C1q |
| Acute rejections per patient-year | 0.5±0.9 | 1.9±1.4 | 0.8±1.0 | 0.9±1.3 | <0.0001† | <0.0001§ | 0.211§ | 0.0018§ |
| CAV | 8 (11.7%) |
19 (51.3%) |
5 (22.7%) |
32 (25.1%) |
0.0001 | <0.0001 | 0.466 * | 0.0256 |
| Graft loss# | 12 (17.6%) |
13 (35.1%) |
2 (9.1%) |
27 (21.2%) |
0.655 | 0.103 | 0.34 * | 0.0323 |
Graft loss due to CAV and/or acute rejections is only used in this analysis.
Note:
† by ANOVA; § by t test; * by Fisher’s Exact Test; Otherwise by Chi Square Test
Table 2B.
Comparisons of adverse outcome of heart transplantation by DSA strength in MFI.
| p valuea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Outcome Variable | No DSA (N=68) |
DSA MFI ≥ 7000 (N=33) |
DSA MFI <7000 (N=26) |
All patients (N=127) |
All patients | No DSA vs DSA MFI ≥7000 | No DSA vs DSA MFI < 7000 | DSA MFI <7000 vs ≥7000 |
| Acute rejections per patient-year | 0.5±0.9 | 2.0±1.4 | 1.0±1.1 | 0.9±1.3 | <0.0001† | <0.0001§ | <0.0372§ | 0.0059§ |
| CAV | 8 (11.7%) |
17 (51.5%) |
7 (26.9%) |
32 (25.1%) |
0.0001 | 0.0002 | 0.107* | 0.1749 |
| Graft loss# | 12 (17.6%) |
10 (39.3%) |
5 (19.2%) |
27 (21.2%) |
0.56 | 0.282 | 0.722 | 0.594 |
Graft loss due to CAV and/or acute rejections is only used in this analysis.
Note:
† by ANOVA; § by t test; * by Fisher’s Exact Test; Otherwise by Chi Square Test
Cardiac Allograft Vasculopathy
A total of 32 (25.1%) patients developed CAV in the entire cohort during the study period. Patients who were C1q+ DSA had significantly higher frequency of CAV compared to patients with C1q− DSA (51.3% vs. 22.7%); p=0.025 (Table 2A). When compared to DSA MFI ≥7000, the development of CAV was 51.5% vs 26.9% in DSA MFI of <7000 (p= 0.174). Freedom from CAV in C1q+ DSA patients was 90%, 70% and 40% at 1, 5 and 10 years compared with 98%, 75% and 75% in C1q− DSA patients (p<0.01, log-rank test) (Figure 2A). When we analyzed the CAV outcome based on DSA cut-off 7000 MFI, freedom from CAV in patients with DSA ≥7000 MFI was 96%, 60% and 36% at 1, 5 and 10 years compared with 98%, 70% and 62% in patients with DSA <7000 MFI (p=0.023, log-rank test) (Figure 2B).
Figure 2A.
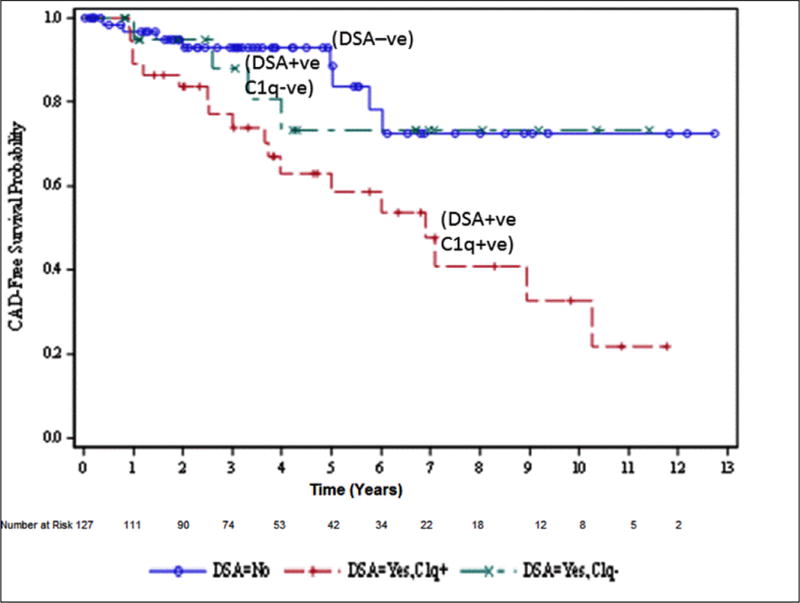
Freedom from CAV. DSA negative patients are presented in the graph with solid line and circles, C1q+ DSA patients are presented with dashed line and cross marks, and C1q− DSA patients are presented with dashed line with straight line marks.
Figure 2B.
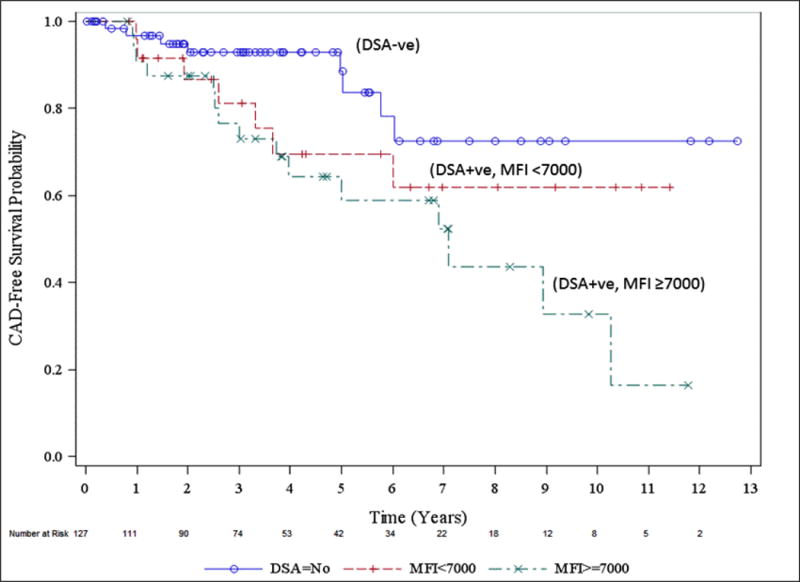
Freedom from CAV based on DSA strength in MFI. DSA negative patients are presented in the graph with solid line with circle, patients with DSA ≥7000 MFI are presented as dashed line with cross marks and those with DSA <7000 MFI are presented with dashed line with straight line marks.
The results of univariate time-dependent Cox regression analysis for CAV are presented in Table 3. It demonstrates that developing C1q+ DSA is significantly associated with the risk of CAV (hazard ratio (HR): 3.32, p=0.004) whereas the risk of development of CAV with DSA MFI is HR 1.19, p=0.53. The final multivariate Cox model identified C1q+ DSA as an independent risk factor for developement of CAV regardless of DSA MFI; HR: 3.25, 95% CI 1.33–7.93, p= 0.0095. There is an association with presence of DSA >7000 MFI and CAV however C1q positivity provides an higher stratification risk regardless of the MFI level.
Table 3.
Clinical and immunological factors associated with CAV (Univariate Analysis).
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Donor age-yr. | 1.01 (0.97–1.05) | 0.55 |
| Donor gender (male) | 0.83 (0.42–1.69) | 0.61 |
| Ischemic time-min | 0.99 (0.99–1.01) | 0.63 |
| Age at HT-yr. | 1.02 (0.95–1.08) | 0.62 |
| Recipient gender (male) | 1.39 (0.68–2.85) | 0.35 |
| Pre-HT diagnosis-CHD | 1.02 (0.49–2.10) | 0.95 |
| Previous cardiac surgery | 1.01 (0.53–2.25) | 0.76 |
| Pre-HT PRA ≥10% | 1.57 (0.69–3.55) | 0.27 |
| Number of acute rejections/patient-year | 1.64 (1.3–2.07) | 0.69 |
| Steroid off at 1-yr post-HT | 0.13 (0.05–0.29) | <0.0001 |
| Pre-HT VAD use | 1.38 (0.28–6.96) | 0.07 |
| Ventilator at HT | 2.13 (0.95–4.81) | 0.06 |
| Renal insufficiency | 0.80 (0.19–3.39) | 0.76 |
| Albumin | 0.78 (0.42–1.45) | 0.43 |
| Bilirubin | 0.95 (0.72–1.26) | 0.73 |
| HLA mismatch | 0.94 (0.76–1.16) | 0.56 |
| Class I DSA | 0.00 (0.0000) | 0.99 |
| Class II DSA | 2.1 (0.84–5.27) | 0.04 |
| Class I and II DSA | 3.67 (1.49–8.98) | 0.04 |
| DSA in MFI (log-transformed) | 1.19 (0.7–2.01) | 0.53 |
| C1q+ DSA | 3.32 (1.44–7.65) | 0.004 |
| C1q− DSA | 1.27 (0.38–4.23) | 0.69 |
| Era of transplant (2006–2010) | 1.82 (0.67–4.92) | 0.17 |
| Era of transplant (2011–2014) | 0.73 (0.18–2.92) | 0.17 |
Graft Loss
Graft loss occurred in 31(24.4%) patients. The cause of graft loss was determined by autopsy reports/study of explanted hearts when retransplantation was performed and clinical events from medical chart review when autopsy was not performed, were CAV/acute rejections (87%, n=27), unknown/sudden death (6.4%, n=2), malignancy like posttransplant lymphoproliferative disease and multisystem failure (6.4%, n=2). C1q+ DSA patients had significantly higher rates of graft loss 13/37(35.12%) compared to C1q− DSA group 2/22(9.1%), p=0.0323. When compared to DSA MFI ≥7000, graft loss was 10/33 (39.3%) vs 5/26 (19.2%) in DSA <7000 MFI (p=0.549) (Table-2B). In univariate Cox regression analysis, covariates significantly associated with graft loss included: female sex of donor, acute rejections per patient year, steroid withdrawal at 1 year post-HT, previous cardiac surgical procedures, ventilator at the time of transplant and renal insufficiency at pretransplantation (Table 4). However in the multivariate analysis only 3 factors were associated with graft loss, namely, 1) C1q+ DSA, 2) pretransplant renal insufficiency, and 3) pretransplant ventilator use (Table 5).
Table 4.
Clinical and immunological factors associated with graft loss (Univariate Analysis).
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Donor age-yr. | 1.0 (0.96–1.05) | 0.97 |
| Donor gender (male) | 0.35 (0.16–0.76) | 0.009 |
| Ischemic time-min | 0.99 (0.99–1.01) | 0.76 |
| Age at HT-yr. | 1.01 (0.94–1.07) | 0.86 |
| Recipient gender (male) | 1.05 (0.51–2.16) | 0.89 |
| Pre-HT diagnosis-CHD | 2.05 (0.99–4.24) | 0.051 |
| Previous cardiac surgery | 2.18 (1.05–4.51) | 0.034 |
| Pre-HT PRA ≥10% | 1.41 (0.62–3.20) | 0.41 |
| Number of acute rejections/patient-year | 1.43 (1.1–1.8) | 0.026 |
| Steroid off at 1-yr post-HT | 0.32 (0.13–0.76) | 0.001 |
| VAD use | 0.3 (0.07–1.30) | 0.27 |
| Inotropes at HT | 0.46 (0.06–3.46) | 0.45 |
| Ventilator at HT | 2.84 (1.32–6.13) | 0.007 |
| Renal insufficiency | 3.52 (1.53–8.08) | 0.0029 |
| Albumin | 0.88 (0.47–1.66) | 0.71 |
| Bilirubin | 1.07 (0.89–1.30) | 0.42 |
| HLA mismatch | 0.86 (0.69–1.07) | 0.18 |
| Class I DSA | 0.00 (0.0000) | 0.99 |
| Class II DSA | 0.6 (0.24–1.50) | 0.28 |
| Class I and II DSA | 1.88 (0.87–4.05) | 0.10 |
| DSA MFI (log-transformed) | 1.02 (0.57–1.84) | 0.94 |
| C1q+ DSA | 1.4 (0.65–3.00) | 0.19 |
| C1q− DSA | 0.37 (0.08–1.65) | 0.19 |
| Era of transplant (2006–2010) | 1.28 (0.50–3.29) | 0.59 |
| Era of transplant (2011–2014) | 0.32 (0.07–1.34) | 0.11 |
Table 5.
Clinical and immunological factors associated with graft loss (Multivariate Analysis).
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| C1q+ DSA | 3.2 (1.34–7.86) | <0.009 |
| Pre-HT renal insufficiency | 11.29 (3.71–34.29) | <0.0001 |
| Pre-HT ventilator use | 3.30 (1.39–7.81) | 0.007 |
Outcomes of Cases that were C1q− and DSA ≥ 7000 Versus those with C1q+ DSA <7000 MFI
There is a total of 11 patients with discrepancy between C1q-binding activity and DSA MFI strength (C1q− DSA ≥7000 MFI (n=3) and C1q+ DSA <7000 (n=8)). Of these 11 patients, total 7 patients had CAV. C1q+ DSA <7000 diagnosed CAV in 3/8 and missed only 1 case, whereas C1q− DSA ≥7000 MFI diagnosed CAV in 1/3 and missed 2 cases of CAV. We compared the adverse outcomes in these 2 groups and various combinations thereof and summarized the data in Table 6. The results showed that C1q+ DSA <7000 MFI and C1q− DSA ≥7000 MFI, both exhibited higher diagnostic value for detection of rejection and graft loss compared to no DSA group. However, the association with CAV is not stronger between C1q+ DSA MFI <7000 and/or C1q−DSA MFI ≥ 7000 versus C1q−DSA MFI <7000 and/or C1q+ DSA MFI ≥ 7000.
Table 6.
Comparing adverse outcomes of transplant between C1q+ DSA MFI <7000 And/or C1q− DSA MFI ≥7000 versus C1q− DSA MFI <7000 and/or C1q+ DSA MFI ≥7000.
| p valuea | ||||||
|---|---|---|---|---|---|---|
| Adverse outcome variable | No DSA (N=68) |
C1q+ DSA MFI <7000 And C1q− DSA MFI ≥7000 (N=11) |
C1q− DSA MFI <7000 And C1q+ DSA MFI ≥7000 (N=48) |
No DSA Vs C1q+ DSA MFI <7000 And/or C1q− DSA MFI ≥7000 | No DSA Vs C1q− DSA MFI <7000 And/or C1q+DSA MFI ≥7000 | C1q+DSA MFI <7000 And/or C1q−DSA MFI ≥ 7000 Vs C1q−DSA MFI <7000 And/or C1q+ DSA MFI ≥ 7000 |
| Acute rejections | 0.5±0.9 | 1.8±1.1 | 1.4±1.4 | <0.0001§ | 0.0002§ | 0.479§ |
| CAV | 8 (11.7%) |
4 (36.3%) |
17 (35.4%) |
0.0574* | 0.0014 | 1.000* |
| Graft Loss (acute rejections and/or CAV) | 12 (17.6%) |
6 (54.5%) |
9 (18.75%) |
0.0259* | 0.893 | 0.0274* |
Note:
§ by t test; * by Fisher’s Exact Test; Otherwise by Chi Square Test
Discussion
This retrospective study demonstrated the worse impact of C1q+ DSA on adverse outcomes such as acute rejections, graft loss and CAV in pediatric HT recipients. Furthermore, our results showed that DSA strength in MFI ≥7000 MFI better correlate with C1q positivity, yet the strength itself are not independently associated with adverse outcome on multivariate analysis, more specifically CAV. Although, DSA strength measured as MFI value and C1q-binding activity may provide equivalent measure of risk of rejection and graft loss, their ability to predict CAV is lower than C1q + DSA ≥7000. Previously, Tambur et al, has shown that high MFI antibodies does not always suggest “high titer antibody” or correlate with clinical outcomes.8 On the other hand, Tyan DB introduced C1q binding as a criterion to stratify pathogenic antibodies utilizing their ability to bind C1q, assuming that this assay identified only those antibodies that have the capacity to bind complement and therefore, those are harmful DSA.12 Chen and colleagues furthermore showed that C1q+ DSA appear to be more highly correlated than those detected by IgG alone by SAB for antibody-mediated rejection in hearts as well as for kidney transplant glomerulpathy and graft failure.13
The relative role of C1q+ and C1q− DSA on the development of CAV in pediatric HT recipients is unknown. Previously, Smith et al, have described that there was no significant correlation between DSA and CAV, but have shown that a significantly higher number of patients with DSA died of CAV/acute rejections in adult HT patients.14 Their study did not evaluate the complement binding (C1q-binding) ability of DSA. In our previous study, although we found DSA had a strong negative impact on posttransplant adverse outcomes after pediatric HT, DSA was not found to be an independent predictor of CAV in multivariable analysis.2 In the present study, we showed that detection of C1q+DSA after HT may support the notion that the complement pathway may play a critical role in acute rejections, CAV and graft loss. However, these findings do not rule out a possible role for complement independent mechanisms in acute rejections, CAV or graft loss mediated by DSA. Identified C1q+ DSA were not subtyped using single antigen beads to determine whether the complement fixing antibodies were IgG, IgM or a combination of both. Previously, Chin et al, have shown that the C1q assay can detect a subset of antibodies capable of fixing complement and predicts AMR early after transplant.3 Similarly, Zeevi et al, has reported that C1q binding can risk stratify for pediatric and adults for positive complement dependent cytotoxicity-crossmatch and early AMR in sensitized heart allograft recipients.4 Therefore, after identifying significant DSA, the addition of C1q assay can be helpful to identify immunologically higher-risk for development of CAV in pediatric HT recipients.
Two studies suggested that the value of reported correlation between C1q binding ability and worse graft outcomes may be an indication of DSA strength and not necessarily reflection of their complement binding abilities.7,15 Recently, a study described some of the technical limitations of the C1q SAB assay and showed that antibody level, interfering factors, and the presence of denatured HLA protein on class I SAB may all affect the clinical interpretation of the C1q assay.16 Tambur et al, suggested that, titration/dilution studies can provide biologically relevant nuances about HLA antigen-antibody dissociation rates and, therefore, are likely to provide better indication of true antibody strength.17 The authors suggested that, by performing dilution studies, several interfering factors in the serum which can lead to an underestimation of the level of DSA are diluted in the process and their effect is unmasked; very high levels of DSA that cause oversaturation of the HLA antigens on SAB are no longer a problem as the serum continues to be diluted until negative responses are achieved; the strength (“affinity’) of antibody binding can be appreciated by the number of dilutions required to remove antibody from its target. But, these findings have not been validated in larger studies. A criticism raised against the dilution approach is the potential increase in cost similar to the criticism for C1q assay given substantial additional cost. EDTA pretreatment of serum sample is proposed as a mean to remove the interfering factors in serum, thus uncovering prozone effect, and provide a more accurate estimate of antibody strength, but this not shown to be effective in studies.16 The clinical utility of C1q assay using previously stored sera which was freeze/thawed for the C1q assay may be effective in reducing some of those interfering factors but this remains to be validated in future studies.
We acknowledge that this study has several important limitations, beginning with the single-center. In our study, we did not perform dilution of serum to test for anti-HLA antibodies. We also did not subclassify the type of IgG HLA-antibodies. We tested the C1q reactivity in all peak serum where high levels of donor specific IgG anti-HLA antibodies were detected using single antigen assay. However, there is a possibility that we may have missed some complement binding DSA in samples that did not contain the iDSAs. In all samples tested using the C1q assay, when C1q positive DSA were identified, the immunodominant DSA was C1q positive. We did not perform repeat C1q testing for all DSA for each patient, but instead tested those sera identified as iDSA, we could not determine if there was any conversion of C1q positive to C1q negative with change in DSA MFI level. We considered that all patients were DSA negative at the time of transplant based on negative retrospective flow cytometric crossmatch results and lack of DSA identified by Luminex LABScreen Single Antigen assay. C1q was not tested pretransplant; presence of IgG DSA would have been detected by SPA but not presence of IgM antibodies. We did not consider the impact of early versus late appearance of DSA and post-HT outcome. Also, we did not study the impact of any change in immunosuppression therapy on DSA or C1q status. In this study, we grouped all types of treated rejection events as acute rejections and because of small number of AMR; we were not able to differentiate between ACR and AMR. Another limitation is that, CAV may be missed by angiography especially when only small vessel disease was present. However, a major strength of this study is that it includes patients exposed to a uniform induction and maintenance immunosuppression therapy protocol and had serial cardiac catheterizations, EMBs, and serum samples for testing DSAs. We did not collect data including noncompliance or change in immunosuppressive medications for each patient to identify the cause of the relatively higher percentage of DSA in our cohort.
In conclusion, our study showed that the C1q assay can potentially identify a clinically relevant subset of DSA and close monitoring of DSA strength (MFI) and function (C1q assay) posttransplant may be useful for identifying pediatric HT recipient at risk of developing CAV. One should consider C1q-binding ability of the DSA while interpreting the result of DSA MFI to identify patients at higher risk for development of CAV in pediatric HT recipients.
Acknowledgments
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- CAV
cardiac allograft vasculopathy
- CDC assay
complement-dependent cytotoxicity assay
- DSA
donor-specific antibodies
- EMB
endomyocardial biopsy
- HLA
human leukocyte antigen
- HR
hazard ratio
- HT
heart transplant
- IgG
immunoglobulin G
- SAB
single antigen bead
- SPA
solid phase assay
Biographies
Bibhuti B Das: Dr Das conceptualized and collected the data, drafted the manuscript, and approved the final manuscript as submitted.
Chantale Lacelle: Dr Lacelle has done all the immunological tests and critically reviewed the manuscript and approved the final manuscript as submitted.
Song Zhang and Ang Gao: Dr Zhang and Gao are the statisticians who have done all the statistical analysis and critically reviewed the manuscript and approved the final manuscript as submitted.
David Fixler: Dr Fixler has coordinated data collection, critically reviewed and revised the manuscript and approved the final manuscript as submitted.
Footnotes
Disclosure
The authors declare no conflicts of interest
References
- 1.Ho EK, Vlad G, Vasilescu ER, et al. Pre- and post-transplantation allosensitization in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol. 2011;72(1):5–10. doi: 10.1016/j.humimm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Tran A, Fixler D, Huang R, Meza T, Lacelle C, Das B. Donor-specific HLA alloantibodies: Impact on cardiac allograft vasculopathy, rejection, and survival after pediatric heart transplantation. J Heart Lung Transplant. 2016;35(1):87–91. doi: 10.1016/j.healun.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Chin C, Chen G, Sequeria F, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30(2):158–63. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatric Transplant. 2012;16(1):12–17. doi: 10.1111/j.1399-3046.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 6.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 7.Yell M, Muth B, Kaufman DB, Djamali A, Elis TM. C1q binding activity of de novo donor-specific HLA antibodies in renal transplant recipients with and without antibody mediated rejection. Transplantation. 2015;99(6):1151–1155. doi: 10.1097/TP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 8.Tambur AR, Herrera ND, Haarberg KMK, et al. Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant. 2015;15(9):2421–2430. doi: 10.1111/ajt.13295. [DOI] [PubMed] [Google Scholar]
- 9.Zeevi A, Marrari M, Feingold B, Weber S, Duquesnoy RJ. Human leukocyte antigen epitope analysis to access complement- and non-complement-binding donor-specific antibody repertoire in a pediatric heart transplant recipient. Human Immunol. 2012;73:48–51. doi: 10.1016/j.humimm.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of the nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013;32(12):1147–62. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Tyan DB. New approaches for detecting complement-fixing antibodies. Cur Opin Organ Transplant. 2012;17(4):409–15. doi: 10.1097/MOT.0b013e328355fb9b. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Sequerira, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin strength on single antigen beads. Hum Immunol. 2011;72(10):849–858. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Smith JD, Banner NR, Hamour IM, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–319. doi: 10.1111/j.1600-6143.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- 15.Peacock S, Kosmoliaptsis V, Bradley AJ, Taylor CJ. Questioning the added value of luminex single antigen beads to detect C1q binding donor HLA-specific antibodies. Transplantation. 2014;98(4):384–86. doi: 10.1097/TP.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 16.Taylor CJ, Kosmopoliaptsis V, Martin J, et al. Technical limitations of the C1q single-antigen bead assay to detect complement binding HLA-specific antibodies. Transplantation. 2017;101(6):1206–14. doi: 10.1097/TP.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tambur AR, Lavee J. Incorporating human leukocyte antibody results into clinical practice. J Heart Lung Transplant. 2016;35(7):851–6. doi: 10.1016/j.healun.2016.05.010. [DOI] [PubMed] [Google Scholar]


