Abstract
Binge alcohol (ethanol) drinking is associated with profound adverse effects on our health and society. Rimonabant (SR141716A), a CB1 receptor inverse agonist, was previously shown to be effective for nicotine cessation and obesity. However, studies using rimonabant were discontinued as it was associated with an increased risk of depression and anxiety. In the present study, we examined the pharmacokinetics and effects of AM4113, a novel CB1 receptor neutral antagonist on binge-like ethanol drinking in C57BL/6J mice using a two-bottle choice drinking-in-dark (DID) paradigm. The results indicated a slower elimination of AM4113 in the brain than in plasma. AM4113 suppressed ethanol consumption and preference without having significant effects on body weight, ambulatory activity, preference for tastants (saccharin and quinine) and ethanol metabolism. AM4113 pretreatment reduced ethanol-induced increase in dopamine release in nucleus accumbens. Collectively, these data suggest an important role of CB1 receptor-mediated regulation of binge-like ethanol consumption and mesolimbic dopaminergic signaling, and further points to the potential utility of CB1 neutral antagonists for the treatment of binge ethanol drinking.
Keywords: Binge drinking, Nucleus accumbens, Cannabinoid receptor, Neutral antagonist, AM4113
INTRODUCTION
Alcohol abuse disorder (AUD) is the most common and chronic mental disorder, and abuse of alcohol (ethanol) increases the risk of developing over 200 diseases and injury-related health conditions leading to about 3.3 million deaths globally (WHO, 2014). Binge alcohol drinking, especially, is a major public health issue due to its long-term adverse effects and is most prevalent (37.5%) in young adults (SAMHSA, 2014). According to National Institute of Alcohol Abuse and Alcoholism (NIAAA), binge drinking is defined as a pattern of drinking that brings blood ethanol concentration (BEC) to 0.08% and above. This generally occurs after consuming 4 drinks (one “standard” drink contains roughly 14 grams of pure ethanol) for women and 5 drinks for men in about 2 hours (NIAAA., 2004).
Several studies have demonstrated the importance of many signaling mechanisms including the endocannabinoid (eCB) system in alcohol-related behaviors (Blednov et al., 2007; Herman and Roberto, 2015; Mangieri et al., 2009; Mitrirattanakul et al., 2007; Naassila et al., 2004; Parsons and Hurd, 2015; Pava and Woodward, 2012; Vinod and Hungund, 2006; Wang et al., 2003). The eCB system consists of two principal cannabinoid (CB) receptors (CB1 and CB2), endogenous cannabinoids (endocannabinoids, eCBs), and enzymes and transporters which are involved in the metabolism of eCBs. This system is thought to mediate several physiological functions by modulating the action of neurotransmitters and neuropeptides, and to regulate reinforcing effects of various substances of abuse including ethanol where there is a reward-supported pathogenic component. For instance, ethanol has been shown to increase brain eCBs and downregulate CB1 receptors (Alvarez-Jaimes et al., 2009a, & 2009b; Caille et al., 2007; Vinod et al., 2006, 2010, 2012). Hyperactivity of brain eCB system might confer a phenotype of high ethanol consumption (Hansson et al., 2007; Vinod et al., 2008b, 2012). Previous studies have also demonstrated the use of the CB1 receptor inverse agonist rimonabant for attenuating ethanol consumption (Colombo et al., 2005; Femenia et al., 2010; Vinod et al., 2008b, 2012; Wang et al., 2003). However, it was withdrawn from the market due to its psychiatric adverse side effect liabilities (Kirilly et al., 2012). Most recent studies revealed a potential use of AM4113, a novel CB1 receptor neutral antagonist as a safer alternative for treating tobacco and cannabis dependence (Gueye et al., 2016; Schindler et al., 2016). AM4113 was shown to exhibit all of rimonabant’s desirable properties and it was clearly differentiated in vivo based on its CNS effects, while showing no nausea-related behavior or depressive and anxiogenic-like effects (Gueye et al., 2016; Kangas et al., 2013; Kirilly et al., 2012; Salamone et al., 2007; Sink et al., 2008; Sink et al., 2010a & 2010b).
Although a significant progress has been made in understanding the neurobiology of alcoholism, the role of CB1 receptor and its mediated mechanism in binge ethanol drinking is not well understood at present. The aim of this study was to examine the effects of AM4113 on binge-like ethanol drinking and ethanol-induced accumbal dopamine (DA) release in C57BL/6J mouse model.
MATERIALS AND METHODS
Animals
All the experiments were conducted using adult male C57BL/6J mice (10–16 weeks old; Jackson Laboratory, USA). Mice were given unlimited access to standard mouse chow during the entire study. All procedures were conducted in accordance with the National Institutes of Health and Nathan Kline Institute Animal Care and Use Committee’s guidelines.
Binge-like ethanol consumption
The effect of systemic blockade of CB1 receptor function on binge-like ethanol consumption was assessed using a two-bottle choice drinking in the dark (DID) paradigm with minor modifications (Patkar et al., 2016; Rhodes et al., 2005; Thiele et al., 2004) as depicted in Figure 1. Mice were housed singly and habituated to reverse dark-light cycle for at least week. Two bottles containing water and 20% ethanol were offered 3 hours into the dark phase for 2 hours for five days a week (Monday to Friday) with two days of abstinence for 4 weeks. Mice were then injected with either AM4113 (1 and 3 mg/kg body wt, i.p.) or vehicle (4% DMSO and 1% Tween 80 in saline, i.p.) once daily for 4 days before the onset of dark cycle. Three hours after drug or vehicle injection, water bottles were replaced with bottles containing water and ethanol (20% v/v). Amounts of ethanol and water consumption were measured over a 2-hour period. The positions of water and ethanol bottles were alternated every day to avoid place preference. Two bottles containing water and 20% ethanol were placed on cage without mouse to control spillage and evaporation.
Figure 1.
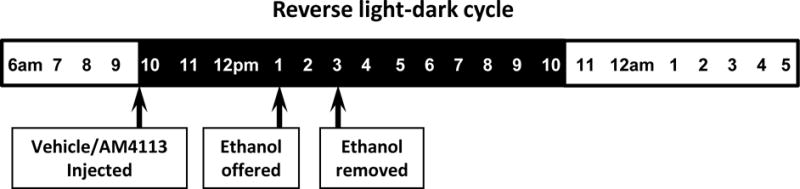
Drinking in the dark (DID) paradigm was used to model binge-like ethanol consumption in mice. Mice were habituated to reverse light-dark cycle for a week and offered two bottles containing water and 20% ethanol 3 hours into the dark phase for 2 hours for five days a week (Monday to Friday) with two days of abstinence.
Effect of AM4113 on locomotor activity and tastants preference
Mice were acclimatized to single housing a week prior to the tests. They were injected once daily with either AM4113 (1 and 3 mg/kg, i.p.) or vehicle (4% DMSO, 1% Tween 80 in saline, i.p.) for 4 days and were used either for assessing the locomotor activity in home cages or for taste preference test. Ambulatory activity (beams broken X-Y) was measured over 2 hours using an automated infrared beam-based system (ATM3; Columbus Instrument, Columbus, OH) in the dark-cycle 3 hours after injections for 4 days.
For the tastants preference test, mice were offered two bottles containing water and saccharin (0.06%) or quinine (0.06 mM) 3 hours into the dark cycle. The consumptions of water and tastants were measured for 2 hours every day for 4 days and preference for tastants was calculated. The positions of water and tastants bottles were alternated every day to avoid place preference. Data were corrected for the loss of liquid due to spillage and evaporation by comparing weights of water and tastants bottles in a control cage without a mouse.
Microdialysis
Ethanol-induced DA release in nucleus accumbens shell (NAcS) was measured by microdialysis. A guide cannula (CMA 7; CMA/Microdialysis, Sweden) was implanted unilaterally into the NAcS region (left hemisphere) of the mouse under isoflurane anesthesia. The coordinates were relative to Bregma for NAcS (AP: +1.5, L: +0.6, V:−4.0) according to the mouse brain atlas (Paxinos and Franklin, 2001). Mice were allowed to recover for a week before microdialysis experiment, which was carried out in awake, freely moving mice using a BAS BeeKeeper™ System (BAS Inc., USA). A microdialysis probe (0.5 mm × 1.0 mm membrane length, CMA 7; CMA/Microdialysis, Sweden) was inserted and after 2 hours wasting period, 4 × 20-min basal samples were collected. AM4113 (1 mg/kg, i.p.) was then injected 120 min before ethanol administration (1.5 g/kg, i.p.). Control mice were administered with vehicle (4% DMSO and 1% Tween 80 in saline). The dialysate samples were collected every 20 min at 1.5 μl/min flow rate. Following microdialysis, probe placements were verified by cresyl violet histology.
High-Performance Liquid Chromatography
Levels of DA were analyzed using a HPLC system with electrochemical detection (ESA CoulArray Detector Model 5600A, with Model 5014B microdialysis cell). The cell potentials were set at +250 mV and at −175 mV for reference. A microbore C18 150× 3.2 mm analytical column (ESA MD-150) and a mobile phase (75 mM sodium dihydrogen phosphate, 1.7 mM 1-octanesulfonic acid sodium salt, 100 μL triethylamine, 25 μM EDTA, and 10% acetonitrile, pH 3.0; Fisher Scientific, Hampton, OH) with a 0.6 mL/min flow rate gave an average elution time for DA at 4.4 min. The dialysate samples (30 μl), collected in 15 mM Na-EDTA and 10% ethanol mixtures, were injected with an autosampler (ESA Model 542) and analyzed for DA levels.
Blood Ethanol Concentration (BEC) Assay
Tail blood samples were collected from mice pretreated with vehicle or AM4113 (1 and 3 mg/kg, i.p.) 120 min prior to ethanol injection (1.5 g/kg., i.p.) to examine if AM4113 has any effect on ethanol metabolism. The trunk blood was collected to measure BEC in mice immediately after a 2-hour drinking session on the last day of AM4113 testing. Blood samples were collected in perchloric acid containing tubes at different time points (40, 80 and 120 min) following ethanol administration. Blood samples were centrifuged at 4°C for 2 min at 13,000 rpm and then plasma was used for BEC assay. Ethanol levels were measured spectrophotometrically using nicotinamide adenine dinucleotide (NAD) and alcohol dehydrogenase (ADH) as described previously (Vinod et al., 2006).
Pharmacokinetics of AM4113
We next conducted pharmacokinetic study of AM4113 to further understand it’s bioavailability. A group of mice were treated intraperitonially with a dose of 1 mg/kg of AM4113 and they were decapitated at various time points (10, 20, 40, 60, 90, 180, 300 and 420 min) under deep anesthesia (isoflurane). Brain and trunk blood were collected for the analysis of AM4113 using liquid chromatography-mass spectrometry (LC-MS) in the positive APCI mode. Following the addition of an internal standard (rimonabant) to the plasma (0.2 ml) or brain homogenate (0.5 ml), AM4113 and the internal standard were extracted by the addition of 0.5 ml of carbonate buffer (pH 9.6) and 3.0 ml of 3% isopropanol in hexane. After a gentle mix for 10 min, the samples were centrifuged at 2000 rpm. The organic phase was taken and evaporated to dryness in a vacuum centrifuge. The residue was then reconstituted with 150 μl of methanol:water (1:1). The extract was injected onto a C-18 column (XTerra MS, 3.0 × 100 mm, 3.5μ, Waters Corp., MA), eluted with an isocratic mobile phase (0.1% formic acid:methanol:acetonitrile [30:35:35]) at a flow rate of 0.5 ml/min, affording a retention time of 5.1 and 9.7 min for AM4113 and rimonabant, respectively. Both AM4113 and rimonabant were detected at their molecular ions. Each analysis was preceded with an eight-point linear calibration curve from 1000 to 7.5 ng/ml.
Statistical analyses
Statistical analyses were performed using Prism software (GraphPad, San Diego, CA) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The group differences in ethanol drinking, ambulatory activity, tastants preference, BEC and DA levels were analyzed using two-way mixed ANOVA followed by Bonferroni post-hoc comparison test when appropriate. The data on body weight and pharmacokinetics were evaluated by one-way ANOVA. Differences were considered statistically significant at p<0.05. All values are presented as mean±SEM.
RESULTS
Effect of AM4113 on binge-like ethanol drinking behavior
The effect of daily treatment of AM4113 (1 and 3 mg/kg, i.p.) on binge-like ethanol consumption and preference was examined for four days. Two-way mixed ANOVA performed on the last cycle of the ethanol drinking data revealed significant main effects of AM4113 [F(2,28)=27.06; p<0.0001], day of treatment [F(3,84)=66.55; p<0.0001] and interaction between AM4113 X day of treatment [F(6,84)=2.97; p=0.011] on ethanol consumption (Fig 2A). Bonferroni post-hoc comparison test revealed a dose-dependent suppression of ethanol consumption. AM4113 significantly reduced ethanol intake at dose 1 mg/kg (n=10) on day 1 (t4.58= −1.19; ***p<0.0001) and day 2 (t2.88= −0.75; *p<0.05) but not on day 3 (t2.08= −0.54) and day 4 (t1.28 = −0.335) compared to the vehicle treated mice (n=10). A robust suppressing effect of AM4113 at dose 3 mg/kg (n=11) was observed on day 1 (t7.11= −1.81; ***p<0.0001), day 2 (t5.56= −1.41; ***p<0.0001), day 3 (t2.82= −0.72; *p<0.05) and day 4 (t2.91= −0.74; *p<0.05) compared to the vehicle treated mice (n=10). There were also overall effects of AM4113 on ethanol preference [F(2,24)=27.51; p<0.0001], day of treatment [F(3,84)=72.0; p<0.0001] and interaction between AM4113 X day of treatment [F(6,84)=4.36; p=0.0007] on ethanol preference (Fig 2B). Bonferroni post-hoc test showed a dose-dependent suppression of ethanol preference. AM4113 at dose 3 mg/kg (n=11) was more effective in reducing the ethanol preference on day 1 (t7.26= −0.579; ***p<0.0001), day 2 (t7.35= −0.586; ***p<0.0001), day 3 (t3.87= −0.309; *p<0.01) and day 4 (t3.45 = −0.275; *p<0.01). However, 1 mg/kg dose (n=10) significantly reduced ethanol preference only on day 1 (t5.08 = −0.415; ***p<0.0001) day 2 (t4.17= −0.341; **p<0.001) but not on day 3 (t1.58= −0.129) and day 4 (t1.76 = −0.144) relative to the vehicle treated mice (n=10).
Figure 2.
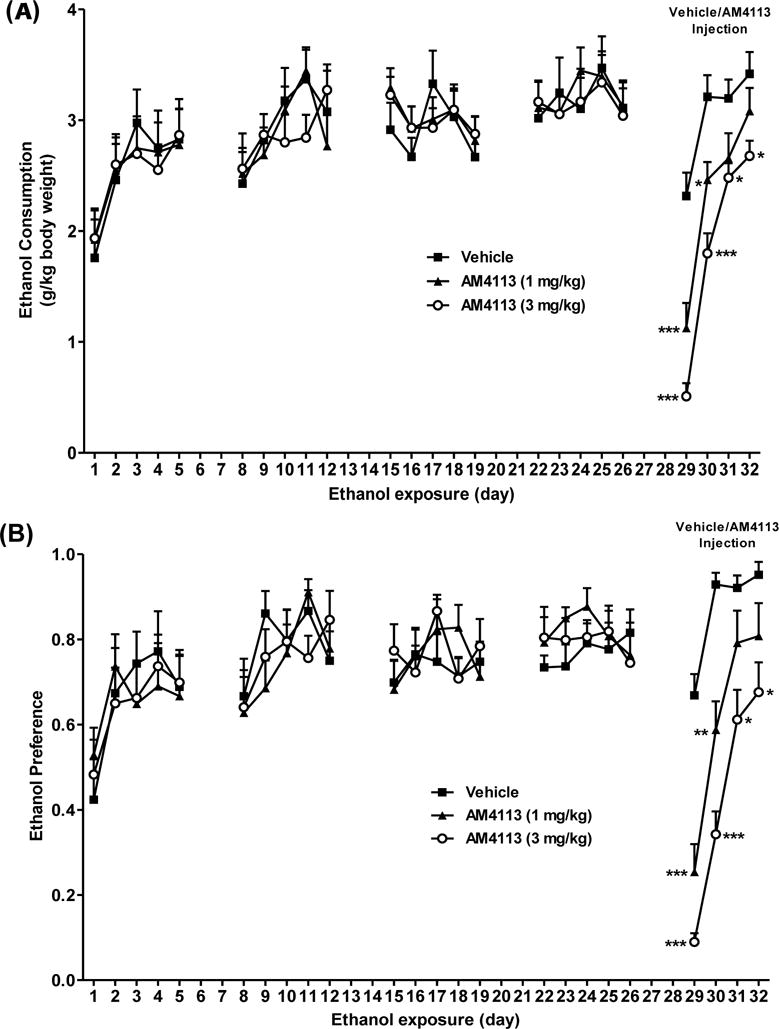
Effect of AM4113 on ethanol consumption and preference was examined using a two-bottle choice paradigm. AM4113 significantly suppressed ethanol consumption at dose 1 mg/kg on day 1 (***p<0.0001) and 2 (*p<0.05), and at dose 3 mg/kg on day 1 (***p<0.0001), day 2(***p<0.0001), day 3 (*p<0.05) and day 4 (*p<0.05). A marked reduction in ethanol preference was observed at dose 1 mg/kg on day 1 (***p<0.0001) and 2 (*p<0.05), and at dose 3 mg/kg on day 1 (***p<0.0001), day 2 (***p<0.0001), day 3 (*p<0.05) and day 4 (*p<0.05). Data are presented as mean ± SEM. (p values are relative to the vehicle treated group (Vehicle = 10; 1 mg/kg =10; 3 mg/kg = 11); Two-way mixed ANOVA with Bonferroni post-hoc test).
Effect of AM4113 on body weight, tastants preference and locomotor activity
The body weights of mice were measured before and after 4 days of AM4113 treatment. One-way ANOVA showed no significant effect of AM4113 on body weight compared to the vehicle group [F(2,15)=0.06; p=0.94; n=6 per group; Fig. 3A]. Two-way mixed ANOVA revealed no significant effect of AM4113 on ambulatory activity relative to the vehicle treated group [F(2,12)=0.32; p=0.73; n=5 per group; Fig. 3B]. Treatment of AM4113 also did not show any significant differences on preference for saccharin [F(2,15)=1.79; p=0.20; n=6 per group] or quinine [F(2,15)=0.34; p=0.71; n=6 per group] compared to the vehicle-treated group (Fig. 3C; n=6 per group).
Figure 3.
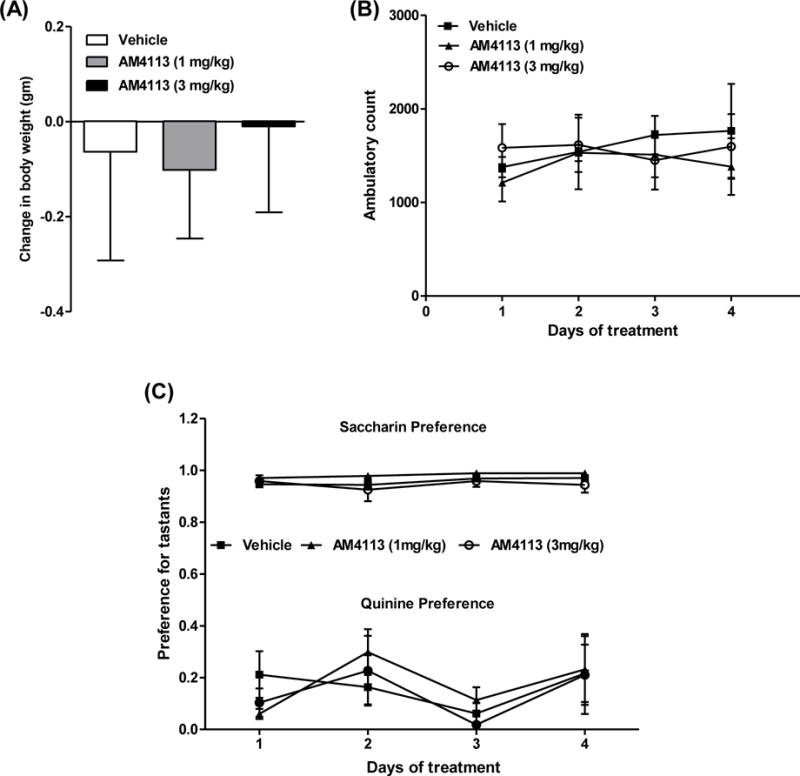
Effect of AM4113 treatment on the body weight, ambulatory activity and preference for tastants. The body weight, measured before and 24 hour after the last injections, was presented as change in body weight. Home cage ambulatory activity (beams broken X-Y) and preference for tastants were measured for 2 hour in the dark-cycle 3 hour after AM4113 and vehicle injections. Treatment of AM4113 for 4 days did not significantly alter (A) body weight [F(2,15) = 0.06; p=0.94; n=6 per group), (B) ambulatory activity [F(2,12) = 0.32; p=0.73; n = 5 per group] and (C) preference for saccharin [F(2,15) = 1.79; p=0.20; n=6 per group] or quinine [F(2,15) = 0.34; p = 0.71; n=6 per group] compared to their respective vehicle treated mice (n = 6 per group). Data are presented as mean ± SEM.
Effect of AM4113 on ethanol-induced DA release in NAcS
The effect of AM4113 on ethanol-induced DA release was examined in NAcS. Ethanol (1.5 g/kg, i.p.) was administered 120 min after the injections of either vehicle (n=5) or AM4113 (1 mg/kg, i.p.; n=8). Ethanol (1.5 g/kg) treatment markedly increased extracellular DA in NAcS after 20 min (t51= −3.49; **p<0.001), 40 min (t51=−5.62; ***p<0.0001) and 60 min (t51= −2.09; *p<0.05) post-injections compared to the vehicle treated group (A). Two-way mixed ANOVA with factors of time and treatment revealed a significant effect of AM4113 on DA levels at 40 min post-injection of ethanol (240th min time point) relative to the vehicle treated group (t51 =3.43; #p=0.0012; A). No significant effect of treatment of AM4113 itself on DA levels was observed (t51= −0.32; p=0.75). The placements of microdialysis probes in NAcS were confirmed by Nissl staining (Fig. 4B).
Figure 4.
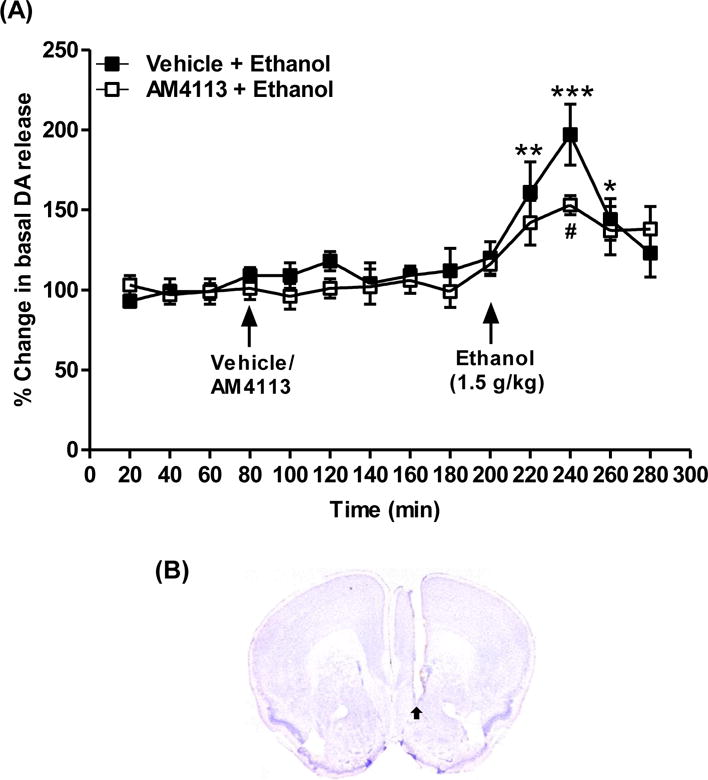
Microdialysis studies were conducted to examine the effect of AM4113 on ethanol-induced on dopamine (DA) release in nucleus accumbens shell (NAcS). Ethanol (1.5 g/kg, i.p.) was administered 120 min after the injections of either vehicle (n=5) or AM4113 (1 mg/kg, i.p.; n=8). Ethanol significantly increased DA release at 20 min (**p<0.001), 40 min (***p<0.0001) and 60 min (*p<0.05) post-injections compared to vehicle treated group (A). Ethanol-induced DA level was suppressed by AM4113 treatment after 40 min of ethanol injection (#p=0.0012; A). The placements of microdialysis probes were verified histologically. A representative image with arrow indicate a probe placement in NAcS (B). Data are presented as mean ± SEM.
Blood ethanol concentration
The BEC was 1.29 ± 0.12 mg/ml (n=5) in the vehicle treated mice at the end of a 2-hour ethanol drinking session on the 4th day of AM4113 treatment. To examine if AM4113 has any effect on ethanol metabolism, the BEC was measured at 40, 80 and 120 min following administration of a single dose of ethanol (1.5 g/kg., i.p.). The BEC was 1.51 ± 0.05 mg/ml at 40 min post-injection of ethanol. Two-way mixed ANOVA showed a significant effect of time on BEC [F(2,24)=221.9; p<0.0001; Fig. 5]. However, no significant effects of either AM4113 treatment [F(2,12)=0.17; p=0.84] or interaction between time and AM4113 treatment [F(4,24)= 0.80; p=0.53] were observed relative to the vehicle treated group (n=5 per group; Fig. 5).
Figure 5.
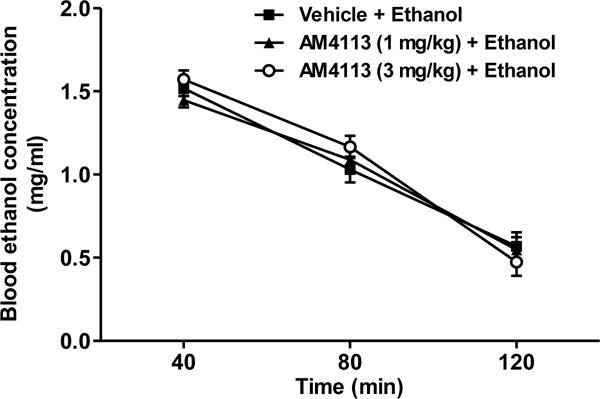
Effect of AM4113 treatment on ethanol metabolism. Blood ethanol concentration (BEC) was measured at 40, 80 and 120 min following ethanol administration (1.5 g/kg., i.p.). There was a significant effect of time on BEC [F(2,24) =2 21.9; p<0.0001]. However, no significant effects of either AM4113 treatment [F(2,12) = 0.17; p = 0.84] or interaction between time and AM4113 treatment [F(4,24)= 0.80; p = 0.53] were observed compared to vehicle treated group. Data are presented as mean ± SEM (n = 5 per group).
Pharmacokinetics of AM4113
A maximum concentration, time to reach maximum concentration and elimination half-life of AM4113 were directly obtained from the data. A maximum concentration of AM4113 in plasma was achieved (159.2 ± 10.1 ng/ml) after 20 min post-injection when dosed intraperitonially at 1 mg/kg (Fig. 6A). One-way ANOVA indicated a time dependent decrease in concentration [F(7,27)=178.1; ***p<0.0001 compared to 40 min [n=5], 60 min [n=5], 90 min [n=5], 180 min [n=5], 300 min [n=4] and 420 min [n=3] post-injections; Fig. 6A]. A peak concentration of AM4113 in brain was detected (91.9 ± 6.9 ng/gm) in 60 min of post-injection [F(7,28)=20.14; ***p<0.0001 compared to 10 [n=4] and 20 min [n=5] time points; Fig. 6B). The brain to plasma ratios for AM4113 were ~0.39 and 1.45 at peak levels of plasma and brain, respectively. The elimination half-life of AM4113 was relatively faster in blood (~ 1 hour) than in brain.
Figure 6.
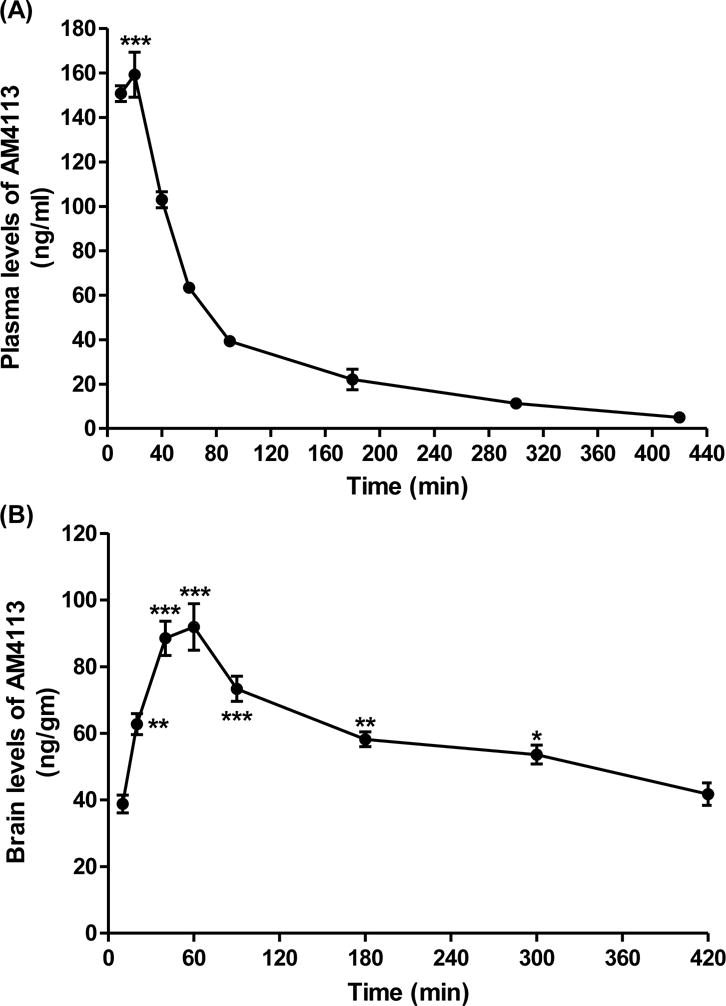
The concentrations of AM4113 in brain and plasma were analyzed by liquid chromatography-mass spectrometry (LC-MS) following intraperitonial injection of mice at dose of 1 mg/kg. Plasma (A; ***p<0.0001 at 20 min [n=4] compared to 40 min [n=5], 60 min [n=5], 90 min [n=5], 180 min [n=5], 300 min [n=4] and 420 min [n=3] post-injection time points) and brain (B; *p<0.05; **p<0.001;***p<0.0001 compared to 10 min post-injection time point) concentrations of AM4113 were significantly higher at 20 min and 60 min, respectively.
DISCUSSION
The present study revealed that a novel CB1 receptor neutral antagonist, AM4113 suppresses binge-like ethanol consumption and preference for ethanol in C57BL/6J mice. Our studies also showed no significant effect of AM4113 on preference for saccharin (sweet) or quinine (bitter), locomotor activity and ethanol metabolism at the doses studied. These results indicate that the effect of AM4113 on ethanol consumption is not due to the alterations in taste reactivity, ambulation and ethanol metabolism but rather likely due to the functional suppression of CB1 receptor signaling in reward-related processes.
Drugs of abuse produce rewarding effects during the acute-phase followed by neurochemical alterations and adaptations in many signaling molecules, which lead to chronic compulsive drug seeking behavior. The motivational effect of ethanol is likely mediated by several reward related brain circuitries. For instance, ethanol has been shown to increase DA levels in nucleus accumbens and activation of accumbal DAergic system appears to mediate some of its effects (Cheer et al., 2007; Di Chiara and Bassareo., 2007; Siciliano et al., 2016). Since AM4113 suppressed ethanol drinking, we next examined if AM4113 modulates ethanol-induced DA release in NAc to further understand the functional consequence of CB1 receptor blockade on DA signaling. An acute ethanol injection significantly increased DA release in NAcS and this effect was suppressed by AM4113 pretreatment. A dose of 1.5 g/kg of ethanol was chosen in this study as it has been shown to alter reward-related behavior (e.g. conditioned place preference) and DA release (Zumlinski et al., 2007; Katner and Weiss, 2001; Kelly et al., 1997; Hungund et al., 2003; Siciliano et al., 2016). This dose yielded BEC of about 0.15% at 40 post-injection at which DA levels significantly increase in nucleus accumbens. We also observed a quite similar BEC (0.129%) in mice following 2-hour drinking session.
Blockade of CB1 receptors specifically in NAc and ventral tegmental area (VTA) has been shown to reduce ethanol self-administration (Alvarez-Jimes et al., 2006; Caille et al., 2007). A previous study showed that the eCB, anandamide and its non-hydrolyzable analog methandamide increase DA release in NAc of rats and this effect was reversed by the CB1 receptor inverse agonist rimonabant, suggesting a critical role for eCB-CB1 mediated regulation of DA release in NAc (Solinas et al., 2006). Most recent studies have also suggested the potential use of AM4113 for treating tobacco dependence (Gueye et al., 2016; Schindler et al., 2016). The suppressing effect of AM4113 on nicotine seeking behavior is associated with reduction in firing and burst rates of DA neurons in VTA (Gueye et al., 2016). Taken together, these studies suggest a functional interaction between eCB and DA systems in mediating the reinforcing effects of drugs of abuse including ethanol.
Previous studies have shown the suppressing effects of the CB1 receptor inverse agonists/antagonists on preference and consumption of ethanol (10% and 12%) in rodent models (Parsons and Hurd, 2015; Vinod and Hungund, 2006; Vinod et al., 2008b; Vinod et al, 2012; Hungund et al., 2003; Wang et al., 2003). However, rimonabant was shown to exert certain psychiatric side-effects that could be attributed to its inverse agonistic activity at the CB1 receptor and its antagonistic activity at the opioid receptor (Kangas et al., 2013; Kirilly et al., 2012). On the contrary, CB1 neutral antagonists such as AM4113 are devoid of intrinsic activity at the cellular level as seen in the cAMP assay, and they suppress the efficacy of eCBs by inhibiting their binding to CB1 receptors. Another explanation for the adverse side effects of rimonabant might be due to its slower pharmacokinetic clearance. The half-life of rimonabant in human is 1–2 weeks depending on body fat content and its functional in vivo half-life in rats is ~15 hours (Turpault et al. 2006; US FDA briefing document, 2007; McLaughlin et al. 2003). We next conducted pharmacokinetic studies to examine the concentrations of AM4113 in brain and plasma and to correlate this with ethanol drinking data. The results indicated a rapid absorbance and elimination of AM4113 in plasma than in brain when dosed at 1 mg/kg intraperitonially. An elimination half-life of AM4113 in plasma was approximately 1 hour while it was about 6 hours in the brain. Interestingly, the elimination half-life was unchanged for a longer period of time in the brain. This could be associated with a sustained suppressing effect of AM4113 on binge-drinking due to a longer duration of action. The rational for pretreating the mice with AM4113 (in light phase) 3 hours prior to the ethanol drinking test was to avoid stress-related effects due to handling, restraining and intraperitonial injections of mice during their active (dark) phase and to determine if this dosing regimen is effective even after few hours of AM4113 administration. While peak concentration of AM4113 was achieved at 60 min post-injection in the brain, the effect of AM4113 could be more robust on binge-drinking and DA release at around this time. One of the limitations of our study is that during microdialysis experiments mice were pretreated with AM4113 two hours prior to the ethanol injection instead of three hours. However, AM4113 could possibly suppress ethanol-induced DA release even after 3-hour pretreatment considering its long unchanged elimination half-life.
Ethanol preferring C57BL/6J mice were utilized in this study to achieve high ethanol consumption instead of non-preferring outbred mice. Mice in the vehicle treated group consumed an average of 3.41 g/kg of pure ethanol and achieved pharmacologically relevant BEC of about 0.129% in a 2-hour drinking session on the 4th day of the 5th week. The neuronal mechanism/s of high drinking phenotype is not clearly understood at present. While direct and indirect agonists of CB1 receptors have been shown to enhance ethanol drinking behavior (Blednov et al., 2007; Colombo et al., 2005; Hansson et al., 2007; Vinod et al., 2008a, 2008b, 2012), the presence of higher levels of CB1 receptor-stimulated G-protein coupling in the striatum of C57BL/6J mice than in ethanol non-preferring DBA/2J mice (Vinod et al., 2008b) might be of one the contributing factors for excessive ethanol drinking phenotype. The effect of binge-like ethanol drinking on the endocannabinoid system is currently not understood. However, previous studies have shown downregulation of CB1 receptors by forced (e.g. chronic ethanol vapor exposure and chronic intermittent exposure) and chronic voluntary ethanol (10% or 12%) consumption (Mitrirattanakul et al., 2006; Pava and Woodward, 2012, Robinson et al., 2006; Vinod et al., 2006, 2010, 2012). Future studies are needed to examine the effects of AM4113 and its withdrawal on different stages of AUD.
Unlike rimonabant and other CB1 receptor inverse agonists, the CB1 neutral antagonists such as AM4113 do not elicit depressive- and anxiogenic-like effects in rats (Gueye et al., 2016; Sink et al., 2010a & 2010b). Additionally, they do not produce nausea or induce conditioned gaping, a marker of nausea, or vomiting in animal models (Limebeer et al., 2010; Salamone et al., 2007; Sink et al., 2008). These studies point to a more favorable pharmacological profile for CB1 neutral antagonists compared to inverse agonists for treating binge ethanol drinking behavior. In summary, our findings suggest an important role of CB1 receptor-mediated signaling in binge alcohol drinking and in modulation of mesolimbic DAergic signaling. These studies also point to a potential utility of CB1 receptor neutral antagonists as an alternative and a new class of medication for treating AUD.
Highlights.
A novel CB1 receptor neutral antagonist, AM4113 suppresses binge-like ethanol drinking.
AM4113 reduces ethanol-induced increase in dopamine release in nucleus accumbens.
AM4113 is rapidly absorbed and eliminated in plasma than in brain.
AM4113 might be useful in the treatment of binge ethanol drinking.
Acknowledgments
This study is supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA-NIH) grants AA021662 (SCP and VKY) and AA018709 (VKY). SCP is also supported by the Department of Veterans Affairs (Senior Research Career Scientist Award). We dedicate this research work to the memory of Dr. Loren (Larry) H. Parsons (1964–2016) for his seminal contributions in the field of endocannabinoid system and alcohol research. We are thankful to Dr. San Hang Lee for assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE/CONFLICT OF INTEREST
There are no conflits of interest to declare.
References
- Alvarez-Jaimes L, Parsons LH. Regional influence of CB1 receptor signaling on ethanol self-administration by rats. Open Neuropsychopharmacol J. 2009;2:77–85. doi: 10.2174/1876523800902020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacol. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: What dopamine does and doesn’t do. Current Opinions in Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Femenía T, García-Gutiérrez MS, Manzanares J. CB1 receptor blockade decreases ethanol intake and associated neurochemical changes in fawn-hooded rats. Alcohol Clin Exp Res. 2010;34:131–141. doi: 10.1111/j.1530-0277.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- Gueye AB, Pryslawsky Y, Trigo JM, Poulia N, Delis F, Antoniou K, Loureiro M, Laviolette SR, Vemuri K, Makriyannis A, Le Foll B. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss, with better psychiatric tolerability. Int J Neuropsychopharmacol. 2016 doi: 10.1093/ijnp/pyw068. e-pub ahead of print 7 Aug 2016. Pii: pyw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. The addicted brain: understanding the neurophysiological mechanisms of addictive disorders. Front Integr Neurosci. 2015;9:18. doi: 10.3389/fnint.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Bermúdez-Silva FJ, Malinen H, Hyytiä P, Sanchez-Vera I, Rimondini R, Rodriguez de Fonseca F, Kunos G, Sommer WH, Heilig M. Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacol. 2007;32:117–126. doi: 10.1038/sj.npp.1301034. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanolpreference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25:198–205. [PubMed] [Google Scholar]
- Kelley BM, Bandy AL, Middaugh LD. A study examining intravenous ethanol-conditioned place preference in C57BL/6J mice. Alcohol Clin Exp Res. 1997;21:1661–1666. [PubMed] [Google Scholar]
- Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, Shukla VG, Makriyannis A, Bergman J. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther. 2013;3:561–567. doi: 10.1124/jpet.112.201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly E, Gonda X, Bagdy G. CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol (Oxf) 2012;205:41–60. doi: 10.1111/j.1748-1716.2012.02402.x. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, Parker LA. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Hong KI, Piomelli D, Sinha R. An endocannabinoid signal associated with desire for alcohol is suppressed in recently abstinent alcoholics. Psychopharmacol. 2009;205:63–72. doi: 10.1007/s00213-009-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and foodreinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–67. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacol. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2016;10:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar OL, Belmer A, Tarren JR, Holgate JY, Bartlett SE. The effect of varenicline on binge-like ethanol consumption in mice is β4 nicotinic acetylcholine receptor-independent. Neurosci Lett. 2016;633:235–239. doi: 10.1016/j.neulet.2016.09.048. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson SL, Alexander NJ, Bluett RJ, Patel S, McCool BA. Acute and chronic ethanol exposure differentially regulate CB1 receptor function at glutamatergic synapses in the rat basolateral amygdala. Neuropharmacol. 2006;108:474–84. doi: 10.1016/j.neuropharm.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Redhi GH, Vemuri K, Makriyannis A, Le Foll B, Bergman J, Goldberg SR, Justinova Z. Blockade of Nicotine and Cannabinoid Reinforcement and Relapse by a Cannabinoid CB1-Receptor Neutral Antagonist AM4113 and Inverse Agonist Rimonabant in Squirrel Monkeys. Neuropsychopharmacol. 2016;41:2283–2293. doi: 10.1038/npp.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, Vemuri VK, Makriyannis A, Salamone JD. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: Comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol. 2010a;2:112–122. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Collins LE, Markus EJ, Vemuri VK, Makriyannis A, Salamone JD. The CB1 inverse agonist AM251, but not the CB1 antagonist AM4113, enhances retention of contextual fear conditioning in rats. Pharmacol Biochem Behav. 2010b;95:479–484. doi: 10.1016/j.pbb.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Peng Y, Olszewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacol. 2008;33:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, Helms CM, Grant KA, Jones SR. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacol. 2016;233:1435–1443. doi: 10.1007/s00213-016-4239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;2:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health (NSDUH) Table 6.89B-Binge alcohol use in the past month among persons aged 18 to 22, by college enrollment status and demographic characteristics: Percentages, 2013 and 2014 2014 [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL., II “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci. 2014;68:9.49.1–12. doi: 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpault S, Kanamaluru V, Lockwood GF, Bonnet D, Newton J. Rimonabant pharmacokinetics in healthy and obese subjects. Clin Pharmacol Ther. 2006;79:P50. [Google Scholar]
- US FDA briefing document. 2007 http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4306b1-fda-backgrounder.pdf.
- Vinod KY, Hungund BL. Cannabinoid-1 receptor: a novel target for the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2006;10:203–210. doi: 10.1517/14728222.10.2.203. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Maccioni P, Garcia-Gutierrez MS, Femenia T, Xie S, Carai MA, Manzanares J, Cooper TB, Hungund BL, Colombo G. Innate difference in the endocannabinoid signaling and its modulation by alcohol consumption in alcohol-preferring sP rats. Addict Biol. 2012;17:62–75. doi: 10.1111/j.1369-1600.2010.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008a;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Ramalhete R, Michaelides M, Thanos PK, Vadasz C, Cooper TB, Volkow ND, Hungund BL. Genetic and pharmacological manipulations of the CB1 receptor alter ethanol consumption and dependence in ethanol preferring and non-preferring mice. Synapse. 2008b;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchilli R, Xie S, Cooper R, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global status report on alcohol and health. 2014:XIII. 45. http://www.who.int/substance_abuse/publications/global_alcohol_report/msb_gsr_2014_1.pdf?ua=1.
- Zumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacol (Berl) 2007;190:415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]


